Abstract
Context:
Polycystic ovary syndrome (PCOS) is an anovulatory disorder characterized by excess androgen production and increased LH secretion. Serum anti-Mullerian hormone (AMH) is also elevated in this disorder. Women with PCOS exhibit a positive correlation between AMH and LH levels and recent in vitro data demonstrate that LH can directly stimulate AMH production by granulosa cells from women with PCOS.
Objective:
The objective of the study was to directly test whether LH increases AMH production in women with PCOS in vivo by assessing responses after recombinant human chorionic gonadotropin (r-hCG) stimulation.
Design:
This was a prospective study.
Setting:
The study was conducted at a research center at an academic medical center.
Participants:
Women with PCOS (n = 28) and normal controls (n = 29) participated in the study.
Interventions:
Blood samples were obtained before and 24 hours after iv administration of 25 μg r-hCG.
Main Outcome Measures:
Basal and stimulated serum AMH, androstenedione, T, and 17-hydroxyprogesterone levels were measured.
Results:
Baseline AMH levels in women with PCOS were greater than in normal controls and correlated with levels of LH as well as androstenedione, T, and 17-hydroxyprogesterone. A rise of serum AMH levels was not observed after r-hCG administration in women with PCOS or normal ovaries.
Conclusion:
These findings are in contrast to in vitro evidence demonstrating that AMH secretion by granulosa cells of PCOS women in response to LH stimulation and suggest AMH regulation in vivo is complex and that the elevated serum AMH in women with PCOS is not a direct effect of the excess LH production characteristic of PCOS.
It has been well documented that women with polycystic ovary syndrome (PCOS) have elevated serum levels of anti-Mullerian hormone (AMH) (1–11). AMH is a member of the TGF-β superfamily of peptides and is produced by granulosa cells (GCs) of growing preantral and early antral follicles. Several studies have shown that AMH levels are strongly correlated with antral follicle number. Accordingly, increased circulating AMH in women with PCOS has generally been attributed to high antral follicle numbers associated with this disorder.
Despite these observations, the precise mechanism of increased AMH production in women with PCOS remains unknown. AMH levels in women with PCOS have generally been found to correlate positively with circulating androstenedione (A4) and T levels and anovulation and negatively with serum FSH and body mass index (BMI) (4, 7, 9, 10, 12–18), although a few studies have not found these associations (17, 19, 20). In addition, studies evaluating the effects of FSH, androgens, or insulin on AMH levels have had either negative or inconsistent findings (2, 3, 9, 16, 20–23).
Recently, however, a large amount of accumulating data, both in vitro and in vivo, strongly suggests that LH acts to stimulate AMH production. Direct LH stimulation of GCs isolated from antral follicles from unstimulated ovaries from women with PCOS resulted in a 4-fold increase in AMH production by the GCs; no significant increase was seen from GCs from normal ovaries (22). Similar increases in AMH expression were also seen after in vitro LH stimulation of luteinized GCs from oligo/anovulatory women with PCOS but again not from GCs from normal ovaries (21, 24). In addition, a close examination of the circadian variation in hormone production in PCOS and normal women revealed a significant positive covariation between AMH and LH levels (but not other hormone measures) and that the circadian secretion of AMH and LH was linked, with a failure of LH and AMH levels to drop overnight in women with PCOS as observed in controls (25). Many other studies have also demonstrated a positive correlation between serum AMH levels and LH levels in women with PCOS (2, 15, 17, 20, 26, 27), although this correlation was not found in some studies (8, 9). Despite the strong in vitro evidence that LH stimulates AMH production and the associative correlations that link these hormones, the possibility of a direct causal effect of LH on AMH production has not been directly tested in women with PCOS.
In the current study, we sought to determine whether LH stimulation of AMH production, as clearly observed in vitro, can also be detected in vivo by assessing serum AMH and androgen responses to recombinant human chorionic gonadotropin (r-hCG) stimulation in women with PCOS and normal women.
Subjects and Methods
Participants
Twenty-eight women with PCOS and 29 normal women were recruited. PCOS subjects exhibited clinical and/or biochemical evidence of hyperandrogenism and were either oligomenorrheic or amenorrheic. Oligomenorrhea was defined as irregular menstrual bleeding occurring less than six times a year. Each PCOS subject had an antral follicle count per ovary of greater than 12, with no follicle that exceeded 9 mm in diameter and the vast majority being 2–5 mm in size. Normal women also had no follicles greater than 10 mm in diameter. Three-dimensional ultrasonography was performed for a subset of the subjects (12 PCOS and 10 controls) before beginning the study to obtain a more accurate antral follicle count than is possible with two-dimensional ultrasound for the calculation of AMH levels per antral follicle. Late-onset congenital adrenal hyperplasia was excluded by serum 17-hydroxyprogesterone (17-OHP) less than 2 ng/mL. Circulating TSH, prolactin, FSH, and estradiol (E2) were normal among all subjects. No subject had received hormone medication for 2 months before the study. The study was approved by the Human Research Protection Program at the University of California, San Diego, and written informed consent was obtained from each participant.
Procedures
Subjects were admitted to the Clinical and Translational Research Institute at the University of California, San Diego, on each day of testing. Each subject received r-hCG as an iv bolus at a dose of 25 μg as previously published (28). In normal subjects, r-hCG was given during the midfollicular phase (cycle days 5–9). In PCOS women, r-hCG was administered on a random day. Blood samples were obtained prior to and 24 hours after r-hCG administration. For a subset of the same and new PCOS (n = 8) and normal (n = 11) subjects, we subsequently evaluated AMH responses to a higher dose of 250 μg of r-hCG iv administered in an identical fashion but with blood samples obtained prior to stimulation as well as at both 24 and 48 hours after stimulation. Two additional PCOS patients and two normal controls received r-hCG as an iv bolus at a dose of 25 μg, with bloods samples obtained at time = 0, 2, 4, 8, 12, and 20 hours after hCG administration. For all studies, none of the PCOS subjects had experienced recent ovulation as evidenced by the absence of menstrual bleeding for 2 months before the study and serum progesterone less than 2.0 ng/mL.
Assays
Serum concentrations of LH and FSH were measured by a RIA with inter- and intraassay coefficients of variation of less than 4% for LH and less than 5% for FSH (Diagnostic Products Corp). Serum concentrations of E2, A4, 17-OHP, and T were measured by well-established RIA assays using column chromatographic separation in assays as previously published (3, 18, 28, 29) with inter- and intraassay coefficients of variation less than 7%. Serum AMH was measured by an ELISA (Beckman Coulter) with inter- and intraassay coefficients of variation less than 4%.
Statistical analysis
Descriptive statistics were calculated by means, medians, and proportions. Hormone values that were not normally distributed were log transformed. If data remained skewed, nonparametric tests were used. Baseline characteristics and circulating hormone levels were compared between women with PCOS and normal controls using the Student's t test or Wilcoxon rank sum test, as appropriate. Paired data were compared by the paired t test or Wilcoxon signed-rank test, as appropriate. Correlations were evaluated by calculation of Spearman's rho. Two-tailed statistical significance was set at P = .05. Statistical analyses were performed with Stata software version 12 (Stata Corp).
Results
Baseline hormone levels and antral follicle counts in women with PCOS and normal controls
Baseline characteristics and circulating hormone levels for each group are shown in Table 1. Women with PCOS and normal controls did not differ significantly in age. As expected, women with PCOS had higher baseline levels of serum LH, T, A4, and 17-OHP as well as a higher BMI and baseline antral follicle count than normal controls. Serum FSH and E2 were similar between groups. Consistent with recent studies, mean baseline serum AMH levels were 2-fold higher in women with PCOS than normal controls, as shown in Figure 1A (9.6 ± 1.2 ng/mL PCOS vs 4.1 ± 0.6 ng/mL controls). No significant difference was detected in the circulating levels of AMH per antral follicle in women with PCOS (0.21 ± 0.02 ng/mL) compared with normal controls (0.19 ± 0.03 ng/mL) (Figure 1B; P = .62).
Table 1.
Baseline Characteristics and Hormone Concentrations of Women With PCOS and Normal Controls
| Controls (n = 29) | PCOS (n = 28) | |
|---|---|---|
| Age, y | 27.5 ± 1.1 | 26.7 ± 0.8 |
| BMI, kg/m2 | 27.2 ± 1.5 | 32.3 ± 1.3a |
| LH, mIU/mL | 3.7 ± 0.4 | 8.5 ± 1.1a |
| A4, ng/mL | 3.3 ± 0.2 | 5.4 ± 0.3a |
| T, ng/mL | 0.8 ± 0.1 | 1.6 ± 0.1a |
| 17-OHP, ng/mL | 1.0 ± 0.2 | 1.8 ± 0.4a |
| FSH, mIU/mL | 6.6 ± 0.4 | 6.6 ± 0.3 |
| E2, pg/mL | 52.5 ± 5.5 | 47.2 ± 2.9 |
| 2- to 9-mm folliclesb | 13.6 ± 1.5 | 26.2 ± 2.3a |
Data are expressed as mean ± SE. To convert to SI units, multiply by the following conversion factors: 17-OHP, 3.03; A4, 3.49; T, 3.47, E2, 3.67.
P < .05 relative to control group.
Average antral follicle count per ovary determined for 12 women with PCOS and 10 normal controls by three-dimensional ultrasonography.
Figure 1.
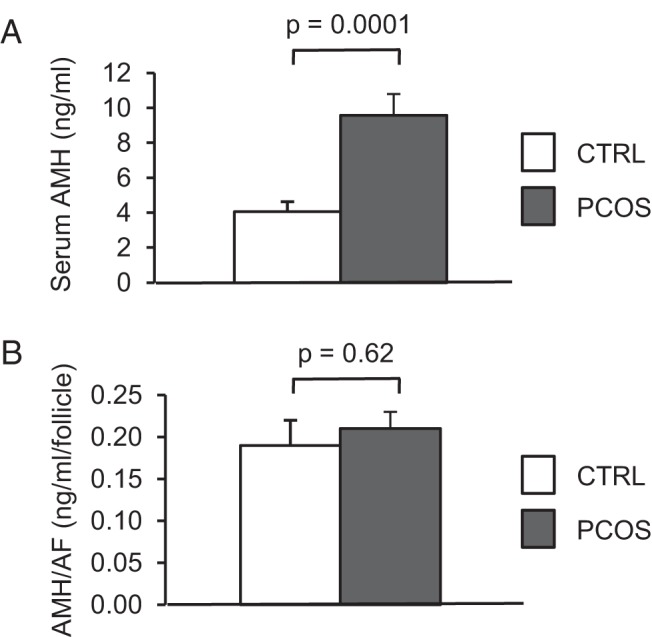
AMH levels are elevated in PCOS subjects before stimulation. A, Graph comparing mean (±SE) serum AMH levels at baseline in PCOS and normal women. B, Graph comparing the mean (±SE) serum AMH per antral follicle in the subset of PCOS (n = 12) and normal (n = 10) women who underwent three-dimensional ovarian ultrasonography for an accurate determination of antral follicle count. CTRL, control.
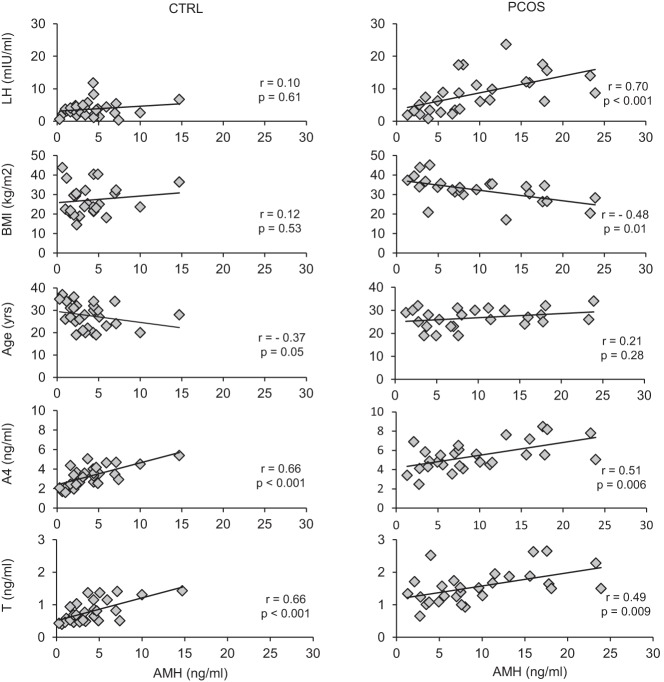
As shown in Figure 2, there was a highly positive correlation between AMH and LH, whereas AMH and BMI were negatively correlated in women with PCOS. These correlations were not present in normal controls. In contrast, AMH was negatively correlated with age in normal controls, but this association was not observed in women with PCOS. Serum AMH was positively correlated with the ovarian androgens T and A4 in both groups, whereas there was no correlation with 17-OHP (controls: r = 0.30, P = .11; PCOS: r = 0.06, P = .74) or FSH (controls: r = −0.03, P = .90; PCOS: r = 0.14, P = .46) in either group. Taken together, these data suggest that additional factors, including LH and BMI, contribute to AMH secretion in women with PCOS compared with normal controls.
Figure 2.
AMH levels positively correlate with LH and androgen levels in PCOS subjects before stimulation. Graphs in which the baseline AMH level for each PCOS subject and normal control is plotted against LH, BMI, age, A4, and total T for each subject at baseline are shown (r = Spearman rho). CTRL, control.
AMH responses to hCG administration
To investigate whether the LH-induced production of AMH seen in PCOS GCs in vitro occurs in PCOS ovaries in vivo, women with PCOS and normal controls were administered 25 μg r-hCG iv. We have previously determined 25 μg r-hCG iv to be an approximate midmaximal stimulatory dose based on 17-OHP responses in normal women and women with PCOS (28).
For both PCOS women and normal controls, r-hCG stimulation resulted in significant increases in serum levels of A4, T, and 17-OHP compared with unstimulated levels (Figure 3). Between-group comparisons revealed that serum A4 and 17-OHP responses were significantly greater in PCOS women than that of normal women, whereas higher T responses in the PCOS group were not statistically different from those of normal women. These findings are similar to results previously reported by our group and provided validation of our study populations and stimulation protocol (28).
Figure 3.

Stimulation with r-hCG results in significant increases in androgen levels in PCOS subjects and normal women. Graphs comparing the mean (±SE) serum 17-OHP, A4, and T levels before and 24 hours after stimulation with 25 μg r-hCG iv in PCOS and normal women. *, P value comparing the change in androgen levels in PCOS vs controls. CTRL, control.
When stimulated AMH levels were evaluated, no significant increase was detected after r-hCG administration in either PCOS or normal controls, as shown in Figure 4A. BMI was not correlated with the percentage change in AMH in either group (controls: r = −0.02, P = .92; PCOS: r = −0.17, P = .37). We also asked whether AMH elevations might occur before 24 hours in vivo. Two PCOS patients received 25 μg of r-hCG iv and had blood samples obtained at time = 0, 2, 4, 8, 12, and 20 hours after the stimulation, but no AMH response was seen. To ask whether a higher dose of hCG and/or an extended interval of response was necessary, a subset of both the PCOS (n = 8) and normal (n = 11) subjects were stimulated with 250 μg of r-hCG, and blood samples were obtained at 24 hours and 48 hours after the injection. However, there was still no significant increase in serum AMH (Figure 4B), although we know androgens are also routinely increased with this stimulation protocol (28).
Figure 4.
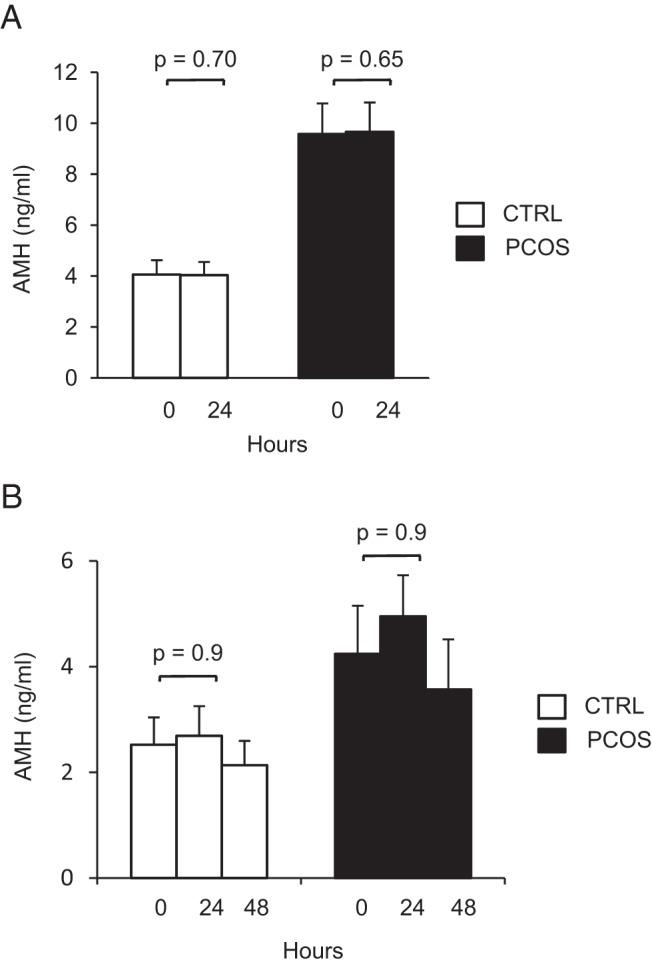
Stimulation with r-hCG does not result in detectable increases in AMH levels in PCOS subjects or normal women. A, Graph comparing the mean (±SE) serum AMH levels before and 24 hours after stimulation with 25 μg r-hCG iv in PCOS and normal women. B, Graph comparing the mean (±SE) serum AMH levels before and 24 and 48 hours after stimulation with a higher dose of 250 μg r-hCG iv in PCOS and normal women. CTRL, control.
Discussion
The results of this study have demonstrated that serum AMH levels do not increase significantly after r-hCG administration in women with PCOS or in normal women. This was true despite both elevated AMH levels and strong correlations between AMH and LH and androgen levels among our PCOS subjects, which is consistent with previous reported findings (2, 4, 7, 9, 10, 12–17, 20, 25–27). Together, these results would suggest that LH is not directly responsible for the elevated serum levels of AMH in vivo.
There are several reasons the in vitro effect of LH on AMH production by GCs from PCOS women may not be observed in vivo. Circulating serum levels may not be a sensitive measure of changes in local concentrations of AMH within the ovary, given its probable paracrine mechanism of action. For example, it has been shown that levels of AMH in the follicular fluid from 4- to 8-mm follicles of women with PCOS were on average 60-fold higher than corresponding serum levels from the same women (29). In further support of this concept, AMH production by GCs is known to vary greatly according to the stage of follicle development, yet serum concentrations were relatively stable when measured throughout the menstrual cycle (30–32). A further decrease in sensitivity would be expected, given that we were limited to sampling steady-state serum levels present in vivo at baseline and 24 hours instead of measuring the accumulated levels of AMH secreted by GCs over 48 hours as was possible in vitro (22). Consistent with this concern, the reported t½ of AMH in vivo is approximately 28 hours (33). A significant increase in AMH levels with measurement at 48 hours instead of 24 hours is unlikely, given that no further increase was seen in the subgroup given 250 μg r-hCG as shown in Figure 4B. Any increase that might occur after 48 hours is unlikely to be a direct effect of hCG stimulation because we know from previous studies that the rise in hCG serum levels after the iv administration of r-hCG is rapid and returns to baseline (25 μg dose) or near baseline (250 μg dose) at 24 hours after the administration and remains at baseline at 48 hours (28).
It is also possible that AMH is maximally stimulated by the elevated levels of LH in PCOS women and that no further increase in AMH levels will be observed with additional stimulation in vivo, whereas the effect of endogenous LH would have been removed in the in vitro studies. Another possibility is that an effect on AMH levels with r-hCG stimulation was obscured by the modestly higher mean BMI in the subjects with PCOS, given that AMH levels are negatively correlated with BMI. Although we cannot rule out this possibility, BMI was not correlated with the percentage change in AMH in women with PCOS or in normal controls. Finally, differences between hCG and LH signaling might also contribute to the disparity of in vitro results and our in vivo findings, despite the ability of the hormones to bind and act through the same receptor (34).
The regulation of AMH production is poorly understood. It is likely that additional intra- and/or extraovarian factors may limit or constrain the production of AMH in vitro but are not evident during in vivo studies. For instance, FSH stimulation has been shown to decrease AMH expression in vitro from GCs isolated from women with PCOS (22, 35) but not from GCs from normal women (22), whereas others have shown an increase in AMH expression with FSH stimulation of GCs from both PCOS and normal women (24). In contrast, we have previously shown that acute FSH stimulation of women with PCOS does not detectably increase or decrease AMH serum levels (3). These conflicting results reiterate the complexity of AMH regulation. Additionally, it has recently been demonstrated that estradiol can regulate AMH production from GCs in vitro in a manner dependent on estrogen receptor (ER) expression; specifically, estradiol up-regulated the AMH expression in the presence of ER-α and inhibited AMH expression in the presence of ER-β (36). There are likely other unidentified factors that impact AMH production in women with PCOS.
Another critical difference between the experiments by Pellat et al (22), and our own was their intentional use of follicles greater than 10 mm to ensure the presence of LH receptors because it had previously been shown that GC responsiveness to LH in normal women was observed only in follicles greater than 10 mm (22, 37). In contrast, in our PCOS subjects, the follicle population predominantly consisted of small antral follicles less than 10 mm. Unfortunately, the role of LH in AMH production on GCs from follicles less than 10 mm in diameter has not yet been tested in vitro. We reasoned, however, that LH might also act on GCs in follicles less than 10 mm in diameter for several reasons. Rigorous studies have demonstrated clear LH responsiveness in GCs from follicles as small as 4 mm in PCOS ovaries (37). In addition, it has been demonstrated that LH receptor expression is prematurely up-regulated in GCs from small antral follicles (4–9 mm) of PCOS ovaries relative to GCs from size matched follicles in normal ovaries (38). If LH signaling stimulates AMH production, we hypothesized that premature LH responsiveness in small antral follicles might explain the 75-fold elevation in AMH production per PCOS GC as well as the 6-fold increase in AMH levels in follicular fluid from PCOS follicles (30). However, the results here do not provide support for this hypothesis.
We did not observe higher AMH levels per antral follicle in women with PCOS at baseline or after r-hCG stimulation, contrary to previously published in vitro (22) and in vivo findings (27). Our results are, instead, consistent with those of Pigny et al (9), in which the ratio of serum AMH to small antral follicles was similar in both normal and PCOS women. However, had we divided the follicle population into those less than and greater than 5 mm because one study demonstrated AMH levels may have correlated with follicles sized 2–5 mm but not with those of 6–9 mm (9). In addition, there is currently no consensus as to an international standard for AMH assays. So although we can accurately make comparisons between AMH levels in PCOS subjects and normal controls and before and after stimulation by using the same assay for all samples, absolute levels may differ among studies according to the assay used. This will remain an issue until a uniform and reliable AMH assay is developed.
In summary, although our findings extend correlative clinical data that suggest an interaction between LH and AMH in this PCOS, we have shown that circulating AMH levels are not significantly increased after r-hCG administration in women with PCOS. The precise reason that the in vitro results are not recapitulated in vivo is unknown. However, it is likely that intraovarian or other mechanisms are present that limit AMH production by GCs in vivo. More studies are needed to elucidate peripheral and intraovarian factors regulating AMH production by GCs.
Acknowledgments
We thank Jeff Wong for his expert technical assistance.
The study had the clinical trial registration number of NCT00747617.
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through Cooperative Agreement U54 HD12303–28 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research; National Institutes of Health Grant T32 HD007203; and in part by National Institutes of Health Grant MO1 RR00827. H.C.-A. was supported by the Women's Reproductive Health Research Grant K12 HD001259.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- A4
- androstenedione
- AMH
- anti-Mullerian hormone
- BMI
- body mass index
- E2
- estradiol
- ER
- estrogen receptor
- GC
- granulosa cell
- 17-OHP
- 17-hydroxyprogesterone
- PCOS
- polycystic ovary syndrome
- r-hCG
- recombinant human chorionic gonadotropin.
References
- 1. Fallat ME, Siow Y, Marra M, Cook C, Carrillo A. Mullerian-inhibiting substance in follicular fluid and serum: a comparison of patients with tubal factor infertility, polycystic ovary syndrome, and endometriosis. Fertil Steril. 1997;67:962–965. [DOI] [PubMed] [Google Scholar]
- 2. Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D. Anti-Mullerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab. 2009;296:E238–E243. [DOI] [PubMed] [Google Scholar]
- 3. Wachs DS, Coffler MS, Malcom PJ, Chang RJ. Serum anti-mullerian hormone concentrations are not altered by acute administration of follicle stimulating hormone in polycystic ovary syndrome and normal women. J Clin Endocrinol Metab. 2007;92:1871–1874. [DOI] [PubMed] [Google Scholar]
- 4. Pigny P, Jonard S, Robert Y, Dewailly D. Serum anti-Mullerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941–945. [DOI] [PubMed] [Google Scholar]
- 5. Dewailly D, Gronier H, Poncelet E, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26:3123–3129. [DOI] [PubMed] [Google Scholar]
- 6. Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Mullerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196–201. [DOI] [PubMed] [Google Scholar]
- 7. Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Mullerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820–1826. [DOI] [PubMed] [Google Scholar]
- 8. Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum mullerian-inhibiting substance and other reproductive hormones in untreated women with polycystic ovary syndrome and normal women. Fertil Steril. 2002;77:141–146. [DOI] [PubMed] [Google Scholar]
- 9. Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957–5962. [DOI] [PubMed] [Google Scholar]
- 10. Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BC. Anti-Mullerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab. 2004;89:318–323. [DOI] [PubMed] [Google Scholar]
- 11. Mulders AG, Laven JS, Eijkemans MJ, de Jong FH, Themmen AP, Fauser BC. Changes in anti-Mullerian hormone serum concentrations over time suggest delayed ovarian ageing in normogonadotrophic anovulatory infertility. Hum Reprod. 2004;19:2036–2042. [DOI] [PubMed] [Google Scholar]
- 12. Eldar-Geva T, Margalioth EJ, Gal M, et al. Serum anti-Mullerian hormone levels during controlled ovarian hyperstimulation in women with polycystic ovaries with and without hyperandrogenism. Hum Reprod. 2005;20:1814–1819. [DOI] [PubMed] [Google Scholar]
- 13. Li Y, Ma Y, Chen X, et al. Different diagnostic power of anti-Mullerian hormone in evaluating women with polycystic ovaries with and without hyperandrogenism. J Assist Reprod Genet. 2012;29:1147–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenfield RL, Wroblewski K, Padmanabhan V, Littlejohn E, Mortensen M, Ehrmann DA. Antimullerian hormone levels are independently related to ovarian hyperandrogenism and polycystic ovaries. Fertil Steril. 2012;98:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin YH, Chiu WC, Wu CH, Tzeng CR, Hsu CS, Hsu MI. Antimullerian hormone and polycystic ovary syndrome. Fertil Steril. 2011;96:230–235. [DOI] [PubMed] [Google Scholar]
- 16. Carlsen SM, Vanky E, Fleming R. Anti-Mullerian hormone concentrations in androgen-suppressed women with polycystic ovary syndrome. Hum Reprod. 2009;24:1732–1738. [DOI] [PubMed] [Google Scholar]
- 17. Skalba P, Cygal A, Madej P, et al. Is the plasma anti-Mullerian hormone (AMH) level associated with body weight and metabolic, and hormonal disturbances in women with and without polycystic ovary syndrome? Eur J Obstet Gynecol Reprod Biol. 2011;158:254–259. [DOI] [PubMed] [Google Scholar]
- 18. Park AS, Lawson MA, Chuan SS, et al. Serum anti-mullerian hormone concentrations are elevated in oligomenorrheic girls without evidence of hyperandrogenism. J Clin Endocrinol Metab. 2010;95:1786–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Mullerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82:970–972. [DOI] [PubMed] [Google Scholar]
- 20. Panidis D, Katsikis I, Karkanaki A, Piouka A, Armeni AK, Georgopoulos NA. Serum anti-Mullerian hormone (AMH) levels are differentially modulated by both serum gonadotropins and not only by serum follicle stimulating hormone (FSH) levels. Med Hypotheses. 2011;77:649–653. [DOI] [PubMed] [Google Scholar]
- 21. Catteau-Jonard S, Jamin SP, Leclerc A, Gonzales J, Dewailly D, di Clemente N. Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:4456–4461. [DOI] [PubMed] [Google Scholar]
- 22. Pellatt L, Hanna L, Brincat M, et al. Granulosa cell production of anti-Mullerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240–245. [DOI] [PubMed] [Google Scholar]
- 23. Crisosto N, Sir-Petermann T, Greiner M, et al. Testosterone-induced downregulation of anti-Mullerian hormone expression in granulosa cells from small bovine follicles. Endocrine. 2009;36:339–345. [DOI] [PubMed] [Google Scholar]
- 24. Pierre A, Peigne M, Grynberg M, et al. Loss of LH-induced down-regulation of anti-Mullerian hormone receptor expression may contribute to anovulation in women with polycystic ovary syndrome. Hum Reprod. 2013;28:762–769. [DOI] [PubMed] [Google Scholar]
- 25. Bungum L, Franssohn F, Bungum M, Humaidan P, Giwercman A. The circadian variation in anti-Mullerian hormone in patients with polycystic ovary syndrome differs significantly from normally ovulating women. PLoS One. 2013;8:e68223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Catteau-Jonard S, Pigny P, Reyss AC, Decanter C, Poncelet E, Dewailly D. Changes in serum anti-mullerian hormone level during low-dose recombinant follicular-stimulating hormone therapy for anovulation in polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:4138–4143. [DOI] [PubMed] [Google Scholar]
- 27. Nardo LG, Yates AP, Roberts SA, Pemberton P, Laing I. The relationships between AMH, androgens, insulin resistance and basal ovarian follicular status in non-obese subfertile women with and without polycystic ovary syndrome. Hum Reprod. 2009;24:2917–2923. [DOI] [PubMed] [Google Scholar]
- 28. Rosencrantz MA, Coffler MS, Haggan A, et al. Clinical evidence for predominance of delta-5 steroid production in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96:1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Das M, Gillott DJ, Saridogan E, Djahanbakhch O. Anti-Mullerian hormone is increased in follicular fluid from unstimulated ovaries in women with polycystic ovary syndrome. Hum Reprod. 2008;23:2122–2126. [DOI] [PubMed] [Google Scholar]
- 30. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006;21:3103–3107. [DOI] [PubMed] [Google Scholar]
- 31. Stubbs SA, Hardy K, Da Silva-Buttkus P, et al. Anti-mullerian hormone protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. J Clin Endocrinol Metab. 2005;90:5536–5543. [DOI] [PubMed] [Google Scholar]
- 32. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057–4063. [DOI] [PubMed] [Google Scholar]
- 33. Griesinger G, Dafopoulos K, Buendgen N, et al. Elimination half-life of anti-Mullerian hormone. J Clin Endocrinol Metab. 2012;97:2160–2163. [DOI] [PubMed] [Google Scholar]
- 34. Casarini L, Lispi M, Longobardi S, Milosa F, et al. LH and hCG action on the same receptor results in quantitatively and qualitatively different intracellular signalling. PLoS One 2012; 7:e46682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Y, Wei LN, Liang XY. Follicle-stimulating hormone suppressed excessive production of antimullerian hormone caused by abnormally enhanced promoter activity in polycystic ovary syndrome granulosa cells. Fertil Steril. 2011;95:2354–2358, 2358.e1. [DOI] [PubMed] [Google Scholar]
- 36. Grynberg M, Pierre A, Rey R, et al. Differential regulation of ovarian anti-mullerian hormone (AMH) by estradiol through α- and β-estrogen receptors. J Clin Endocrinol Metab. 2012;97:E1649–E1657. [DOI] [PubMed] [Google Scholar]
- 37. Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. J Clin Endocrinol Metab. 1998;83:3984–3991. [DOI] [PubMed] [Google Scholar]
- 38. Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab. 2001;86:1318–1323. [DOI] [PubMed] [Google Scholar]