Abstract
Background
Emerging data suggest that reduced exposure to ultraviolet (UV) radiation is associated with increased mortality in the general population. To date, there has not been examination of the association between UV exposure and mortality in dialysis patients.
Methods
We examined the association between UV index, a proxy of UV exposure, and all-cause mortality among 47,286 US dialysis patients (entry period 2001–2006, with follow-up through 2009) from a large national dialysis organization using multivariable Cox regression. The UV index was ascertained by linkage of individual patients’ residential zip codes to National Oceanic and Atmospheric Administration data, and was categorized as low (0–<3), moderate (3–<5), moderate-high (5–<6), high (6–<7), and very-high (≥7). In secondary analyses, we examined the UV index—mortality association within subgroups of age (<65 vs. ≥ 65 years old), sex, and race (white vs. non-white).
Results
The study population’s mean±SD age was 60±16 years old and included 46% women and 56% diabetics. Compared to patients residing in moderate-high UV index regions, those residing in high and very-high UV index regions had lower mortality risk: adjusted HRs 0.84 (95% CI) 0.81–0.88 and 0.83 (95% CI) 0.75–0.91, respectively. A similar inverse association between UV index and mortality was observed across all subgroups, although there was more pronounced reduction in mortality among whites vs. non-whites.
Conclusion
These data suggest that dialysis patients residing in higher UV index regions have lower all-cause mortality compared to those living in moderate-high UV regions. Further studies are needed to determine mechanisms underlying the UV index—mortality association.
Keywords: Dialysis, environment, mortality, ultraviolet radiation, vitamin D
INTRODUCTION
There has been increasing interest in the impact of environmental exposures such as ultraviolet (UV) radiation exposure on human health. UV radiation has been classified as a human carcinogen by the U.S. Department of Health and Human Services and the World Health Organization given its causal associations with skin cancer.[1] However, emerging data from the general population suggest that increased UV exposure is associated with decreased risk of certain non-dermatologic malignancies (e.g., prostate, breast, non-Hodgkin’s lymphoma) as well as reduced cardiovascular and all-cause mortality.[2–4] It has been hypothesized that increased endogenous synthesis of vitamin D stimulated by UV exposure may be a mechanistic link for this paradoxical association. Indeed, UV irradiance is an important determinant of vitamin D biosynthesis and status in the general population.[5, 6]
Vitamin D deficiency is highly prevalent in chronic kidney disease (CKD) patients including those who are dialysis-dependent,[7–9] and it is associated with increased risk of adverse cardiovascular surrogates (e.g., coronary artery calcification,[10] atherosclerosis, [11] endothelial dysfunction[11]), cardiovascular events,[12] and mortality.[13, 14] Emerging data suggest that, despite impaired conversion of inactivated to activated vitamin D in dialysis patients, both solar UV exposure[15–18] and artificial UV exposure[19] may be important predictors of vitamin D status in this population. Notwithstanding the known impact of vitamin D on hard outcomes in dialysis patients, there has not been examination of the association between UV exposure and mortality risk in this context.
We hypothesized that dialysis patients residing in areas of high UV exposure have decreased mortality risk compared to those living in areas of low UV exposure. To better inform the field, we sought to examine the association between UV exposure and all-cause mortality within a large, contemporary cohort of US dialysis patients with comprehensive capture of sociodemographic, comorbidity, and laboratory data.
MATERIALS AND METHODS
Study Population
We examined administrative data from all end-stage renal disease patients who underwent hemodialysis or peritoneal dialysis in one of the DaVita Healthcare Partners Inc. outpatient dialysis facilities during an entry period of July 1, 2001 to June 30, 2006, with follow-up through June 30, 2009. The creation of this cohort has been previously described.[20] The study was approved by the Institutional Review Committees of the Los Angeles Biomedical Research Institute at Harbor-UCLA and DaVita Clinical Research. The requirement for a written consent form was waived because of the large sample size, anonymity of the patients studied, and the non-intrusive nature of the research.
The first (baseline) study quarter was the calendar quarter in which each patient’s dialysis vintage was >45 days during at least half of that quarter. Patients were included provided that they were ≥18 years old during the baseline quarter, and had available residential zip code and UV exposure data.
Ultraviolet Exposure Ascertainment
We sought to examine the association between forecasted ambient UV radiation, quantified by the UV index, and mortality. The UV index was first proposed by Environment Canada and subsequently adopted and standardized by the World Health Organization and the World Meteorological Organization in 1994. It is used to predict the UV radiation intensity reaching the earth’s surface, and has been used as a proxy of UV exposure in prior epidemiologic studies.[21–23] The calculation of UV index takes into account stratospheric ozone concentration, cloud coverage, altitude, sun’s position, surface albedo, tropospheric aerosol loading[24], and it is additionally adjusted for the variations in skin sensitivity to different wavelengths of light, based on the McKinlay-Diffey erythema action spectrum.[25] The resulting value is scaled by a numerical factor and rounded to the nearest whole number, ranging from zero to the mid-teens. A higher UV index corresponds to more intense UV radiation incident at the surface of a particular location.[26]
The National Oceanic and Atmospheric Administration (NOAA) National Weather Center provides daily UV index forecasts for 58 major US cities. Each US state is represented by at least one of these 58 cities, with additional representation in California, Florida, New York, Pennsylvania, and Texas. UV data for each city was applied to all zip codes within the cities’ county boundaries, for an aggregate of 5,425 zip codes. UV index values were linked to each individual DaVita dialysis patient using their residential zip codes, ascertained at baseline and updated quarterly. While UV index may be forecasted for any time of the day or year, in this study, each patient’s UV index represents the average of the annual noon-time UV index values estimated over his/her respective follow-up period. In primary analyses, UV indices were stratified into five groups, adapted from the Environmental Protection Agency’s UV index categories: low (0–<3), moderate (3–<5), moderate-high (5–<6), high (6–<7), and very-high (≥7).
Outcome Ascertainment
The primary outcome of interest was all-cause mortality which was ascertained from the DaVita database and through United States Renal Data System database linkage. Patients were followed for the outcome of interest until death, or censoring for kidney transplantation or end of the study period (June 30, 2009).
Dialysis Treatment and Laboratory Covariates
Dialysis vintage was defined as the duration of time between the first day of dialysis treatment and the first day that the patient entered the cohort. To minimize measurement variability and to address the effect of short-term variation in dietary and fluid intake on weight and laboratory measurements, we averaged all repeated measures for each patient during any given calendar quarter (i.e., over 13 consecutive weeks or 3 months). Blood samples were drawn using uniform techniques in all dialysis clinics and were transported to the DaVita Laboratory in Deland, Florida typically within 24 hours, and were measured by automated and standardized methods. Most laboratory values were measured monthly, and serum ferritin and intact parathyroid hormone (PTH) levels were measured at least quarterly. Hemoglobin was measured weekly to bi-weekly in most patients. Most blood samples were collected pre-dialysis with the exception of the post-dialysis serum urea nitrogen.
Statistical Methods
Baseline characteristics between UV index categories were compared using non-parametric tests for linear trend. The associations between UV index and mortality were evaluated using Cox proportional hazards regression models. For each analysis, three models were examined with incremental multivariable adjustment for baseline covariates:
Model 1 which included the entry calendar quarter;
Model 2 which included covariates from Model 1, as well as age, sex, dialysis modality, race/ethnicity (African–Americans, non-Hispanic Caucasians, Hispanics, Asians, and other), diabetes mellitus, dialysis vintage, primary insurance, marital status, and dialysis dose (i.e., single pool kt/v);
Model 3 which included covariates from Model 2, as well as body mass index, baseline comorbidities (alcohol dependence, active smoking, cardiac failure, chronic-obstructive pulmonary disorder, cerebrovascular disease, and peripheral vascular disease), and the following laboratory covariates: serum albumin, calcium, bicarbonate, creatinine, ferritin, hemoglobin, lymphocyte percentage, normalized protein catabolic rate, phosphorus, white blood cell count, alkaline phosphatase, and PTH.
UV index-mortality associations were examined within subgroups of age (<65 years vs. ≥65 years), sex, and race (white vs. non-white). We employed a complete case analysis approach in which analyses were restricted to patients with available data for all covariates used in each respective model, although missing data was <1% for most laboratory and demographic variables. All p-values were 2-tailed (p<0.05). Analyses were conducted using Stata version 10.1 (Stata Corporation, College Station, Texas).
RESULTS
Baseline Characteristics
After excluding patients who were <18 years old or who had missing age data; those who did not maintain ≥45 days of thrice-weekly dialysis treatment during the baseline calendar quarter; and those with missing zip code-linked UV index data, the final source cohort consisted of 47,286 dialysis patients. Supplementary Figure 1 shows the distribution of dialysis patients according to their respective UV index categories, with the majority of patients residing in areas of moderate to high UV regions.
Baseline demographic, clinical and laboratory data of dialysis patients across the five UV index categories were examined (Table 1). Compared to patients residing in the lowest UV index category, those residing in the highest category were more likely to be African-American, Hispanic, divorced, treated with hemodialysis, and of shorter dialysis vintage; had a higher prevalence of diabetes, atherosclerotic heart disease, cardiac failure, and peripheral vascular disease; and had higher mean residual renal function, ferritin, and PTH levels.
Table 1.
Baseline characteristics among dialysis patients stratified by residential ultraviolet (UV) index category.
| UV Index | |||||||
|---|---|---|---|---|---|---|---|
| Total n= 47,286 | <3 (n=595) | 3–<5 (n= 19,941) | 5–<6 (n= 18,630) | 6–<7 (n= 7,206) | 7+ (n= 914) | p-value | |
| Age, mean (SD) | 60 (16) | 60 (16) | 60 (16) | 59 (16) | 60 (16) | 61 (16) | 0.2 |
| Female (%) | 46 | 40 | 45 | 50 | 44 | 41 | 0.9 |
| Diabetes mellitus (%) | 56 | 49 | 54 | 49 | 58 | 55 | <0.001 |
| Dialysis Modality (%) | |||||||
| Hemodialysis | 95 | 93 | 96 | 94 | 94 | 99 | 0.01 |
| Peritoneal Dialysis | 5 | 7 | 4 | 6 | 6 | 1 | |
| Race and Ethnicity (%) | |||||||
| White | 27 | 50 | 31 | 22 | 30 | 13 | <0.001 |
| Black | 41 | 22 | 50 | 35 | 30 | 39 | <0.001 |
| Hispanic | 21 | 4 | 9 | 28 | 31 | 45 | <0.001 |
| Asian | 4 | 10 | 2 | 7 | 4 | ~0 | <0.001 |
| Other | 6 | 12 | 6 | 7 | 5 | 3 | <0.001 |
| Vintage (%) | |||||||
| <6 months | 12 | 6 | 12 | 8 | 21 | 15 | <0.001 |
| 6–<24 months | 29 | 25 | 29 | 22 | 46 | 32 | <0.001 |
| 2–<5 years | 35 | 41 | 35 | 40 | 22 | 30 | <0.001 |
| ≥5 years | 24 | 28 | 24 | 29 | 11 | 23 | <0.001 |
| Primary Insurance (%) | |||||||
| Medicare | 58 | 49 | 61 | 55 | 60 | 52 | <0.001 |
| Medicaid | 8 | 3 | 7 | 10 | 8 | 5 | <0.001 |
| Private Insurance | 10 | 24 | 8 | 13 | 4 | 11 | <0.001 |
| Other | 14 | 16 | 15 | 10 | 23 | 28 | <0.001 |
| Marital Status (%) | |||||||
| Married | 36 | 44 | 34 | 35 | 44 | 41 | <0.001 |
| Divorced | 6 | 4 | 7 | 6 | 7 | 10 | 0.007 |
| Single | 28 | 26 | 30 | 25 | 29 | 26 | <0.001 |
| Widowed | 11 | 10 | 12 | 11 | 12 | 10 | 0.09 |
| spKt/V, mean (SD) | 1.50 (0.35) | 1.61 (0.32) | 1.48 (0.33) | 1.49 (0.35) | 1.53 (0.39) | 1.59 (0.36) | <0.001 |
| Residual Renal Function (ml/min), mean (SD) | 2.77 (2.5) | 1.99 (1.7) | 2.64 (2.50) | 2.60 (2.25) | 3.26 (2.90) | 3.30 (2.39) | <0.001 |
| Comorbidities (%) | |||||||
| Active Smoking | 4 | 4 | 5 | 3 | 3 | 2 | <0.001 |
| AHD | 16 | 19 | 18 | 13 | 16 | 24 | <0.001 |
| Alcohol Dependence | 1 | 1 | 1 | 1 | 1 | 1 | <0.001 |
| Cardiac Failure | 23 | 20 | 24 | 22 | 24 | 30 | 0.09 |
| COPD | 4 | 5 | 4 | 3 | 4 | 4 | <0.001 |
| CVD | 6 | 7 | 7 | 5 | 6 | 7 | 0.004 |
| Drug Use | 1 | 1 | 2 | 1 | 1 | 1 | <0.001 |
| Non-ambulatory State | 3 | 1 | 3 | 3 | 3 | 4 | <0.001 |
| Malignancy | 3 | 3 | 4 | 2 | 3 | 5 | <0.001 |
| PVD | 8 | 10 | 9 | 6 | 9 | 16 | 0.002 |
| Serum or Blood Levels, mean (SD) | |||||||
| Ferritin (ng/mL) | 526 (504) | 519 (411) | 497 (479) | 566 (533) | 481 (483) | 582 (521) | <0.001 |
| Hemoglobin (g/dL) | 11.9 (1.4) | 12.0 (1.2) | 11.9 (1.4) | 11.9 (1.3) | 12.1(1.4) | 12.2 (1.2) | <0.001 |
| TIBC (mg/dL) | 208 (46) | 211 (46) | 208 (47) | 206 (46) | 214 (46) | 204 (41) | <0.001 |
| Albumin (g/dL) | 3.67 (0.47) | 3.71 (0.44) | 3.67 (0.48) | 3.69 (0.46) | 3.65 (0.48) | 3.72 (0.45) | 0.3 |
| Calcium (mg/dL) | 9.2 (0.7) | 9.3 (0.8) | 9.2 (0.7) | 9.2 (0.7) | 9.2 (0.7) | 9.3 (0.7) | 0.5 |
| Intact PTH (pg/ml) | 375 (391) | 340 (347) | 384 (405) | 369 (380) | 366 (377) | 447 (493) | 0.006 |
| Phosphorus (mg/dL) | 5.6 (1.5) | 5.8 (1.5) | 5.6 (1.5) | 5.6 (1.5) | 5.5 (1.5) | 5.6 (1.4) | 0.5 |
| Creatinine (mg/dL) | 8.5 (3.5) | 8.9 (3.3) | 8.6 (3.5) | 8.7 (3.6) | 7.6 (3.3) | 8.8 (3.5) | <0.001 |
| nPCR (g/kg/day) | 0.96 (0.26) | 0.97 (0.26) | 0.94 (0.25) | 0.97 (0.26) | 0.94 (0.26) | 1.00 (0.27) | 0.002 |
| BMI (kg/m2) | 26.6 (6.9) | 26.7 (8.0) | 26.8 (7.0) | 26.4 (6.9) | 26.6 (6.5) | 26.6 (6.3) | 0.1 |
| WBC (×103/ul) | 7.3 (2.4) | 7.7 (2.8) | 7.3 (2.5) | 7.4 (2.4) | 7.5 (2.3) | 7.4 (2.3) | <0.001 |
| Lymphocyte % | 21 (8) | 20 (8) | 21 (8) | 21 (8) | 21 (8) | 23 (8) | <0.001 |
| Bicarbonate (mg/dL) | 22.4 (3.1) | 23.2 (3.4) | 22.2 (3.1) | 22.5 (3.0) | 22.9 (3.2) | 22.1 (3.1) | <0.001 |
p-values were calculated using non-parametric tests of linear trend.
Abbreviations: SD: standard deviation; AHD: atherosclerotic disease; COPD: chronic-obstructive pulmonary disease; CVD: cerebrovascular disease; PVD: peripheral vascular disease; TIBC: total iron binding capacity; PTH: parathyroid hormone; nPCR: normalized protein catabolic rate; BMI: body mass index; WBC: white blood cell count
Ultraviolet Index and Mortality in Dialysis Patients
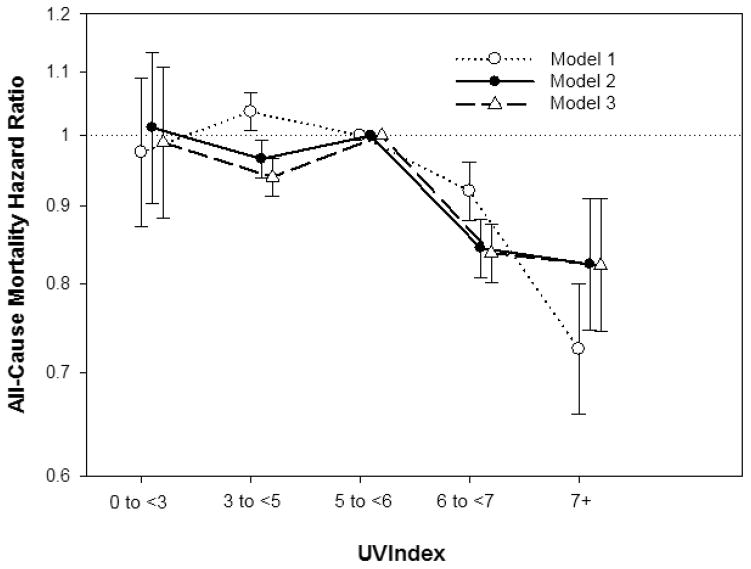
The association between residential UV index divided into five categories and all-cause mortality was examined in three Cox regression models with incremental levels of multivariable adjustment (Figure 1 and Table 2). Compared to patients living in moderate-high UV index areas, those living in high to very-high UV index areas had decreased mortality risk, whereas patients residing in moderate and low UV index areas had decreased and similar mortality risk, respectively.
Figure 1. Association between residential ultraviolet (UV) index divided into five categories (low: <3; moderate: 3–<5; moderate-high: 5–<6; high: 6–<7; and very-high: ≥7) with all-cause mortality in dialysis patients (reference group: UV index 5–<6).
Model 1 included UV index category and entry calendar quarter. Model 2 included covariates from Model 1, as well as age, sex, dialysis modality, race/ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, and dialysis dose (i.e., single pool kt/v). Model 3 included covariates from the Model 2, baseline comorbidities (alcohol dependence, active smoking, cardiac failure, chronic-obstructive pulmonary disorder, cerebrovascular disease, and peripheral vascular disease), body mass index, serum albumin, calcium, bicarbonate, creatinine, ferritin, hemoglobin, lymphocyte percentage, normalized protein catabolic rate, phosphorus, white blood cell count, alkaline phosphatase, and parathyroid hormone.
Table 2.
All-cause mortality hazard ratios (95% confidence intervals) in dialysis patients across ultraviolet (UV) index categories.
| UV index | n=47,286 | Model 1* HR (95% CI) |
Model 2† HR (95% CI) |
Model 3†† HR (95% CI) |
|---|---|---|---|---|
|
| ||||
| <3 | 595 | 0.98 (0.87, 1.09) | 1.01 (0.90, 1.13) | 0.99 (0.88, 1.11) |
| 3–<5 | 19,941 | 1.04 (1.01, 1.07) | 0.97 (0.94, 0.99) | 0.94 (0.91, 0.97) |
| 5–<6 | 18,630 | 1.00 | 1.00 | 1.00 |
| 6–<7 | 7,206 | 0.92 (0.88, 0.96) | 0.84 (0.81, 0.88) | 0.84 (0.80, 0.88) |
| 7+ | 914 | 0.73 (0.66, 0.80) | 0.82 (0.75, 0.91) | 0.82 (0.74, 0.91) |
Model 1 included UV index category and entry calendar quarter.
Model 2 included covariates from Model 1, as well as age, sex, dialysis modality, race/ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, and dialysis dose (i.e., single pool kt/v).
Model 3 included covariates from Model 2, baseline comorbidities (alcohol dependence, active smoking, cardiac failure, chronic-obstructive pulmonary disorder, cerebrovascular disease, and peripheral vascular disease), body mass index, serum albumin, calcium, bicarbonate, creatinine, ferritin, hemoglobin, lymphocyte percentage, normalized protein catabolic rate, phosphorus, white blood cell count, alkaline phosphatase, and parathyroid hormone.
Subgroup Analyses
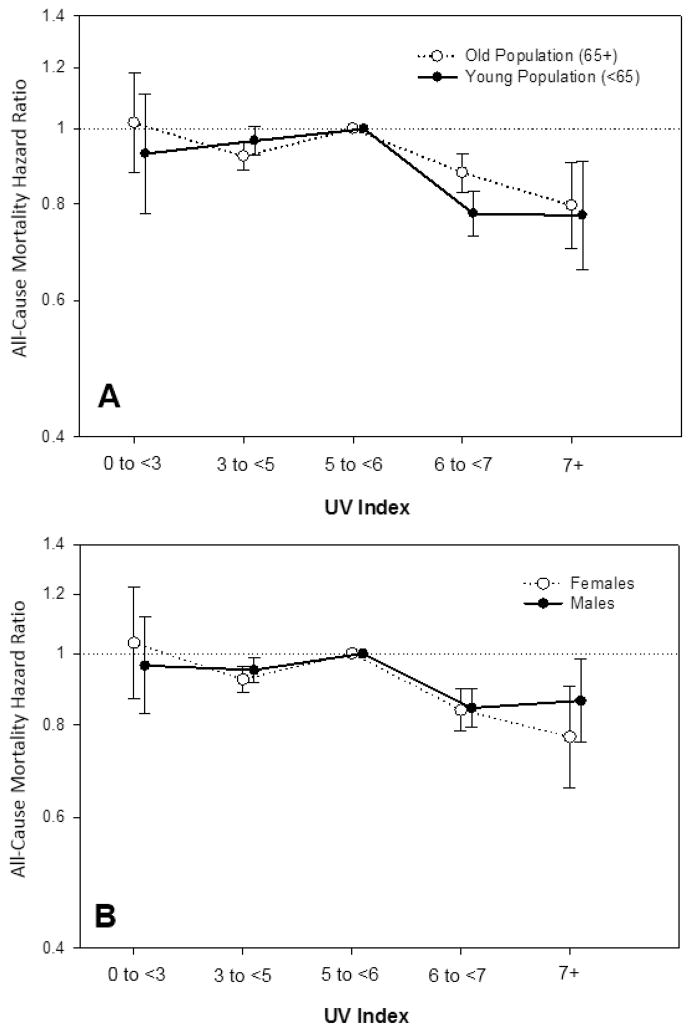
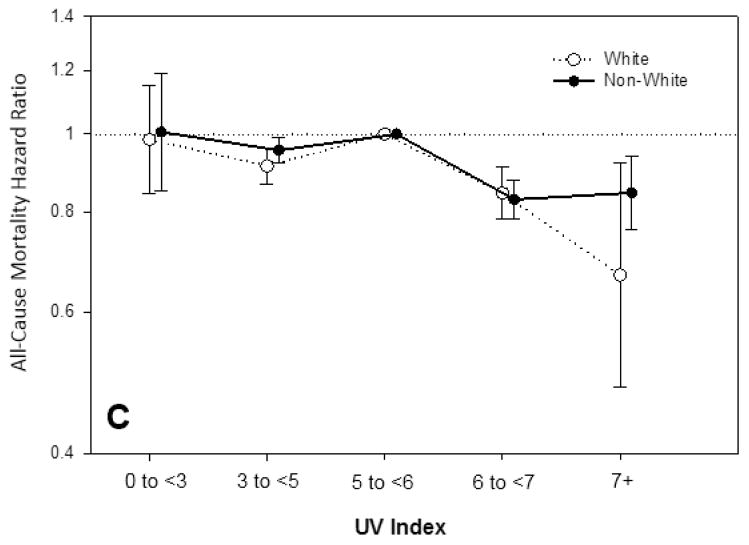
The UV index—mortality association was examined in patients stratified by age (<65 vs. ≥ 65 years old), sex, and race (white vs. non-white). A similar inverse association between UV index and mortality was observed in all subgroups, although there appeared to be a more pronounced reduction in mortality among whites compared to non-whites (Figure 2 and Supplementary Table 1).
Figure 2. Association between residential ultraviolet (UV) index divided into five categories (low: <3; moderate: 3–<5; moderate-high: 5–<6; high: 6–<7; and very-high: ≥7) with all-cause mortality in dialysis patients within subgroups of age (<65 years vs. ≥65 years), sex (female vs. male), and race (white vs. non-white).
Models included UV index category, entry calendar quarter, age, sex, dialysis modality, race/ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, dialysis dose (i.e., single pool kt/v), baseline comorbidities (alcohol dependence, active smoking, cardiac failure, chronic-obstructive pulmonary disorder, cerebrovascular disease, and peripheral vascular disease), body mass index, serum albumin, calcium, bicarbonate, creatinine, ferritin, hemoglobin, lymphocyte percentage, normalized protein catabolic rate, phosphorus, white blood cell count, alkaline phosphatase, and parathyroid hormone.
DISCUSSION
To our knowledge, this is the first study to examine the association between UV exposure and mortality in a contemporary cohort of US dialysis patients. In the general population, there is evidence that solar UV radiation is causally associated with skin cancer (e.g., malignant melanoma, basal and squamous cell cancer) via direct induction of DNA damage and indirect effects on immune suppression,[27, 28] and it has been deemed to be a carcinogen by the International Agency for Research on Cancer.[29] However, emerging data suggest that UV radiation may also have potential health benefits. A number of ecologic and cohort studies have suggested that there is an inverse association between UV radiation (defined as UV index, solar UV-B radiation, and erythemogenic UV radiation in these studies) with cancer incidence and mortality (renal,[4, 30] prostate,[3, 4, 31] breast,[4, 31, 32] colon,[4, 30–32], and rectal[4, 30]). Furthermore, in a recent prospective cohort study of 38,472 Swedish women, participants who reported higher levels of prior natural and artificial UV exposure (i.e., prior history of sunburn, sunbathing, and solarium use) had decreased all-cause and cardiovascular mortality.[2] In our study, UV index was employed as an objective metric of forecasted solar radiation, and we observed that individuals living in higher UV exposure regions had decreased mortality risk compared to those living in low to moderate UV exposure regions.
Increased biosynthesis of vitamin D has been suggested as a potential mechanistic link between higher UV indices and decreased cancer incidence and cardiovascular mortality. In terms of the former outcome, vitamin D has been associated with decreased cancer risk vis-a-vis improved cell differentiation and apoptosis, anti-angiogenesis, decreased metastases, and decreased risk of viral infection-associated malignancies in some[33–35], but not all studies[36]. In terms of the latter outcome, multiple observational studies have reported an inverse association between vitamin D levels and greater risk of adverse cardiovascular surrogates, cardiovascular events and mortality in populations with[12–14] and without[37–39] chronic kidney disease (CKD). Solar UV-B radiation promotes the conversion of 7-dehydrocholesterol to pre-vitamin D3 in skin tissue, which then isomerizes to vitamin D3 (i.e., cholecalciferol). Vitamin D3 is then transported to the liver where it undergoes hydroxylation by cytochrome p450 enzymes to become 25-hydroxy-vitamin D (i.e., calcidiol), the major circulating form of vitamin D. Inactive 25-hydroxy-vitamin D is then converted into active 1,25-dihydroxy-vitamin D (i.e., calcitriol), by the 1-α hydroxylase enzyme present in various parts of the nephron.[40] Although 1,25-dihydroxy-vitamin D production is downregulated in advanced CKD as a result of hyperphosphatemia,[41] uremia,[42, 43] metabolic acidosis,[44] and elevated fibroblast growth factor-23,[45, 46] experimental and clinical studies have shown that administration of 25-hydroxy-vitamin D, even in anephric individuals,[18] is associated with significant increases in activated vitamin D. This may be due to the presence of 1-α hydroxylase in other tissues (e.g., skin, lymph nodes, gastrointestinal tract, pancreas, heart, and adrenal glands) as an extra-renal source of activated vitamin D.[47, 48]
Cardiovascular disease is the leading cause of death in CKD, and vitamin D deficiency is associated with an increased risk of cardiovascular risk factors (e.g., albuminuria[49]), adverse cardiovascular surrogates (e.g., coronary artery calcification,[10] atherosclerosis,[11] endothelial dysfunction[11]) as well as increased risk of cardiovascular events[12] and all-cause mortality in this population.[13, 14] Observational studies have suggested that treatment with activated vitamin D reduces all-cause and cardiovascular mortality in patients with CKD, including those who are non-dialysis dependent and those receiving dialysis.[50–54] Although several studies suggest 25-hydroxy-vitamin D treatment may have benefits on cardiovascular surrogates in CKD patients,[55, 56] there have not been any well-designed randomized controlled trials or large observational cohort studies examining hard outcomes in this context.
Emerging data suggest that reduced sunlight exposure may be a predictor of vitamin D deficiency in dialysis patients,[17] and that artificial UV radiation may be an alternative source for vitamin D repletion in this context. In an observational cohort study of 15 dialysis patients, narrow-band UV-B treatment over a 3-week period resulted in a significant increase in serum 25-hydroxy and 1,25-dihydroxy-vitamin D levels.[19] Although dialysis patients may be less likely to participate in outdoor physical activity and hence have reduced natural UV exposure,[57, 58] even brief durations of solar or artificial UV exposure have been shown to significantly increase vitamin D levels in the general population[59–62]; further study is needed to determine if natural UV radiation may be a viable source of vitamin D repletion in dialysis patients. Due to data limitations, we were not able to directly ascertain patients’ frequency or duration of outdoor activity, other factors that modify UV exposure (e.g., photosensitizing medications or photo-protective clothing), or vitamin D levels in our study; however, it is plausible that patients residing in higher UV index regions experience greater solar UV radiation exposure and subsequent synthesis of vitamin D. Future studies directly measuring solar UV radiation exposure, serum vitamin D levels, and cardiovascular outcomes in CKD patients are needed.
When we examined associations between UV index and mortality across subgroups of race, higher UV index categories were associated with a more potent survival benefit among white patients compared to non-whites in the highest UV index category (≥7). While genetic polymorphisms may partially account for variations in total vitamin D level across racial groups [63], light-skinned individuals experience greater UV-B absorption and subsequent vitamin D synthesis as a result of their reduced melanin skin content, compared to those who are non-white.[64, 65]
Strengths of our study include the examination of a large, contemporary US dialysis population with extended follow-up; comprehensive availability of clinical data allowing for adjustment of multiple confounders; examination of individual patients’ residential UV indices; and use of a validated assessment of UV exposure that accounts for potential confounders such as altitude. However, several limitations of our study bear mention. First, our analyses examined quarterly residential UV index only, and did not account for patients who may have migrated over time within that period. Second, we are unable to confirm that an individual’s residential UV index, which is a forecast, directly correlates with the patient’s actual UV exposure. Third, given that the National Oceanic and Atmospheric Administration measures UV index in major US cities only, our study cohort may not be representative of dialysis patients living in outlier or rural regions. Fourth, while we attempted to adjust for broad markers of nutritional status (e.g., normalized protein catabolic rate, serum albumin) in our multivariable models, due to data limitations we were unable to account for more granular nutritional variables (e.g., diet, nutritional supplements) that may confound the UV index—mortality association. For example, there may be regional variation in dietary intake (including foods that are supplemented with vitamin D), or utilization of vitamin D supplements that are also associated with mortality.[66] Lastly, as with all observational studies, we cannot confirm that there is a causal association between UV index and mortality.
Our data suggest that higher UV index is associated with survival benefit, and that these associations may be even more pronounced among dialysis patients vs. the US general population. Further studies are needed to confirm findings, and to determine the mechanistic pathways by which UV index is associated with mortality risk in dialysis patients.
Supplementary Material
Acknowledgments
Support: The authors are supported by research grants from the NIH/NIDDK including K24-DK091419 (KKZ), K23-DK102903 (CMR), R01-DK078106 (KKZ), and philanthropist grants from Mr. Harold Simmons and Mr. Louis Chang.
Portions of these data have been presented in an abstract published in the Journal of the American Society of Nephrology; a poster presentation at the American Society of Nephrology annual conference, October 27–31, 2012, San Diego, CA; and an oral abstract presentation at the Annual Dialysis Conference, March 9–13, 2013, Seattle, WA.
Footnotes
Conflicts of Interest:
KKZ was medical director of the DaVita Harbor-UCLA Long Beach from 2007–2012. BBS, ES, JLTC, CPK, and CMR have nothing to declare.
References
- 1.Young C. Solar ultraviolet radiation and skin cancer. Occup Med (Lond) 2009;59(2):82–8. doi: 10.1093/occmed/kqn170. [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Lof M, Veierod MB, Sandin S, Adami HO, Weiderpass E. Ultraviolet exposure and mortality among women in Sweden. Cancer Epidemiol Biomarkers Prev. 2011;20(4):683–90. doi: 10.1158/1055-9965.EPI-10-0982. [DOI] [PubMed] [Google Scholar]
- 3.Colli JL, Grant WB. Solar ultraviolet B radiation compared with prostate cancer incidence and mortality rates in United States. Urology. 2008;71(3):531–5. doi: 10.1016/j.urology.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 4.Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993–2002. BMC Cancer. 2006;6:264. doi: 10.1186/1471-2407-6-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holick MF. McCollum Award Lecture, 1994: vitamin D--new horizons for the 21st century. Am J Clin Nutr. 1994;60(4):619–30. doi: 10.1093/ajcn/60.4.619. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 7.Pilz S, Tomaschitz A, Friedl C, Amrein K, Drechsler C, Ritz E, Boehm BO, Grammer TB, Marz W. Vitamin D status and mortality in chronic kidney disease. Nephrol Dial Transplant. 2011;26(11):3603–9. doi: 10.1093/ndt/gfr076. [DOI] [PubMed] [Google Scholar]
- 8.Bansal B, Bansal S, Mithal A, Kher V, Marwaha R. Vitamin D deficiency in hemodialysis patients. Indian J Endocrinol Metab. 2012;16(2):270–3. doi: 10.4103/2230-8210.93749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saab G, Young DO, Gincherman Y, Giles K, Norwood K, Coyne DW. Prevalence of vitamin D deficiency and the safety and effectiveness of monthly ergocalciferol in hemodialysis patients. Nephron Clin Pract. 2007;105(3):c132–8. doi: 10.1159/000098645. [DOI] [PubMed] [Google Scholar]
- 10.de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20(8):1805–12. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.London GM, Guerin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, Metivier F. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18(2):613–20. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 12.Wang AY, Lam CW, Sanderson JE, Wang M, Chan IH, Lui SF, Sea MM, Woo J. Serum 25-hydroxyvitamin D status and cardiovascular outcomes in chronic peritoneal dialysis patients: a 3-y prospective cohort study. Am J Clin Nutr. 2008;87(6):1631–8. doi: 10.1093/ajcn/87.6.1631. [DOI] [PubMed] [Google Scholar]
- 13.Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, Mallamaci F, Zoccali C. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75(1):88–95. doi: 10.1038/ki.2008.501. [DOI] [PubMed] [Google Scholar]
- 14.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Jr, Tonelli M, Thadhani R. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72(8):1004–13. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 15.Petchey WG, Johnson DW, Hawley CM, Isbel NM. Predictors of vitamin D status in predialysis chronic kidney disease patients: a cross-sectional analysis in a high ultraviolet climate. J Ren Nutr. 2012;22(4):400–8. doi: 10.1053/j.jrn.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Cuppari L, Carvalho AB, Draibe SA. Vitamin D status of chronic kidney disease patients living in a sunny country. J Ren Nutr. 2008;18(5):408–14. doi: 10.1053/j.jrn.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Del Valle E, Negri AL, Aguirre C, Fradinger E, Zanchetta JR. Prevalence of 25(OH) vitamin D insufficiency and deficiency in chronic kidney disease stage 5 patients on hemodialysis. Hemodial Int. 2007;11(3):315–21. doi: 10.1111/j.1542-4758.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen OH, Lund B, Thode JD, Storm TL, Lund B, Brahm M, Friedberg M, Holmegaard SN. Effect of sunlight exposure on circulating 1,25-dihydroxyvitamin D in hemodialyzed patients and of exogenous parathyroid hormone in anephric patients. Acta Med Scand. 1986;219(2):215–9. doi: 10.1111/j.0954-6820.1986.tb03301.x. [DOI] [PubMed] [Google Scholar]
- 19.Ala-Houhala MJ, Vahavihu K, Hasan T, Kautiainen H, Snellman E, Karisola P, Dombrowski Y, Schauber J, Saha H, Reunala T. Narrow-band ultraviolet B exposure increases serum vitamin D levels in haemodialysis patients. Nephrol Dial Transplant. 2012;27(6):2435–40. doi: 10.1093/ndt/gfr700. [DOI] [PubMed] [Google Scholar]
- 20.Lukowsky LR, Kheifets L, Arah OA, Nissenson AR, Kalantar-Zadeh K. Patterns and predictors of early mortality in incident hemodialysis patients: new insights. Am J Nephrol. 2012;35(6):548–58. doi: 10.1159/000338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eide MJ, Weinstock MA. Association of UV index, latitude, and melanoma incidence in nonwhite populations--US Surveillance, Epidemiology, and End Results (SEER) Program, 1992 to 2001. Arch Dermatol. 2005;141(4):477–81. doi: 10.1001/archderm.141.4.477. [DOI] [PubMed] [Google Scholar]
- 22.Walls AC, Han J, Li T, Qureshi AA. Host Risk Factors, Ultraviolet Index of Residence, and Incident Malignant Melanoma In Situ Among US Women and Men. Am J Epidemiol. 2013;177(9):997–1005. doi: 10.1093/aje/kws335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei-Passanese EX, Han J, Lin W, Li T, Laden F, Qureshi AA. Geographical variation in residence and risk of multiple nonmelanoma skin cancers in US women and men. Photochem Photobiol. 2012;88(2):483–9. doi: 10.1111/j.1751-1097.2012.01077.x. [DOI] [PubMed] [Google Scholar]
- 24.Sin C, Beauchet A, Marchal A, Sigal ML, Mahe E. Understanding and use of the global solar UV index (“UV index”) by French dermatologists. Ann Dermatol Venereol. 2013;140(1):15–20. doi: 10.1016/j.annder.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 25.McKinlay AF, Diffey BL. A reference action spectrum for ultraviolet induced erythema in human skin. CIE Journal. 1987;6:17–22. [Google Scholar]
- 26.Kinney JP, Long CS. The Ultraviolet Index: a useful tool. Dermatol Online J. 2000;6(1):2. [PubMed] [Google Scholar]
- 27.Marrot L, Meunier JR. Skin DNA photodamage and its biological consequences. J Am Acad Dermatol. 2008;58(5 Suppl 2):S139–48. doi: 10.1016/j.jaad.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Ullrich SE. Sunlight and skin cancer: lessons from the immune system. Mol Carcinog. 2007;46(8):629–33. doi: 10.1002/mc.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balk SJ. Ultraviolet radiation: a hazard to children and adolescents. Pediatrics. 2011;127(3):e791–817. doi: 10.1542/peds.2010-3502. [DOI] [PubMed] [Google Scholar]
- 30.Grant WB, Garland CF. The association of solar ultraviolet B (UVB) with reducing risk of cancer: multifactorial ecologic analysis of geographic variation in age-adjusted cancer mortality rates. Anticancer Res. 2006;26(4A):2687–99. [PubMed] [Google Scholar]
- 31.Robsahm TE, Tretli S, Dahlback A, Moan J. Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway) Cancer Causes Control. 2004;15(2):149–58. doi: 10.1023/B:CACO.0000019494.34403.09. [DOI] [PubMed] [Google Scholar]
- 32.Freedman DM, Dosemeci M, McGlynn K. Sunlight and mortality from breast, ovarian, colon, prostate, and non-melanoma skin cancer: a composite death certificate based case-control study. Occup Environ Med. 2002;59(4):257–62. doi: 10.1136/oem.59.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grant WB. How strong is the evidence that solar ultraviolet B and vitamin D reduce the risk of cancer?: An examination using Hill’s criteria for causality. Dermatoendocrinol. 2009;1(1):17–24. doi: 10.4161/derm.1.1.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majewski S, Skopinska M, Marczak M, Szmurlo A, Bollag W, Jablonska S. Vitamin D3 is a potent inhibitor of tumor cell-induced angiogenesis. J Investig Dermatol Symp Proc. 1996;1(1):97–101. [PubMed] [Google Scholar]
- 35.Nakagawa K, Kawaura A, Kato S, Takeda E, Okano T. 1 alpha,25-Dihydroxyvitamin D(3) is a preventive factor in the metastasis of lung cancer. Carcinogenesis. 2005;26(2):429–40. doi: 10.1093/carcin/bgh332. [DOI] [PubMed] [Google Scholar]
- 36.Lin SW, Wheeler DC, Park Y, Spriggs M, Hollenbeck AR, Freedman DM, Abnet CC. Prospective study of ultraviolet radiation exposure and mortality risk in the United States. Am J Epidemiol. 2013;178(4):521–33. doi: 10.1093/aje/kws589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168(12):1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 39.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nigwekar SU, Bhan I, Thadhani R. Ergocalciferol and cholecalciferol in CKD. Am J Kidney Dis. 2012;60(1):139–56. doi: 10.1053/j.ajkd.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 41.Li YC. Renoprotective effects of vitamin D analogs. Kidney Int. 2010;78(2):134–9. doi: 10.1038/ki.2009.175. [DOI] [PubMed] [Google Scholar]
- 42.Vanholder R, Patel S, Hsu CH. Effect of uric acid on plasma levels of 1,25(OH)2D in renal failure. J Am Soc Nephrol. 1993;4(4):1035–8. doi: 10.1681/ASN.V441035. [DOI] [PubMed] [Google Scholar]
- 43.Hsu CH, Vanholder R, Patel S, De Smet RR, Sandra P, Ringoir SM. Subfractions in uremic plasma ultrafiltrate inhibit calcitriol metabolism. Kidney Int. 1991;40(5):868–73. doi: 10.1038/ki.1991.287. [DOI] [PubMed] [Google Scholar]
- 44.Kawashima H, Kraut JA, Kurokawa K. Metabolic acidosis suppresses 25-hydroxyvitamin in D3-1alpha-hydroxylase in the rat kidney. Distinct site and mechanism of action. J Clin Invest. 1982;70(1):135–40. doi: 10.1172/JCI110586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429–35. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 46.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Juppner H, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205–15. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 47.Adams JS, Hewison M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch Biochem Biophys. 2012;523(1):95–102. doi: 10.1016/j.abb.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qazi RA, Martin KJ. Vitamin D in kidney disease: pathophysiology and the utility of treatment. Rheum Dis Clin North Am. 2012;38(1):115–23. doi: 10.1016/j.rdc.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 49.de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2007;50(1):69–77. doi: 10.1053/j.ajkd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 50.Kalantar-Zadeh K, Kovesdy CP. Clinical outcomes with active versus nutritional vitamin D compounds in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(9):1529–39. doi: 10.2215/CJN.02140309. [DOI] [PubMed] [Google Scholar]
- 51.Kalantar-Zadeh K, Miller JE, Kovesdy CP, Mehrotra R, Lukowsky LR, Streja E, Ricks J, Jing J, Nissenson AR, Greenland S, Norris KC. Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J Bone Miner Res. 2010;25(12):2724–34. doi: 10.1002/jbmr.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kovesdy CP. Survival benefits with vitamin D receptor activation: new insights since 2003. Clin J Am Soc Nephrol. 2010;5(9):1704–9. doi: 10.2215/CJN.02590310. [DOI] [PubMed] [Google Scholar]
- 53.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008;168(4):397–403. doi: 10.1001/archinternmed.2007.110. [DOI] [PubMed] [Google Scholar]
- 54.Shinaberger CS, Kopple JD, Kovesdy CP, McAllister CJ, van Wyck D, Greenland S, Kalantar-Zadeh K. Ratio of paricalcitol dosage to serum parathyroid hormone level and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2008;3(6):1769–76. doi: 10.2215/CJN.01760408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bucharles S, Barberato SH, Stinghen AE, Gruber B, Piekala L, Dambiski AC, Custodio MR, Pecoits-Filho R. Impact of cholecalciferol treatment on biomarkers of inflammation and myocardial structure in hemodialysis patients without hyperparathyroidism. J Ren Nutr. 2012;22(2):284–91. doi: 10.1053/j.jrn.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Matias PJ, Jorge C, Ferreira C, Borges M, Aires I, Amaral T, Gil C, Cortez J, Ferreira A. Cholecalciferol supplementation in hemodialysis patients: effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol. 2010;5(5):905–11. doi: 10.2215/CJN.06510909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johansen KL, Chertow GM, Kutner NG, Dalrymple LS, Grimes BA, Kaysen GA. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int. 2010;78(11):1164–70. doi: 10.1038/ki.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agarwal R, Light RP. Sleep and activity in chronic kidney disease: a longitudinal study. Clin J Am Soc Nephrol. 2011;6(6):1258–65. doi: 10.2215/CJN.10581110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 60.Reid IR, Gallagher DJ, Bosworth J. Prophylaxis against vitamin D deficiency in the elderly by regular sunlight exposure. Age Ageing. 1986;15(1):35–40. doi: 10.1093/ageing/15.1.35. [DOI] [PubMed] [Google Scholar]
- 61.Rhodes LE, Webb AR, Fraser HI, Kift R, Durkin MT, Allan D, O’Brien SJ, Vail A, Berry JL. Recommended summer sunlight exposure levels can produce sufficient (> or =20 ng ml(−1)) but not the proposed optimal (> or =32 ng ml(−1)) 25(OH)D levels at UK latitudes. J Invest Dermatol. 2010;130(5):1411–8. doi: 10.1038/jid.2009.417. [DOI] [PubMed] [Google Scholar]
- 62.Sato Y, Iwamoto J, Kanoko T, Satoh K. Amelioration of osteoporosis and hypovitaminosis D by sunlight exposure in hospitalized, elderly women with Alzheimer’s disease: a randomized controlled trial. J Bone Miner Res. 2005;20(8):1327–33. doi: 10.1359/JBMR.050402. [DOI] [PubMed] [Google Scholar]
- 63.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, Thadhani R. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brenner M1 Hearing VJ. Photochem Photobiol. The protective role of melanin against UV damage in human skin. 2008 May-Jun;84(3):539–49. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farrar MD, Kift R, Felton SJ, Berry JL, Durkin MT, Allan D, Vail A, Webb AR, Rhodes LE. Recommended summer sunlight exposure amounts fail to produce sufficient vitamin D status in UK adults of South Asian origin. Am J Clin Nutr. 2011;94(5):1219–24. doi: 10.3945/ajcn.111.019976. [DOI] [PubMed] [Google Scholar]
- 66.Freisling H, Fahey MT, Moskal A, Ocké MC, Ferrari P, Jenab M, Norat T, Naska A, Welch AA, Navarro C, Schulz M, Wirfält E, Casagrande C, Amiano P, Ardanaz E, Parr C, Engeset D, Grioni S, Sera F, Bueno-de-Mesquita B, van der Schouw YT, Touvier M, Boutron-Ruault MC, Halkjaer J, Dahm CC, Khaw KT, Crowe F, Linseisen J, Kröger J, Huybrechts I, Deharveng G, Manjer J, Agren A, Trichopoulou A, Tsiotas K, Riboli E, Bingham S, Slimani N. Region-specific nutrient intake patterns exhibit a geographical gradient within and between European countries. J Nutr. 2010 Jul;140(7):1280–6. doi: 10.3945/jn.110.121152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.