Abstract
BACKGROUND
The potential of gemcitabine to interact with carboplatin was explored in a phase II trial in ‘platinum-resistant’ ovarian cancer. Peripheral blood lymphocytes (PBLs) were sampled after drug administration to measure DNA interstrand crosslink formation and repair.
PATIENTS AND METHODS
40 patients received carboplatin AUC4 followed by gemcitabine 1000 mg/m2 with a second dose of gemcitabine on day 8. PBLs were obtained in 12 patients before, and at intervals during the first cycle of chemotherapy. DNA crosslink formation and repair (unhooking) were measured by the single cell gel electrophoresis (comet) assay following ex vivo incubation.
RESULTS
The global response rate was 47% (RECIST rate: 29%; CA125 rate: 63%). Delays in treatment were seen in 24% of cycles largely due to myelosuppression; 15% of day 8 administration was omitted. Peak carboplatin-induced DNA crosslinking was seen by 24 hours. Significant reduction was seen in the repair of in vivo carboplatin-induced DNA crosslinks following administration of gemcitabine.
CONCLUSION
An enhanced activity of carboplatin in ‘platinum-resistant’ ovarian cancer may be due to synergy with gemcitabine through inhibition of repair of DNA crosslinks. Future studies should explore co-administration of these drugs, as this may be a more effective schedule.
Introduction
Platinum containing drugs are the most active compounds for treating ovarian cancer. They are often reused for recurrent disease but their activity diminishes as tumors becoming increasingly resistant. The working definition of ‘platinum resistance’, based on observations made many years ago (1, 2), refers to tumors relapsing or progressing within 6 months of previous therapy. In this situation the probability of a further response to platinum compounds is low and most patients are offered non-platinum drugs. However, the activity of these agents in this situation is modest and usually short lived.
Gemcitabine, (2′,2′-difluorodeoxycytidine) is one of many non-platinum drugs that has some activity in recurrent, ‘platinum-resistant ovarian’ cancer(3). Interestingly, preclinical studies suggest that gemcitabine may have an additive or synergistic effect when combined with cisplatin (4) and clinical studies in a group of women who had previously received multiple lines of therapy support this notion (5, 6). The cytotoxicity of platinum drugs results from the formation of DNA adducts. Intrastrand crosslinks constitute the majority (>80%) of lesions formed on cellular DNA and these distorting lesions are repaired by nucleotide excision repair (7). Interstrand crosslinks, which link the two complementary strands of DNA together, comprise less than 5% of the total DNA lesions but are highly cytotoxic and difficult to repair (8). We have recently demonstrated enhanced repair of DNA interstrand crosslinks in ovarian cancer cells from patients following treatment with platinum-based chemotherapy (9). In vitro studies with cell lines have demonstrated a direct inhibitory effect of gemcitabine on the repair of cisplatin-induced crosslinks and suggested a mechanistic basis for the cytotoxic synergy between the two drugs (10) although this had not been studied in the clinic.
In this phase II study in ‘platinum-resistant’ ovarian cancer we explored the possibility that gemcitabine might have a synergistic effect on carboplatin therapy resulting in a higher tumor response than might be expected from either drug on its own. Additionally, a potential mechanism of interaction was studied by examining the effect of gemcitabine on the formation and repair of carboplatin-induced DNA interstrand crosslinks in peripheral blood lymphocytes (PBLs).
Methods
Between June 2004 and June 2006, 40 women gave consent to enter this phase II open labelled trial from four UK hospitals following ethical and regulatory approval.
Patient eligibility
Patients aged 18 years or older with known epithelial ovarian carcinoma, primary peritoneal carcinoma of serous type or fallopian tube carcinoma were included. All women relapsed or progressed within 6 months of previous platinum-based chemotherapy and started carboplatin and gemcitabine when there was a clinical indication for chemotherapy. Patients had an ECOG performance score of 0-2, a life expectancy > 12 weeks and assessable disease, either radiologically measurable, or evaluable by serum CA125.
Treatment plan
All patients received carboplatin at AUC 4 (or AUC 5 if the GFR was calculated) intravenously on day 1 as a 30 minute infusion followed by gemcitabine 1000mg/m2 intravenously over 30 minutes on days 1 and 8 for a maximum of six cycles, every three weeks. Treatment was delayed for up to two weeks if the neutrophil count was < 1.5 ×109/l or platelets < 100 × 109/l. Granulocyte colony stimulating factors were not used in this study. Gemcitabine was reduced to 800 mg/m2 for delays of more than one week, presence of neutropenic fever or grade 4 thrombocytopenia. The dose of gemcitabine was reduced by 50% on day 8 if neutrophils were between 1.0 and 1.4 × 109/l, and/or the platelets between 75-99 × 109/l. For values lower than this gemcitabine on day 8 was omitted.
Assessments
Patients were assessed at baseline, prior to each chemotherapy cycle and then six weekly for the first six months and then three monthly until progression or death. Response was assessed either by CT or CA125. CT scans were performed at baseline and after the third, sixth or final chemotherapy cycle. Serum CA125 was measured at baseline and prior to each chemotherapy cycle. A CA125 response was defined according to the Gynaecological Cancer Intergroup as at least a 50% decrease, confirmed with a further sample at least 28 days later (11). Patients stopped treatment after three cycles for disease progression, defined either by a > 30% increase in measurable lesions, development of new lesions or if the CA125 rose beyond twice the lowest previous level. Toxicity was assessed according to the NCI Common Terminology Criteria for Adverse Events, Version 3.0.
Statistical analysis
The primary outcome measure was a RECIST response rate to gemcitabine and carboplatin; secondary measures included toxicity, CA125 response, time to disease progression and (in selected centres only) measurement of DNA damage in PBLs before, during and after therapy. We also assessed the global response rate based on having a response under either the RECIST or CA125 criteria. A two-stage Simon design (12) was used assuming the response rate of gemcitabine was 15%, and the acceptable response rate of combination chemotherapy was 30%, 10% statistical significance and 80% power. It was concluded that the regimen would be worthy of further study if 9 or more responses were observed from a total of 39 patients. Overall and progression-free survival were calculated from date of registration to date of death, and progression or death, respectively.
Translational research
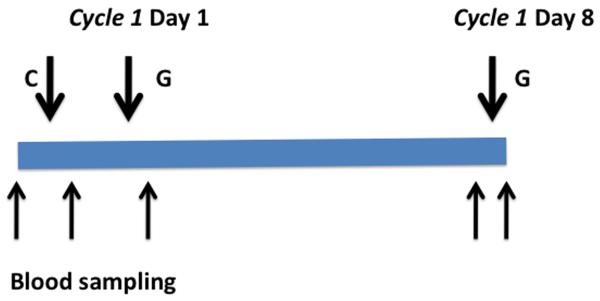
Blood samples were taken from 12 patients before chemotherapy, immediately after carboplatin infusion and immediately after gemcitabine on the first cycle of chemotherapy. This sequencing allowed measurement of DNA damage and repair of PBLs following carboplatin and after carboplatin and gemcitabine in the same patient. On day 8, blood samples were taken before and after gemcitabine infusion (Figure 1). PBLs were isolated using the Vacutainer® CPT™ system. Following centrifugation and removal of the mononuclear layer, cells were washed once and re-suspended. PBLs were then incubated ex vivo for 0, 4, 24 and 48 hours and carboplatin-induced DNA interstrand crosslink formation and repair (unhooking) were assessed using the single cell gel electrophoresis (Comet) assay, as previously described (13, 14). Briefly, PBL samples were irradiated (12.5Gy) to deliver a fixed number of random DNA strand breaks. After embedding cells in 1% low gelling temperature agarose on a precoated microscope slide, the cells were lysed (pH 10.5) for 1 hour, washed, then incubated in alkali buffer (pH 12.5) for 45 minutes followed by electrophoresis. After drying, the slides were stained with propidium iodide. The images were visualised using an inverted fluorescent microscope and captured using a digital camera. For each duplicate slide 25 cells were analysed. The tail moment for each image was calculated using the Komet 5.5 Analysis software (Andor Technology, Kinetic Imaging, U.K.) based on the definition of Olive et al (15).
Figure 1. Treatment and Blood sampling schema. C= Carboplatin; G= Gemcitabine.
In patients receiving carboplatin and gemcitabine, DNA interstrand crosslinking was expressed as percentage decrease in tail moment compared to irradiated controls calculated by the formula below. This formula was used to compensate for any additional single strand breaks induced by gemcitabine in addition to those produced by the irradiation step. The greater the percentage decrease in tail moment, the greater the level of DNA interstrand crosslinking.
where TMdi = tail moment of drug-treated irradiated sample; TMdu = tail moment of drug-treated unirradiated sample; TMcu = tail moment of untreated, unirradiated control; TMci = tail moment of untreated, irradiated control.
Results
Baseline characteristics of patients are given in Table 1. All had developed progressive disease within 6 months of previous treatment but in some the start of carboplatin and gemcitabine was delayed beyond this time, until there was a clinical indication to start treatment.
Table 1. Patient baseline characteristics.
| No. of patients (%) | |
|---|---|
| ECOG performance status: | |
| 0 | 16 (40) |
| 1 | 18 (45) |
| 2 | 6 (15) |
| Primary site: | |
| Ovary | 35 (88) |
| Primary peritoneal | 4 (10) |
| Missing | 1 (3) |
| Age at registration (years): | |
| <60 | 10 (25) |
| 60-69.9 | 23 (58) |
| 70+ | 7 (18) |
| Median age: 64.3 (range: 47.5-80.1) years | |
| Measurable disease, according to RECIST criteria: | |
| Yes | 35 (88) |
| No (no measured target lesions) | 5 (13) |
| Previous anticancer therapy: | |
| No. of prior platinum-contained regimens: | |
| 1 | 27 (68) |
| 2 | 10 (25) |
| 3+ | 2 (5) |
| Missing | 1 (3) |
Toxicity
Twenty-one patients received six cycles of chemotherapy and only six (15%) received less than 3 complete cycles. The main reason for early cessation of treatment was disease progression. Treatment administration is summarised in Table 2. Nineteen patients had a dose delay due to toxicity and in 36% this occurred at cycle 2. Three patients did not have a second cycle. Twenty-four percent of all chemotherapy cycles were delayed. Most of the toxicity was asymptomatic hematological toxicity and 30 patients required a dose reduction of gemcitabine or omission of the drug on Day 8. Twenty eight (15%) of all cycles were given without day 8 gemcitabine. Grade 3/4 neutropenia was seen in 15 (38%) patients but there were only four cases of neutropenic fever. Grade 3/4 thrombocytopenia occurred in seven patients (18%). Other non-hematological grade 3-4 toxicities such as nausea, vomiting or fatigue each occurred in 5% or less of patients.
Table 2. Treatment Summary.
| Compliance with chemotherapy 1 | No. of patients (%) |
|---|---|
| No. of chemotherapy cycles received | |
| ≤ 2 | 6 (15) |
| 3 | 6 (15) |
| 4 | 5 (13) |
| 5 | 2 (5) |
| 6 | 21 (53) |
| Chemotherapy dose reductions 2 | |
| Cycle 1 | 9/40 (23) |
| Cycle 2 | 16/36 (44) |
| Cycle 3 | 14/34 (41) |
| Cycle 4 | 16/28 (57) |
| Cycle 5 | 15/23 (65) |
| Cycle 6 | 14/20 (70) |
| Any cycle | 30/40 (75) |
Not all patient records are complete with respect to chemotherapy dates and dosages. Therefore the number of patients at risk of having a reduction may not match the number of cycles received.
Chemotherapy reductions are calculated as dose reductions > 10% compared to Day 1, Cycle 1 or a Day 8 omission.
Response
Response assessments were made after 3 cycles in 34 patients with measurable disease, and after 6 cycles in 19 patients. Five patients had no target lesions measured and one patient had missing RECIST response data. Five out of 34 patients died or were too ill to be assessed by the end of cycle 3 and were considered to have progressive disease by cycle 3. Ten of 34 (29%) patients had a measurable response (one complete response and nine partial responses) by the end of chemotherapy. Seventeen out of 39 patients (44%) had progressive disease while on treatment.
Response was assessed by CA125 measurement in 27 of the 40 patients. Five patients were not assessable because of an initially low CA125 level, seven patients had less than three CA125 measurements and one patient did not have a CA125 assessment within the specified time from the start of chemotherapy. Overall, 17/27 (63%) patients had a 50 % or greater fall in CA125. The global response rate based on either RECIST or CA125 was 47% (18/38).
Survival
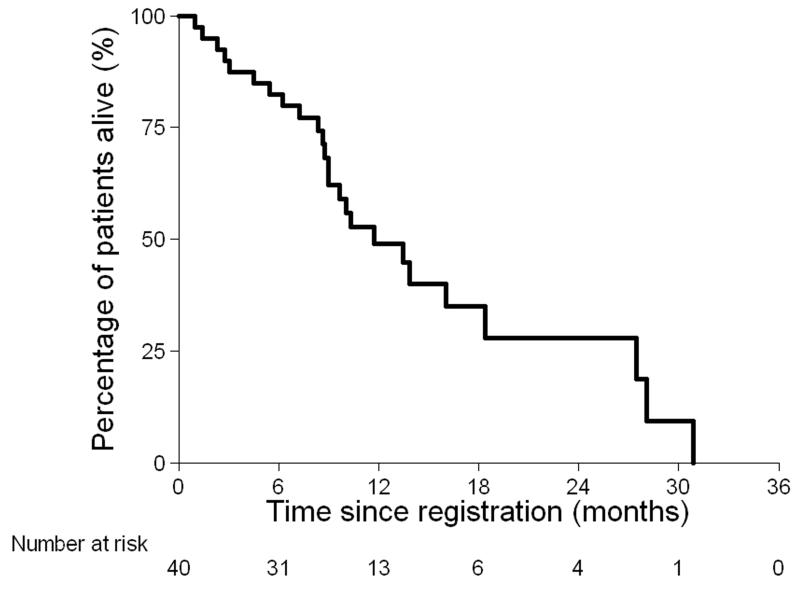
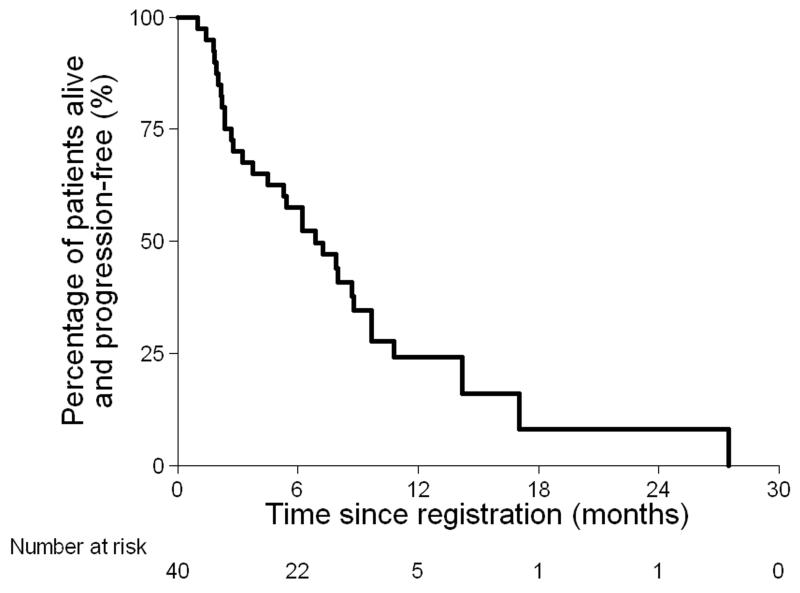
At the time of analysis 25 out of 40 (63%) patients had died (Figure 2a). Median survival was 11.7 months (95% CI: 9.0 – 18.4 months). Thirty-one of the 40 (78%) patients had progressive disease, or died (Figure 2b). The median progression-free survival was 6.9 months (95% CI 3.7-8.8 months). When measured by CA125, progression-free survival was 8.1 months (95% CI 6.2-10.1 months).
Figure 2. Overall survival (a) and Progression-free survival (b).
Effect of gemcitabine on repair of carboplatin-induced DNA damage
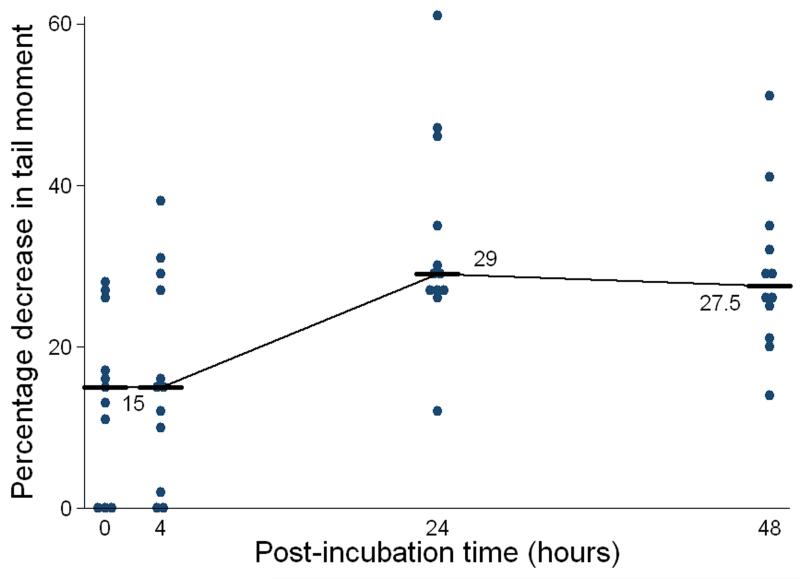
The crosslink data for the 12 patients, from whom PBL samples were taken, are shown in Figure 3. Carboplatin–induced DNA interstrand crosslink formation was observed to increase over 24 hours post carboplatin (Figure 3a) and in the sample taken after carboplatin and gemcitabine (Figure 3b). In the majority of patients (75%) complete repair (unhooking) of DNA interstrand crosslinks was observed at 48 hours post-treatment following carboplatin alone (Figure 3a). However, in the same patients following treatment in combination with gemcitabine, the level of repair of carboplatin-induced interstrand crosslinks at 48 hours was significantly reduced. The Wilcoxon Signed Rank Test indicates no treatment difference at 0 and 4 hours, but at 24 and 48 hours there is evidence that after the addition of gemcitabine there is a larger decrease in tail moment (p=0.006 and 0.002, respectively), with no patient sample showing complete repair (Figure 3b).
Figure 3. Formation and repair of carboplatin-induced DNA interstrand crosslinks in PBLs taken from 12 patients following treatment with carboplatin (a) and then in a sample taken after the administration of gemcitabine following carboplatin (b).
Results are expressed as percentage decrease in tail moment and the horizontal bars and numbers indicate the median values.
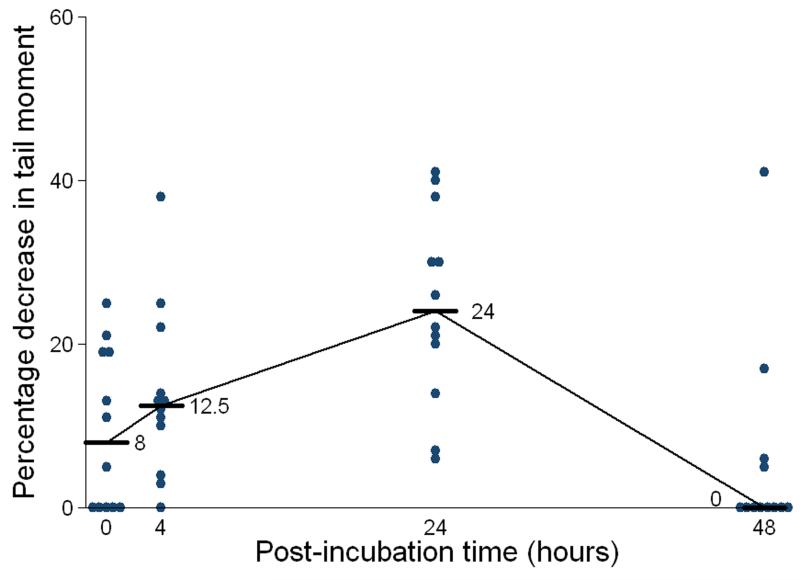
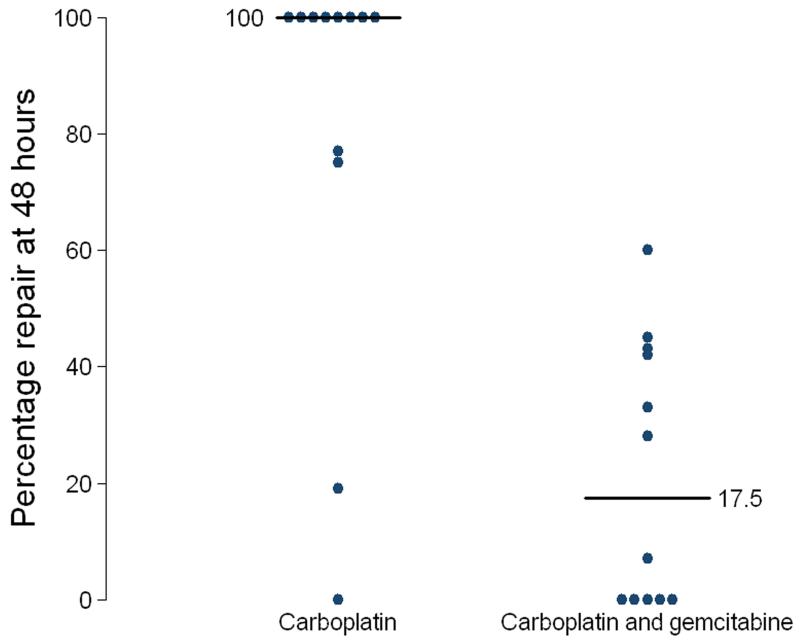
With carboplatin alone, median repair of DNA interstrand crosslinks at 48 hours was 100% (Figure 4). Eight of twelve patients demonstrated 100% repair at 48 hours following carboplatin alone, with the remaining four patients demonstrating repair ranging from 0 to 75%. However, following combination with gemcitabine in the same patients, repair of carboplatin-induced DNA interstrand crosslinks was significantly reduced (Non-parametric Wilcoxon Signed Rank test p-value = 0.003) with a median repair of 17.5% (Figure 4). Five patients showed no repair at 48 hours. Seven patients showed repair ranging from 7 to 60%. No single strand breaks were seen in any patient following gemcitabine alone. These data demonstrate that the addition of gemcitabine significantly inhibits the repair (unhooking) of carboplatin-induced DNA interstrand crosslinks in vivo.
Figure 4. Percentage repair of carboplatin-induced DNA interstrand crosslinks in patient PBLs at 48 hours in the blood samples taken after treatment with carboplatin alone and after treatment with the combination of carboplatin and gemcitabine.
Horizontal bars indicate median values.
Discussion
This study further supports the view that in patients gemcitabine has a synergistic effect on platinum therapy. Firstly, in patients with ‘platinum resistance’ the global response rate was 45 % (18/40). Platinum resistance is not absolute; some tumors respond and others are truly resistant. Historic data suggests the response rate in ‘platinum resistant’ tumors is less than 20% (1, 2). Thus, some caution is needed in interpreting the results of a non randomised study due to the heterogeneity of ‘platinum resistance’. Similar reasoning applies to single-agent gemcitabine where the response rate in this population was reported to be 17% (16).
Nevertheless, our results using carboplatin and gemcitabine are in keeping with other reported studies of cisplatin and gemcitabine, all of which used cisplatin in a day1 and day 8 schedule (5, 6, 17-19). Dividing the cisplatin schedule into a day 1 and day 8 administration exploits the knowledge from in vitro studies that have suggested the effect of the two drugs is synergistic. The initial preclinical report by Peters et al (20) did not identify a mechanism but suggested the effects on platinum-sensitive and resistant cell lines were schedule dependent. It was not due to accumulation of gemcitabine triphosphate, or increase in DNA strand breaks. In vitro studies have shown that gemcitabine impairs repair of platinum-induced crosslinks (10). The initial clinical reports of activity of the combination of cisplatin and gemcitabine in a heterogeneous group of patients including those with clear ‘platinum-resistant’ disease supported this hypothesis, and included some patients who failed to respond to single agent gemcitabine but responded when cisplatin was added (6).
Secondly, the key finding in our study is that we have demonstrated a mechanism of synergy between carboplatin and gemcitabine in patients, supporting the observations with cisplatin and gemcitabine. Although we were not able to study the effect of the drugs on tumor tissue, the interaction of carboplatin and gemcitabine was demonstrated in vivo, by studying the real-time effect of DNA crosslink formation and repair in PBLs removed from patients at varying time points. The maximum formation of interstrand crosslinks was seen 24 hours after carboplatin administration. Following incubation of cells removed after carboplatin was administered, the repair of crosslinks was efficient in the majority of patients, with 8 out of 12 showing complete repair at 48 hours. We have previously shown significant repair of crosslinks in ovarian cancer cells from patients who had received platinum-based therapy (9). In contrast, in samples taken after gemcitabine administration, repair of the crosslinks produced by carboplatin was significantly impaired at 48 hours (Figure 4). The slightly higher DNA crosslink formation at 24 hours after carboplatin and gemcitabine compared to carboplatin alone (Figure 3) is likely to be due to the balancing effects of crosslink formation and repair. It has been shown in a lung cancer cell line that there were more platinum-DNA adducts in the presence of gemcitabine (21). No strand breaks were observed following gemcitabine alone in PBLs in the present study under the assay conditions employed, although a previous in vitro study has demonstrated the formation of DNA strand breaks following gemcitabine (22).
Gemcitabine is believed to inhibit nucleotide excision repair by incorporation into repair patches thereby causing chain termination (10). Repair of interstrand crosslinks is complex and is thought to involve components of the nucleotide excision repair pathway and recombination (8). A modified comet assay was employed in the current study to measure interstrand crosslinking. The disappearance of crosslinks measured using this assay reflects an early step in the repair process, the unhooking of the crosslink from one strand. This study demonstrates this step is clearly inhibited in vivo for carboplatin-induced crosslinks by gemcitabine. One possible mechanism for this is that the nucleotide excision repair of carboplatin-induced intrastrand adducts is inhibited by incorporation of gemcitabine into repair patches resulting in sequestering of repair proteins, including those required for the initial unhooking step of DNA interstrand crosslinks.
This study supports the notion that ‘platinum-resistance’ is usually not absolute and that patients in this group may continue to benefit from platinum-based therapy. First-line studies have not shown that carboplatin-gemcitabine combinations are superior to standard chemotherapy with carboplatin and paclitaxel (23) but progression-free survival is prolonged when gemcitabine is added to carboplatin in patients with ‘platinum-sensitive’ relapse (24). A reason for this may be that in this group of patients there is a reduction in platinum sensitivity that is partially overcome through the synergistic effect of gemcitabine. In the standard three weekly schedule of carboplatin and gemcitabine, used frequently in lung and ovarian cancer, carboplatin is given only on day1 of each cycle and gemcitabine on day 1 and 8. In recurrent ovarian cancer we and others (24) have found that this regimen has significant but asymptomatic hematological toxicity, frequently requiring a dose reduction or omission of day 8 gemcitabine.
If the improved antitumor effect results from synergy between the drugs as shown in PBLs there is a strong rationale for changing the schedule to combine each dose of gemcitabine with carboplatin, as is the case with cisplatin and gemcitabine (5, 6, 17-19). Our in vivo data in PBLs supports this alternative approach to therapy, dividing the dose of carboplatin between day 1 and day 8, always giving carboplatin with gemcitabine. Reports of using dose-fractionated therapies with, for example, carboplatin and paclitaxel in both ‘platinum-resistant’ and newly diagnosed ovarian cancer have been encouraging (25, 26). Regimens of dose-fractionated carboplatin with gemcitabine are now being explored (27) and these should be more extensively studied in ovarian cancer. This will make best use of synergy between the drugs and this schedule may be less myelosuppressive.
Translational Statement.
We have shown that the combination of gemcitabine with carboplatin is synergistic by demonstrating in patients’ lymphocytes that gemcitabine, administered following carboplatin leads to inhibition in the repair of carboplatin-induced DNA interstrand crosslinks. These findings support the view that the combination of carboplatin and gemcitabine is rational, and that clinically useful synergy may occur. The combination should in future be given as a doublet to make best use of the interaction, and further studies should be performed with this schedule in tumors where these drugs are currently used.
Acknowledgements
This work was supported in part by Cancer Research UK (grant C2259/A9994), UCL Experimental Cancer Medicine Centre, UCLH Comprehensive Biomedical Research Centre, and an educational study grant from Eli Lilly Limited.
Footnotes
Presented in part at European Cancer Conference ECCO 14, Barcelona September 2007
References
- 1.Blackledge G, Lawton F, Redman C, Kelly K. Response of patients in phase II studies of chemotherapy in ovarian cancer: implications for patient treatment and the design of phase II trials. Br J Cancer. 1989;59:650–3. doi: 10.1038/bjc.1989.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markman M, Rothman R, Hakes T, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991;9:389–93. doi: 10.1200/JCO.1991.9.3.389. [DOI] [PubMed] [Google Scholar]
- 3.Lund B, Hansen OP, Theilade K, Hansen M, Neijt JP. Phase II study of gemcitabine (2′,2′-difluorodeoxycytidine) in previously treated ovarian cancer patients. J Natl Cancer Inst. 1994;86:1530–3. doi: 10.1093/jnci/86.20.1530. [DOI] [PubMed] [Google Scholar]
- 4.Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Peters GJ. Synergistic interaction between cisplatin and gemcitabine in vitro. Clin Cancer Res. 1996;2:521–30. [PubMed] [Google Scholar]
- 5.Nagourney RA, Brewer CA, Radecki S, et al. Phase II Trial of Gemcitabine plus Cisplatin Repeating Doublet Therapy in Previously Treated, Relapsed Ovarian Cancer Patients. Gynecologic Oncology. 2003;88:35–9. doi: 10.1006/gyno.2002.6855. [DOI] [PubMed] [Google Scholar]
- 6.Rose PG, Mossbruger K, Fusco N, Smrekar M, Eaton S, Rodriguez M. Gemcitabine Reverses Cisplatin Resistance: Demonstration of Activity in Platinum- and Multidrug-Resistant Ovarian and Peritoneal Carcinoma. Gynecologic Oncology. 2003;88:17–21. doi: 10.1006/gyno.2002.6850. [DOI] [PubMed] [Google Scholar]
- 7.Fichtinger-Schepman AM, van Dijk-Knijnenburg HC, van der Velde-Visser SD, Berends F, Baan RA. Cisplatin- and carboplatin-DNA adducts: is PT-AG the cytotoxic lesion? Carcinogenesis. 1995;16:2447–53. doi: 10.1093/carcin/16.10.2447. [DOI] [PubMed] [Google Scholar]
- 8.McHugh PJ, Spanswick VJ, Hartley JA. Repair of DNA interstrand crosslinks: molecular mechanisms and clinical relevance. Lancet Oncol. 2001;2:483–90. doi: 10.1016/S1470-2045(01)00454-5. [DOI] [PubMed] [Google Scholar]
- 9.Wynne P, Newton C, Ledermann JA, Olaitan A, Mould TA, Hartley JA. Enhanced repair of DNA interstrand crosslinking in ovarian cancer cells from patients following treatment with platinum-based chemotherapy. Br J Cancer. 2007;97:927–33. doi: 10.1038/sj.bjc.6603973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moufarij MA, Phillips DR, Cullinane C. Gemcitabine potentiates cisplatin cytotoxicity and inhibits repair of cisplatin-DNA damage in ovarian cancer cell lines. Mol Pharmacol. 2003;63:862–9. doi: 10.1124/mol.63.4.862. [DOI] [PubMed] [Google Scholar]
- 11.Rustin GJ, Quinn M, Thigpen T, et al. New guidelines to evaluate the response to treatment in solid tumors (ovarian cancer) J Natl Cancer Inst. 2004;96:487–8. doi: 10.1093/jnci/djh081. [DOI] [PubMed] [Google Scholar]
- 12.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 13.Hartley JM, Spanswick VJ, Gander M, et al. Measurement of DNA cross-linking in patients on ifosfamide therapy using the single cell gel electrophoresis (comet) assay. Clin Cancer Res. 1999;5:507–12. [PubMed] [Google Scholar]
- 14.Spanswick V, Hartley J, Ward T, Hartley J. Measurement of drug-induced DNA interstrand crosslinking using the single cell gel electrophoresis (comet) assay. In: Brown R, Boger-Brown U, editors. Methods in Molecular Medicine. Humana Press; New York: 1999. pp. 143–54. [DOI] [PubMed] [Google Scholar]
- 15.Olive PL, Banath JP, Durand RE. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the “comet” assay. Radiat Res. 1990;122:86–94. [PubMed] [Google Scholar]
- 16.D’Agostino G, Amant F, Berteloot P, Scambia G, Vergote I. Phase II study of gemcitabine in recurrent platinum-and paclitaxel-resistant ovarian cancer. Gynecol Oncol. 2003;88:266–9. doi: 10.1016/s0090-8258(03)00011-8. [DOI] [PubMed] [Google Scholar]
- 17.Bozas G, Bamias A, Koutsoukou V, et al. Biweekly gemcitabine and cisplatin in platinum-resistant/refractory, paclitaxel-pretreated, ovarian and peritoneal carcinoma. Gynecol Oncol. 2007;104:580–5. doi: 10.1016/j.ygyno.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Brewer CA, Blessing JA, Nagourney RA, Morgan M, Hanjani P. Cisplatin plus gemcitabine in platinum-refractory ovarian or primary peritoneal cancer: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2006;103:446–50. doi: 10.1016/j.ygyno.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Tewari D, Monk BJ, Hunter M, Falkner CA, Burger RA. Gemcitabine and cisplatin chemotherapy is an active combination in the treatment of platinum-resistant ovarian and peritoneal carcinoma. Invest New Drugs. 2004;22:475–80. doi: 10.1023/B:DRUG.0000036690.14585.a3. [DOI] [PubMed] [Google Scholar]
- 20.Peters GJ, Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Braakhuis BJ. Interaction between cisplatin and gemcitabine in vitro and in vivo. Semin Oncol. 1995;22:72–9. [PubMed] [Google Scholar]
- 21.van Moorsel CJ, Pinedo HM, Veerman G, et al. Mechanisms of synergism between cisplatin and gemcitabine in ovarian and non-small-cell lung cancer cell lines. Br J Cancer. 1999;80:981–90. doi: 10.1038/sj.bjc.6690452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plunkett W, Huang P, Gandhi V. Preclinical characteristics of gemcitabine. Anticancer Drugs. 1995;6(Suppl 6):7–13. doi: 10.1097/00001813-199512006-00002. [DOI] [PubMed] [Google Scholar]
- 23.Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27:1419–25. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfisterer J, Plante M, Vergote I, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 25.Havrilesky LJ, Alvarez AA, Sayer RA, et al. Weekly low-dose carboplatin and paclitaxel in the treatment of recurrent ovarian and peritoneal cancer. Gynecol Oncol. 2003;88:51–7. doi: 10.1006/gyno.2002.6859. [DOI] [PubMed] [Google Scholar]
- 26.Katsumata N, Yasuda M, Takahashi F, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009 doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez RD, Mannel R, Garcia AA, et al. Fixed-dose rate gemcitabine plus carboplatin in relapsed, platinum-sensitive ovarian cancer patients: results of a three-arm Phase I study. Gynecol Oncol. 2009;115:389–95. doi: 10.1016/j.ygyno.2009.09.013. [DOI] [PubMed] [Google Scholar]