Abstract
Extra-group paternity (EGP) occurs commonly among group-living mammals and plays an important role in mating systems and the dynamics of sexual selection; however, socio-ecological and genetic correlates of EGP have been underexplored. We use 23 years of demographic and genetic data from a high-density European badger (Meles meles) population, to investigate the relationship between the rate of EGP in litters and mate availability, mate incompatibility and mate quality (heterozygosity). Relatedness between within-group assigned mothers and candidate fathers had a negative quadratic effect on EGP, whereas the number of neighbouring-group candidate fathers had a linear positive effect. We detected no effect of mean or maximum heterozygosity of within-group candidate fathers on EGP. Consequently, EGP was associated primarily with mate availability, subject to within-group genetic effects, potentially to mitigate mate incompatibility and inbreeding. In badgers, cryptic female choice, facilitated by superfecundation, superfoetation and delayed implantation, prevents males from monopolizing within-group females. This resonates with a meta-analysis in group-living mammals, which proposed that higher rates of EGP occur when within-group males cannot monopolize within-group females. In contrast to the positive meta-analytic association, however, we found that EGP associated negatively with the number of within-group assigned mothers and the number of within-group candidate fathers; potentially a strategy to counter within-group males committing infanticide. The relationship between the rate of EGP and socio-ecological or genetic factors can therefore be intricate, and the potential for cryptic female choice must be accounted for in comparative studies.
Keywords: breeding density, European badger, extra-pair paternity, group composition, heterozygosity, inbreeding, mate incompatibility, mating system, promiscuity
Introduction
In socially monogamous pair-breeding and group breeding species, offspring may be fathered by males from outside of the female's pair or group. This is termed extra-group or extra-pair paternity (EGP/EPP; hereafter EGP), where all, or just a proportion, of litters (broods) may have a nonresident father. EGP is a widespread phenomenon, occurring in 90% of socially monogamous bird species (Griffith et al., 2002) and more than two-thirds of the 26 social group-living mammalian species investigated so far (Isvaran & Clutton-Brock, 2007; Soulsbury, 2010). EGP can play an important role in the mating system and the socio-genetic structuring of otherwise socially segregated populations (Young et al., 2007; Schubert et al., 2011), yet there is limited understanding of the interaction between socio-ecological and genetic factors in determining the frequency of EGP (Cohas et al., 2006; Isvaran & Clutton-Brock, 2007).
Although males may engage in EGP to increase breeding success (Westneat et al., 1990), for example gaining kleptogamous mating opportunities despite subordinate breeding status (Young et al., 2007), the advantages of EGP to females are less clear (Clutton-Brock & McAuliffe, 2009). In species where females are also able to reassess choices through cryptic mechanisms such as superfecundation, superfoetation, delayed implantation, selective implantation and embryonic re-absorption (Yamaguchi et al., 2006), mating with multiple partners might have a selective advantage. For example, extra group mating combined with within-group mating might mask extra-group paternity, as a counter-strategy to infanticide (Agrell et al., 1998).
Mate selection is predicated upon mixed criteria, such as: (i) which mating partners are available or accessible; (ii) mate compatibility (i.e. the rejection of unsuitable mates or unviable embryos); and (iii) mate quality (e.g. mate heterozygosity, when this is associated with increased offspring or grand-offspring fitness) (Jennions & Petrie, 2000; Kokko & Rankin, 2006; Kempenaers, 2007).
The availability of potential (candidate) partners may be an important determinant of mate selection. Mate availability is affected by the rate at which individuals encounter each other (e.g. Connor & Whitehead, 2005), the potential for female coercion (Smuts & Smuts, 1993), and the extent to which access to females might be defended by co-resident males (e.g. Treves, 1998). For example, EGP could arise if females mated randomly with whomever they encountered and, by chance, therefore mated with extra-group males (Kokko & Rankin, 2006). Under this mechanism, encounter rates between individuals from different groups will affect mating opportunities, as a function of group-range overlap or the rate of intergroup transgression. Higher encounter rates with neighbouring groups than with more isolated groups would therefore be expected to lead to higher rates of EGP with these neighbouring males.
Without further mate selection refinement (or post-copulatory selection), EGP might be nonadaptive; in fact, extra-group (or extra-pair) paternity occurs in many species without advantages being apparent (e.g. Forstmeier et al., 2011). There are, however, risks associated with EGP, such as agonistic encounter with a same-sex competitor from the group (or pair) visited, or the possible rejection of extra-group young (Westneat & Stewart, 2003) if EGP can be detected. EGP can provide an adaptive tactic through which females seek to increase offspring production, for example, avoidance of infanticide when neighbouring-group/immigrant males pose a threat to infants (Agrell et al., 1998; Borries et al., 2011) or fertility assurance (Sheldon, 1994; Vedder et al., 2011).
If social mate choice is limited, individuals might settle for a social group mate with less optimal compatibility, but mitigate this with extra group matings (Richardson et al., 2005). Mate incompatibility may lead to inbreeding depression (Moore & Ali, 1984; Pusey & Wolf, 1996), and inbreeding avoidance through EGP has been shown empirically in some mammal (e.g. Sillero-Zubiri et al., 1996) and bird populations (e.g. Brouwer et al., 2011). Moreover, dispersing from the natal territory involves risk (Van Vuren & Armitage, 1994), with the potential to exacerbate inbreeding. EGP may therefore have evolved to mitigate inbreeding (Durrant & Hughes, 2005) as a facet of promiscuity (Brooker et al., 1990), especially when offspring dispersal is delayed, or over a short distance (Pusey & Wolf, 1996). As a consequence, EGP frequency might correlate positively with the relatedness of breeding females to the mates available within their group (e.g. Kingma et al., 2013).
Alternatively, the advantages of EGP for a breeding population might be due to genetic benefits, such as the ‘Good-genes-as-heterozygosity Hypothesis’ (Brown, 1997). This posits that ‘general’ allelic diversity increases fitness, consequently individuals should select mates to produce the most heterozygous offspring. The relationship between fitness and heterozygosity, however, can vary (Britten, 1996; David, 1998; Hansson & Westerberg, 2002; Coltman & Slate, 2003; Annavi et al., 2014). In studies that show positive effects, heterozygosity has been associated with higher offspring survival rates (Cohas et al., 2009; Mainguy et al., 2009; Annavi et al., 2014), breeding success (Harrison et al., 2011), disease resistance (Coltman et al., 1999; Whiteman et al., 2006) and developmental stability (reviewed in Kempenaers, 2007). In circumstances where mate heterozygosity confers fitness benefits to offspring (Fromhage et al., 2009), EGP rates would be predicted to correlate with the level of heterozygosity among within-group males (Cohas et al., 2006).
European badgers (Meles meles) provide an informative species to address the adaptive benefits of extra-group paternity. They exhibit a variety of traits that can lead to multiple-paternity litters (Carpenter et al., 2005; Dugdale et al., 2007). In the study population examined here, which typifies populations in south-western England, badgers have a polygynandrous mating system (i.e. they do not have one exclusive social mate; Dugdale et al., 2007, 2011); up to seven males and females breed within a social group per year, with a mean of 1.9 breeders of each sex (95% confidence interval: 1.8–2.0; range = 1–7, Dugdale et al., 2007). Badgers also have low fecundity (i.e. 1–4 cubs once per year; Macdonald & Newman, 2002; Carpenter et al., 2005; Dugdale et al., 2007), and extra-group paternity accounts for > 40% of offspring in our study population, which has been assigned mainly to males in neighbouring groups (Dugdale et al., 2007; see also Carpenter et al., 2005). An individual's social group and neighbouring groups therefore contain close relatives (Dugdale et al., 2008). Badgers typically have two mating peaks, a major peak immediately post-partum and a secondary peak in the late summer/autumn (Cresswell et al., 1992; see also Ahnlund, 1980). Females are induced ovulators, and gestation involves several months of embryonic diapause, where delayed implantation uncouples mating and parturition (Thom et al., 2004), and then they give birth fairly synchronously around February (Yamaguchi et al., 2006). Wandeler & Graf (1982) discovered that ova produced during delayed implantation may also be fertilized, resulting in superfoetation promoted by superfecundation (Yamaguchi et al., 2006). This extends the opportunity for females to select the most suitable mates, through pre- and post-copulatory mate choice (Andersson & Simmons, 2006; Fisher et al., 2006). Furthermore, a proportion of males extend testicular activity late into autumn – prolonging the mating season (Woodroffe & Macdonald, 1995; Buesching et al., 2009). Crucially, males provide no paternal care to litters (Fell et al., 2006; Dugdale et al., 2010).
Badgers in this population also exhibit high group fidelity, through natal philopatry (Woodroffe et al., 1995). Macdonald et al. (2008) report that 19% of the badgers captured at least four times in this population were found to have dispersed, mainly to adjacent neighbouring social groups. The extent to which each sex solicits extra-group mating is not known in badgers (Dugdale et al., 2011). Whether within-group males actively defend their group territory and/or within-group females is highly equivocal in our study population (Stewart et al., 1997; Kilshaw et al., 2009). Badgers forage solitarily, and both sexes make incursions (Bohm et al., 2009) and temporary visits to other groups (Macdonald et al., 2008), which demonstrates that within-group males are not able to control female access to extra-group males effectively.
Based on a genetic pedigree spanning 23 years of data, here we examine the effects of local socio-ecological (breeding group size and numbers, and proportions of sexes per group) and genetic (breeding group relatedness) factors on extra-group paternity rates. We test whether EGP is more likely when there is [1a] a larger number of neighbouring-group candidate fathers (86% of EGP were assigned to neighbouring-group fathers; Dugdale et al., 2007), [1b] a larger number of within-group candidate or assigned mothers and [1c] a lower number of within-group candidate fathers. We then test whether: [2a] EGP increases with the mean pairwise relatedness between within-group assigned mothers and candidate fathers, consistent with inbreeding avoidance and [2b] EGP correlates negatively with the mean or maximum heterozygosity of within-group candidate fathers (offspring first-year survival probability is positively correlated with paternal heterozygosity in years with low food availability in this population; Annavi et al., 2014).
Materials and methods
Study site and field methods
This study was based on a high-density population of badgers inhabiting Wytham Woods; a 424-ha site situated 5 km north-west of Oxford, England (51°46′26 N; 1°19′19 W), which has been studied intensively since the 1970s (Kruuk, 1978a,b). A detailed description of the study site (e.g. soil, microclimates and vegetation) is provided elsewhere (Morecroft et al., 1998; Savill et al., 2010). At this study site, there was a mean [95% confidence interval] of 19 ([17, 21]; range = 14–26; Dugdale et al., 2008) mixed-sex social groups (Johnson et al., 2002; Newman et al., 2011), with a mean of 13 ([12–14]; range: 2–51) individuals (including annual cubs) per social group per year (hereafter social-group-year).
Since 1987, this study has attempted to mark all individuals in the population, following a systematic capture–mark–recapture regime (Macdonald & Newman, 2002; Macdonald et al., 2009). Live-trapping was conducted three to four times per annum; generally over 2 weeks in June, September and November, with 1 week of trapping in January of some years (Macdonald et al., 2009). Badgers were caught in mesh-traps, baited with peanuts (Macdonald & Newman, 2002; Macdonald et al., 2009), placed near the entrances of active communal badger dens, termed setts (Noonan et al., 2014). Captured badgers were then transferred to holding cages and transported to a central handling facility and sedated by an intramuscular injection using ketamine hydrochloride at 0.2 mL kg−1 body weight (McLaren et al., 2005). Upon their first capture, all badgers were tattooed with a unique number on the left inguinal region for permanent individual identification. The sex, age-class (cub or adult, based on body size and previous trapping history) and capture location (social group name) of each badger were recorded. For genetic analysis, hair samples and/or blood from the jugular vein (ca 3 mL) were collected from all individuals.
Social group ranges were established using a ‘bait-marking’ technique approximately every 2 years (Kilshaw et al., 2009). The number of social group ranges within this study site has increased steadily (Macdonald et al., 2004) with population density (Macdonald & Newman, 2002; Macdonald et al., 2009). We defined the social group of residence of each individual per year (N = 1165; five unmarked individuals found dead on local roads, for which social group could not be assigned, were excluded from analyses) based on their trapping history, according to the following rules:
Badgers first caught as cubs (N = 839) were considered to be resident in the social group in which they were first caught (natal group; N = 709/839), unless they subsequently satisfied the dispersal rules (N = 130/839) provided by Macdonald et al. (2008).
Badgers first caught as adults (N = 326) were assigned to a social group based on the site where they were trapped most frequently (N = 273/326), unless: clear dispersal events (Macdonald et al., 2008) were recorded (N = 43/326), or they exhibited equal affiliation to two social groups over their lifetime, in which case they were assigned to the social group in which they were captured initially (N = 10/326).
In years when females were assigned maternity, they were allocated to the social group in which their cubs were born subsequently, if this differed from the previous historical trapping data (N = 3).
Badger social groups often include more than one sett in their group territory and therefore territories can undergo fission, that is the splitting of a social group into two (or more) new distinct social units (da Silva et al., 1993; Macdonald et al., 2004), dividing the physical range occupied by the former group (defined by bait-marking). Badgers trapped in the new social group were assigned accordingly after the group split (N = 41), unless they were subsequently retrapped in the former group (N = 173).
Genotyping and parentage analysis
Details of the DNA extraction, microsatellite characterization and genotyping methods are presented elsewhere (Dugdale et al., 2007; Annavi et al., 2011, 2014). We genotyped 1170 individuals trapped during 1987–2010, at 35 microsatellite loci, of which 813 were cubs born between 1988 and 2010. Means are provided with their lower and upper 95% confidence values, unless otherwise stated. Mean observed heterozygosity was estimated at 0.45 [0.39, 0.51], with 4.46 [3.79, 5.13] alleles per locus. No locus, or pair of loci, departed consistently from Hardy–Weinberg equilibrium or linkage equilibrium (Annavi et al., 2011). Two types of genotyping error, allelic drop-out rate (ε1 = 0.005) and stochastic error rate (ε2 = 0.005), were estimated using PEDANT 1.0 (Johnson & Haydon, 2007).
Parentage was determined sequentially using MasterBayes 2.47 (Hadfield et al., 2006) implemented in the R statistics programme 2.12.2 (R Development Core Team, 2011) and in Colony 2.0 (Wang & Santure, 2009). For detailed description about the selection of candidate parents and their social group assignment, see Dugdale et al. (2007); for the MasterBayes and Colony analyses, see Annavi et al. (2014). We were unable to assign a mother to 16% (N = 130) of cubs and a father to 19% (N = 158).
Offspring that were fathered by males residing within their natal group in the year of conception were categorized as within-group offspring (WGO, N = 340; assigned to 125 within-group males), whereas offspring fathered by males that did not reside in their natal group were categorized as extra-group offspring (EGO, N = 315; assigned to 140 extra-group males). Cubs with unassigned paternity (N = 158) were excluded from this analysis.
Heterozygosity and pairwise relatedness
Individual standardized heterozygosity was estimated as the proportion of loci that were heterozygous, divided by the population mean heterozygosity for these loci (Coltman et al., 1999) using GENHET 2.2 (Coulon, 2010) in R 2.12.2. The mean and maximum standardized heterozygosity of within-group adult males (candidate fathers) was then derived per social-group-year. Models with mean and maximum within-group candidate fathers' heterozygosity (SH) produced comparable results overall; therefore, we present results from mean SH models in the main text (see Tables S1–S4 for the results from models including maximum SH).
Pairwise relatedness values between females that were assigned maternity, and their candidate within-group mates, were estimated using Coancestry 1.0.0.1 (Wang, 2011) and averaged per social-group-year. We computed and compared two marker-based pairwise relatedness estimators, to take a comprehensive approach: the commonly used Queller and Goodnight's pairwise estimator (QG; Queller & Goodnight, 1989), and the Lynch and Ritland's pairwise estimator (LR; Lynch & Ritland, 1999), which performs well for most population compositions in simulations (Csillery et al., 2006). The QG and LR pairwise relatedness estimators were highly correlated (Spearman's rank correlation coefficient = 0.91, P < 0.001) and yielded similar results generally. Consequently, we used the QG estimator for our main analyses (see Tables S3–S6 for the LR estimator).
Distribution of EGO and extra-group mate pairs (EGMP) across litters
We tested whether the distribution of EGO within each litter corresponded to an expected distribution, generated through binomial processes, using a chi-square goodness-of-fit test. For this analysis, we included only litters in which all cubs were assigned paternity (N = 378). The number of litters expected to include EGO was calculated as:
where nCX = n!/[(n–X)!X!], p = proportion of EGO in the population, q = 1–p, n = litter size, X = number of EGO per litter and N = number of litters of size n (Sokal & Rohlf, 1995; Perreault et al., 1997). Using this formula, we also tested whether the distribution of EGMPs (each female's litter could be sired by EGMPs, within-group mate pairs (WGMPs), or both; where multiple EGMPs or WGMPs, or both a WGMP and an EGMP occurred, the litter was attributable to multiple fathers) differed from that expected under the binomial distribution.
Statistical analyses
All analyses were run in R 2.13.2 (R Development Core Team, 2011), and the rate of EGP was investigated at the litter level. We fitted generalized linear mixed models (GLMMs), using the lmer function with Laplace approximation in the lme4 0.999375-42 package (Bates & Sarkar, 2007).
We used two measures for EGP (expressed, throughout, as per litter): (i) the number of EGO, and (ii) the number of EGMP. We also investigated EGP from two perspectives: (i) the relative proportion of EGPs (EGO: NEGO/[NEGO + NWGO]; EGMP: NEGMP/[NEGMP + NWGMP]) in relation to the fixed effects, using a binomial error distribution and a logit link function, and (ii) the absolute number of EGP per litter in relation to the fixed effects by controlling for the total number of cubs (in models including EGO metrics), or the number of mate pairs involved in each litter (in models including EGMP metrics), using Poisson error structure and log link function. Here, we present results from the relative proportion models; these are compared to the absolute models, the results of which are presented in Tables S1–S6 and S8–S10.
We examined the socio-ecological effects of the number of females and males in proximity to the assigned mother, by including the following fixed effects: (i) the number of within-group assigned mothers (or within-group candidate mothers; see Tables S9–S10), (ii) the number of within-group candidate fathers, (iii) the number of neighbouring-group candidate fathers, and (iv) all two-way interactions of the three previous terms.
We included two genetic estimates of within-group candidate parents: (i) the mean (or maximum, Tables S1–S4) heterozygosity of within-group candidate fathers as a fixed effect and (ii) the mean pairwise relatedness of within-group assigned mothers and candidate fathers, as a linear and quadratic effect. To interpret main effects in the presence of interactions and quadratic effects when model averaging (Schielzeth, 2010; Grueber et al., 2011), all fixed effects were standardized to a mean of zero and a standard deviation (SD) of two (Gelman, 2008). Female identity, social group and year were included as random effects in all models.
We employed an information-theoretic (IT) approach to select sets of plausible models and to estimate the overall importance of each fixed effect (Burnham et al., 2011). Models were ranked by their QAICc value, such that the top model had the lowest QAICc value (Burnham et al., 2011). If the difference in QAICc between the top model and the second ranked model (∆QAICc) was ≥ 7, we considered the top model to be the only plausible model. A model's relative Akaike weight (ω) was calculated as the model's relative likelihood (exp [−0.5 * ∆QAICc]), divided by the sum of the likelihoods for all models considered (whether plausible or not).
We used the ‘natural average method’ (averaged over all plausible models in which the given parameter was included, weighted by the summed weights (ω) of these models; Burnham & Anderson, 2002) to estimate model-averaged parameters. Estimates of fixed effects were averaged over the plausible models, including models with and without the parameter estimates as an interaction and/or quadratic effect. Unconditional standard errors for model-averaged parameter estimates were calculated using the model.avg function in R. The relative importance of each fixed effect was calculated as the total ω of all plausible models that included the fixed effect of interest.
To investigate the rate of EGP per litter, measured as EGO and EGMP, we used an unrestricted data set that included all social-group-year data for which all trapped within-group candidate parents were genotyped, comprising 549 cubs [297 = WGO; 252 = EGO], although not all offspring were necessarily assigned both parents (unrestricted data set: no of litters = 386 [from 198 mothers]; 205 litters were assigned only to WGMP, 170 only to EGMP and 11 both WGMP + EGMP).
We also performed these same analyses using a restricted data set, including only social-group-years (as group compositions differed between years) in which all individuals were genotyped and all offspring were assigned both parents (restricted data set: no. of litters = 239 [from 147 mothers], comprising 345 cubs [174 = WGO; 171 = EGO]; 119 litters were assigned only to WGMP, 112 only to EGMP and 8 both WGMP + EGMP). The restricted data set was smaller than the unrestricted data set and thus had reduced statistical power, but including cubs that were not assigned paternity could bias the EGP rate (all within-group candidate parents were genotyped, consequently cubs that were not assigned a father were likely to be EGO). These analyses ultimately yielded very similar results to the unrestricted data set (Tables S1–S10).
Results
Patterns of EGP
Of the 502 candidate mothers and 612 candidate fathers trapped between 1987 and 2010, only 228 females (45%) and 201 males (33%) were assigned offspring. The mean litter size was 1.46 [1.43, 1.49] (range = 1–5). Forty-eight per cent of assigned paternities were extra-group (315 of 655 cubs), of which 85% were attributable to neighbouring-group fathers (268 of 315 cubs). EGP was detected in 64% of 225 social-group-years and 47% (178 of 378, Table1a, Fig.1) of litters, considering only those litters for which all offspring were assigned fathers. Of these 178 litters, 64% included one EGO (94% of them with litter size of 1), 32% included two and 4% three (Table1a).
Table 1.
Distribution of (a) extra-group offspring (EGO) and (b) extra-group mate pairs (EGMP) within litters that include only cubs that had both parents assigned. The numbers of litters expected from binomial probabilities are shown in parentheses
| Litter Size | No. of EGO per litter | Total litters | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| (a) | ||||||
| 1 | 132 (129.1) | 107 (109.9) | – | – | – | 239 |
| 2 | 57 (34.1) | 6 (58.1) | 54 (24.8) | – | – | 117 |
| 3 | 10 (3.2) | 1 (8.0) | 2 (6.9) | 7 (1.9) | – | 20 |
| 4 | 1 (0.2) | 0 (0.6) | 1 (0.7) | 0 (0.4) | 0 (0.1) | 2 |
| Total | 200 (166.6) | 114 (176.6) | 57 (32.4) | 7 (2.3) | 0 (0.1) | 378 |
| Number of mates | No. of EGMP per litter | Total litters | ||||
| 0 | 1 | 2 | ||||
| (b) | ||||||
| 1 | 188 (180.2) | 152 (159.8) | - | 340 | ||
| 2 | 12 (10.7) | 10 (18.9) | 16 (8.4) | 38 | ||
| Total | 200 (190.9) | 162 (178.7) | 16 (8.4) | 378 | ||
Figure 1.
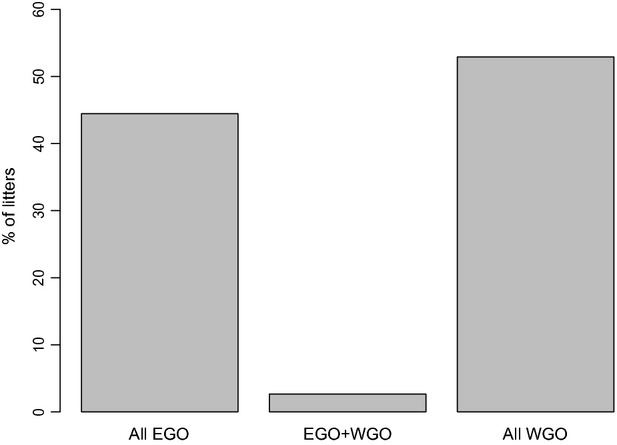
Percentage of litters with only within-group offspring (WGO), only extra-group offspring (EGO) and having both WGO and EGO. Data were restricted to litters that include only cubs with both parents assigned (N = 378). Values at the top of each bar represent the numbers of offspring.
The number of EGO within a litter ranged from 0 to 4, with a mean of 0.65 [0.61, 0.69] in the unrestricted data set (restricted data set: 0–3, mean = 0.72 [0.67, 0.77]). Considering only litters that included EGO, 26 litters involved two fathers (16 had two extra-group fathers and 10 had one extra-group and one within-group father). No litters were fathered by more than two extra-group males. The remaining 152 litters were fathered exclusively by one extra-group male (Table1b).
The number of assigned EGMP per litter ranged from 0 to 2 with a mean of 0.51 [0.48, 0.54] in the unrestricted data (restricted data set: 0–2, mean = 0.54 [0.50, 0.58]). EGO were not distributed evenly among litters: attributing a probability of 0.48 (the mean population EGP rate) to each offspring being assigned as EGO, we observed significant differences in the number of EGO according to litter sizes (per assigned mother, per social-group-year) than expected, according to the binomial probability distribution (χ2 = 75.25, d.f. = 13, P < 0.001, Table1a). The number of EGMP within a litter had a random distribution (χ2 = 5.54, d.f. = 4, P = 0.236, Table1b). Sixty-four per cent of females (unrestricted data set; 65% in restricted data set) mated with an extra-group male during their lifetime, with a maximum of five and a mode of one different mating partner pairs.
Socio-ecological effects of breeding group size
The number of neighbouring-group candidate fathers had a positive effect on both the absolute number and relative proportion of EGP (measured as EGO or EGMP per litter), with both the unrestricted and restricted data sets (Table2, Fig.2, Tables S7 and S8 and Fig. S1). Higher numbers of within-group candidate fathers were associated with a lower proportion of EGO and EGMP per litter (Table2); this ceased to be the case, however, when litters with incomplete paternity assignment were excluded, showing that this effect differs when restricted data are used (Table S7). We also found no associations between the number of within-group candidate fathers and the absolute numbers of EGO and EGMP (Table S8).
Table 2.
Model-averaged parameter estimates over all submodels with ∆AICc< 7, testing the relative proportion of extra-group offspring (EGO) and extra-group mate pairs (EGMP) in a litter in relation to local group density and composition using the unrestricted data set. WGO = within-group offspring. M = No. of within-group assigned mothers. WGCF = No. of within-group candidate fathers. NGCF = No. of neighbouring-group candidate fathers. Mean SH = Mean standardized heterozygosity of within-group candidate fathers. QG = Queller and Goodnight's mean pairwise relatedness estimator between within-group assigned mothers and candidate fathers. QG^2 = quadratic effect of QG. * = Interaction term. REML = Restricted maximum likelihood. All fixed effects were standardized to a mean of zero and a standard deviation of two. Bold estimates have a confidence interval that does not overlap with zero
| Explanatory variable | The relative proportion of EGO | The relative proportion of EGMP | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Unconditional SE | 95% Confidence Interval | Relative importance | Estimate | Unconditional SE | 95% Confidence Interval | Relative importance | |
| (Intercept) | 0.37 | 0.41 | (−0.43, 1.18) | – | 0.14 | 0.29 | (−0.43, 0.70) | |
| Mean SH | −0.74 | 0.42 | (−1.56, 0.08) | 0.57 | −0.44 | 0.34 | (−1.11, 0.24) | 0.42 |
| WGCF | −1.04 | 0.44 | (−1.90, −0.18) | 0.94 | −0.87 | 0.35 | (−1.56, −0.18) | 0.94 |
| M | −0.98 | 0.35 | (−1.66, −0.3) | 0.98 | −0.61 | 0.30 | (−1.19, −0.03) | 0.90 |
| NGCF | 1.34 | 0.48 | (0.41, 2.28) | 1.00 | 1.18 | 0.37 | (0.46, 1.91) | 1.00 |
| QG | 1.51 | 0.49 | (0.55, 2.48) | 0.98 | 1.19 | 0.37 | (0.47, 1.90) | 1.00 |
| QG^2 | −1.65 | 0.66 | (−2.93, −0.36) | 1.00 | −1.04 | 0.51 | (−2.04, −0.05) | 0.73 |
| M*WGCF | 1.08 | 0.73 | (−0.35, 2.51) | 0.44 | 0.70 | 0.65 | (−0.56, 1.97) | 0.32 |
| M*NGCF | 0.85 | 0.68 | (−0.49, 2.19) | 0.33 | 0.94 | 0.58 | (−0.20, 2.07) | 0.49 |
| WGCF*NGCF | −0.87 | 0.84 | (−2.52, 0.78) | 0.41 | −0.74 | 0.68 | (−2.07, 0.59) | 0.35 |
Full models:
Model EGO
y < – cbind(EGO,WGO)
Model < – lmer (y ∼ Litter Size + (1|Year) + (1|Social Group) + (1|Mother ID) + M + WGCF + NGCF + Mean SH + QG + QG^2 + M*WGCF + M*NGCF + WGCF*NGCF, family=binomial, REML = FALSE, data=Unrestricted)
Model EGMP
y < – cbind (EGMP, WGMP)
Model < – lmer (y ∼ Number of mates + (1|Year) + (1|Social Group) + (1|Mother ID) + M + WGCF + NGCF + Mean SH + QG + QG^2 + M*WGCF + M*NGCF + WGCF*NGCF, family=binomial, REML = FALSE, data=Unrestricted).
Figure 2.
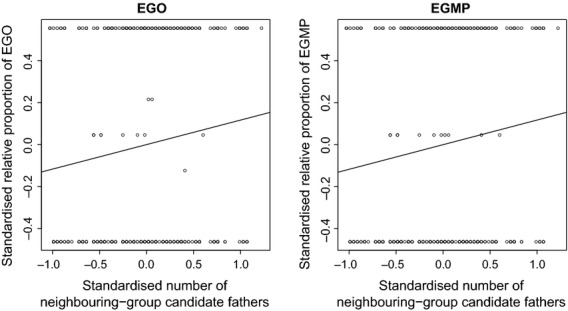
The relationship between the number of neighbouring-group candidate fathers and the relative proportion of extra-group paternity (EGP) measured as extra-group offspring (EGO) and extra-group mate-pairs (EGMP) per litter using the unrestricted data set. Data points represent the standardized (mean of zero and a standard deviation of two) raw data from which the regression lines are derived.
A higher number of assigned mothers in the natal group was associated with lower relative proportions of EGO and EGMP per litter (Table2), when using the unrestricted data set. Using the restricted data set, this effect was only detected in the EGO analysis (Table S7). Models that included candidate mothers, rather than assigned mothers, yielded similar results overall; however, the number of candidate mothers was not significant in the restricted analyses of the relative proportion of EGO and EGMP (Tables S9 and S10).
Genetic factors
The mean pairwise relatedness between within-group assigned mothers and candidate fathers was associated positively with both the relative proportion and absolute number of EGP across litters (Tables2, S7 and S8). We observed a negative quadratic effect of pairwise relatedness on the absolute number of EGO (but not on EGMP; Fig. S2 and Table S8A) and the relative proportion of EGO and EGMP per litter (Fig.3 and Table2). This quadratic relationship remained significant even after exclusion of the two outliers. There was no quadratic relationship, but a positive first-order association in the restricted data set (Tables S7 and S8B). The mean heterozygosity of within-group candidate fathers was not associated with either the absolute or relative proportion of EGP (Tables2 and S7–S8).
Figure 3.
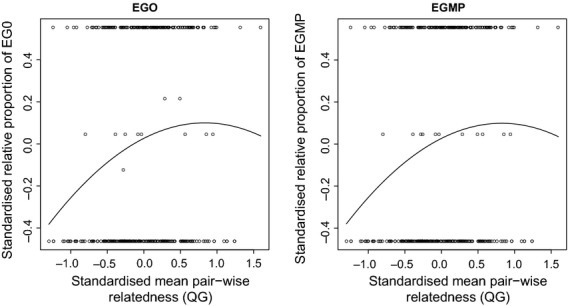
The relationship between the mean pairwise relatedness (Queller and Goodnight's estimator) of assigned mothers and candidate fathers within each social-group-year, and the relative proportion of extra-group paternity (EGP) measured as extra-group offspring (EGO) and extra-group mate pairs (EGMP) per litter using the unrestricted data set. Data points represent the standardized (mean of zero and a standard deviation of two) raw data from which the regression lines are derived.
Discussion
In reality, sexual selection is not predicated upon free choice (Millstein, 2002; Walsh et al., 2002). Although mate selection may relate to perceived quality and compatibility, with realized offspring further honed by post-copulatory mechanisms, the actual availability of mates is also a major contributing factor (Eshel, 1979; Clutton-Brock & McAuliffe, 2009). Capacity to access mates may simply be determined by encounter rate, or it may be limited (actively or passively). Furthermore, although females are typically the choosy sex (Clutton-Brock & McAuliffe, 2009), males can also exhibit prudence (Wedell et al., 2002), and intermale or interfemale competition can restrict free access to mating partners (Preston et al., 2003). As a consequence, ecological, sociological and genetic factors interact to constrain the pace of evolution (Emlen & Oring, 1977).
Extra-group (or extra-pair) copulation is well documented in birds (e.g. Schwartz et al., 1999; Pryke et al., 2010) and mammals (e.g. Cohas et al., 2006). We observed that 48% of offspring in this badger population were assigned extra-group fathers, and the majority of EGP (85%) were attributed to candidate fathers from neighbouring groups. This demonstrates that the social group unit does not correspond to a breeding unit (Carpenter et al., 2005; Dugdale et al., 2007). We also showed that the rate of EGP was associated with the number of each sex in each group, as well as genetic parameters.
Socio-ecological factors
We found that, when the number of candidate males in neighbouring groups was greater, there was a higher rate of EGP. Furthermore, when the number of within-group candidate fathers and also within-group candidate or assigned mothers was higher, the relative proportions, but not the absolute numbers, of both EGO and EGMP per litter were lower in the unrestricted, but generally not in the restricted, analyses. That the rates of EGO and EGMP were lower in the unrestricted analyses probably arose because cubs with unassigned parents were present in these groups (which were likely to be extra-group parents, because both analyses only included groups in which all resident candidate parents were genotyped); however, including these groups added statistical power to the analyses. The smaller sample size of the restricted data set produced effects in the same direction, but the 95% confidence intervals around these estimates overlapped zero.
The largest socio-ecological model-averaged parameter estimate was attributed to the number of neighbouring-group males (Tables2 and S7–S8). The number of within-group candidate fathers and candidate/assigned mothers, however, also affected the rate of EGP. The lower rate of EGP in groups with more within-group candidate fathers might be a tactic to deter within-group males from killing EGO. In contrast, females could use EGP as a counter-strategy to infanticide committed by extra-group males (Agrell et al., 1998).
Our finding of lower EGP with greater numbers of candidate/assigned mothers in a group is in contrast to Isvaran & Clutton-Brock (2007). Their meta-analysis of 26 mammal species found that higher rates of EGP were associated with larger numbers of within-group females and that lower rates of EGP occurred in species with longer mating seasons. They concluded that within-group males are less able to mate guard when more within-group females are present and when the breeding season is extended. Following a post-partum mating peak in February/March, badgers can continue to mate throughout the summer, occasionally up until December, yet 48% of cubs were EGO. There is little evidence that male badgers mate guard; rather, noncompetitive serial copulations with different partners underscore a freely promiscuous system (Dugdale et al., 2007). Moreover, female badgers are able to reject matings (Dugdale et al., 2010), preventing individual males from monopolizing females. Delayed and selective implantation, superfoetation and superfecundation then facilitate cryptic female choice, which should be accounted for in meta-analyses.
In socially paired species, the constraints of limited social mate choice, due to a lack of breeding territories, will lead to unpaired females pairing with any available unpaired male possessing a breeding territory (Richardson et al., 2005). In this high-density badger population, contact occurs frequently between groups (Macdonald et al., 2008) comprising multiple (breeding) males and females (Dugdale et al., 2007, 2008). Females mate promiscuously (Dugdale et al., 2011) and delayed implantation allows them to mate whenever a male is available (Yamaguchi et al., 2006), thus females are unlikely to be constrained by the availability of mating partners. Furthermore, we have found no evidence for clear mating hierarchies in these badgers; that is, male mounting frequency is not related to dominance rank or body condition index, and male mounting frequency does not correlate with paternity success (Dugdale et al., 2011).
If females mate indiscriminately with whomever they encounter, and thus by chance mate with extra-group males (Kokko & Rankin, 2006), then EGP would not necessarily be adaptive. Nevertheless, the correlation we observed with relatedness precludes parentage from being totally random. When the relative proportion of neighbouring-group candidate fathers was higher, the rate of EGP was greater. We assume that greater neighbouring-group mate availability implies a greater likelihood of contact. This type of mating system could be due to nonexclusive use of feeding ranges (Stewart et al., 1997) or facilitated by the relatively high rate of temporary intergroup movements observed in this population, even from a maximum of just four trapping events per year (see Macdonald et al., 2008; Huck et al., 2008; see also Stewart et al., 1997). These visits might serve to ingratiate males with females in adjacent groups and exploit EGMP encounter rate, to broaden mate selection options. Encounter rate-based mating could arise if the genetic basis of a female's response to her social mate (within-group males) and extra-group males is the same (Within-sex Genetic Correlation Hypothesis; Forstmeier et al., 2011). Alleles for resistance to (group-) infidelity may, however, also convey resistance to (group-) fidelitous copulations, leading to infertility (Arnqvist & Kirkpatrick, 2005).
Genetic factors
We found no evidence that badgers were seeking heterozygosity in their within-group mating partners (heterozygous advantage; Jennions & Petrie, 2000), although we did not test for an environmental interaction. The propensity for males and females to engage in promiscuous mating may be affected by the same set of alleles (Halliday & Arnold, 1987). There is likely to be strong positive selection for alleles that enhance promiscuous behaviour in males (Albrecht et al., 2007). If promiscuous behaviour is heritable (Reid et al., 2011), pleiotropic effects on these sexually selected alleles could also cause promiscuous behaviour to evolve in females, despite antagonistic selection (Rice, 1992) known as the between-sex genetic correlation hypothesis (Halliday & Arnold, 1987; Forstmeier et al., 2011).
The mean pairwise relatedness between within-group assigned mothers and candidate fathers had high relative importance, for both the relative proportion and the absolute number of EGP (Tables2 and S7 and S8). The rate of EGP increased with greater relatedness between within-group assigned mothers and candidate fathers, showing a negative quadratic effect such that the rate of EGP plateaued with high relatedness. Small sample size, however, prevented us from testing this effect (Fig.3).
Female preference for immigrant males over residents, when the residents are relatives, is theoretically predicted to occur when inbreeding is costly (Lehmann & Perrin, 2003). Badgers might avoid inbreeding; Annavi et al. (2014) report positive correlations between offspring first-year survival probability and paternal heterozygosity in this same population in summers with a good food supply (from the proxy of wet conditions, yielding abundant earthworm food). Additionally, assigned within-group parent pairs in this population were less related than randomly assigned within-group pairs (Sin, 2012), but simulations including all potential parent pairs are required to determine whether inbreeding avoidance occurs. Intergroup promiscuity, facilitated by frequent temporary visits between groups (Macdonald et al., 2008), seems a plausible mechanism to avoid inbreeding, circumventing the need for permanent dispersal, and the associated risks.
Conclusions
Studies of the ecological correlates of EGP in mammals have focused on the spatial and temporal grouping of females and males within groups (Isvaran & Clutton-Brock, 2007). We highlight that the number of neighbouring males is an important and overlooked parameter, along with the potential for cryptic female choice. From the combined socio-ecological and genetic correlates of EGP in badgers, it is possible that the genetic effects we observed could occur through cryptic female choice, superimposed on a backcloth of random mating. Further research is required to elucidate the costs and benefits of EGP in mammals; for example, the survival, recruitment or life-time reproductive success of WGO versus EGO, and whether there is a genetic basis to EGP.
Acknowledgments
We thank Pierre Nouvellet and Stephen Ellwood for their support in the field, Andy Krupa, Deborah Dawson and Gavin Horsburgh for advice in the laboratory and Jarrod Hadfield for help with the program MasterBayes. We are also grateful to Jan Komdeur, Marta Szulkin and two anonymous reviewers for comments on the manuscript. Trapping protocols were subject to ethical review and were performed under Natural England Licence (currently 20104655) and UK Home Office (Animals [Scientific Procedures] Act, 1986) Licence (PPL 30/2835). This research was supported by the UK Natural Environment Research Council (NERC) Biomolecular Analysis Facility, Sheffield; People's Trust for Endangered Species (DWM); Ministry of Higher Education, Malaysia (GA); Netherlands Organisation for Scientific Research (Visitor's Travel Grant 040.11.232; HLD); NERC (fellowship NE/I021748/1; HLD); and the Croucher Foundation, Hong Kong (YWS).
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1 The relationship between the number of neighbouring-group candidate fathers and the mean absolute number of extra-group paternity (EGP) measured as extra-group offspring (EGO) and extra-group mate-pairs (EGMP) per litter using the unrestricted data set.
Figure S2 The relationship between the mean pair-wise relatedness (Queller and Goodnight's estimator) of assigned mothers and candidate fathers within each social-group-year, and the mean absolute number of extra-group paternity (EGP) measured as extra-group offspring (EGO) and extra-group mate-pairs (EGMP) per litter using the unrestricted data set.
Table S1 Model-averaged parameter estimates over all sub-models with ΔAICc < 7, testing the relative proportion and absolute number of extra-group offspring (EGO) in a litter in relation to local group density and composition.
Table S2 Model-averaged parameter estimates over all sub-models with ΔAICc < 7, testing the relative proportion and absolute number of extra-group mate-pairs (EGMP) in a litter in relation to local group density and composition.
Table S3 Model-averaged parameter estimates over all sub-models with ΔAICc < 7, testing the relative proportion and absolute number of extra-group offspring (EGO) in a litter in relation to local group density and composition.
Table S4 Model-averaged parameter estimates over all sub-models with ΔAICc < 7, testing the relative proportion and absolute number of extra-group mate-pairs (EGMP) in a litter in relation to local group density and composition.
Table S5 Model-averaged parameter estimates over all sub-models with ΔAICc < 7, testing the relative proportion and absolute number of extra-group offspring (EGO) in a litter in relation to local group density and composition.
Table S6 Model-averaged parameter estimates over all sub-models with ΔAICc < 7, testing the relative proportion and absolute number of extra-group mate-pairs (EGMP) in a litter in relation to local group density and composition.
Table S7 Model-averaged parameter estimates over all submodels with ΔAICc < 7, testing the relative proportion of extragroup offspring (EGO) and extra-group mate-pairs (EGMP) in a litter in relation to local group density and composition using the restricted data set.
Table S8 Model-averaged parameter estimates over all submodels with ΔAICc < 7, testing the absolute number of extra-group offspring (EGO) and extra-group mate-pairs (EGMP) in a litter in relation to local group density and composition using the (A) unrestricted and (B) restricted data set.
Table S9 Model-averaged parameter estimates over all sub-models with ΔAICc < 7, testing the relative proportion and absolute number of extra-group offspring (EGO) in a litter in relation to local group density and composition.
Table S10 Model-averaged parameter estimates over all sub-models with ΔAICc < 7, testing the relative proportion and absolute number of extra-group mate-pairs (EGMP) in a litter in relation to local group density and composition.
References
- Agrell J, Wolff JO, Ylonen H. Counter-strategies to infanticide in mammals: costs and consequences. Oikos. 1998;83:507–517. [Google Scholar]
- Ahnlund H. Sexual maturity and breeding season of the badger, Meles meles in Sweden. J. Zool. 1980;190:77–95. [Google Scholar]
- Albrecht T, Schnitzer J, Kreisinger J, Exnerova A, Bryja J, Munclinger P. Extrapair paternity and the opportunity for sexual selection in long-distant migratory passerines. Behav. Ecol. 2007;18:477–486. [Google Scholar]
- Andersson M, Simmons LW. Sexual selection and mate choice. Trends Ecol. Evol. 2006;21:296–302. doi: 10.1016/j.tree.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Annavi G, Dawson DA, Horsburgh GJ, Greig C, Dugdale HL, Newman C, et al. Characterisation of twenty-one European badger (Meles meles) microsatellite loci facilitates the discrimination of second-order relatives. Conserv. Genet. Resour. 2011;3:515–518. [Google Scholar]
- Annavi G, Newman C, Buesching CD, Macdonald DW, Burke T, Dugdale HL. Heterozygosity–fitness correlations in a wild mammal population: accounting for parental and environmental effects. Eco. Evol. 2014;4:2594–2609. doi: 10.1002/ece3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnqvist G, Kirkpatrick M. The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. Am. Nat. 2005;165(suppl):S26–S37. doi: 10.1086/429350. [DOI] [PubMed] [Google Scholar]
- Bates D, Sarkar D. 2007. lme4: linear mixed-effects models using S4 classes. R package version 0.9975-12, http://CRAN.R-project.org/
- Bohm M, Hutchings MR, White P. Contact networks in a wildlife-livestock host community: identifying high-risk individuals in the transmission of Bovine TB among badgers and cattle. PLoS ONE. 2009;4:e5016. doi: 10.1371/journal.pone.0005016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borries C, Savini T, Koenig A. Social monogamy and the threat of infanticide in larger mammals. Behav. Ecol. Sociobiol. 2011;65:685–693. [Google Scholar]
- Britten H. Meta-analyses of the association between multilocus heterozygosity and fitness. Evolution. 1996;50:2158–2164. doi: 10.1111/j.1558-5646.1996.tb03606.x. [DOI] [PubMed] [Google Scholar]
- Brooker MG, Rowley I, Adams M, Baverstock PR. Promiscuity: an inbreeding avoidance mechanism in a socially monogamous species? Behav. Ecol. Sociobiol. 1990;26:191–199. [Google Scholar]
- Brouwer L, Van de Pol M, Atema E, Cockburn A. Strategic promiscuity helps avoid inbreeding at multiple levels in a cooperative breeder where both sexes are philopatric. Mol. Ecol. 2011;20:4796–4807. doi: 10.1111/j.1365-294X.2011.05325.x. [DOI] [PubMed] [Google Scholar]
- Brown J. A theory of mate choice based on heterozygosity. Behav. Ecol. 1997;8:60–65. [Google Scholar]
- Buesching CD, Heistermann M, Macdonald DW. Seasonal and inter-individual variation in testosterone levels in badgers Meles meles: evidence for the existence of two endocrinological phenotypes. J. Comp. Physiol. A. 2009;195:865–871. doi: 10.1007/s00359-009-0465-0. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd edn. Berlin: Springer; 2002. [Google Scholar]
- Burnham KP, Anderson DR, Huyvaert KP. AIC model selection and multimode linference in behavioral ecology: some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011;65:23–35. [Google Scholar]
- Carpenter PJ, Pope LC, Greig C, Dawson DA, Rogers LM, Erven K, et al. Mating system of the European badger, Meles meles, in a high density population. Mol. Ecol. 2005;14:273–284. doi: 10.1111/j.1365-294X.2004.02401.x. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH, McAuliffe K. Female mate choice in mammals. Q. Rev. Biol. 2009;84:3–27. doi: 10.1086/596461. [DOI] [PubMed] [Google Scholar]
- Cohas A, Yoccoz NG, da Silva A, Goossens B, Allaine D. Extra-pair paternity in the monogamous alpine marmot (Marmota marmota): the roles of social setting and female mate choice. Behav. Ecol. Sociobiol. 2006;59:597–605. [Google Scholar]
- Cohas A, Bonenfant C, Kempenaers B, Allaine D. Age-specific effect of heterozygosity on survival in alpine marmots, Marmota marmota. Mol. Ecol. 2009;18:1491–1503. doi: 10.1111/j.1365-294X.2009.04116.x. [DOI] [PubMed] [Google Scholar]
- Coltman D, Slate J. Microsatellite measures of inbreeding: a meta-analysis. Evolution. 2003;57:971–983. doi: 10.1111/j.0014-3820.2003.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Coltman DW, Pilkington JG, Smith JA, Pemberton JM. Parasite-mediated selection against inbred Soay sheep in a free-living, island population. Evolution. 1999;53:1259–1267. doi: 10.1111/j.1558-5646.1999.tb04538.x. [DOI] [PubMed] [Google Scholar]
- Connor R, Whitehead H. Alliances II: rates of encounter during resource utilization: a general model of intrasexual alliance formation in fission–fusion societies. Anim. Behav. 2005;69:127–132. [Google Scholar]
- Coulon A. GENHET: an easy-to-use R function to estimate individual heterozygosity. Mol. Ecol. Resour. 2010;10:167–169. doi: 10.1111/j.1755-0998.2009.02731.x. [DOI] [PubMed] [Google Scholar]
- Cresswell WJ, Harris S, Cheeseman CL, Mallinson PJ. To breed or not to breed: an analysis of the social and density-dependent constraints on the fecundity of female badgers (Meles meles. Philos. Trans. R. Soc. Lond. B. 1992;338:393–407. doi: 10.1098/rstb.1992.0157. [DOI] [PubMed] [Google Scholar]
- Csillery K, Johnson T, Beraldi D, Clutton-Brock TH, Coltman D, Hansson B, et al. Performance of marker-based relatedness estimators in natural populations of outbred vertebrates. Genetics. 2006;173:2091–2101. doi: 10.1534/genetics.106.057331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David P. Heterozygosity-fitness correlations: new perspectives on old problems. Heredity. 1998;80:531–537. doi: 10.1046/j.1365-2540.1998.00393.x. [DOI] [PubMed] [Google Scholar]
- Dugdale HL, Macdonald DW, Pope LC, Burke T. Polygynandry, extra-group paternity and multiple-paternity litters in European badger (Meles meles) social groups. Mol. Ecol. 2007;16:5294–5306. doi: 10.1111/j.1365-294X.2007.03571.x. [DOI] [PubMed] [Google Scholar]
- Dugdale HL, Macdonald DW, Pope LC, Johnson PJ, Burke T. Reproductive skew and relatedness in social groups of European badgers, Meles meles. Mol. Ecol. 2008;17:1815–1827. doi: 10.1111/j.1365-294X.2008.03708.x. [DOI] [PubMed] [Google Scholar]
- Dugdale HL, Elwood SA, Macdonald DW. Alloparental care and long-term costs of mothers tolerating other members of the group in a plurally breeding mammal. Anim. Behav. 2010;84:721–735. [Google Scholar]
- Dugdale HL, Griffiths A, Macdonald DW. Polygynandrous and repeated mounting behaviour in European badgers, Meles meles. Anim. Behav. 2011;82:1287–1297. [Google Scholar]
- Durrant K, Hughes J. Differing rates of extra-group paternity between two populations of the Australian magpie (Gymnorhina tibicen. Behav. Ecol. Sociobiol. 2005;57:536–545. [Google Scholar]
- Emlen ST, Oring LW. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- Eshel I. Sexual selection, population density, and availability of mates. Theor. Popul. Biol. 1979;16:301–314. doi: 10.1016/0040-5809(79)90019-4. [DOI] [PubMed] [Google Scholar]
- Fell RJ, Buesching CD, Macdonald DW. The social integration of European badger (Meles meles) cubs into their natal group. Behaviour. 2006;143:683–700. [Google Scholar]
- Fisher DO, Double MC, Blomberg SP, Jennions MD, Cockburn A. Post-mating sexual selection increases lifetime fitness of polyandrous females in the wild. Nature. 2006;444:89–92. doi: 10.1038/nature05206. [DOI] [PubMed] [Google Scholar]
- Forstmeier W, Martin K, Bolund E, Schielzeth H, Kempenaers B. Female extrapair mating behavior can evolve via indirect selection on males. Proc. Natl. Acad. Sci. USA. 2011;108:10608–10613. doi: 10.1073/pnas.1103195108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromhage L, Kokko H, Reid JM. Evolution of mate choice for genome wide heterozygosity. Evolution. 2009;63:684–694. doi: 10.1111/j.1558-5646.2008.00575.x. [DOI] [PubMed] [Google Scholar]
- Gelman A. Scaling regression inputs by dividing by two standard deviations. Stat. Med. 2008;27:2865–2873. doi: 10.1002/sim.3107. [DOI] [PubMed] [Google Scholar]
- Griffith SC, Owens IPF, Thuman KA. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. [DOI] [PubMed] [Google Scholar]
- Grueber CE, Waters JM, Jamieson IG. The imprecision of heterozygosity-fitness correlations hinders the detection of inbreeding and inbreeding depression in a threatened species. Mol. Ecol. 2011;20:67–79. doi: 10.1111/j.1365-294X.2010.04930.x. [DOI] [PubMed] [Google Scholar]
- Hadfield JD, Richardson DS, Burke T. Towards unbiased parentage assignment: combining genetic, behavioural and spatial data in a Bayesian framework. Mol. Ecol. 2006;15:3715–3730. doi: 10.1111/j.1365-294X.2006.03050.x. [DOI] [PubMed] [Google Scholar]
- Halliday T, Arnold SJ. Multiple mating by females - a perspective from quantitative genetics. Anim. Behav. 1987;35:939–941. [Google Scholar]
- Hansson B, Westerberg L. On the correlation between heterozygosity and fitness in natural populations. Mol. Ecol. 2002;11:2467–2474. doi: 10.1046/j.1365-294x.2002.01644.x. [DOI] [PubMed] [Google Scholar]
- Harrison XA, Bearhop S, Inger R, Colhoun K, Gudmundsson GA, Hodgson D, et al. Heterozygosity fitness correlations in a migratory bird - an analysis of inbreeding and single-locus effects. Mol. Ecol. 2011;20:4786–4795. doi: 10.1111/j.1365-294X.2011.05283.x. [DOI] [PubMed] [Google Scholar]
- Huck M, Frantz A, Dawson D, Burke T, Roper T. Low genetic variability, female-biased dispersal and high movement rates in an urban population of Eurasian badgers Meles meles. J. Anim. Ecol. 2008;77:905–915. doi: 10.1111/j.1365-2656.2008.01415.x. [DOI] [PubMed] [Google Scholar]
- Isvaran K, Clutton-Brock TH. Ecological correlates of extra-group paternity in mammal. Proc. R. Soc. Lond. B. 2007;274:219–224. doi: 10.1098/rspb.2006.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennions MD, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 2000;75:21–64. doi: 10.1017/s0006323199005423. [DOI] [PubMed] [Google Scholar]
- Johnson PCD, Haydon DT. Maximum-likelihood estimation of allelic dropout and false allele error rates from microsatellite genotypes in the absence of reference data. Genetics. 2007;175:827–842. doi: 10.1534/genetics.106.064618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DD, Jetz W, Macdonald DW. Environmental correlates of badger social spacing across Europe. J. Biogeogr. 2002;29:411–425. [Google Scholar]
- Kempenaers B. Mate choice and genetic quality: a review of the heterozygosity theory. Adv. Stud. Behav. 2007;37:189–278. [Google Scholar]
- Kilshaw K, Newman C, Buesching CD, Bunyan J, Macdonald DW. Coordinated latrine use by European badgers, Meles meles: potential consequences for territory defense. J. Mammal. 2009;90:1188–1198. [Google Scholar]
- Kingma SA, Hall ML, Peters A. Breeding synchronization facilitates extrapair mating for inbreeding avoidance. Behav. Ecol. 2013;24:1390–1397. [Google Scholar]
- Kokko H, Rankin DJ. Lonely hear ts or sex in the city? Density-dependent effects in mating systems. Philos. Trans. R. Soc. Lond. B. 2006;361:319–334. doi: 10.1098/rstb.2005.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruuk H. Foraging and spatial organisation of the European Badger, Meles meles L. Behav. Ecol. Sociobiol. 1978a;4:75–89. [Google Scholar]
- Kruuk H. Spatial organisation and territorial behaviour of the European badger, Meles meles. J. Zool. 1978b;184:1–19. [Google Scholar]
- Lehmann L, Perrin N. Inbreeding avoidance through kin recognition: choosy females boost male dispersal. Am. Nat. 2003;162:638–652. doi: 10.1086/378823. [DOI] [PubMed] [Google Scholar]
- Lynch M, Ritland K. Estimation of pairwise relatedness with molecular markers. Genetics. 1999;152:1753–1766. doi: 10.1093/genetics/152.4.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald DW, Newman C. Population dynamics of badgers (Meles meles) in Oxfordshire, U.K.: numbers, density and cohort life histories, and a possible role of climate change in population growth. J. Zool. 2002;256:121–138. [Google Scholar]
- Macdonald DW, Newman C, Dearr J, Buesching CD, Johnson PJ. The distribution of Eurasian badger, Meles meles, setts in a high density area: field observations contradict the sett dispersion hypothesis. Oikos. 2004;106:295–307. [Google Scholar]
- Macdonald DW, Newman C, Buesching CD, Johnson PJ. Male-biased movement in a high-density population of the European badger (Meles meles. J. Mammal. 2008;89:1077–1086. [Google Scholar]
- Macdonald DW, Newman C, Nouvellet PM, Buesching CD. An analysis of Eurasian badger (Meles meles) population dynamics: implication for regulatory mechanisms. J. Mammal. 2009;90:1392–1403. [Google Scholar]
- Mainguy J, Cote S, Coltman D. Multilocus heterozygosity, parental relatedness and individual fitness components in a wild mountain goat, Oreamnos americanus population. Mol. Ecol. 2009;18:2297–2306. doi: 10.1111/j.1365-294X.2009.04197.x. [DOI] [PubMed] [Google Scholar]
- McLaren GW, Thornton PD, Newman C, Buesching CD, Baker SE, Mathews F, et al. The use and assessment of ketamine–medetomidine–butorphanol combinations for field anaesthesia in wild European badgers (Meles meles. Vet Anaesth. Analg. 2005;32:367–372. doi: 10.1111/j.1467-2995.2005.00206.x. [DOI] [PubMed] [Google Scholar]
- Millstein RL. Are random drift and natural selection conceptually distinct? Biol. Philos. 2002;17:33–53. [Google Scholar]
- Moore AJ, Ali R. Are dispersal and inbreeding avoidance related? Anim. Behav. 1984;32:94–112. [Google Scholar]
- Morecroft M, Taylor M, Oliver H. Air and soil microclimates of deciduous woodland compared to an open site. J. Agric. For. Meteorol. 1998;90:141–156. [Google Scholar]
- Newman C, Zhou Y, Buesching CD, Kaneko Y, Macdonald DW. Contrasting sociality in two widespread, generalist, Mustelid genera, Meles and Martes. Mammal Study. 2011;36:169–188. [Google Scholar]
- Noonan MJ, Markham A, Newman C, Trigoni N, Buesching CD, Ellwood SA, et al. Climate and the individual: inter-annual variation in the autumnal activity of the European Badger (Meles meles. PLoS ONE. 2014;9:e83156. doi: 10.1371/journal.pone.0083156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault S, Lemon RE, Kuhnlein U. Patterns and correlates of extrapair paternity in American redstarts (Setophagaruticilla. Behav. Ecol. 1997;8:612–621. [Google Scholar]
- Preston BT, Stevenson IR, Pemberton JM, Coltman DW, Wilson K. Overt and covert competition in a promiscuous mammal: the importance of weaponry and testes size to male reproductive success. Proc. Roy. Soc. B. 2003;270:633–640. doi: 10.1098/rspb.2002.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryke S, Rollins L, Griffith S. Females use multiple mating and genetically loaded sperm competition to target compatible genes. Science. 2010;329:964–967. doi: 10.1126/science.1192407. [DOI] [PubMed] [Google Scholar]
- Pusey A, Wolf M. Inbreeding avoidance in animals. Trends Ecol. Evol. 1996;11:201–206. doi: 10.1016/0169-5347(96)10028-8. [DOI] [PubMed] [Google Scholar]
- Queller DC, Goodnight KF. Estimating relatedness using genetic-markers. Evolution. 1989;43:258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. ISBN 3-900051-07-0, URL http://www.R-project.org. [Google Scholar]
- Reid JM, Arcese P, Sardell RJ, Keller L. Heritability of female extra-pair paternity rate in song sparrows (Melospiza melodia. Proc. Roy. Soc. B. 2011;278:1114–1120. doi: 10.1098/rspb.2010.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W. Sexually antagonistic genes: experimental evidence. Science. 1992;256:1436–1439. doi: 10.1126/science.1604317. [DOI] [PubMed] [Google Scholar]
- Richardson DS, Komdeur J, Burke T, von Schantz T. MHC-based patterns of social and extra-pair mate choice in the Seychelles warbler. Proc. Roy. Soc. B. 2005;272:759–767. doi: 10.1098/rspb.2004.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill PS, Perrins CM, Kirby KJ, Fisher N. Wytham Woods: Oxford's Ecological Laboratory. Oxford, UK: Oxford University Press; 2010. [Google Scholar]
- Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 2010;1:103–113. [Google Scholar]
- Schubert G, Stoneking CJ, Aranjelovi M, Boesch C, Eckhardt N, Hohmann G, et al. Male-mediated gene flow in patrilocal primates. PLoS ONE. 2011;6:21514. doi: 10.1371/journal.pone.0021514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Boness D, Schaeff C, Majluf P, Perry E, Fleischer R. Female-solicited extrapair matings in Humboldt penguins fail to produce extrapair fertilizations. Behav. Ecol. 1999;10:242–250. [Google Scholar]
- Sheldon B. Male phenotype, fertility, and the pursuit of extra-pair copulations by female birds. Proc. Roy. Soc. B. 1994;257:25–30. [Google Scholar]
- Sillero-Zubiri C, Gottelli D, Macdonald DW. Male philopatry, extra-pack copulations and inbreeding avoidance in Ethiopian wolves (Canis simensis. Behav. Ecol. Sociobiol. 1996;38:331–340. [Google Scholar]
- da Silva J, Woodroffe R, Macdonald DW. Habitat, food availability and group territoriality in the European badger, Meles meles. Oecologia. 1993;95:558–564. doi: 10.1007/BF00317441. [DOI] [PubMed] [Google Scholar]
- Sin YW. 2012. The major histocompatibility complex, mate choice and pathogen resistance in the European badger Meles meles. D.Phil. Thesis, University of Oxford, UK.
- Smuts BB, Smuts RW. Male aggression and sexual coercion of females in nonhuman primates and other mammals: evidence and theoretical implications. Adv. Stud. Behav. 1993;22:1–63. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: The Principles and Practices of Statistics in Biological Research. New York: W.H Freeman and Company; 1995. [Google Scholar]
- Soulsbury C. Genetic patterns of paternity and testes size in mammals. PLoS ONE. 2010;5:e9581. doi: 10.1371/journal.pone.0009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PD, Anderson C, Macdonald DW. A mechanism for passive range exclusion: evidence from the European badger (Meles meles. J. Theor. Biol. 1997;184:279–289. doi: 10.1006/jtbi.1996.0248. [DOI] [PubMed] [Google Scholar]
- Thom MD, Johnson DDP, Macdonald DW. The evolution and maintenance of delayed implantation in the Mustelidae (Mammalia: Carnivora) Evolution. 2004;58:175–183. doi: 10.1111/j.0014-3820.2004.tb01584.x. [DOI] [PubMed] [Google Scholar]
- Treves A. Primate social systems: conspecific threat and coercion-defense hypotheses. Foli. Primatol. 1998;69:81–88. [Google Scholar]
- Van Vuren D, Armitage KB. Survival of dispersing and philopatric yellow-bellied marmots: what is the cost of dispersal? Oikos. 1994;69:179–181. [Google Scholar]
- Vedder O, Komdeur J, Van der Velde M, Schut E, Magrath MJL. Polygyny and extra-pair paternity enhance the opportunity for sexual selection in blue tits. Behav. Ecol. Sociobiol. 2011;65:741–752. doi: 10.1007/s00265-010-1078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Lewens T, Ariew A. The trials of life: natural selection and random drift. Philos. Sci. 2002;69:429–446. [Google Scholar]
- Wandeler AI, Graf M. Der Geschlechtszyklus weiblicher Dachse (Meles meles L.) in der Schweiz. Rev. Suisse Zool. 1982;89:1009–1016. [Google Scholar]
- Wang J. COANCESTRY: a program for simulating, estimating and analysing relatedness and inbreeding coefficients. Mol. Ecol. Resour. 2011;11:141–145. doi: 10.1111/j.1755-0998.2010.02885.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Santure AW. Parentage and sibship inference from multilocus genotype data under polygamy. Genetics. 2009;181:1579–1594. doi: 10.1534/genetics.108.100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedell N, Gage MJ, Parker GA. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 2002;17:313–320. [Google Scholar]
- Westneat D, Stewart I. Extra-pair paternity in birds: causes, correlates, and conflict. Ann. Rev. Ecol. Evol. S. 2003;34:365–396. [Google Scholar]
- Westneat D, Sherman PW, Morton ML. The evolution of extra-pair copulations in birds. Current Ornithol. 1990;7:331–369. [Google Scholar]
- Whiteman N, Matson K, Bollmer J, Parker P. Disease ecology in the Galápagos Hawk (Buteo galapagoensis): host genetic diversity, parasites, and natural antibodies. Proc. Roy. Soc. B. 2006;273:797–804. doi: 10.1098/rspb.2005.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodroffe R, Macdonald DW. Costs of breeding status in the European badger, Meles meles. J. Zool. 1995;235:237–245. [Google Scholar]
- Woodroffe R, Macdonald DW, da Silva J. Dispersal and philopatry in the European badger, Meles meles. J. Zool. 1995;237:227–239. [Google Scholar]
- Yamaguchi N, Dugdale HL, Macdonald DW. Female receptivity, embryonic diapause, and superfetation in the European badger (Meles meles): implications for the reproductive tactics of males and females. Q. Rev. Biol. 2006;81:33–48. doi: 10.1086/503923. [DOI] [PubMed] [Google Scholar]
- Young A, Spong G, Clutton-Brock TH. Subordinate male meerkats prospect for extra-group paternity: alternative reproductive tactics in a cooperative mammal. Proc. Roy. Soc. B. 2007;274:1603–1609. doi: 10.1098/rspb.2007.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The relationship between the number of neighbouring-group candidate fathers and the mean absolute number of extra-group paternity (EGP) measured as extra-group offspring (EGO) and extra-group mate-pairs (EGMP) per litter using the unrestricted data set.
Figure S2 The relationship between the mean pair-wise relatedness (Queller and Goodnight's estimator) of assigned mothers and candidate fathers within each social-group-year, and the mean absolute number of extra-group paternity (EGP) measured as extra-group offspring (EGO) and extra-group mate-pairs (EGMP) per litter using the unrestricted data set.
Table S1 Model-averaged parameter estimates over all sub-models with ΔAICc < 7, testing the relative proportion and absolute number of extra-group offspring (EGO) in a litter in relation to local group density and composition.
Table S2 Model-averaged parameter estimates over all sub-models with ΔAICc < 7, testing the relative proportion and absolute number of extra-group mate-pairs (EGMP) in a litter in relation to local group density and composition.
Table S3 Model-averaged parameter estimates over all sub-models with ΔAICc < 7, testing the relative proportion and absolute number of extra-group offspring (EGO) in a litter in relation to local group density and composition.
Table S4 Model-averaged parameter estimates over all sub-models with ΔAICc < 7, testing the relative proportion and absolute number of extra-group mate-pairs (EGMP) in a litter in relation to local group density and composition.
Table S5 Model-averaged parameter estimates over all sub-models with ΔAICc < 7, testing the relative proportion and absolute number of extra-group offspring (EGO) in a litter in relation to local group density and composition.
Table S6 Model-averaged parameter estimates over all sub-models with ΔAICc < 7, testing the relative proportion and absolute number of extra-group mate-pairs (EGMP) in a litter in relation to local group density and composition.
Table S7 Model-averaged parameter estimates over all submodels with ΔAICc < 7, testing the relative proportion of extragroup offspring (EGO) and extra-group mate-pairs (EGMP) in a litter in relation to local group density and composition using the restricted data set.
Table S8 Model-averaged parameter estimates over all submodels with ΔAICc < 7, testing the absolute number of extra-group offspring (EGO) and extra-group mate-pairs (EGMP) in a litter in relation to local group density and composition using the (A) unrestricted and (B) restricted data set.
Table S9 Model-averaged parameter estimates over all sub-models with ΔAICc < 7, testing the relative proportion and absolute number of extra-group offspring (EGO) in a litter in relation to local group density and composition.
Table S10 Model-averaged parameter estimates over all sub-models with ΔAICc < 7, testing the relative proportion and absolute number of extra-group mate-pairs (EGMP) in a litter in relation to local group density and composition.


