Abstract
Context:
Familial hyperaldosteronism type III (FH-III) is a rare and clinically heterogeneous condition, that can display mild as well as severe phenotypes. Point mutations in the KCNJ5 gene, affecting the ion selectivity of the inward rectifier K+ channel 4 (Kir3.4), underlie the molecular basis of FH-III.
Objective:
The objective of the study was to investigate the effects of a de novo germline KCNJ5 mutation.
Patients and Methods:
We describe the case of a girl who came to medical attention at the age of 2 years because of polydipsia, polyuria, and failure to thrive. The patient, affected by hypertension and hypokalemia, was diagnosed with primary aldosteronism on the basis of extremely high aldosterone levels and suppressed plasma renin activity. Genomic DNA was isolated and KCNJ5 sequenced. Human adrenocortical cells were used as an in vitro model for the functional characterization of the mutant channel.
Results:
KCNJ5 sequencing in the index case and her parents revealed a de novo p.Glu145Gln germline mutation. The substitution resulted in Na+-dependent depolarization of adrenal cells and increased intracellular calcium concentration, which activated the transcription of NR4A2 and, in turn, CYP11B2. Pharmacological studies revealed that the mutant channel was insensitive to tertiapin-Q and calcium-channel blocker verapamil.
Conclusions:
Herein we report the identification of a novel KCNJ5 germline mutation responsible for severe hyperaldosteronism that presented in infancy with symptoms of diabetes insipidus. The findings of this study further elucidate the etiology of FH-III and expand our knowledge of this rare condition.
Primary aldosteronism (PA) is the most frequent cause of secondary hypertension (1). In addition to the predominant sporadic forms of PA there are at least three distinct familial variants, named familial hyperaldosteronism (FH) types I, II, and III. FH-III is an autosomal-dominant disease clinically distinct from the first two familial forms (2) caused by heterozygous mutations in the KCNJ5 gene encoding the G protein–activated inward rectifier K+ channel 4 (Kir3.4) (3). So far, five germline mutations in the KCNJ5 gene have been identified and functionally characterized in patients with FH-III (4, 5). All these missense mutations are located near or within the conserved glycine-tyrosine-glycine motif of the selectivity filter of the channel. Here we report and characterize a germline missense mutation affecting another conserved residue located N-terminal to the GlyTyrGly signature sequence. This substitution, p.Glu145Gln, previously reported as somatic mutation in sporadic aldosterone producing adenomas (APAs) (6), was identified in a patient with severe hyperaldosteronism that presented in infancy. Interestingly, the importance of this p.Glu145Gln substitution for Kir3.4 function had been predicted and confirmed in vitro (7) a decade before the KCNJ5 mutations were linked to FH-III.
Materials and Methods
Detailed Materials and Methods are available on The Endocrine Society's Journals Online web site at http://press.endocrine.org/journal/jcem.
Case Report
The index case is a Caucasian girl, born to nonconsanguineous parents. At the age of 8 weeks poor weight gain was noticed. At the age of 8 months she displayed failure to thrive, polydipsia, and polyuria. At the age of 2 years, her height was 80 cm (SDS = −2.1); weight, 12 kg (SDS = −2.7); blood pressure (BP), 115/65 mm Hg (systolic BP > 99th height-corrected percentile for age). The urinary output was 3 L/d, urine-specific gravity ranged from 1000–1003, serum potassium was 2.3 mmol/L, sodium 140 mmol/L. PA was diagnosed on the basis of extremely elevated serum aldosterone (> 5000 pmol/L; normal range, 111–830 pmol/L) and suppressed plasma renin activity (PRA) (0.2 ng/ml/h; normal range, 0.98–4.18 ng/ml/h). Aldosterone remained elevated (> 5000 pmol/L) after dexamethasone suppression test (4 ug/d for 2 d). Treatment with spironolactone (50 mg/d) and potassium supplements were started.
At the age of 4 years no difference in the aldosterone/PRA ratio before (>2632) and after (>2849) orthostatic exercise was observed. Computed tomography and magnetic resonance imaging failed to reveal adrenal masses. At the age of 8 years, the patient was admitted to the Warren Magnuson Clinical Center (National Institutes of Health [NIH], Bethesda, Maryland). At admission she was off spironolactone for 2 weeks. Her height was 134.7 cm (75th percentile); weight, 34.4 kg (90th percentile); and pubertal stage Tanner B2P2. She was hypertensive (135/100 mm Hg, >99th height-corrected percentile for age) and serum potassium was 2.3 mmol/L. Serum createnine was 0.6 mg/dl (0.7–1.33 mg/dl). Serum aldosterone was 8365 pmol/L; PRA, 0.6 ng/ml/h; and serum 18OH-corticosterone, 27.0 nmol/L (0.2–2.3). Intravenous saline loading test confirmed nonsuppressibilty of aldosterone production (8815 pmol/L after saline infusion). A 2-day dexamethasone suppression test showed a decrease of serum aldosterone from 16 537–9086 pmol/L excluding glucocorticoid-remediable aldosteronism. Adrenal vein sampling was performed displaying bilateral aldosterone overproduction.
Subsequently, she has been treated with spironolactone (50–400 mg/d), potassium chloride and calcium-channel blockers. She remained hypertensive and hypokalemic (serum potassium 2.7–3.9 mmol/L). The patient underwent pubertal development starting at 11 years and progressed to Tanner stage B4P4 but no menarche occurred. Because of the low effectiveness of the therapy, bilateral adrenalectomy was recommended, but the family denied consent.
At the age of 19 years she was re-evaluated at NIH. She was on four antihypertensive medications and potassium supplements; serum creatinine was 2.19 mg/dl; potassium, 3.2 mmol/L; and urine protein/creatinine ratio, 2.5 mg/mg. Transthoracic echocardiography revealed mildly dilated aortic root and ascending aorta without left ventricular hypertrophy. The patient consented to bilateral adrenalectomy, which was performed laparoscopically (Supplemental Figure 1).
Identification of a novel KCNJ5 mutation
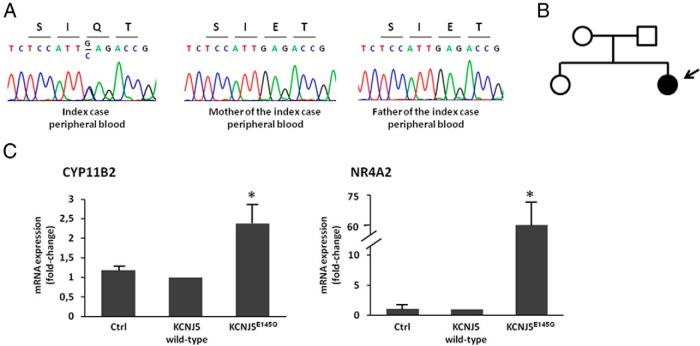
Sequencing of the KCNJ5 gene from peripheral blood DNA identified a novel germline heterozygous c.433G>C substitution (Figure 1A), which causes a glutamic acid to glutamine substitution at position 145 (p.Glu145Gln). The mutation was not detected in the parents (Figure 1, A and B), thereby indicating a de novo mutation in our patient. An amino acid alignment of Kir3.4 showed that Glu145 is highly conserved among orthologs and paralogs (Supplemental Figure 2).
Figure 1.
A, Sequencing of peripheral blood DNA showing the KCNJ5 c.433G>C substitution resulting in the p.Glu145Gln (p.E145Q) mutation in the index case, but not in the parents. B, Pedigree of kindred with germline KCNJ5 mutation; affected individual is shown as filled symbol. C, CYP11B2 and NR4A2 gene expression study in HAC15 adrenocortical cells overexpressing empty expression vectror (Ctrl) or human wild-type (WT) KCNJ5 (KCNJ5 WT) or KCNJ5 cDNA encoding the p.Glu145Gln substitution (KCNJ5E145Q). Gene expression levels were quantified by TaqMan real-time PCR using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as endogenous control. Each bar represents the mean ± SD of relative fold change (over KCNJ5 WT) of gene expression in four independent experiments. Each assay was performed in triplicate.*, P < .05 compared with KCNJ5 WT. CYP11B2 = cytochrome P450, family 11, subfamily B, polypeptide 2; NR4A2, nuclear receptor subfamily 4, group A, member 2.
Gene expression study in HAC15 cells
To define the effect of KCNJ5 c.433G>C substitution in adrenal cell function the mutant channel p.Glu145Gln was overexpressed in HAC15 cells and gene expression evaluated by real-time PCR. We observed a significant up-regulation of both CYP11B2 (2.4 ± 0.5 fold; P < .001) and its transcriptional regulator NR4A2 (60.8 ± 13–fold; P < .001) in Kir3.4 p.Glu145Gln–overexpressing cells, compared with cells expressing the wild-type (WT) protein (Figure 1C).
Na+-dependent depolarization of adrenal cells expressing Kir3.4 p.Glu145Gln
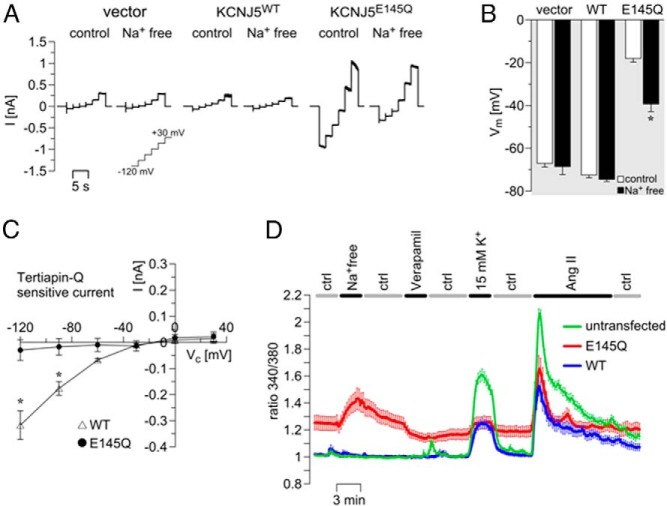
Whole-cell currents in NCI-H295R cells expressing WT Kir3.4 were very small and did not change after removal of Na+ from the extracellular solution (Figure 2A). In contrast, cells expressing Kir3.4 p.Glu145Gln showed larger inward and outward currents (Figure 2A) under control conditions compared with KCNJ5WT-transfected cells. The inward current was reduced under Na+-free conditions, demonstrating the Na+ permeability of the mutant channel. Accordingly, mutant channel–expressing cells were strongly depolarized under resting conditions, and hyperpolarized under Na+-free conditions (Figure 2B).
Figure 2.
The Kir3.4 p.Glu145Gln mutation induced a Na+ influx and increased intracellular Ca2+ levels in adrenal NCI-H295R cells. Representative whole-cell currents, A; and membrane voltage, B, of NCI-H295R cells transfected with empty vector (n = 5), WT KCNJ5 (WT, n = 10), or mutant KCNJ5 encoding the p.Glu145Gln substitution (E145Q, n = 16). Currents and voltage were measured under control (ctrl, normal Ringer solution) and Na+-free (using N-methyl-D-glucamine chloride, NMDG+, instead of NaCl) conditions. C, The tertiapin-Q (1μM) sensitive current of cells expressing the KCNJ5 WT (WT; n = 12) or mutant KCNJ5 encoding the p.Glu145Gln substitution (E145Q; n = 6) is shown. To stimulate currents in cells expressing the WT channel, the pipet solution was supplemented with Na+ (30mM) and GTP (0.5mM) and bath K+ was increased (50mM K+). D, Fura-2 Ca2+ measurements: Traces show mean values of 340 nm/380 nm ratios ± SEM as a measure of intracellular Ca2+ concentration (WT in black, E145Q in red, and untransfected cells in green; n = 17–26 per group) under control and Na+-free conditions. Verapamil (10μM) and angiotensin II (Ang II, 20nM) were applied in control solution. In the solution containing 15mM K+, the Na+ concentration was reduced to the same extent. *, P < .05 comparing B, control and Na+-free; or D, WT and Kir3.4- p.Glu145Gln-expressing cells.
Pharmacology of Kir3.4 p.Glu145Gln
Kir3.4 is activated by G protein–coupled receptors and by high intracellular Na+ concentrations (8). Accordingly, an inward current was activated in cells expressing WT Kir3.4 when 30 mM Na+ and 0.5mM GTP were present in the pipet solution, and when extracellular K+ was increased to 50mM. Under these conditions, the inward current in KCNJ5WT-transfected cells was inhibited by application of 1μM tertiapin-Q (9) to the bath (Figure 2C). The mutant p.Glu145Gln channel was insensitive to tertiapin-Q (Figure 2C). The L-type Ca2+-channel blocker verapamil, previously shown to inhibit other Kir3.4 mutants (10) had no significant effect on Kir3.4 p.Glu145Gln (81 ± 10% remaining inward current, P = .25, n = 10).
Kir3.4 p.Glu145Gln increased cytosolic Ca2+ levels in adrenal cells
Under control conditions, Ca2+ concentration was increased in NCI-H295R cells expressing the mutant channel, compared with cells expressing WT Kir3.4 (Figure 2D). In Na+-free extracellular solution (a condition where the Na+/Ca2+ exchanger [NCX] is inhibited or even works in reversed mode), Ca2+ concentration was further increased in cells expressing Kir3.4 p.Glu145Gln. Application of verapamil under control conditions led to a reduction of Ca2+ concentration in cells expressing the mutant, although Ca2+ was not normalized to WT levels (Figure 2D). Stimulation of cells with 15mM K+ increased Ca2+ in WT Kir3.4–expressing cells but had a minor effect on cells expressing the p.Glu145Gln channel, mainly because the mutant-expressing cells were already strongly depolarized under control conditions (Figure 2B). Calcium increase in WT Kir3.4–expressing cells was smaller than in untransfected cells, indicating that the WT channel was activated under these conditions, therefore reducing depolarization and subsequent activation of voltage-activated Ca2+ channels. The effect of stimulation with angiotensin II was similar in WT and mutant KCNJ5-transfected cells (Figure 2D).
Discussion
An important breakthrough in the understanding of the molecular mechanisms underlying autonomous aldosterone production in PA has been made recently by the discovery of germline and somatic mutations in genes that affecting calcium entry in adrenal zona glomerulosa cells (3, 11, 12). KCNJ5 gene mutations, which cause both FH-III and a consistent proportion of sporadic APAs (3, 4), impair the selectivity filter of the Kir3.4 K+ channel, leading to angiotensin II–independent depolarization of adrenal cells and constitutive aldosterone production.
Ion selectivity of all K+ channels (including Kir3.4) was shown to be dependent on the presence of specific amino acids within the P-loop domain (13). In addition to the GlyTyrGly motif, which coordinates dehydrated K+ ions as they move through the channel (14), further structural features of the channels are necessary to confer K+ selectivity (15). Yang et al (16) demonstrated that two oppositely charged conserved residues in Kir2.1, Glu138 and Arg148, could form a salt bridge and subsequently Dibb et al (7) mutated the equivalent glutamate residue in Kir3.4, Glu145, and confirmed that the p.Glu145Gln substitution resulted in loss of ion selectivity and inward rectification.
The p.Glu145Gln mutation was first reported by Åkerström et al (6) as a somatic mutation in two APAs. Herein we describe the first case of FH-III associated with this molecular defect and characterize the functional consequences of the mutation in adrenal cells. Electrophysiological studies using adrenocortical NCI-H295R cells revealed that loss of ion selectivity of the p.Glu145Gln-mutated channel led to cell membrane depolarization as observed in Xenopus oocytes (7). Ca2+ measurements in adrenocortical cells disclosed pathologically increased cytosolic Ca2+ levels, which were further increased upon removal of extracellular Na+. The latter Ca2+ increase was likely caused by the reversed mode of NCX that was promoted by Na+ loading of the cells. Besides the activation of voltage-gated Ca2+ channels and the Ca2+ influx through the mutated Kir3.4 channel itself (17), impaired NCX-mediated Ca2+ extrusion or even reversed transport mode could account for the severe phenotype of cells expressing mutant Kir3.4. Taken together, the effects of the p.Glu145Gln mutant expressed in adrenal cells were reminiscent of those reported for other, mostly somatic mutations of Kir3.4 (10, 18) Interestingly, expression of the Kir3.4 WT channel diminished the Ca2+ signal provoked by high K+, indicating a physiological function of Kir3.4 in limiting overwhelming aldosterone secretion rather than setting the baseline activity of glomerulosa cells.
In accordance with an increase in Ca2+ influx, expression of the mutated channel in HAC15 adrenal cells increased NR4A2 and CYP11B2 transcript levels compared with WT KCNJ5–expressing cells, supporting a mechanistic role for this mutation in the activation of aldosterone production.
Based on the limited number of cases reported to date, the clinical severity of FH-III varies significantly, ranging from early to late manifestations with mild to severe hypertension and different responsiveness to medical therapy (4, 5). So far it is not clear whether a genotype–phenotype correlation exists in FH-III. The patient described herein is one of the most severe cases reported so far. The girl presented with severe hyperaldosteronism in the first months of life with profound hypokalemia mimicking nephrogenic diabetes insipidus. Interestingly, despite the extreme hyperaldosteronism and hypokalemia, BP was only slightly elevated. This is in agreement with other reports of congenital mineralocorticoid excess in children, showing that overt hypertension is not uniformly present in younger patients (19, 20).
Treatment with large doses of spironolactone and potassium supplementation in our patient was only partially effective. Addition of calcium channel blockers did not result in a significant improvement and had no effect on plasma aldosterone concentration in agreement with the experimental data showing no effect of the L-type calcium-channel blocker verapamil on the mutated channel.
In conclusion, we report the identification of a novel KCNJ5 germline mutation responsible for severe hyperaldosteronism that presented in infancy with symptoms of diabetes insipidus. This p.Glu145Gln substitution, as for other KCNJ5 mutations, causes Na+-dependent cell membrane depolarization and disturbed intracellular Ca2+ homeostasis in adrenocortical cells. Indeed, the findings of this study further elucidate the etiology of FH-III and expand our knowledge of this rare condition.
Acknowledgments
We thank Christina Sterner (Medical Cell Biology, University of Regensburg, Germany) for her expert assistance. We acknowledge the support by the Intramural Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, and its staff (Elena Belyavskaya, MD; and Charalambos Lyssikatos, among others).
This work was supported by 2013 by a fellowship from the Società Italiana dell'Ipertensione Arteriosa to S.M.; P.M. is in receipt of a grant from the Italian Ministry of the Instruction, University and Research (grant ex-60%-2013); S.B. was supported by Deutsche Forschungsgemeinschaft (FOR 1086). This work was supported in part by Alfa-Endo Program of Charities Aid Foundation Russia funded by Alfa-Group and by the Intramural Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH), Bethesda, Maryland.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- APAs
- aldosterone producing adenomas
- BP
- blood pressure
- FH
- familial hyperaldosteronism
- NIH
- National Institutes of Health
- Kir3.4
- K+ channel 4
- NCX
- Na+/Ca2+ exchanger
- PA
- Primary aldosteronism
- PRA
- plasma renin activity
- WT
- wild type.
References
- 1. Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF, Jr., Montori VM. Case detection, diagnosis, and treatment of patients with primary aldosteronism: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. [DOI] [PubMed] [Google Scholar]
- 2. Geller DS, Zhang J, Wisgerhof MV, Shackleton C, Kashgarian M, Lifton RP. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 2008;93:3117–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi M, Scholl UI, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mulatero P, Monticone S, Rainey WE, Veglio F, Williams TA. Role of KCNJ5 in familial and sporadic primary aldosteronism. Nat Rev Endocrinol. 2013;9:104–112. [DOI] [PubMed] [Google Scholar]
- 5. Monticone S, Hattangady NG, Penton D, et al. A novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III. J Clin Endocrinol Metab. 2013;98:E1861–E1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Åkerström T, Crona J, Delgado Verdugo A, et al. Comprehensive re-sequencing of adrenal aldosterone producing lesions reveal three somatic mutations near the KCNJ5 potassium channel selectivity filter. PLoS One. 2012;7:e41926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dibb KM, Rose T, Makary SY, et al. Molecular basis of ion selectivity, block, and rectification of the inward rectifier Kir3.1/Kir3.4 K(+) channel. J Biol Chem. 2003;278:49537–49548. [DOI] [PubMed] [Google Scholar]
- 8. Yamada M, Inanobe A, Kurachi Y. G protein regulation of potassium ion channels. Pharmacol Rev. 1998;50:723–760. [PubMed] [Google Scholar]
- 9. Jin W, Klem AM, Lewis JH, Lu Z. Mechanisms of inward-rectifier K+ channel inhibition by tertiapin-Q. Biochemistry. 1999;38:14294–14301. [DOI] [PubMed] [Google Scholar]
- 10. Tauber P, Penton D, Stindl J, et al. Pharmacology and pathophysiology of mutated KCNJ5 found in adrenal aldosterone-producing adenomas. Endocrinology. 2014;155:1353–1362. [DOI] [PubMed] [Google Scholar]
- 11. Beuschlein F, Boulkroun S, Osswald A, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45:440–444, 444e1–2. [DOI] [PubMed] [Google Scholar]
- 12. Scholl UI, Goh G, Stölting G, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45:1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doyle DA, Morais Cabral J, et al. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. [DOI] [PubMed] [Google Scholar]
- 14. Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001;414:43–48. [DOI] [PubMed] [Google Scholar]
- 15. Korn SJ, Ikeda SR. Permeation selectivity by competition in a delayed rectifier potassium channel. Science. 1995;269:410–412. [DOI] [PubMed] [Google Scholar]
- 16. Yang J, Yu M, Jan YN, Jan LY. Stabilization of ion selectivity filter by pore loop ion pairs in an inwardly rectifying potassium channel. Proc Natl Acad Sci U S A. 1997;94:1568–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scholl UI, Nelson-Williams C, Yue P, et al. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc Natl Acad Sci U S A. 2012;109:2533–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuppusamy M. A Novel KCNJ5-insT149 Somatic Mutation Close to, but Outside, The Selectivity Filter Causes Resistant Hypertension by Loss of Selectivity for Potassium. J Clin Endocrinol Metab. 2014;99:E1765–E1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zachmann M, Tassinari D, Prader A. Clinical and biochemical variability of congenital adrenal hyperplasia due to 11 beta-hydroxylase deficiency. A study of 25 patients. J Clin Endocrinol Metab. 1983;56:222–229. [DOI] [PubMed] [Google Scholar]
- 20. Mulatero P, di Cella SM, Williams TA, et al. Glucocorticoid remediable aldosteronism: Low morbidity and mortality in a four-generation italian pedigree. J Clin Endocrinol Metab. 2002;87:3187–3191. [DOI] [PubMed] [Google Scholar]