Abstract
Context:
Ectopic ACTH/CRH syndrome is a rare cause of Cushing syndrome (CS), especially in children. The localization, work-up, and management of ACTH/CRH-secreting tumors are discussed.
Setting:
A retrospective study was conducted of patients under 21 years of age evaluated at the National Institutes of Health (NIH) for CS and diagnosed with ectopic ACTH/CRH-secreting tumors during the period 2009–2014.
Patients:
Seven patients with ectopic ACTH/CRH CS are included in this study with a median age 13.6 years (range 1–21), and 3 are female.
Measurements:
Clinical, biochemical, radiological features, treatment, and histological findings are described.
Results:
Seven patients were found to have ACTH/CRH-secreting tumors, all with neuroendocrine features. The site of the primary lesion varied: pancreas (3), thymus (2), liver (1), right lower pulmonary lobe (1). Patients underwent biochemical evaluation for CS, including diurnal serum cortisol and ACTH levels, urinary free cortisol levels (UFC), and CRH stimulation tests. All patients underwent radiological investigations including MRI, CT, and PET scan; imaging with octreotide and 68 gallium DOTATATE scans were performed in individual cases. Five patients underwent inferior petrosal sinus sampling; 4 patients had sampling for ACTH and CRH levels from additional sites. Three patients underwent trans-sphenoidal surgery (TSS), and 3 patients required bilateral adrenalectomy. Three patients (43%) died due to metastatic disease, demonstrating the high mortality rate. One of the unique findings in these seven patients is that in each case, their neuroendocrine tumors were ultimately proven to be co-secreting ACTH and CRH. This explains the enigmatic presentation, in which 3 patients initially thought to have Cushing's disease (CD) with corresponding pituitary hyperplasia underwent TSS prior to the correct localization of the causative tumor.
Conclusions:
Ectopic ACTH/CRH co-secreting tumors are extremely rare in children and adolescents. The diagnosis of this condition is frequently missed and is sometimes confused with CD due to the effect of CRH on the pituitary.
Cushing's syndrome (CS) is a rare disease, especially in children, with an overall incidence of 2–5 new cases per million people per year, from which only 10% concern children (1, 2). The most common endogenous cause for CS is adrenocorticotropic hormone (ACTH) overproduction from the pituitary, also known as Cushing's disease (CD), which accounts for 75% of all cases of CS in children over 7 years of age, whereas autonomous secretion of cortisol from the adrenal glands, or ACTH-independent CS accounts for 15% of all CS in childhood (2). Ectopic ACTH-producing tumors occur rarely in young children and their prevalence is less than 1% of all adolescents with CS (2). There have been many cases of ACTH-secreting ectopic neuroendocrine tumors reported in the literature (3, 4). However, there are only a few case reports associated with ectopic ACTH/CRH co-secreting, or exclusively corticotropin-releasing hormone (CRH)-secreting tumors. In 1993, Saeger et al studied 13 tumors causing ectopic CS and by immunohistochemistry, it was found that 4 of them were CRH-secreting and 2 were ACTH/CRH co-secreting tumors (5): CRH without ACTH secretion, was found in a small cell carcinoma of the bronchus, a bronchial carcinoid, a prostatic cancer, and a choristoma of the sellar region. ACTH along with CRH secretion was present in one bronchial carcinoid and in one islet cell carcinoma. There are also case reports of pancreatic neuroendocrine tumors (NET), a malignant gastrinoma, a ganglioneuroblastoma in an infant, and two cases of metastatic small cell carcinoma associated with CRH or ACTH/CRH co-secretion (6–9). Thus, ectopic ACTH/CRH-secreting tumors are very rare, particularly in children. More et al reported the largest case series of childhood ACTH-secreting tumors, five bronchial carcinoids, one thymic carcinoid, one mediastinal lymph node, one thymic carcinoma, one Ewing's sarcoma, and one liver stromal epithelial tumor (4). Here we describe the evaluation and management of ectopic ACTH/CRH-secreting tumors in children and adolescents from our experience at the NIH, over the last two decades.
Patients and Methods
Patients
A retrospective study was conducted which included children and adolescents evaluated for CS at the National Institutes of Health (NIH) during the period 2009–2014. The criteria for inclusion were ACTH- and CRH-dependent hypercortisolism, evidence of ectopic source from imaging and confirmed by histopathology, and age of patients under 21. Seven patients were included in this case series, 6 of whom underwent both diagnostic evaluation and management at NIH. In one patient, only a pathological evaluation was performed and the remaining information was retrieved from the institute where the patient had been hospitalized. All studies were conducted under clinical protocols, 97-CH0076 and 00-CH-180, that were approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board. Informed consent from the patients' parents (and assent from older children) was obtained for all patients. Patient 3 was reported previously in a study focused on diagnosis (3).
Clinical evaluation
Patients were diagnosed with CS as per standard criteria. Data were also retrieved from outside hospitals where patients had undergone prior evaluations.
Biochemical evaluation
Patients were evaluated for their 24-h urine free cortisol (UFC), serum cortisol before and after high-dose dexamethasone suppression test (HDDST), plasma ACTH and CRH levels. Two assays were used for the determination of UFC. One was performed by the Department of Laboratory Medicine, NIH, Bethesda with a normal range of 4–56 mcg/24 h and the other by the Mayo Clinic Department of Laboratory Medicine and Pathology with a normal range from 2.6 to 37 mcg/24 h. Due to the different normal ranges for UFC from patient to patient, the results were expressed relative to the normal limits (NL). Concerning serum cortisol and ACTH levels, the normal range was from 5 to 25 mcg/dL and 0–46 pg/mL, respectively. Diurnal ACTH and cortisol tests were also performed and two specimens were needed. The diurnal AM specimen included the average ACTH/cortisol levels of time points 07.30 am and 08.00 am, and the diurnal PM specimen, included the average of ACTH/cortisol levels of time point 23.30 pm and 23.59 pm. The AM and PM diurnal levels were then compared and evaluated. The HDDST was conducted as an overnight procedure of one time dose administration of 8 mg by mouth, adjusted for weight in children. Baseline and stimulated ACTH and cortisol were measured during the CRH stimulation test. The CRH test was conducted by administration of corticorelin ovine triflutate injection (1 mcg/kg of body weight). Plasma CRH levels were obtained by the Inter Science Institute, where the normal range was below 10 pg/mL; in one patient, CRH levels were obtained by Quest Diagnostics Nichols Institute, where the normal range was below 34 pg/mL. Inferior petrosal sinus sampling (IPSS) was performed in all but two before and after CRH administration. In the case of inadequate biochemical data that could not lead to a diagnosis and/or the presence of a supporting radiological investigation, patients underwent venous sampling for ACTH/CRH from specific locations that were indicated from the imaging results.
Radiological evaluation
Imaging including abdominal ultrasound, computed tomography (CT) scans of the chest and abdomen, as well as magnetic resonance imaging (MRI) of the chest, abdomen, and pituitary were performed. Five patients underwent 18F-FDG PET/CT and octreotide scan, whereas in one of them the 68Ga-DOTATATE scan was also performed.
Pathological analysis
Immunostaining for CRH, ACTH, and hematoxylin and eosin (H&E) was performed in all cases. Some samples also had additional staining, such as chromogranin A, synaptophysin, and reticulin.
Results
Patients
Six patients were evaluated at the NIH and were finally diagnosed with ectopic ACTH/CRH CS. Their age ranged from 10 to 21 years, with a median of 13.6. Three were females, and two of the patients were African-American. One male patient age 20 months had only a pathological evaluation at the NIH.
Tumors (Table 1)
Table 1.
Demographic Characteristics of the Patients; Tumor Type; Biochemical Results
| Patient | Sex | Race | Age at Diagnosis | Tumors | TSS | Adrenalectomy |
|---|---|---|---|---|---|---|
| 1 | F | African-American | 13.9 | Metastatic hepatic neuroendocrine tumor (12 cm) | No | No |
| 2 | M | White | 10.5 | Metastatic pancreatic NET (primary tumors were 2 lobular masses above the pancreatic tail and next to the spleen, measuring 2.4 × 2.3 cm, 3.5 × 2 cm) | No | Yes |
| 3 | M | African-American | 13.6 | Poorly differentiated thymic carcinoid | Yes | No |
| 4 | F | White | 11.2 | Metastatic pancreatic neurorendocrine tumor (primary tumor at distal pancreatic tail measuring 1.4 cm) | Yes | Yes |
| 5 | F | White | 13.6 | Bronchogenic carcinoid tumor of right lower pulmonary lobe (1.3 cm) | No | No |
| 6 | M | White | 21.3 | Atypical thymic carcinoid (6 mm) | Yes | Yes |
| 7 | M | White | 1.8 | Pancreatoblastoma (7 cm) | No | No |
| Patient | Midnight Cortisol (ug/dL) | Morning Cortisol (ug/dL) | Midnight ACTH (pg/mL) | Morning ACTH (pg/mL) | Pre-op ACTH (pg/mL) | Post-op ACTH (pg/mL) | Pre-op UFC (ug/24 h) | Pre-op UFC (× NL) | Post-op UFC (ug/24 h) | Post-op UFC (× NL) | Pre-op Plasma CRH (pg/mL) | Post-op Plasma CRH (pg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NA | NA | NA | NA | NA | NA | 793.00 | 14.16 | NA | NA | 2.4 | NA |
| 2 | 9.5 | 13 | 40.7 | 44.4 | 79.70 | 8.5 | 64.00 | 1.73 | 0.7 | 0.27 | 248 | 2.7 |
| 3 | 29.4 | 30.4 | 141.5 | 222.5 | 217.00 | 27.1 | 939.00 | 16.77 | 334 | 5.96 | 2.8 | NA |
| 4 | 23.2 | 26.1 | 51 | 41.9 | 47.00 | 46.7 | 834.00 | 22.54 | 1.9 | 0.73 | 2.6 | NA |
| 5 | 23.8 | 32 | 65.2 | 66.8 | 103.00 | 5 | 817.50 | 14.60 | 18.8 | 1 | 2.7 | NA |
| 6 | 17.5 | 22.5 | 89.4 | 121 | 230.00 | 86.1 | 1013.50 | 22.52 | NA | NA | 3.3 | 2.4 |
| 7 | NA | NA | NA | NA | 101.00 | NA | NA | NA | NA | NA | 4.4 | NA |
| Patient | IPS/P Baseline ACTH Ratio | IPS/P Post- CRH ACTH Ratio | Baseline Cortisol (ug/dL) | Post-CRH Cortisol (ug/dL) | % Cortisol Change | Baseline ACTH (pg/mL) | Post-CRH ACTH (pg/mL) | % ACTH Change | Baseline Serum Cortisol (ug/dL) | Post-dexa Serum Cortisol (ug/dL) | Serum cortisol Change (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NA | NA | 59.8 | 58.6 | −1.92 | 57.5 | 59.8 | 4.09 | NA | NA | NA |
| 2 | 1.17 | 1.45 | 21.3 | 23.1 | 8.45 | 65.1 | 61.4 | −5.61 | 11.1 | 3.8 | −65.77 |
| 3 | 1.23 | 1.02 | 29.8 | 31.4 | 5.20 | 246.0 | 212.5 | −13.62 | 28.9 | 24.3 | −15.92 |
| 4 | 7.62 | 10.87 | 28.5 | 32.7 | 14.56 | 45.7 | 54.5 | 19.39 | NA | NA | NA |
| 5 | 1.04 | 1.40 | 18.3 | 18.7 | 2.19 | 57.3 | 68.85 | 20.26 | 20.6 | 6 | −70.87 |
| 6 | 1.46 | 1.52 | 53.8 | 61.1 | 13.67 | 119.5 | 213.5 | 78.66 | 18.1 | 15 | −17.13 |
| 7 | NA | NA | 135.9 | NA | NA | NA | NA | NA | NA | NA | NA |
In three patients the primary tumor was located in the pancreas, ranging in size from 1.4 to 7 cm. In one patient a 12 cm primary tumor was located in the liver. Two patients had thymic carcinoids with sizes ranging from 6 mm to 11.5 cm, while one patient was found to have a 1.3 cm bronchogenic carcinoid tumor of the right pulmonary lobe. All tumors displayed features of NET. Metastases were identified in four of these patients.
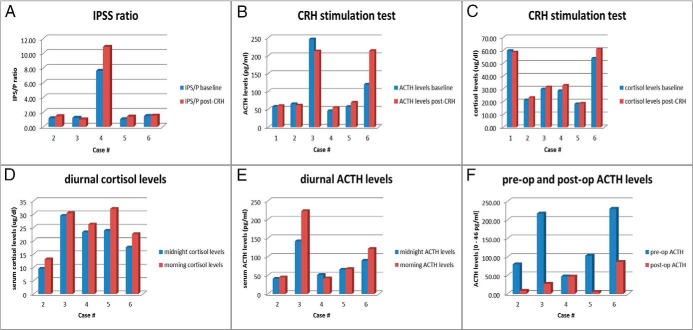
Biochemical evaluation (Figure 1 and Table 1)
Figure 1.
Biochemical results of patients. A, IPSS ACTH ratio pre- and post-CRH administration; B, ACTH levels pre- and post-CRH stimulation test; C, cortisol levels pre- and post-CRH stimulation test; D, diurnal (midnight and morning) cortisol levels; E, diurnal (midnight and morning) ACTH levels; F, ACTH levels pre- and postoperatively.
All patients were evaluated for hypercortisolemia. Regarding UFC, 6 patients preoperatively had approximately 2–23-fold elevated levels compared to the upper limit of normal. Postoperatively three patients remained hypercortisolemic and ultimately required adrenalectomies. After HDDST was performed in 4 patients, there was a suppression in serum cortisol levels ranging from 16% to 71%, with only 1 patient reaching suppression >69% consistent with CD. A CRH stimulation test was performed in all six patients evaluated at the NIH. None of the patients reached the criteria for cortisol response to the CRH that would be expected in CD, with all patients showing <20% increase in cortisol (ranging from 2% to 15% elevation of cortisol from baseline). Regarding ACTH response to CRH testing, only 1 patient out of 6 met the criteria consistent with CD with a mean increase of >35% above baseline (Table 1). This relatively flat cortisol and ACTH response is consistent with ectopic ACTH/CRH-producing tumors. Interestingly, 5 patients had inappropriately normal, and one had elevated plasma CRH levels, whereas we would expect undetectable levels due to the negative feedback from hypercortisolemia. Five patients showed blunted diurnal variation for both their cortisol and ACTH levels (midnight to morning). In 4 patients, ACTH was significantly decreased postoperatively, but in one patient it remained unchanged. Five patients underwent BIPSS with CRH administration and the inferior petrosal sinus/peripheral (IPS/P) ACTH ratio was calculated. Only 1 out of 5 patients who underwent IPSS met the criteria consistent with CD, with a ≥2:1 central to peripheral gradient at baseline and a≥3:1 gradient after CRH stimulation (Table 1). Finally, in 4 patients, venous sampling of ACTH and CRH was performed in order to verify the source of CS from anatomical sites suggested on imaging. In patient 4, there were elevated ACTH levels from sampling of the main portal, proximal, and midsplenic vein both before and after CRH administration (levels ranged from 51.5 to 79.6 pg/mL). Patient 6 had elevated ACTH levels from sampling of the right hepatic (61.3 pg/mL), left and middle hepatic vein, and even more from the thymic vein (88.4 pg/mL), thymic orifice (249 pg/mL), and peripheral jugular vein (66 pg/mL).
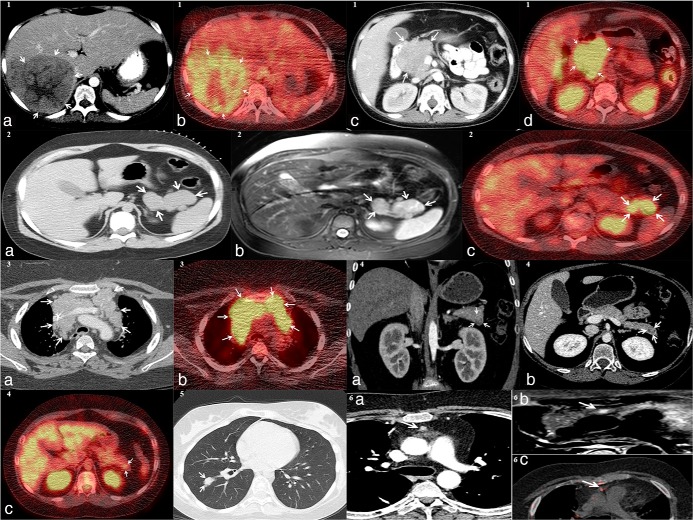
Imaging studies (Figures 2 and 3)
Figure 2.
1(a), axial CT, 1(b), axial 18F-FDG PET/CT, demonstrating liver NET in patient 1, 1(c), axial CT, 1(d), axial 18F-FDG PET/CT, both showing NET in the head of the pancreas from patient 1; 2(a), axial CT, 2(b), axial MRI STIR technique, 2(c), axial 18F-FDG PET/CT, all visualizing the NET in the pancreatic tail of patient 2; 3(a), axial CT, 3(b), axial 18F-FDG PET/CT, both showing thymic carcinoid from patient 3; 4(a), coronal CT, 4(b), axial CT, 4(c), axial 18F-FDG PET/CT, all demonstrating NET of the pancreatic tail of patient 4; 5, axial CT showing bronchogenic carcinoid in right lower lung of patient 5; 6(a), axial CT, 6(b), axial MRI STIR technique, 6(c), axial 68Ga-DOTATE PET/CT, demonstrating thymic carcinoid from patient 6
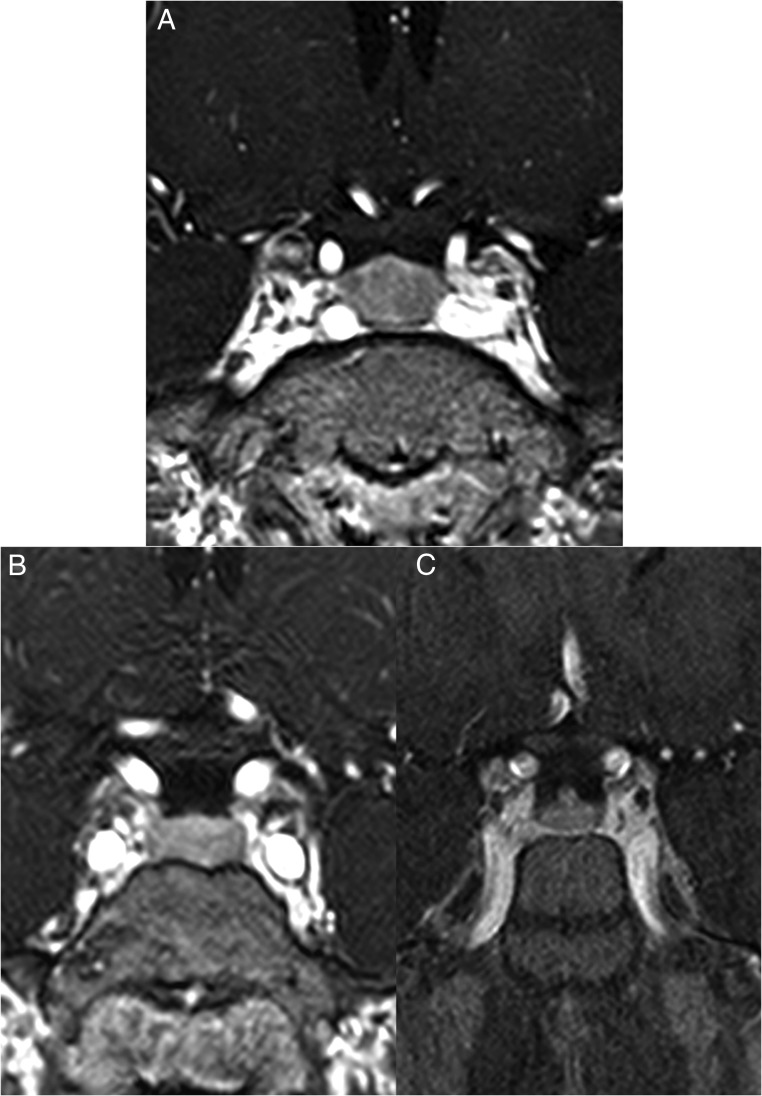
Figure 3.
A, Coronal post contrast T1-weighted 3D gradient echo MRI scan of patient 3. The pituitary gland is slightly enlarged with the vertical height measuring 10.5 mm. There is homogeneous enhancement throughout the pituitary parenchyma. These findings suggested diffuse hypertrophy. No focal space occupying lesion was present within the gland; (B, C) Two coronal post contrast T1-weighted 3D gradient echo MRI scans performed before (B) and after (C) surgical resection of the CRH/ACTH-secreting tumor of patient 2. After resection of the tumor, there is obvious reduction in the size of the pituitary (C).
Six patients had pituitary MRI imaging and in 2 of them, hypoenhancing segments suggestive of macroadenomas were identified and led one of them to TSS (see Figure 3). The other patient that did not undergo TSS, had a second pituitary MRI scan after the resection of his pancreatic neuroendocrine tumor, and a decrease in size of his pituitary focus was noticed from 3.6 to 2.6 mm (Figure 3, B and C). The remaining 4 patients had negative pituitary imaging. Abdominal or chest MRI and CT scans were performed and all 7 patients had findings consistent with NET. Five patients underwent 18F-FDG PET/CT scans; in 4, radiotracer uptake was shown from the locations that were finally proven to be the cause of the disease. Octreotide scans were performed in 5 patients. In 3 patients uptake from the responsible location was shown, however, in two patients the scan was negative for the location that was ultimately found to be the source of CRH/ACTH secretion. Finally, one patient underwent a 68Ga-DOTATATE scan that showed uptake from his neuroendocrine tumor.
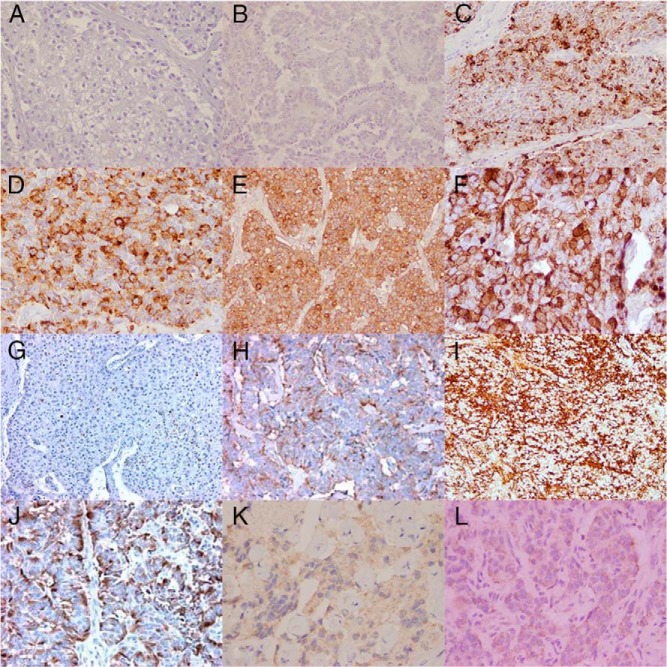
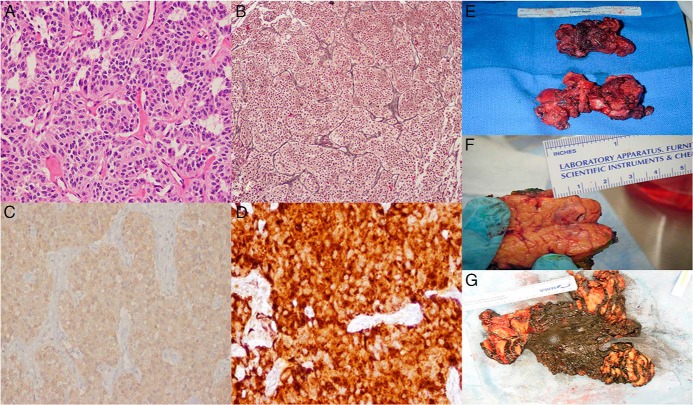
Histopathology (Figures 4 and 5)
Figure 4.
A, B, C, E, NET with ACTH staining from patients 1, 2, 3, and 5 respectively (magnification, ×20); D, F, ACTH staining from patients 3 and 6 respectively (magnification, ×40); G–J, CRH staining from patients 1–4 respectively (magnification, ×20); K, bronchogenic carcinoid tumor with CRH staining from patient 5 (magnification, ×40); L, thymic carcinoid tumor with CRH staining from patient 6 (magnification, ×60).
Figure 5.
A, pancreatic NET with hematoxylin and eosin staining (magnification, ×20); B, pancreatic NET with reticulin staining (magnification, ×10); C, bronchogenic carcinoid tumor with synaptophysin staining (magnification, ×20); D, thymic carcinoid tumor with chromogranin A staining (magnification, ×40) from patients 2, 4, 5, and 6 respectively; E, thymic carcinoid tumor; F, pancreatic NET; G, thymic carcinoid tumor from patients 3, 4, and 6 respectively.
All patients stained positive for ACTH and CRH. Some of the patients additionally had chromogranin A, reticulin, and synaptophysin staining and were found to be strongly positive for these markers.
Genetics
All patients were screened for MEN1 mutations; only patient 4 was found to have a germline de novo menin mutation, heterozygous for the c.1546dupC mutation of the MEN1 gene. In addition, this patient had an associated chromosome 8p23.2 duplication that was present in unaffected members of the family and hence, is considered an incidental finding. None of the patients including the one with MEN1 mutation, had any family history of multiple neoplasias.
Management
Surgeries
Out of the seven patients, three underwent trans-sphenoidal surgery. Two of them had the surgery based on the IPSS interpretation and the other due to his pituitary MRI findings. All patients finally had their CRH/ACTH-secreting tumor resected. Patient 1 initially had a liver biopsy and then underwent Whipple's procedure (pancreaticoduodenectomy) which included resection of his liver tumor. The second patient had a distal pancreatectomy and a splenectomy. Patient 3 had a median sternotomy with bilateral clamshell thoracotomy (thymectomy) and extensive central and lateral neck dissection. Patient 4 underwent partial pancreatectomy, followed by splenectomy, cholecystectomy, left retroperitoneal mass, and lymph node resection. Patient 5 had a right lower pulmonary lobe wedge resection, followed by a right thoracotomy with completion of the lower lobectomy. Patient 6 underwent fiberoptic bronchoscopy, right video-assisted thoracoscopy, and median sternotomy with complete thymectomy. Finally, patient 7 had a pancreatic and omental mass excision consistent with pancreatoblastoma. In cases 2, 4, and 6, bilateral adrenalectomy had to be performed due to persistent hypercortisolemia secondary to metastatic disease.
Outcome
All patients were followed-up for 1–57 months (median, 11) after undergoing their first operation. In two patients however (patients 5, 6), the follow-up was very short (6, 7 months) due to the recent occurrence of the disease (patients 5, 6), and unfortunately, three patients (patients 1, 3, 7) died due to metastatic disease. Specifically, in patient one, 3 months after the excision of the liver and the pancreatic tumor, the CT scan revealed new liver lesions with pancreatic involvement and chemotherapy was initiated. Five months after chemotherapy, a new CT scan showed enlargement of both tumors and the patient underwent radiation therapy. In patient 2, there was recurrence of the disease 3 years after the resection of the primary pancreatic NET tumor, which led to bilateral adrenalectomy due to cortisol excess. Patient 3 underwent resection of a high grade thymic carcinoid, with diffuse infiltration into the adjacent soft tissues and significant vascular and perineural invasion. He underwent octreoscan and 18F-FDG PET scan, 3 and 6 months postoperatively, respectively, and tumor regrowth was found. He was placed on ketoconazole as adrenolytic therapy. After having multiple post-op complications, the patient died due to multi-organ system failure 1 year later. In patient 4, a year after partial pancreatectomy, a PET scan showed abdominal metastatic lesions, and the patient was started on octreotide. Two years later, the patient underwent left retroperitoneal mass and lymph node removal, splenectomy, and cholecystectomy, and was later started on a tyrosine-kinase inhibitor. Patient 5, three months after having lower lobe wedge resection, underwent right thoracotomy with completion of the lower lobectomy. Patient 6 underwent thymectomy, revealing a multifocal NET grade 2 with extensive lymphovascular invasion and multiple small calcifications. There was CS and persistent elevation of cortisol and ACTH, which led to bilateral adrenalectomy, with the recommendation of adjuvant radiation therapy and octreotide administration. While undergoing excision of his pancreatoblastoma, patient 7 was found to have carcinomatosis throughout his abdomen, and total resection of the tumor could not be completed. Chemotherapy (etoposide and cisplatin) was then initiated, as well as spironolactone for his hypercortisolemia, since ketoconazole was contraindicated due to elevated LFTs. The patient died a week later, due to acute GI bleed and other complications from his extensive pancreatoblastoma.
Discussion
Ectopic ACTH/CRH co-secreting tumors are very rare in children and adolescents. Over the last 20 years at the NIH, we had the opportunity of encountering 7 such patients. Both the work-up and the management are challenging and can possibly lead to an erroneous diagnosis. In our patients 3 out of the total 7 patients underwent unnecessary TSS, due to the false assumption of CD (two in other institutions). This is because CRH secretion stimulates the pituitary gland causing hyperplasia, as shown in Figure 3A.
Thus, localization of ectopic ACTH/CRH-secreting tumors is a challenging process. Four patients had abdominal tumors (pancreas, liver) and three chest tumors (thymus, lung). In some cases, imaging studies, such as octreotide or 68Ga-DOTATE PET/CT scans, as well as sampling from specific veins and special biochemical tests, such as measurement of plasma CRH levels were performed, in order to detect the tumors. The lack of these imaging, biochemical, and invasive techniques in most hospitals, as well as the rarity of an ectopic ACTH/CRH-producing tumor were reasons for the decision of a TSS, after having excluded an adrenal source for the unnecessary pituitary surgery in 3 of our patients. In all patients the primary tumors were eventually detected and resected. However, three patients finally underwent bilateral adrenalectomies due to persistent hypercortisolemia, which was because of residual disease.
It is important to note the biochemical features of this ectopic form of CS, since the findings apparently depend strongly on the ratio of ACTH/CRH secretion. According to the literature, relatively flat ACTH levels pre and post- CRH administration are consistent with non-pituitary disease, something that was identified in 4 of our patients (10). In addition, only one had an inferior pertrosal sinus to peripheral ratio of ACTH greater than 2. Reimondo et al have shown data which suggest that the criterion for a diagnosis of EAS is an ACTH percentage increment lower than 50% (11). Five of the six patients in our series had an ACTH increase after CRH lower than 50%, but one patient stimulated more than that. Cortisol levels after CRH stimulation were more helpful since none of our patients stimulated more than 20%, the criterion for CD (12). Plasma CRH level were helpful in that they were measurable and in one case elevated, whereas we expected them to be undetectable due to suppression by increased cortisol.
There are only a few publications in the literature describing ACTH/CRH co-secreting tumors, and this is the first case series of patients, and especially of children and adolescents, with this extremely rare disease. On the other hand, there are a number of good studies of ACTH-producing tumors in the literature such as the one described by Ilias et al (3) and Ejaz et al at MD Anderson (14). The second study included 43 patients and the mortality rate was high at 62.8%. We conclude that ectopic CS due to combined ACTH/CRH production is a rare form of CS that presents significant challenges in terms of diagnosis. The ratio of ACTH to CRH secretion by the tumor largely determines the response to diagnostic tests and the disease can easily be confused with a pituitary ACTH-producing adenoma. It is critical to carefully follow the existing algorithms for the diagnosis of CS, in addition to performing functional imaging and measurement of peripheral CRH levels.
Acknowledgments
We thank the patients and their families for their consent to participate in this study and their collaboration. We are also grateful to all the staff at NICHD that contributed to the work-up, the management, the collection of the patients' data, and made this work possible.
This work was supported by the intramural programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, and the Randall Children's Hospital at Legacy Emanuel, Children's Diabetes and Endocrine Center, Portland, OR.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CT
- computed tomography
- H&E
- hematoxylin and eosin
- IPS/P
- inferior petrosal sinus/peripheral
- IPSS
- inferior petrosal sinus sampling
- MRI
- magnetic resonance imaging
- TSS
- trans-sphenoidal surgery.
References
- 1. Magiakou MA, Mastorakos G, Oldfield EH, Gomez MT, Doppman JL, Cutler GB, Jr, et al. Cushing's syndrome in children and adolescents. Presentation, diagnosis, and therapy. N Engl J Med. 1994;331(10):629–636. [DOI] [PubMed] [Google Scholar]
- 2. Stratakis CA. Cushing syndrome in pediatrics. Endocrinology and Metabolism Clinics of North America. 2012;41(4):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing's syndrome due to ectopic corticotropin secretion: twenty years' experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005;90(8):4955–4962. [DOI] [PubMed] [Google Scholar]
- 4. More J, Young J, Reznik Y, Raverot G, Borson-Chazot F, Rohmer V, et al. Ectopic ACTH syndrome in children and adolescents. J Clin Endocrinol Metab. 2011;96(5):1213–1222. [DOI] [PubMed] [Google Scholar]
- 5. Saeger W, Reincke M, Scholz GH, Lüdecke DK. [Ectopic ACTH- or CRH-secreting tumors in Cushing's syndrome]. Zentralbl Pathol. 1993;139(2):157–163. [PubMed] [Google Scholar]
- 6. Park SY, Rhee Y, Youn JC, Park YN, Lee S, Kim DM, et al. Ectopic Cushing's syndrome due to concurrent corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) secreted by malignant gastrinoma. Exp Clin Endocrinol Diabetes. 2007;115(1):13–16. [DOI] [PubMed] [Google Scholar]
- 7. Sauer N, Zur Wiesch CS, Flitsch J, Saeger W, Klutmann S, Zustin J, et al. Cushing's Syndrome Due to a Corticotropin-Releasing Hormone- and Adrenocorticotrophic Hormone-Producing Neuroendocrine Pancreatic Tumor. Endocr Pract. 2014;20(4):e53–e57. [DOI] [PubMed] [Google Scholar]
- 8. Shahani S, Nudelman RJ, Nalini R, Kim HS, Samson SL. Ectopic corticotropin-releasing hormone (CRH) syndrome from metastatic small cell carcinoma: a case report and review of the literature. Diagnostic Pathology. 2010;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zangeneh F, Young WF, Jr, Lloyd RV, Chiang M, Kurczynski E, Zangeneh F. Cushing's syndrome due to ectopic production of corticotropin-releasing hormone in an infant with ganglioneuroblastoma. Endocr Pract. 2003;9(5):394–399. [DOI] [PubMed] [Google Scholar]
- 10. Findling JW, Kehoe ME, Shaker JL, Raff H. Routine inferior petrosal sinus sampling in the differential diagnosis of adrenocorticotropin (ACTH)-dependent Cushing's syndrome: early recognition of the occult ectopic ACTH syndrome. J Clin Endocrinol Metab. 1991;73(2):408–413. [DOI] [PubMed] [Google Scholar]
- 11. Reimondo G, Paccotti P, Minetto M, Termine A, Stura G, Bergui M, et al. The corticotrophin-releasing hormone test is the most reliable noninvasive method to differentiate pituitary from ectopic ACTH secretion in Cushing's syndrome. Clin Endocrinol (Oxf). 2003;58(6):718–724. [DOI] [PubMed] [Google Scholar]
- 12. Batista DL, Riar J, Keil M, Stratakis CA. Diagnostic tests for children who are referred for the investigation of Cushing syndrome. Pediatrics. 2007;120(3):e575–e586. [DOI] [PubMed] [Google Scholar]
- 13. Hashimoto K, Nishioka T, Numata Y, Ogasa T, Kageyama J, Suemaru S. Plasma levels of corticotropin-releasing hormone in hypothalamic-pituitary-adrenal disorders and chronic renal failure. Acta Endocrinol (Copenh). 1993;128(6):503–507. [DOI] [PubMed] [Google Scholar]
- 14. Ejaz S, Vassilopoulou-Sellin R, Busaidy NL, Hu MI, Waguespack SG, Jimenez C, et al. Cushing syndrome secondary to ectopic adrenocorticotropic hormone secretion: the University of Texas MD Anderson Cancer Center Experience. Cancer. 2011;117(19):4381–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]