Abstract
Background:
Acyl-ghrelin is a 28-amino acid peptide released from the stomach. Ghrelin O-acyl transferase (GOAT) attaches an 8-carbon medium-chain fatty acid (MCFA) (octanoate) to serine 3 of ghrelin. This acylation is necessary for the activity of ghrelin. Animal data suggest that MCFAs provide substrate for GOAT and an increase in nutritional octanoate increases acyl-ghrelin.
Objectives:
To address the question of the source of substrate for acylation, we studied whether the decline in ghrelin acylation during fasting is associated with a decline in circulating MCFAs.
Methods:
Eight healthy young men (aged 18–28 years, body mass index range, 20.6–26.2 kg/m2) had blood drawn every 10 minutes for acyl- and desacyl-ghrelin and every hour for free fatty acids (FFAs) during the last 24 hours of a 61.5-hour fast and during a fed day. FFAs were measured by a highly sensitive liquid chromatography-mass spectroscopy method. Acyl- and desacyl-ghrelin were measured in an in-house assay; the results were published previously. Ghrelin acylation was assessed by the ratio of acyl-ghrelin to total ghrelin.
Results:
With the exception of MCFAs C8 and C10, all other FFAs, the MCFAs (C6 and C12), and the long-chain fatty acids (C14–C18) significantly increased with fasting (P < .05). There was no significant association between the fold change in ghrelin acylation and circulating FFAs.
Conclusions:
These results suggest that changes in circulating MCFAs are not linked to the decline in ghrelin acylation during fasting and support the hypothesis that acylation of ghrelin depends at least partially on the availability of gastroluminal MCFAs or the regulation of GOAT activity.
Acyl-ghrelin is a 28-amino acid peptide released from the stomach (1, 2). The acylation of ghrelin is necessary for its orexigenic and GH-releasing effects (3). Ghrelin is acylated by ghrelin O-acyl transferase (GOAT), a polytopic membrane-bound enzyme, which is the only member of a family of 16 hydrophobic membrane-bound acyltransferases that is able to octanoylate ghrelin (4, 5). Studies in rodents and humans suggest that ghrelin acylation can be modified by nutritional status and that the availability of medium-chain fatty acids (MCFAs) is the rate-limiting step for the acylation (6). Therefore, ghrelin may act as an energy sensor to inform the central nervous system about the presence of lipid-rich food to optimize fat storage (6, 7). Previously, we have shown that extended fasting decreases circulating acyl-ghrelin levels by suppressing the fraction of ghrelin that is acylated (8). The mechanism for this decline in acylation is unclear. We tested the hypothesis that the decline in circulating acyl-ghrelin levels during fasting is accompanied by a decrease in circulating MCFAs.
Subjects and Methods
This study was approved by the institutional review board of the University of Virginia and the General Clinical Research Center. All volunteers gave written informed consent.
We studied eight young men with a mean (± SD) age of 24.8 ± 3.4 years (range, 18–28 years) and mean body mass index of 24 ± 2 kg/m2 (range, 20.6–26.2 kg/m2). Acyl-ghrelin results in the young men were reported previously (8) and are shown in Supplemental Figure 1 to demonstrate the effects of fasting.
Protocol
Fed admission
Volunteers were admitted for dinner and allowed to sleep after 9:00 pm. In the morning, 2 forearm indwelling venous cannulae were placed at 6:00 am. Starting at 8:00 am, blood was drawn every 10 minutes for 26.5 hours for acyl-ghrelin and every hour for free fatty acids (FFAs). Sampling ended at 10:30 am, 2.5 hours after breakfast (8:00 am) on the second day, however, only the FFA data from the 24-hour sampling period from 8:00 am to 8:00 am are reported here. Standardized meals (20% protein, 30% fat, and 50% carbohydrate) were served at 8:00 am, 1:00 pm, and 6:00 pm and 8:00 am (next day).
Fasting admission
The same procedures described for the fed admission were carried out, except that after the meal on the evening of admission, the subjects fasted (only water was permitted) for 59 hours, and blood sampling occurred between 37.5 and 61.5 hours. A breakfast was served 2.5 hours before the end of sampling (8:00 am).
Ghrelin sandwich assays
Samples were collected to preserve ghrelin acylation as described previously (8). Plasma acyl-ghrelin and desacyl-ghrelin were measured with in-house 2-site sandwich ELISAs specific for the full-length peptide (8).
FFA measurements
Serum FFA concentrations were measured by a liquid chromatography/mass spectrometry methodology developed for the quantitative determination of MCFAs (C6–C12) and long-chain fatty acids (LCFAs) (C1–C18). Acidified serum samples were extracted with ethyl acetate and separated using a reverse-phase HPLC column, coupled with a mass spectrometer operated in negative atmospheric pressure chemical ionization mode. Seven saturated fatty acids and 1 unsaturated (C16:1) fatty acid were detected using the selected ion monitoring technique and quantified in ratios to 5 stable isotope–labeled internal standards. Assays were performed at Eli Lilly Research Laboratories (by A.N.).
Statistical analysis
Unless otherwise stated, data are expressed as means ± SEM. For each 24-hour FFA time profile, the trapezoid rule was used to compute the area under the curve (AUC). AUC measurements were rescaled to the natural logarithmic scale and analyzed via a linear mixed-effects model with one source of variation (fed vs fast). The comparison of log(AUC) under the “fed” and the “fast” conditions represented a within-subject comparison of log(AUC).
A linear contrast of means was used to test the null hypothesis that the mean log(AUC) was the same under both study conditions. The criterion for rejection was a value of P ≤ .05. To obtain an estimate for the ratio of AUC geometric means, as well as a 95% confidence interval (CI) for the ratio of AUC geometric means, the logarithmic estimates were rescaled back to the original scale of measure. Correlation analyses were performed to assess the relationship between the fold changes with fasting in ghrelin acylation (assessed by the average of the mean acyl-ghrelin to total ghrelin ratio) and the fold changes in FFA.
The statistical software package SAS version 9.3 (SAS Institute Inc) was used to conduct most of the statistical analyses. The correlation analysis was performed in Excel 2007.
Results
Ghrelin
Fasting and fed acyl-ghrelin levels were published previously (8) and are shown in Supplemental Figure 1. Acyl-ghrelin levels were significantly decreased by 58% during the fasting admission and desacyl-ghrelin was increased by 19%, whereas the sum of acyl- and desacyl-ghrelin was not different. The percent acylation dropped by 9.9%.
FFAs
The 24-hour means for each FFA, and the results of the analyses of the effects of treatment are shown in Figure 1 and Supplemental Figure 2 and Table 1. During the long-term fasting condition, the AUC geometric mean of the MCFA C6 was increased by 17% (95% CI, 0.4%–30.6%; P = .046) and C12 was increased 36% (95% CI, 4%–58%; P = .034), whereas C8 and C10 were −1% (95% CI, −30% to 23%; P = .96) and −5% (−45% to 24%; P = .74) compared with those for the fed condition, respectively. The LCFAs C14, C16, C16:1, and C18 AUC geometric means were increased by 42% (95% CI, 15%–60%; P = .009), 48% (95% CI, 35%–57%; P < .001), 68% (95% CI, 55%–77%; P < .001), and 42% (95% CI, 29%–53%; P < .001), respectively.
Figure 1.
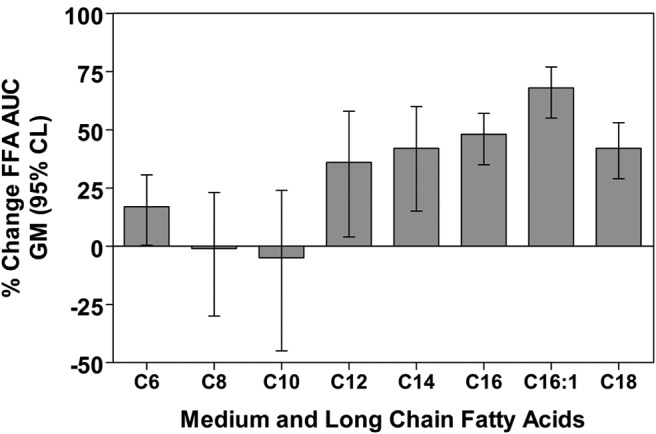
Bar graphs indicate percent changes in geometric means (GM) of FFA (MCFAs and LCFAs) 24-hour AUCs (± 95% confidence limits [CL]) in 8 healthy young adults during the last 24 hours of a 61.5 hours fast.
Table 1.
24-Hour Arithmetic FFA Concentrations
| FFA Concentration, μM |
||||||||
|---|---|---|---|---|---|---|---|---|
| C6 | C8 | C10 | C12 | C14 | C16 | C16:1 | C18 | |
| Fed | 4.34 ± 0.19 | 2.80 ± 0.09 | 2.58 ± 0.14 | 3.88 ± 0.26 | 42.7 ± 2.7 | 381.0 ± 13.2 | 17.3 ± 1.0 | 108.7 ± 3.0 |
| Fast | 5.19 ± 0.20 | 2.72 ± 0.08 | 2.28 ± 0.05 | 5.84 ± .17 | 68.9 ± 1.6 | 709.6 ± 14.7 | 48.9 ± 1.2 | 187.2 ± 3.6 |
| Pa | .046 | .95 | .74 | .034 | .009 | <.001 | <.001 | <.001 |
n = 7 for C6 and C14; n = 8 for all other FFAs during long-term fasting.
Statistical significance is for the fed to fast comparison of the geometric mean of the 24-hour FFA AUC.
Relationship between the FFA changes and the acyl-ghrelin/total ghrelin ratio
There was no significant association between the average fold change of the acyl-ghrelin/total ghrelin ratio between the fed and fasting admission and the fold change of the FFAs during the long-term fasting. In particular, the correlations between the changes in ghrelin acylation and the changes in different FFAs were as follows: C6, r = −0.004; C8, r = −0.133; C10, r = 0.063; C12, r = 0.152; C14, r = 0.238; C16:1, r = 0.433; C16, r = 0.220; and C18, r = 0.223. None of these correlations were significant.
Discussion
During long-term fasting, the C8 and C10 MCFAs, known to be used in the acylation of ghrelin, did not change in the circulation. In contrast, LCFAs (C14–C18) increased during fasting, with the greatest increase in C16:1. The increase in LCFAs is probably explained by the well-known rise in GH, a known lipolytic hormone, and by the decrease in insulin levels. We have previously reported that fasting decreases circulating acyl-ghrelin levels but not total ghrelin levels, suggesting that ghrelin acylation is inhibited without inhibition of ghrelin secretion (8). This decreased acylation during fasting could be due to either decreased availability of GOAT substrate (MCFAs) and/or decreased GOAT activity. Potential sources of the fatty acid substrate used by GOAT for ghrelin acylation include circulating MCFAs or MCFAs present in the gut lumen. The daily MCFA intake equals about 2% of the daily fat intake, and the specifics of MCFA absorption, hepatic portal transportation and carnitine-independent mitochondrial metabolism facilitate greater and faster oxidation of MCFAs than LCFAs, making MCFAs a more likely candidate to be a substrate for GOAT (9).
In this study, we found that the long-term fasting–induced decline in acyl-ghrelin levels is not associated with a decrease in 24-hour circulating MCFA levels. Based on unpublished observations from a separate study (manuscript in preparation), circulating acyl-ghrelin and FFA concentrations show similar changes during short-term fasting (37.5 hours fast). These observations suggest that the intraluminal availability of octanoate could be important for the acylation of ghrelin, whereas the circulating octanoate seems to play no role. The anatomy of a subtype of ghrelin-secreting cells in the duodenum, ileum, cecum, and colon supports the hypothesis that intraluminal octanoate is at least partially the substrate source for acyl-ghrelin (10). How the substrate for GOAT reaches the gastric ghrelin cells remains to be determined. For example, because microbiota of the lower gastrointestinal tract can produce MCFAs (11), it is possible that microbiota are involved in providing substrate for GOAT, either from carbohydrates or short-chain fatty acids. During long-term fasting, the substrate availability for the microbiota may decline resulting in a decrease in MCFA production in the gastrointestinal tract.
Another possible explanation is that fasting inhibits GOAT enzyme activity. Some studies show an increase in GOAT message, GOAT protein, and increased enzymatic activity in states of nutritional deficits and suppression by nutritional sufficiency (12–14). GOAT expression is highest in rodent stomach under ad libitum conditions, and it decreased significantly with fasting for 36 hours (15). Rodent studies support the concept that circulating acyl-ghrelin levels are at least in part dependent on nutritional availability of MCFAs (16). This observation is supported by studies of Nishi et al (17), who showed that the amount of C8 or C10 acyl-ghrelin content in the stomach depends on nutritional conditions. Ingestion of the medium-chain triglyceride, glycerol triheptanoate, which is not naturally synthesized by mammalian cells, resulted in the production of n-heptanoyl-ghrelin, which was found both in the circulation and in the stomach of the animals (16). These findings were confirmed by Kirchner et al (6) in genetic animal models with a specific loss (GOAT-null) or gain of GOAT function.
Based on the indirect nature of our study, we cannot rule out that the decline in ghrelin acylation is due to the inhibition of GOAT and not dependent on the availability of intraluminal MCFAs. In addition, we tested only men in our study, and we cannot exclude possible sex-specific effects in the regulation of MCFA substrate use of GOAT during fasting, albeit the role of estrogens in the regulation of ghrelin is controversial (18, 19).
Oral administration of MCFAs increases the concentration of acyl-ghrelin levels and the fraction of circulating ghrelin that is acylated in humans, supporting a role for intraluminal MCFAs as the rate-limiting step for the process of ghrelin acylation (20).
Our studies demonstrate that during long-term fasting, the FFAs either increase (C14 and higher) or remain unchanged, and changes in ghrelin acylation are not correlated with FFA changes. These results suggest that acylation of ghrelin is independent of circulating octanoate and may be regulated by the availability of luminal octanoate and/or the inhibition of GOAT during fasting.
Acknowledgments
We thank the University of Virginia Clinical Research Unit (CRU) nursing staff for their excellent support in the conduction of this study and the CRU Core Laboratory staff and our volunteers who made this work possible.
This work was supported in part by the National Institutes of Health Grants K23RR018770 (to R.N.), 1R01DK32632 and 1R01DK076037 (to M.O.T.), M01 RR00847 (General Clinical Research Center at the University of Virginia), and R01 DK082805 (to L.S.F.).
Disclosure Summary: A.N. is an employee of Eli Lilly. M.H. is chief scientific officer at MicroBiome Therapeutics LLC. M.O.T. is the founder and chief scientific officer of Ammonett Pharma, which will be developing MK-0677, a GH secretagogue receptor agonist; has received grant support from Novo Nordisk; and is an advisory group member for Pfizer, Ipsen, and Chaisma. The other authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- CI
- confidence interval
- FFA
- free fatty acid
- GOAT
- ghrelin O-acyl transferase
- LCFA
- long-chain fatty acid
- MCFA
- medium-chain fatty acid.
References
- 1. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. [DOI] [PubMed] [Google Scholar]
- 2. Tschoep M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. [DOI] [PubMed] [Google Scholar]
- 3. Sun Y, Wang P, Zheng H, Smith RG. 2004 Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA. 2004;101:4679–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. [DOI] [PubMed] [Google Scholar]
- 5. Gutierrez JA, Solenberg PJ, Perkins DR, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105:6320–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kirchner H, Gutierrez JA, Solenberg PJ, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15:741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al Massadi O, Tschop MH, Tong J. Ghrelin acylation and metabolic control. Peptides. 2011;32:2301–2308. [DOI] [PubMed] [Google Scholar]
- 8. Liu J, Prudom CE, Nass R, et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab. 2008;93:1980–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikeda Y, Okamura-Ikeda K, Tanaka K. Purification and characterization of short-chain, medium-chain, and long-chain acyl-CoA dehydrogenases from rat liver mitochondria. Isolation of the holo- and apoenzymes and conversion of the apoenzyme to the holoenzyme. J Biol Chem. 1985;260:1311–1325. [PubMed] [Google Scholar]
- 10. Sakata I, Sakai T. Ghrelin cells in the gastrointestinal tract. Int J Pept. 2010;2010:945056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saerens SM, Verstrepen KJ, Van Laere SD, et al. The Saccharomyces cerevisiae EHT1 and EEB1 genes encode novel enzymes with medium-chain fatty acid ethyl ester synthesis and hydrolysis capacity. J Biol Chem. 2006;281:4446–4456. [DOI] [PubMed] [Google Scholar]
- 12. Gahete MD, Cordoba-Chacon J, Salvatori R, Castano JP, Kineman RD, Luque RM. Metabolic regulation of ghrelin O-acyl transferase (GOAT) expression in the mouse hypothalamus, pituitary, and stomach. Mol Cell Endocrinol. 2010;317:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gonzalez CR, Vazquez MJ, Lopez M, Dieguez C. Influence of chronic undernutrition and leptin on GOAT mRNA levels in rat stomach mucosa. J Mol Endocrinol. 2008;41:415–421. [DOI] [PubMed] [Google Scholar]
- 14. Xu G, Li Y, An W, et al. Gastric mammalian target of rapamycin signaling regulates ghrelin production and food intake. Endocrinology. 2009;150:3637–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reimer RA, Maurer AD, Lau DC, Auer RN. Long-term dietary restriction influences plasma ghrelin and GOAT mRNA level in rats. Physiol Behav. 2010;99:605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nishi Y, Hiejima H, Hosoda H, et al. Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology. 2005;146:2255–2264. [DOI] [PubMed] [Google Scholar]
- 17. Nishi Y, Mifune H, Yabuki A, et al. Changes in subcellular distribution of n-octanoyl or n-decanoyl ghrelin in ghrelin-producing cells. Front Endocrinol (Lausanne). 2013;4:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kellokoski E, Poykko SM, Karjalainen AH, et al. Estrogen replacement therapy increases plasma ghrelin levels. J Clin Endocrinol Metab. 2005;90:2954–2963. [DOI] [PubMed] [Google Scholar]
- 19. Dafopoulos K, Chalvatzas N, Kosmas G, Kallitsaris A, Pournaras S, Messinis IE. The effect of estrogens on plasma ghrelin concentrations in women. J Endocrinol Invest. 2010;33:109–112. [DOI] [PubMed] [Google Scholar]
- 20. Ashitani J, Matsumoto N, Nakazato M. Effect of octanoic acid-rich formula on plasma ghrelin levels in cachectic patients with chronic respiratory disease. Nutr J. 2009;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]


