Abstract
The cornea is one of the most densely innervated structures of the body. In the developing chicken embryo, nerves from the ophthalmic trigeminal ganglion (OTG) innervate the cornea in a series of spatially and temporally regulated events. However, little is known concerning the signals that regulate these events. Here we have examined the involvement of the axon guidance molecules Semaphorin3A and Slit2, and their respective receptors, Neuropilin-1 and Robo2. Expression analyses of early corneas suggest an involvement of both Semaphorin3A and Slit2 in preventing nerves from entering the corneal stroma until the proper time (i.e., they serve as negative regulators), and analyses of their receptors support this conclusion. At later stages of development the expression of Semaphorin3A is again consistent with its serving as a negative regulator – this time for nerves entering the corneal epithelium. However, expression analyses of Robo2 at this stage raised the possibility that Slit2 had switched from a negative regulator to a positive regulator. In support of such a switch, functional analyses – by addition of recombinant Slit2 protein or immunoneutralization with a Slit2 antibody – showed that at an early stage Slit2 negatively regulates the outgrowth of nerves from the OTG, whereas at the later stage it positively regulated the growth of nerves by increasing nerve branching within the corneal epithelium.
Keywords: cornea, innervation, trigeminal ganglion, Slit2, Semaphorin3A
INTRODUCTION
The cornea is one of the most densely innervated tissues in the body (Marfurt et al., 1989; Muller et al., 2003; Rozsa and Beuerman, 1982). The majority of this innervation is sensory and in the chicken originates in the ophthalmic lobe of the trigeminal ganglion (OTG) (Kubilus and Linsenmayer, 2009; Marfurt et al., 1989; Morgan et al., 1978). Within the cornea, the nerves function to transduce thermal, chemical and mechanical stimuli as sensations of pain, and also to maintain the cornea in a healthy state – through avoidance of these painful stimuli (Chen et al., 1997) as well as through the release of trophic factors which promote the survival of corneal epithelial (CE) cells (Baker et al., 1993; Garcia-Hirschfeld et al., 1994). Loss of corneal innervation through trauma, surgical complications or viral infection can lead to vision impairment due to loss of both of these functions of innervation (Wilson and Ambrosio, 2001).
Previous studies have demonstrated that in the chicken embryo corneal innervation occurs through a precise temporal and spatial series of stages (Bee, 1982). The first of these stages involves the growth of nerves from the OTG and their attraction towards the cornea. However, when the nerves reach the periphery of the cornea, initially they do not enter it, and instead form a ring that surrounds the cornea. Although the role of this “peri-corneal nerve ring” is unknown, a likely possibility is that its formation ensures that when innervation of the cornea subsequently does occur – which is by the simultaneous centripetal growth of nerves from this ring into the corneal stroma (CS) – the resulting innervation is uniform throughout the cornea. The final stages of innervation ensure that the nerves from the CS subsequently enter the CE and reach its surface. This involves their turning towards the CE, penetration through Bowman’s layer and entrance into the CE, and growth towards the corneal surface.
In the developing nervous system, neurons extend axons which must be directed to their appropriate targets while avoiding inappropriate targets. To accomplish this, the nervous system employs both positive and negative guidance cues (Kettunen et al., 2005; O’Leary et al., 1990).
Within the cornea, it is thought that positive cues, provided by the neurotrophins, serve to attract nerves during development (Bennett et al., 1999; de Castro et al., 1998; You et al., 2000); they also provide trophic stimuli promoting neuronal survival (Ebendal and Persson, 1988; Tucker et al., 2001). In the human, the expression of all four neurotrophins [i.e., nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), and neurotrophins-3 (NT-3) and 4 (NT-4)] have been detected in the cornea (You et al., 2000). Also, in the corneas of transgenic mice (expressing beta-galactosidase under the control of the BDNF or NT-3 promoters), the expression of BDNF was detected in both the CE and the corneal endothelium; while NT-3 was detected in the CE (Bennett et al., 1999).
In addition to the neurotrophins, four other families of regulators and their receptors have been reported to be involved in axonal guidance [reviewed by (Carmeliet and Tessier-Lavigne, 2005)]. These are the semaphorins, Slits, netrins and ephrins – with their respective receptors being the Neuropilins (Nrp), Roundabout (Robo), UNC/DCC, and eph receptors. Two of these, Sema3A and Slit2 – and their respective receptors neuropilin-1 (Nrp1) and Roundabout (Robo2) – constitute the main focus of the current study.
Sema3A is one of the best characterized negative regulators of innervation (Luo et al., 1993; Yazdani and Terman, 2006). Sema3A is secreted as a diffusible molecule that signals through its receptor Nrp1; it was originally called collapsin for its ability to collapse the growth cones of neurons in vitro (Luo et al., 1993). As Sema3A has been shown to inhibit innervation, release from this inhibition (i.e., permitting innervation to occur), can be effected either by down-regulating the production of the Sema3A at its source (e.g. the cornea) (Fu et al., 2000; Puschel et al., 1996; Shepherd et al., 1997; Wright et al., 1995) or by down-regulating its receptor, NRP-1 (in the target nerves) (Pond et al., 2002). For the cornea, knockout mice for Sema3A show premature and abnormal innervation of the anterior eye, including nerves entering the lens – which normally is not innervated (Taniguchi et al., 1997). Also, recent work (Lwigale and Bronner-Fraser, 2007) has suggested that lens-derived Sema3A is involved in regulating the initial stages of peri-corneal nerve ring formation by inhibiting the growth of nerves into the cornea.
Slits are secreted proteins that signal through the transmembrane receptor Robo. There are three forms of Slit (Slit1, 2 and 3) as well as four forms of Robo (Robo1, 2, 3 and 4). Mouse Slit2-Slit3 double mutants, Slit1-Slit2-Slit3 triple mutants, and Robo1-Robo2 double mutants exhibit defects in axon guidance (Bagri et al., 2002; Long et al., 2004; Plump et al., 2002). These defects can be either repulsive or attractive depending on the neuron type and developmental timing (Ma and Tessier-Lavigne, 2007; Ozdinler and Erzurumlu, 2002). Also, it has been reported that proteolytic cleavage of Slit2 may be involved in this role reversal, with the full length molecule inhibiting the growth of axons and an N-terminal cleavage fragment of Slit2, Slit2N, promoting nerve branching (Ma and Tessier-Lavigne, 2007; Wang et al., 1999). Slit2 has previously been detected in the cornea and lens of the chicken (Conrad et al., 2009; Holmes and Niswander, 2001), however its effects on nerve guidance in this structure during development are unknown.
While recent studies (Lwigale and Bronner-Fraser, 2007) have suggested that Sema3A is involved in the initial stages of corneal innervation (i.e., formation of the peri-corneal nerve ring) little is known as to what regulates the later stages (e.g., when nerves enter the CE and when they reach their target – the apical cells of the CE (Kubilus and Linsenmayer, 2009).
In the present study, through each of the stages of corneal innervation we have analyzed the nerve guidance molecules Sema3A and Slit2, and their receptors Nrp1 and Robo2. At each stage, we have observed that major changes occur in the expression of one or more of these regulatory components. In addition, using a function-blocking antibody against Slit2, we have obtained evidence that this regulator does have disparate roles in corneal innervation – initially acting as a negative regulator that prevents nerves from entering the corneal stroma – thus participating in the formation of the peri-corneal nerve ring – and subsequently acting as a positive regulator that promotes branching of nerves once they have entered the CE – thus facilitating expansion of the nerve network that forms between Bowman’s layer and the basal CE cells.
MATERIAL AND METHODS
Animals
White leghorn chicken eggs (Hy-line, Elizabethtown, PA) were incubated at 38°C. Embryos were removed, rinsed in Hank’s balanced saline solution (Invitrogen, Carlsbad, CA), and staged both by chronological time of incubation and by the criteria of Hamburger and Hamilton (Hamburger and Hamilton, 1951).
Immunohistochemistry
For immunohistochemistry (IHC) of tissue sections, tissues were fixed (4% PFA in 0.1M PBS pH 7.4) for 1 hour to overnight (depending on the tissue thickness), and then were processed for either frozen or paraffin sectioning as previously described (Kubilus and Linsenmayer, 2009). TuJ-1 antibody (R&D Systems, Minneapolis, MN) was diluted to 5μg/ml (in 1% blocking solution). Control corneas were incubated in either blocking solution without primary antibody or in the AC-9 antibody to type X collagen (Linsenmayer et al., 1988). Following incubation in primary antibody slides were incubated in anti-mouse northern lights red secondary antibody (R&D Systems, Minneapolis, MN) diluted 1:200 in PBS. Nuclei were stained with Hoechst and slides were then mounted in 95% glycerol in PBS.
Whole mount immunohistochemistry
For whole mount IHC the methods of Sillitoe and Hawkes (Sillitoe and Hawkes, 2002) were adapted and performed as previously described (Kubilus and Linsenmayer, 2009).
Scanning Electron microscopy
For scanning electron microscopy of Bowman’s layer, E10 corneas were dissected into HBSS. Then, to remove the CE the corneas were transferred to 0.5M EDTA (pH 8.0) for 24 hours at 4°C, followed by transfer to HBSS and mechanical removal with fine forceps. This left the CS with Bowman’s layer exposed on its apical surface. The samples were then fixed for two hours at 4°C in 2.5% gluteraldehyde in 0.1 M sodium cacodylate, pH 7.3. After rinsing in cacodylate buffer, they were postfixed in 1% osmium tetroxide in cacodylate buffer, rinsed again, and dehydrated in a graded series of ETOH to 100%, and then critical point dried (Samdri 790). The samples were mounted on aluminum stubs, sputter coated with gold/palladium, and imaged with an ISI-DS-130 scanning electron microscope.
RT-PCR
For RT-PCR analyses of potential nerve guidance molecules in the cornea, mRNA was isolated from OTG and from corneal tissue using a Microfast-track mRNA isolation kit (Invitrogen, Carlsbad, CA). cDNA was reverse transcribed using SuperScript reverse transcriptase (Invitrogen, Carlsbad, CA). The primers used for corneal RT-PCR are shown in Table 1. Primers were used to amplify products by PCR, followed by electrophoresis on an ethidium bromide agarose gel and visualization by UV illumination.
Table 1.
Primers used for RT-PCR analyses and cloning in situ probes.
| RT-PCR Primers | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| BDNF | ACTTCAGTTGCATGAAAGCTGCCC | ACCAGACATGTCCACTGCAGTCTT |
| NGF | AAGACCCTTCTCAGGAGCAGCTTT | CTTGCTTTGCCCTCCATGGTCAAT |
| NT-3 | TAAGGCAGCAGAGACGCTACAACT | TTCTTGACAATGCACACACGCAGG |
| Sema3A | TTCCATATCCACGGCCAGGAACTT | AGGCATCGGCGTCTTCTTCCTTAT |
| Sema3F | TGGGATCGAGACGCACTTTGATGA | AAGCTGTTGCCTCTT GGA GGA GAT |
| Slit1 | TGTACGACAACCAGATCAGCACCA | TATCGTTGCCATGGAGAGACAGCA |
| Slit2 | AGAAACGCATTGTGACGGGAAACC | CTGCACAAGTGCACCAGAAACCAT |
| Slit3 | TGTGAAACTCCTCCACCGATGGTT | TGGGTCTCCAATTTCCTGGTCACA |
| Netrin1 | AACATGGAGCTGTACAAGCTGTCG | TTGGAGGCTTTGCAGTACGAGTCA |
| Netrin2 | CTGGCAAGACCTGCAACCAAACAA | AGGGCTTCACACACTTCCCTTTCT |
| Netrin4 | TCCAACCAACACTCAACGAGAGCA | GCTGGGACTTCAGTGCAAATGCTT |
| Ephrin-b2 | TCTACTCAACTGTGCCAAGCCAGA | GGCTCAGAACCATTGTTGTTGCCA |
| Nrp1 | TGTCCCTACTGTTTCCGAAGGCAA | TTGCCTGGTTTCCTGGAGATGTCA |
| Nrp2 | TCCCGCTCCTTCGTGAAGAAGTTT | CCTGCGTGGCCATTGCGTATAAAT |
| Robo1 | AAGGAAGGCCAACACCAACCATTG | ACTGTGGAGGCTCAGACACAACTT |
| Robo2 | ATTGCTACGAGATGACTTCCGCCA | TGCCTTCATCTGTGCTCATAGCCT |
| DCC | AATCTAACGCTGGAGGCACGGAAT | ATTGGCTCAGGATGAAGCGTGACT |
| Neogenin | ATCGCTGCGTCATTGAAAGTGGTG | TGTAGGACAGTGGTTCCCGTTTGT |
| Unc-5b | ACCTGGTCATCAACAAGGCAGAGA | AAGTTTCTGGGCGAGAAGTCTCCA |
| Unc-5c | GCACTGCATTTGGGACCTTCAACT | AGTTCAGCTGGAAGATCTGCCCTT |
| eph-A1 | CCGCCCAATGTTCATCAAGCTCAA | ACAGACTGAGCTGTGTGCCAGTAA |
In situ Hybridization
The PCR products produced using the RT-PCR primers for Sema3A, Slit2, Nrp1 and Robo2 were cloned into the pCR4 vector by TOPO-TA cloning (Invitrogen, Carlsbad, CA). Cloned sequences were amplified and sequenced using M13 forward and reverse primers to assure that the proper sequence was cloned and then anti-sense RNA probes as well as sense control probes were synthesized from dsDNA templates using either T3 or T7 polymerase and Digoxigenin-labeled nucleotides. Hybridization was performed as previously described (Bandyopadhyay et al., 2008).
Quantitative Real-Time PCR
For qRT-PCR E7-E14 OTG and corneas were dissected at daily time points and for the corneas the CE and CS were separated by dispase treatment at daily time points. Tissues from approximately six dozen embryos were collected and pooled for each daily time point three separate times. mRNAs for each collection were isolated using a Microfast-track mRNA isolation kit (Invitrogen, Carlsbad, CA). cDNA was reverse transcribed using SuperScript reverse transcriptase (Invitrogen, Carlsbad, CA). Quantitative Real-time PCR was then performed on a Stratagene Mx3000 using the QuantiTect SYBR Green PCR kit (Qiagen) and the primers shown in Table 2. Due to differences in the expression of housekeeping genes across the different tissues it was not possible to use the same normalizing gene for the CE, CS or OTG. Thus, the CE, CS and OTG Ct values are normalized to L19, TAPBP and Cyclophilin-A, respectively, and the fold change was calculated as the delta-delta-Ct relative to E14.
Table 2.
Primers used for qRT-PCR.
| qRT-CR Primers | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Sema3A | AATCTCAACGTGCCATAGTCT | GCTCTTCCAAGTGGTCAGTA |
| Slit2 | TCCAGTTGCTTTCTGAACTGCTCT | TGTCTACTGCTCCACGAAATGCCT |
| L-19 | CAAGCTCAAGGCAGACAAAG | TACACAAGACAGCCACCGTT |
| TAPBP | GGGGCTCTTCTTGGTGGC | TCTTGGTTTCCTCTTTGGGTC |
| Nrp1 | ATCTGGGACGGATTCCCTGATGTT | TTCTGATACACTGCTCTGCGACAC |
| Robo2 | AGATGCAGCCATTCGGTCTGTAGT | ATGACAACTTCGTTACGGCCTCCA |
| Cyclophilin-A | CGGCTCCCAGTTCTTCATCT | TGCTTGCTCGTCTTGCGT |
Western Blot
CE, CS and lens tissues were isolated and proteins were extracted in ice cold RIPA buffer containing Complete Protease Inhibitor Cocktail (Roche). SDS-PAGE and western blot were performed as previously described (Beazley et al., 2009). A rabbit polyclonal antibody against Sema3a (Abcam, Cambridge, MA) was used at a dilution of 1:1000 followed by anti-rabbit horse-radish conjugated secondary antibody (Sigma, St. Louis, MO) used at 1:2000. Following the initial immunoprobe, the blots were stripped using Restore stripping buffer (Thermo-Scientific, Waltham, MA) and reprobed with an antibody against α-tubulin (Anaspec Inc., Fremont, CA) as a control to verify equal loading of samples in all wells.
In vitro co-culture
In vitro co-cultures of ophthalmic trigeminal ganglia (OTG) with either cornea or lens were done as previously described (Lwigale and Bronner-Fraser, 2007), with minor changes. Briefly, tissues from E7 chicks were dissected into sterile HBSS. OTG were co-cultured with lens or corneas in collagen matrix (Vitrogen) as described by (Keynes et al., 1997). Explants were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, GIBCO) supplemented with 10% fetal bovine serum and antibiotics (100 units Penicillin; 100μg/ml Streptomycin). Rabbit polyclonal Slit2 antibody (Abcam) or rabbit IgG (Sigma) were used at various concentrations [0.01 (n=8), 0.1 (n=8), 1 (n=8), and 10μg/ml (n=36)]; recombinant mouse Slit2 (rmSlit2; R&D Systems) was used at a concentration of 3μg/ml (n=16). N=16 for rmSlit2 (3μg/ml) plus Slit2 antibody (10μg/ml) co-cultures and untreated controls (n=16) were cultured in medium alone. Explant cultures were incubated at 37°C in a humidified CO2 incubator for 24 hours. Then, to visualize axons, the cultures were fixed in 4% paraformaldehyde and immunolabeled with the TuJ-1 antibody followed by donkey anti-mouse Northern lights Red fluorescent secondary antibody (R&D Systems)
Images were captured using a fluorescent stereo dissecting microscope (SMZ 1500, Nikon Instruments, Melville, NY) equipped with a SPOT Flex real time CCD camera (Diagnostic Instruments, Inc., Sterling Heights, MI) and neuron numbers and lengths were analyzed using ImageJ software.
In vivo Slit2 Antibody Injection
To access the embryos, E3 chicken eggs were windowed; the windows were sealed with parafilm, and the eggs were returned to incubator for an additional 6 days. Then, for each embryo (E9) one of the eyes was injected in the anterior chamber (between the lens and cornea) with 20μl of a 10μg/ml solution of the Slit2 antibody (n=20), or, as a control rabbit IgG (n=20) (both in PBS). In a second set of experiments 3μg/ml rmSlit2 (n=10) was injected into the anterior chamber. To ensure that the injection was into the anterior chamber, the injection solutions also contained 5% DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Molecular Probes, Eugene, OR) which was visualized with a fluorescence dissecting microscope. For each embryo, the contralateral eye was used for comparison. Then the eggs were resealed with parafilm, and were returned to the incubator for an additional for 48 hours. The corneas were then removed and processed for whole mount TuJ-1 labeling (as described above). Confocal imaging was performed as described above, with 1μm thick optical sections taken en face through the central cornea at 0, 5.6, 11.1, 16.7, 22.3, 27.8 and 33.4μm from the apical surface. Nerve branch points were analyzed using ImageJ software, as previously described (Ozdinler and Erzurumlu, 2002).
RESULTS
Developmental timecourse of corneal innervation
A previous study describing the innervation of the developing chicken cornea employed a gold-based histochemical procedure for staining the nerves (Bee, 1982). These studies established that in the chicken embryo corneal innervation occurs in a precise series of spatial and temporal stages, including: 1) attraction of the nerves towards the cornea and formation of the peri-corneal nerve ring, 2) invasion of nerves into the corneal stroma (CS) and 3) growth of nerves into the CE. However, as our studies on the regulation of corneal innervation (described later) required precise knowledge of the timing of these events, and as we felt that the currently available IHC procedures might be more sensitive than the histochemical procedure used previously, we reexamined the temporal and spatial occurrence of these events by IHC using an antibody directed against neuronal class III β-tubulin [the TuJ-1 antibody; (Lee et al., 1990)] to label the corneal nerves. For visualization of both whole mounts and sectioned material we employed a fluorescently-conjugated secondary antibody.
In general, our analyses confirmed the spatial sequence of events in innervation previously described by (Bee, 1982). However, we observed that certain of the events occur much earlier than this previous study would suggest. As the temporal and spatial appearance of these stages is requisite for evaluating the potential involvement of factors in their regulation, the salient features of these events are presented next.
Figure 1 shows representative results of TuJ-1 labeled whole mount preparations during the initial stages of corneal innervation. At E4 (the earliest stage examined) the nerves have grown from the OTG – which our recent analyses have shown to be the source of all the corneal nerves in this embryo (Kubilus and Linsenmayer, 2009) – and entered the anterior eye. At E5, the nerves (arrows) have approached the periphery of the cornea where they turn and branch dorsally and ventrally, forming the peri-corneal ring that surrounds the cornea. Through E6 the nerves continue to encircle the cornea (arrows), and by E7 the ring is nearly complete. Also at E7, the nerves that comprise the ring have begun to give off fine branches (arrowheads) that are growing towards the cornea but have not yet entered it. By E8 formation of the ring is complete, and, as can be seen in figure 2A (which is a quadrant of a cornea), nerve branches have begun to enter the cornea (arrowheads). The timing of these events in innervation is at least two days earlier than previously reported (Bee, 1982), and is consistent with a recent report (Lwigale and Bronner-Fraser, 2007).
Figure 1. Formation of the peri-corneal nerve ring.

Anterior eyes from embryonic ages E4-E7 as brightfield images – overlayed by fluorescent images showing nerves (arrows) labeled red with the TuJ-1 antibody and growing toward the cornea. In E4 the cornea is designated with a “C”. At E7 arrowheads designate small nerve branches growing towards the cornea, but not into it. The scale in all figures is the same, as shown by the bar with the E4 image, which is 1mm.
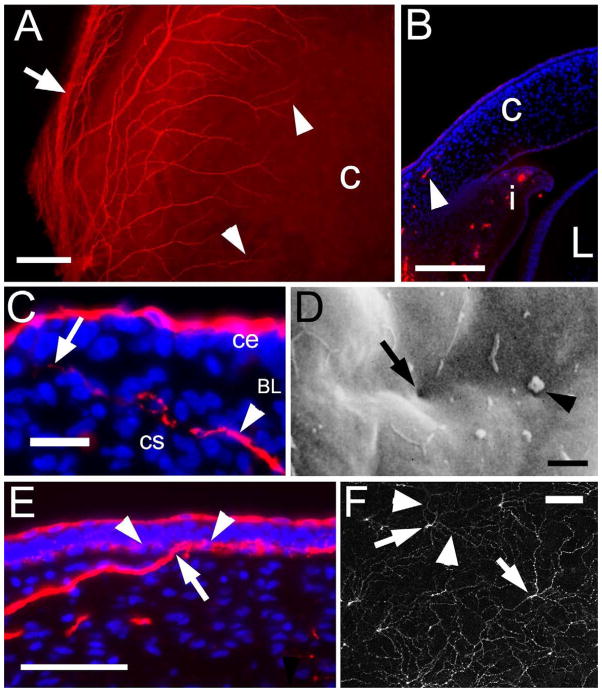
Figure 2. Corneal nerve growth into the CS and CE.
(A) Quadrant of an E8 anterior eye labeled with the TuJ-1 antibody and visualized en face, showing nerves (arrowheads) growing in to the cornea “c” from the large nerve bundles of the peri-corneal nerve ring (arrow) at the periphery. (B) Section through the anterior eye of an E8 embryo, labeled with the TuJ-1 antibody (red) and nuclei stained with Hoechst (blue) showing a nerve growing into the cornea “c” (arrowhead) as well as the iris “i” but not the lens “L”. (C) Higher magnification image of an E10 cornea section with a nerve (arrowhead) in the anterior stroma “cs” and penetrating (arrow) Bwoman’s layer (BL) but not within the epithelium (ce). (D) Scanning EM at E10 of the anterior surface of Bowman’s layer – exposed by removal of the CE cell layer by treatment with EDTA – showing pores through Bowman’s layer (left arrow) – some of which contain processes (right arrow). (E) E11 cornea showing a large nerve bundle penetrating Bowman’s layer (arrow), and branching to form the web-like plexus within the CE just apical to Bowman’s layer (arrowheads). (F) 1μm confocal optical section of a TuJ-1 labeled E11 cornea imaged en face at the basal layer of the CE just adjacent to Bowman’s layer. The arrows designate the large nerve bundles that have penetrated through Bowman’s layer and then are branching (arrowheads) to form the sub-basal nerve plexus. scale bars in A=10μm, B=20μm, C=20μm, D=2μm, E=50μm and F=50μm.
To examine the later stages of innervation (i.e., growth into the CS and penetration into the CE) the IHC analyses were also performed on frozen sections. Figure 2B (arrowhead) confirms that at E8 innervation of the cornea has occurred, and in addition, shows that this is restricted to the anterior third of the CS. In this figure nerves can also be seen in the iris (i), but not the lens (L).
Functionally, for sensory perception, the nerves must enter the CE (MacIver and Tanelian, 1993). The initial step in this event occurs at E10, at which time the nerves have turned towards the CE and have penetrated Bowman’s layer (which is an acellular matrix that separates the CS from the CE). This can be seen in the E10 cornea shown in figure 2C, at which time Bowman’s layer (BL) begins to be penetrated by nerves (arrow). In this figure, it can be seen that the TuJ-1 antibody also reacts with the apical-most cells of the CE itself, which constitute a unique population of cells we have termed TuJ-1 positive apical CE cells [described in detail in (Kubilus and Linsenmayer, 2009)].
This penetration of Bowman’s layer by nerves can also be seen in figure 3D, which is a scanning electron micrograph of an E10 cornea, from which the CE has been removed with EDTA (Spurr and Gipson, 1985). This treatment exposes the basal lamina associated with the apical surface of Bowman’s layer. At this stage – but not earlier – pores through Bowman’s layer can be seen (arrow), and many of these show projections (arrowhead) that likely represent the growing tips of the invading nerves. Again, this event in innervation at day 10 is two days earlier than was previously observed (Bee, 1982).
Figure 3. Sema3A expression during corneal innervation.
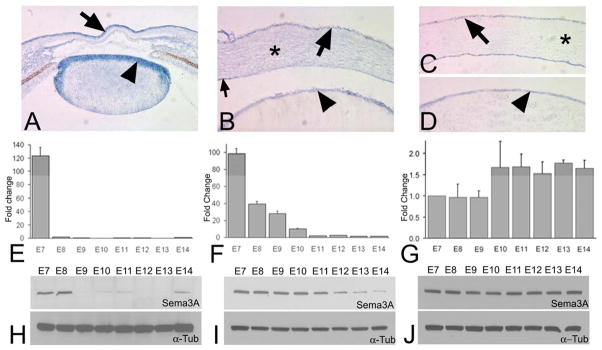
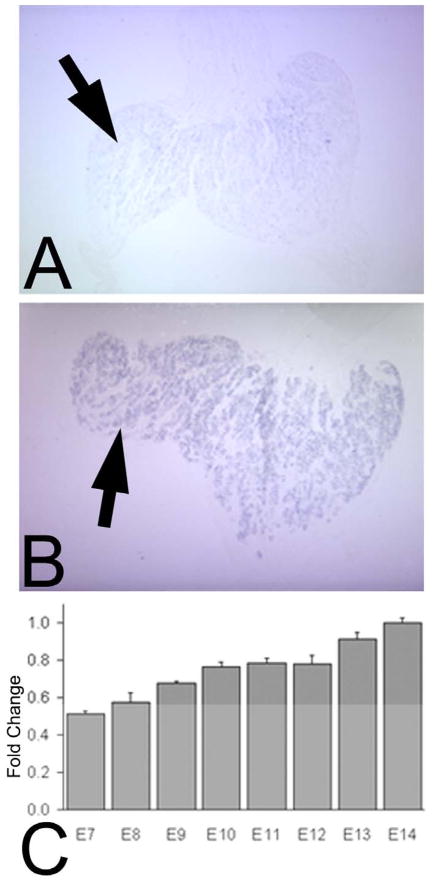
(A) in situ hybridization on a section of an E5 anterior eye for Sema3A in cornea (arrow) and the lens epithelium (arrowhead). (B) In situ hybridization of an E7 anterior eye showing Sema3A in the CE (arrow), CS (*) and lens epithelium (arrowhead), as well as in the corneal endothelium. (C) in situ hybridization of E14 cornea showing Sema3A in CE (arrow) and E14 lens (D) showing Sema3A in the lens epithelium (arrowhead). qRT-PCR of E7-14 tissues for Sema3A in the CS (E) and the CE (F), and lens (G). The data for E and F are presented as the fold change compared to E14 and G compared to E7, with the error bars representing the standard error of the mean. Western blot for Sema3A in the CS (H), CE (I) and lens (J), with alpha-tubulin serving as a loading control.
Also, in figure 2E which is a section of an E11 cornea, it can be seen that after the nerves penetrate Bowman’s Layer (arrow), they rapidly undergo branching along the interface between Bowman’s Layer and the basal surface the basal CE cells (arrowheads) – forming a plexus termed the sub-basal nerve plexus (Muller et al., 2003). As in this image the nerve branches are cut in cross section, the degree of branching is difficult to appreciate. However, this branching can be clearly visualized by confocal microscopy – as shown in figure 2F which is an optical section of a TuJ-1 labeled E11 cornea imaged en face. In this image the optical section is just apical to Bowman’s layer, and shows the large nerve bundles (arrows) that have penetrated Bowman’s Layer branching into a web like plexus of smaller nerves in the basal layer of the CE. The final stages of innervation involve these nerve branches turning and extending projections towards the apical surface of the CE, where they terminate at the baso-lateral borders of the TuJ-1 positive apical CE cells [not shown here but previously described in detail (Kubilus and Linsenmayer, 2009)].
Molecular Regulators of corneal innervation
Developmental innervation frequently involves both positive and negative regulation. To identify in the developing cornea factors that may perform these functions, we performed RT-PCR as an initial screen on corneas from two developmental stages – an early stage when formation of the peri-corneal ring is occurring but before innervation of the cornea itself has been initiated (E7), and a later stage when all the events of innervation have occurred, or are occurring (E13). As positive regulators we analyzed for NGF, BDNF and NT-3; as negative regulators we analyzed for semaphorins 3A and 3F, slits 1–3, netrins 1, 2, and 4, and ephrin B2. These factors were chosen based on both previous reports of their effects on nerve behavior as well as the availability of their genetic sequence in the chicken. The results (summarized in Table 3) showed that a number of these molecules are expressed in the cornea – including NGF, BDNF, NT-3, Sema3A and F, Slits 1–3, Netrin2, and Ephrin B2. So, these may all be involved in innervation. However, to choose those for further study, we applied another criterion, which was a large differential in their expression at the two time points examined. The only two molecules that fulfilled this criterion were Sema3A and Slit2 – both of whose expression was clearly greater at E7 than at E13.
Table 3.
RT-PCR analysis of nerve guidance molecule expression in E7 and E13 cornea.
| E7 Cornea | E13 Cornea | |
|---|---|---|
| NGF | + | + |
| BDNF | + | + |
| NT-3 | + | + |
| Sema3A | + | − |
| Sema3F | + | + |
| Slit1 | + | + |
| Slit2 | + | − |
| Slit3 | + | + |
| Netrin1 | − | − |
| Netrin2 | + | + |
| Netrin4 | − | − |
| Ephrin B2 | + | + |
As both Sema3A and Slit2 are thought to act primarily as negative cues (Dickson 2002), their high levels of expression in the cornea at E7 raised the possibility that they initially might function in preventing precocious nerve growth into the cornea until the peri-corneal nerve ring has formed. Their subsequent decrease in expression might then allow the later stages of innervation, i.e., nerve growth into the CS and then into CE, to occur. Therefore, both of these factors were analyzed further by in situ hybridization on sections of anterior eye between E5 and E14 and qRT-PCR performed on isolated corneal tissues between E7 and E14 at daily intervals. This time-span encompasses the developmental periods when nerves have reached the periphery of the cornea and are forming the peri-corneal ring (E5-E7); when they have begun to enter the stroma (E8); and when they have entered CE and are undergoing growth and branching (E10-14). These analyses were also performed for the Sema3A receptors Nrp1 and 2, and the Slit2 receptors ROBO1, 2 and 3 in the OTG.
Semaphorin3A expression decreases in the cornea during development
For Sema3A, the in situ hybridizations performed on E5 anterior eyes (Fig. 3A) showed strong Sema3A expression in the primary corneal epithelium (arrow) and the lens epithelium (arrowhead) as well as the mesenchyme of the iris and ciliary body. This is consistent with what has previously been described (Lwigale and Bronner-Fraser, 2007). At E7, anterior eyes (Fig. 3B) showed a signal in the CE (arrow), the CS (asterisk) and the endothelium (small arrow). In addition there was also a signal in the lens epithelium (arrowhead). At E8 through E13, for the corneal tissues there was a noticeable diminution in the signals for Sema3A in the corneal tissues – similar to that shown for the E14 tissues in figure 3C. However for the lens epithelium, over this time period the diminution in the signal for Sema3A was if at all slight – being similar to that of the E14 lens shown in figure 3D (arrowhead).
Studies by others (Tang et al., 2004) have shown that the axon repulsive properties of Sema3A occur in a dose-dependent manner, and the in situ hybridization results just described suggest that during development, within the anterior eye there is a temporal and tissue-specific decrease in the expression of Sema3A. However, as expression analyses by in situ hybridization is at best semi-quantitative, we also analyzed Sema3A in selected tissues using qRT-PCR performed at daily intervals over the same time period as the in situ hybridizations (from E7-E14). The tissues examined were the CE, the CS (to which the endothelium remained attached), and the lens.
In both the CS (Fig. 3E) and the CE (Fig. 3F), Sema3A mRNA is at its highest level at E7 – which is approximately 100 fold greater than at E14. Then, between E7 and E8 the CS undergoes a precipitous decrease in its Sema3A mRNA (p<0.0001). In the CE (Fig. 3F) there is a concomitant decrease, that is somewhat less dramatic but still highly significant (P<0.0001). As the timing of these decreases correlate precisely with the growth of nerves into the CS (at E8), this suggests a model in which corneal Sema3A functions as a repulsive cue that prevents nerve growth into the cornea until the peri-corneal nerve ring has formed. Subsequently, the precipitous decrease in Sema3A (at E8) could remove this inhibition, allowing nerves from the peri-corneal ring to grow into the cornea.
Although in the CE the decreased expression of Sema3A between E7 and E8 is less dramatic than in the CS, in the CE the decease continues progressively through E10-E11 – at which time it reaches a level that is essentially he same as E14 (the oldest time examined). As E10 is when the nerves enter the CE (described earlier), this suggests that Sema3A might also function in regulating the timing of this event.
To verify that these patterns of expression of Sema3A mRNA in the CS and CE reflect changes in the protein itself, we also performed Western blot for Sema3A in both the CS (Fig. 3H) and CE (Fig. 3I) with α–tubulin as a loading control. The temporal patterns of these parallel those of the qRT-PCR described above.
Lastly, concerning the Sema3A in the lens, both we (described earlier), and others (Chilton and Guthrie, 2003; Lwigale and Bronner-Fraser, 2007) have detected this by in situ hybridization. Therefore, to examine whether developmental changes occur in lens Sema3A that would fit into the patterns of innervation we examined the lens by qRT-PCR (Fig. 3G) and western blot (Fig. 3J). The results do not show any significant change in the level of Sema3A expression over the course of corneal innervation (see also Discussion).
Neuropilin-1 expression decreases in the ophthalmic trigeminal ganglion during development
As the physiological action of a regulatory factor also requires the presence of an appropriate receptor in the target tissue, we examined whether during innervation developmental changes occur in the receptor for Sema3A – Nrp1. For this we analyzed Nrp1 in the OTG by in situ hybridization and qRT-PCR – performed over the same time frame as for the Sema3A. By in situ hybridization (Fig. 4A and B), from E7 through E14, Nrp1 is expressed broadly throughout the OTG. However, the expression appears to decrease in intensity. This decrease was confirmed by qRT-PCR (Fig. 4C), which showed a significant decrease in Nrp-1 mRNA (p=0.003) from E7 to E14. This decrease occurs concomitant with that of Sema3A mRNA in the corneal tissues (Fig. 3E and 3F), suggesting that changes in Nrp1 by the nerves themselves is also likely to be involved in regulating corneal innervation (see discussion).
Figure 4. Nrp1 expression in the OTG.

in situ hybridizations for Nrp1 in the trigeminal ganglion, with Nrp1 present at E7 (A) in the OTG (arrow), and Nrp1 still present at E14 (B; arrow). (C) qRT-PCR for Nrp1 in the OTG between E7 and E14, shown as fold change compared to E14 with the error bars representing the standard error of the mean.
Slit2 expression decreases in the cornea during development
Slit2 generally functions as a negative regulator of innervation (Bagri et al., 2002), but it has been reported in mice and in vitro to also act as a positive regulator that stimulates nerve branching (Ma and Tessier-Lavigne, 2007; Wang et al., 1999). For corneal innervation, our expression and functional analyses suggest that both types of regulation by Slit2 are involved.
By in situ hybridization, at E5 (Fig. 5A) when the peri-corneal nerve ring is forming, Slit2 was present in the lens epithelium (arrowhead) and the cornea (arrow), as well as in the retina. At E7, after the CS has swelled but while the peri-corneal ring is still forming, Slit2 (Fig. 5B) was present in the CE (arrow), the CS (asterisk), and the lens epithelium (arrowhead). However, at E14 (Fig. 5C), Slit2 was barely detectable in the CE (arrow) and the CS (asterisk), but in the lens it was still present (Fig. 5D, arrowhead). Thus the tissue distributions are similar to those of Sema3A.
Figure 5. Slit2 expression during corneal innervation.
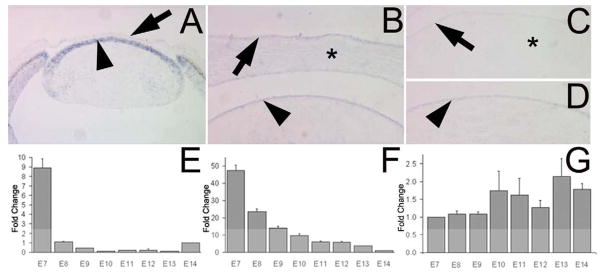
(A) in situ hybridization on a section of an E5 anterior eye for Slit2 in cornea (arrow) and the lens epithelium (arrowhead). Note at this time the cornea is closely apposed to the lens epithelium. (B) in situ hybridization on a section of an E7 cornea for Slit2 in CE (arrow), CS (*) as well as the corneal endothelium and the lens epithelium (arrowhead). (C) in situ hybridization of E14 cornea showing Slit2 in CE (arrow) but only slightly detectable in the CS (*), and E14 lens (D) showing Slit2 present in the lens epithelium (arrowhead). qRT-PCR of E7-14 tissues for Slit2 in the CS (E), the CE (F), and the lens (G). The data for E and F are presented as the fold change compared to E14 while G is compared to E7, with the error bars representing the standard error of the mean.
Analyses by qRT-PCR confirmed the high levels of Slit2 at E7 in both the CS (Fig. 5E) and the CE (Fig. 5F). In the CS there was a large and significant (p<0.0001) decrease between E7 and E8, precisely when the nerves begin to enter the CS and similar to what was observed for Sema3A. Also similar to what was observed for Sema3A, expression of Slit2 mRNA in the lens (Fig. 5G) did not significantly change over the developmental time period examined.
In the CE, while there is a significant decrease between E7 and E8 (p<0.0001), the mRNA levels of Slit2 begin to decrease gradually between E8 and E14. Again this pattern is similar to that observed for Sema3A. However, for Sema3A the decrease is complete between E10 - E11, when nerves are entering the CE and beginning to branch. For Slit2, the decrease extends throughout the period examined. As this protracted expression of Slit2 in the CE encompasses the time when the nerves form the sub-basal nerve plexus, it raised the possibility that during this later time the Slit2 in the CE has changed its role from a negative regulator (of innervation), to a positive stimulator (of branching).
Additional support for this role change by Slit2 was obtained by expression analysis of Robo2 – which is one of the receptors for Slit2 – and by functional analyses of Slit 2 itself. The chicken has three forms of the Robo receptor. However, in a preliminary screen of the OTG by RT-PCR, Robo2 was the only one to show clear temporal changes (data not presented). Therefore, we further analyzed Robo2 (from E7-E14) by in situ hybridization and qRT-PCR. By in situ hybridization, Robo2 is expressed in the OTG throughout this time period. However, as can be seen in figures 6A and 6B, with time the intensity of the labeling increases. This is the reverse of what would be expected to affect a release from inhibition as was observed with Nrp1, the receptor for Sema3A. qRT-PCR for Robo2 in the OTG (Fig. 6C), taken at daily intervals, confirmed this progressive increase (from E7-E14; p<0.0001).
Figure 6. Robo2 expression during corneal innervation.
in situ hybridizations for Robo2 in the trigeminal ganglion show expression in the OTG at E7 (arrow in A), with an increase at E14 (arrow in B). (C) qRT-PCR for Robo2 in the OTG showing the fold change compared to E14. Error bars represent the standard of the mean.
Functional analyses of Slit2 by immunoneutralization and addition of rmSlit2
Using a Slit2 function-blocking antibody, we examined the role(s) of Slit2 on corneal innervation, and whether temporally these switched from negative regulation (at pre-innervation stage E7), to positive regulation (during formation of the sub-basal nerve plexus at E10).
Slit2 negatively regulates early trigeminal neurite growth in vitro
For analyses, at the early time point (E7), we employed three dimensional collagen co-cultures of OTG (as a source of neurons) and either cornea or lens (as sources of potential regulatory factors). In a recent study (Lwigale and Bronner-Fraser, 2007) using this assay it was observed that both of these tissues inhibited nerve outgrowth, which we have also observed (data not presented). They also observed that one factor likely to be involved in this inhibition was Sema3A – as an inhibitory peptide against this regulator promoted nerve outgrowth.
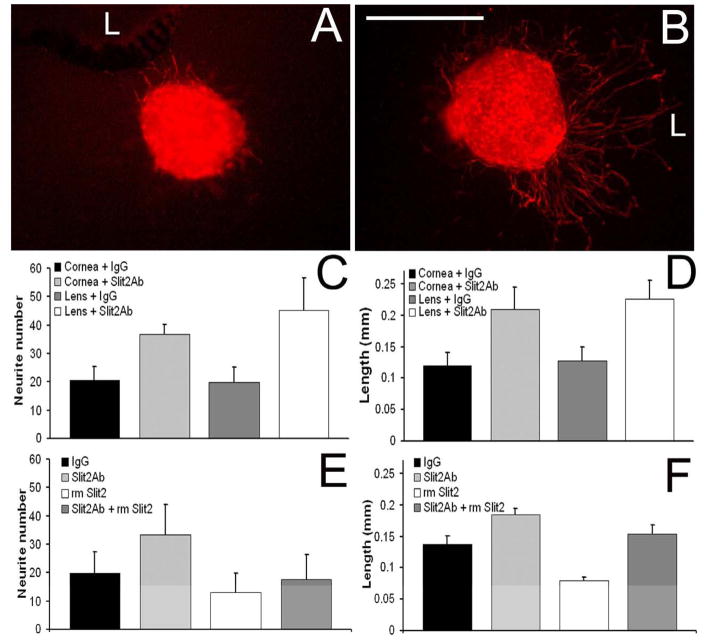
To determine whether Slit2 might also be involved in this early negative regulation, we examined neurite outgrowth in the presence of a chicken-specific Slit2 antibody when added to E7 co-cultures of OTG, plus either cornea or lens (Figure 7). The antibody was used at a concentration of 10μg/ml – which we determined to have maximum activity (data not shown) – or the same concentration of rabbit IgG (as a control). The results showed that addition of the Slit2 antibody to the co-cultures resulted in increased neurite outgrowth – as can be seen in a control culture (Fig. 7A) versus one treated with the antibody (Fig. 7B). These increases were statistically significant for both cornea and lens, and were observed both for the numbers of neurites (Fig. 7C) and for the lengths of neurites (Fig. 7D) extending from the OTG. These experiments suggested that Slit2, at E7 was negatively regulating the outgrowth of neurites from the OTG.
Figure 7. Effects of Slit2 on OTG neurons in vitro.
(A and B) Fluorescent images of E7 TuJ-1 labeled OTG co-cultured with an E7 lens “L” in the presence of (A) 10μg/ml rabbit IgG antibody or (B) 10μg/ml Slit2 antibody. (C) Neurite numbers and (D) neurite lengths from OTG co-cultured with lens or cornea and treated with 10μg/ml of either Slit2 antibody or control rabbit IgG. Neurite numbers (E) and neurite lengths (F) from co-cultures of E7 OTG with E7 cornea treated with 10μg/ml rabbit IgG antibody, 10μg/ml Slit2 antibody, 3μg/ml recombinant mouse Slit2, or 10μg/ml Slit2 antibody and 3μg/ml recombinant mouse Slit2. Error bars in C - F show the SEM. Scale bar in B = 0.3mm.
We further tested this hypothesis by a second set of experiments in which rmSlit2 was added to in vitro co-cultures of E7 OTG and E7 cornea. Consistent with our previous results, the addition of rmSlit2 significantly decreased the length of outgrowing neurites from the OTG (Fig. 7F). rmSlit2 also slightly decreased the number of neurites growing out from the OTG (Fig. 7E), however this decrease was not significant.
These results confirm that at this early stage of innervation both the lens and cornea are capable of negatively regulating axon growth and that Slit2 is one of these regulators – most likely acting in concert with Sema3A. However, it remains to be determined during normal development, which of the sources of Slit2 – and Sema3A – is responsible for regulating this innervation (see discussion).
Slit2 blocking antibody decreases branching into the CE in vivo
To examine functionally whether Slit2 signaling also plays a role in the later stages of corneal innervation, and whether this involves a switch to positive regulation, we again employed the Slit2 antibody for immunoneutralization. However, to examine these later stages of development we injected the antibody in ovo into the anterior chamber of the eye (the cavity between the cornea and lens). This was done on E9 embryos – a stage after the nerves are within the CS but have not yet entered the CE. Control injections consisted of substituting rabbit IgG for the Slit2 antibody, with the uninjected, contralateral eye serving for comparison. Also, to verify that the injections were into the anterior chamber, the injection solutions also contained the lipophilic fluorescent dye, DiI, which was visualized with a fluorescent dissecting microscope.
Two days following the injection (at E11) – when the nerves have penetrated through Bowman’s Layer and formation of the adjacent sub-basal nerve plexus is occurring – the corneas were removed and their nerves visualized en face by confocal microscopy of whole mounts labeled with the TuJ-1 antibody. Then, data on the numbers of nerves and branch points were obtained from 1μm optical sections, starting at the apical surface of the CE and progressing downwards through the CE and into the CS (as presented in figure 8).
Figure 8. in ovo Slit2 immunoneutralization.

Effect of the Slit 2 antibody on the branching of nerves within the CE in ovo examined quantitatively (A) and qualitatively (B and C) by confocal microscopy of TuJ-1 labeled corneas from embryos that two days earlier had received injections (into the anterior chamber) of either the Slit2 antibody, control rabbit IgG, or were left untreated. (A) Quantitative analysis of the number of nerve branch points per 450μm × 450μm × 1μm field in confocal optical sections starting at the apical surface in Slit2 antibody, rabbit IgG or untreated E11 corneas. (B and C) Representative images from Slit2 antibody treated (B) and rabbit IgG treated embryos (C) taken 5.6μm from the corneal surface showing large nerve bundles (arrowheads) with radial branches. Error bars in (A) are standard error of means; in (B) and (C) the scale bars = 50um.
Within the CS – where the nerves are in thick bundles (e.g., at depths from 11.1 μm to 33.4μm) – in Slit2 antibody-containing injections versus the controls no difference was detected in the number of nerve branch points (Fig. 8A), or the number of nerves (not shown). This suggests that Slit2 is not involved in regulating the branching or number of nerves within the CS – at least not at this stage. Likewise, in the apical-most optical sections at the surface of the CE (0.0μm) the number of branches in the antibody treated corneas was not statistically different compared to controls (p=0.69). But, it should be noted that when these corneas were analyzed (at E11) innervation of this apical region has just begun.
However, at a depth of 5.6 μm the number of branch points in the antibody-treated corneas was significantly less than in the controls treated with rabbit IgG (p<0.01) – or the untreated contralateral eyes. 5.6 μm is within the CE and just apical to Bowman’s layer – which is where nerve bundles, having penetrated through Bowman’s layer, are branching to form the sub-basal nerve plexus. That this is where the antibody is effecting major changes can be visualized by comparing the reduced number of branch points in the corneas from the antibody injected eyes (Fig. 8B), versus the number in the control injected eyes (Fig. 8C). The corneas from the antibody injected eyes also had a corresponding, reduction in the number of nerves within this region (statistically-significant but data not shown) – all of which is consistent with the Slit2 at this stage of development converting to a positive regulator of innervation.
In a second set of experiments (data not shown) rmSlit2 was injected into the anterior chamber of the eye at E9, and again nerve behavior was analyzed in optical sections of the cornea at E11. However, addition of rmSlit2 did not result in any significant change as compared to sham injected controls in either the number of nerves or their branching pattern.
DISCUSSION
The cornea is one of the most densely innervated tissues in the body, yet little is known concerning how it is innervated during development. Here, we have defined the spatiotemporal stages through which the cornea becomes innervated, and have provided evidence that these stages are likely to be regulated by multiple factors, two of which are Sema3A and Slit2. Furthermore, Slit2 appears to have opposite roles at different stages of innervation.
Spatiotemporal stages of corneal innervation during development
In the embryonic chicken, corneal nerves originate from the OTG (Kubilus and Linsenmayer, 2009), and during development these nerves innervate the cornea in a series of spatiotemporal stages, as originally described by Bee using histological staining (Bee, 1982). Here, to determine more precisely the timing of each of these stages, we have used IHC with the TuJ-1 antibody on whole mounts and sectioned samples – visualized by conventional fluorescence and confocal microscopy, and by scanning EM. Using these more sensitive techniques we observed that the formation of the peri-corneal nerve ring is completed by E7 and nerves enter the CS at E8 – events described by Bee as not occurring until E10. Also the growth of nerves into the CE begins at E10 – rather then at E12 as described by Bee (Bee, 1982). That both of these events occur two days earlier than previously thought, is critical in determining the expression and potential roles of regulatory factors that may be involved in the developmental behavior of the nerves.
Using these more sensitive techniques we have also observed developmental behaviors of nerves that are responsible for certain of their spatial patterns that have been previously described by others in adult corneas of a number of species [reviewed by (Muller et al., 2003)]. For example, in the adult corneal nerves have been described in the basal CE that run parallel to Bowman’s membrane and give off branches (Muller et al., 1996; Rozsa and Beuerman, 1982). The networks that form from these branches have been referred to as epithelial nets (ZANDER and WEDDELL, 1951) or the sub-basal nerve plexus (Beuerman and Schimmelpfennig, 1980; Muller et al., 1996). In the present study, using en face confocal optical sectioning, we have observed that formation of this plexus is an early event in the innervation of the CE, which begins to develop just after nerve bundles from the CS have penetrated through Bowman’s layer. At this time (E10–E11) nerves within the bundles begin to undergo radial branching and growth in a plane that parallels Bowman’s layer – thus forming this sub-basal plexus.
Functionally, formation of this plexus may ensure that the corneal surface becomes uniformly innervated, as subsequently these nerves, or branches from them, turn and grow towards the apical surface of the CE, where they interact with specialized apical CE cells in the formation of unique nerve endings (Kubilus and Linsenmayer, 2009; MacIver and Tanelian, 1993). Although this potential role in patterning of the nerves remains to be experimentally tested, we have obtained evidence that the radial branching itself is likely to involve regulation by the axon guidance molecule Slit2, as discussed next.
Slit2 in corneal innervation
Our studies on the expression and function of Slit2 during developmental innervation of the embryonic chicken cornea are consistent with this regulator having dual roles – an early one which is inhibitory and participates in formation of the peri-corneal nerve ring by inhibiting nerves from entering the CS until the ring is complete, and a later one in which it promotes the branching of the nerves that have penetrated through Bowman’s layer thus participating in formation of the basal nerve plexus.
Such a dual role for Slit2 is suggested by its expression during development – especially when coupled with those of its receptor, Robo2. For Slit2, in situ hybridization of anterior eyes shows it to be present in both corneal tissues, the CS and CE, as well as in the lens epithelium, but absent from the scleral region where the peri-corneal ring forms at from E4-E7; qRT-PCR shows developmental changes in the corneal tissues, whereas in the lens Slit2 remains constant.
For the corneal tissues the highest Slit2 levels are when the peri-corneal nerve ring is forming (E7), which is consistent with it inhibiting nerve growth into the CS. Subsequently, at the time when nerves begin to enter the CS, the expression of Slit2 deceases, which is consistent with a release from the negative regulation being involved in this event.
For the CS, this decrease is precipitous, but for CE it is more protracted, gradually continuing throughout the entire time period examined. Thus, when the next stage of innervation occurs (i.e., when nerves enter the CE and begin to branch and form the web-like network at E10) the CE itself is still expressing considerable Slit2. In addition, during this period the expression of mRNA for the receptor of Slit2 (i.e. Robo2) in the OTG, is undergoing a progressive increase, rather than the decrease that would be predicted for a release from negative regulation. These results, when taken together, raise the possibility that at this time Slit2 functions to promote axon branching within the CE – rather than functioning as an inhibitor of innervation.
This possibility was functionally tested by immunoneutralization using an antibody against chicken Slit2. As the peptide sequence used to generate the antibody was within the N-terminal portion of the molecule (Nguyen-Ba-Charvet and Chedotal, 2002), the antibody should bind to both the inhibitory form of Slit2 – which is the full length molecule – as well as the form that is proposed to promote the branching of sensory nerves – which is an N-terminal proteolytic cleavage product (Slit2N) (Ma and Tessier-Lavigne, 2007).
The results showed that at the early time point the antibody promoted neurite outgrowth from OTG co-cultured with either cornea or lens (i.e., it reduced the negative regulation of the Slit2 produced by these tissues). Consistent with this, addition of rmSlit2 to early time point co-cultures decreased nerve outgrowth compared to controls. While these analyses do not elucidate whether the source of Slit2 responsible for this regulation is the cornea, the lens or both, when combined with the expression data showing the developmental changes within cornea suggests the importance of corneal derived Slit2.
Slit2 is generally thought to serve as a negative cue that inhibits axons from growing into inappropriate targets – a function that is conserved in flies, worms, and vertebrates (Fricke et al., 2001; Hao et al., 2001; Kidd et al., 1999; Long et al., 2004; Plump et al., 2002; Zallen et al., 1998). However, several recent studies have shown the N-terminal cleavage product of Slit2 also functions to promote branching and arborization of sensory nerves both in vitro and vivo. This was originally observed in cultures of dorsal root ganglion cells from rat embryos – in which an N-terminal fragment of Slit2 (Slit2N) stimulated branching and elongation of axons (Wang et al., 1999). Supporting evidence for this stimulation of branching has been obtained in cultures of mouse and rat trigeminal ganglia (Ozdinler and Erzurumlu, 2002), in somites of zebrafish in which overexpression of Slit2 induced nerve branching (Yeo et al., 2004), and in mouse models in which trigeminal sensory nerve branching was decreased in Slit2-Slit3 double knock-outs (Ma and Tessier-Lavigne, 2007).
To examine functionally the role(s) of Slit2 in later stages of innervation, the antibody was injected in ovo into the anterior chamber of eyes at a time (E9) when nerves are within the CS but have not yet entered the CE. This did not noticeably alter the penetration of the large nerve bundles nerves through Bowman’s layer and into the CE. However, the subsequent branching of these nerves within the CE was greatly diminished – thus decreasing the formation of a normal sub-basal nerve plexus, and demonstrating that Slit2 was now acting as a positive cue that promotes axon branching. For the Slit2 antibody to alter the branching of the growing nerves at this interface between the CE and Bowman’s layer, requires that the antibody diffuse from the anterior chamber through the CS. In support of this possibility, in other of our studies (unpublished) we have observed that IgG antibodies can rapidly diffuse through the cornea. Also, studies by others have observed effects on corneal innervation when a blocking peptide against of Sema3A is injected into the anterior chamber (Lwigale and Bronner-Fraser, 2007), and intravitreal injections of antibodies against VEGF have been shown to decrease corneal vascularization in humans (Costagliola et al., 2008).
We also examined the effect of rmSlit2 when injected into the anterior chamber, and failed to detect an effect on nerve branching within the epithelium. This suggests that, in this system, the potential negative regulatory role of the rmSlit2 was incapable of overriding the branch-inducing capabilities of the Slit2N. However, this essentially negative result will require further studies to clarify the relative regulation between these two forms of Slit [see for example (Ozdinler and Erzurumlu, 2002)].
These functional studies lend strong support to Slit2 playing a dual role during corneal innervation – initially acting as a negative regulator of early innervation, and subsequently as positive regulator that promotes branching. Consistent with these conclusions, trigeminal axons of Slit1:Slit2 double knock-out mice (Ma and Tessier-Lavigne, 2007) initially innervate improper targets, and later show decreased arborization at the sites that were innervated. However, as these mice were double knockouts, no conclusions could be made whether Slit1, Slit2, or both were responsible for the observed types of regulation. Also, it is unclear if the improper arborization is due to the improper initial innervation. Our analyses show for the cornea that both types of regulation are effected by Slit2. However, they do not elucidate whether this switch from negative to positive regulation involves a proteolytic conversion of intact Slit2 to Slit2N, (Ozdinler and Erzurumlu, 2002), or whether some other mechanism is involved.
Although the major regulation of innervation is thought to be effected through the actions of extrinsic regulatory factors, it has also been proposed that within the neurons themselves, intrinsic factors may also be involved in determining whether a cue is interpreted as being positive or negative. For the Slits, it has been suggested that the level of cGMP within a responding neuron can modulate whether a cue is interpreted as being repulsion or as a promoter of branching (Nguyen-Ba-Charvet and Chedotal, 2002). Likewise, the levels of receptors within a growth cone has been suggested to modify the response to signaling molecules (Dickson, 2002; Tessier-Lavigne and Goodman, 1996). In the present study we have obtained evidence – although indirect – that the receptor for Slit2 (i.e., Robo2) may be involved in the switch of Slit2 from negative to positive regulation as our qRT-PCR results show that the levels of Robo2 mRNA increase between the times when Slit2 exerts its negative effect, to when it acts as a positive regulator of branching. However, it remains to be determined whether these changes in Robo2 play active roles in this switch.
Role of Sema3A
The other major regulator that our expression analyses suggest may be involved in corneal innervation is Sema3A. Previous studies by others have shown that Sema3A – functioning through its receptor Nrp1 – acts as a negative cue in the innervation of many tissues and organs (Dickson, 2002), including the cornea (Chilton and Guthrie, 2003; Lwigale and Bronner-Fraser, 2007).
It was recently shown that Sema3A is likely to be involved in formation of the peri-corneal nerve ring in the developing chicken embryo (Lwigale and Bronner-Fraser, 2007). The in situ hybridization results in this study for Sema3A as well as those here for both Sema3A and Slit2, show an expression pattern consistent with both molecules guiding the formation of the peri-corneal nerve ring. In the previous study it was proposed that the source of the Sema3A regulating this event was the lens – as both lentectomy and the injection of a Sema3A blocking peptide into the lens or anterior chamber resulted in nerves growing directly into the central anterior eye without formation of the peri-corneal ring. However, previous work by others (Beebe and Coats, 2000) have shown that if lentectomy is performed very early in development, a bona fide cornea fails to form. Instead, what forms is a disorganized aggregate of mesenchymal cells beneath an epithelium – with no recognizable CE, CS, or endothelium. Also the studies using the Sema3A blocking peptide did not report whether the lens itself also became innervated.
During development, Sema3A knockout mice show precocious growth of nerves directly into the cornea without formation of a peri-corneal nerve ring as well as innappropriate growth of nerves into the lens (Taniguchi et al., 1997). As the lens has been reported to produce a number of neurotrophic factors (Bennett et al., 1999), this raises the possibility that the Sema3A produced by the lens functions to prevent its own innervation (as proposed by Chilton and Guthrie, 2003).
Our quantitative analyses on the developmental expression of Sema3A by the CS, CE and lens provide additional information in support of the involvement of corneal-derived Sema3A in regulating corneal innervation – at least for the stages we examined. Between the time when the peri-corneal ring is forming (E7), and when the nerves begin to enter the cornea (E8), the mRNA for Sema3A by the CS decreases more than 120 fold, with a corresponding decrease in the protein to essentially non-detectible levels. This suggests that release from inhibition by Sema3A produced by the CS itself is involved in allowing nerves to grow into the CS – possibly working in concert with a similar decrease in inhibition by CS-derived Slit2 (as described above). Likewise, for the CE during this time period, the expression of Sema3A also decreases. However this decrease is not as abrupt as in the CS, and it continues to occur progressively until E10-11, which is when innervation of the CE begins. For the lens, however, no such temporal decrease was observed, and if anything there was a slight increase in expression.
Lastly, regulation by the Sema3A receptor (Nrp1) may also be involved. Previous studies by others in innervated tissues such as tongue, tooth pulp and muscle have suggested that the timing of this innervation is dependent on the downregulation of both Sema3A by the target tissue and Nrp1 in the innervating nerve (Dillon et al., 2004; Kettunen et al., 2005). And we have observed that throughout the entire period of corneal innervation examined the expression of Nrp1 in the OTG also decreases. So as is the case in many systems, the regulation of corneal innervation may be multifactorial, and may include both the regulatory cues and their receptors.
Acknowledgments
Supported by National Institutes of Health grant 1R01EY018889 The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Eye Institute or the National Institutes of Health.
The authors wish to thank Christopher Talbot for assistance with qRT-PCR analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- Baker KS, Anderson SC, Romanowski EG, Thoft RA, SundarRaj N. Trigeminal ganglion neurons affect corneal epithelial phenotype. Influence on type VII collagen expression in vitro. Invest Ophthalmol Vis Sci. 1993;34:137–144. [PubMed] [Google Scholar]
- Bandyopadhyay A, Kubilus JK, Crochiere ML, Linsenmayer TF, Tabin CJ. Identification of unique molecular subdomains in the perichondrium and periosteum and their role in regulating gene expression in the underlying chondrocytes. Dev Biol. 2008;321:162–174. doi: 10.1016/j.ydbio.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beazley KE, Canner JP, Linsenmayer TF. Developmental regulation of the nuclear ferritoid-ferritin complex of avian corneal epithelial cells: roles of systemic factors and thyroxine. Exp Eye Res. 2009;89:854–862. doi: 10.1016/j.exer.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bee JA. The development and pattern of innervation of the avian cornea. Dev Biol. 1982;92:5–15. doi: 10.1016/0012-1606(82)90145-2. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Coats JM. The lens organizes the anterior segment: Specification of neural crest cell differentiation in the avian eye. Dev Biol. 2000;220:424–431. doi: 10.1006/dbio.2000.9638. [DOI] [PubMed] [Google Scholar]
- Bennett JL, Zeiler SR, Jones KR. Patterned expression of BDNF and NT-3 in the retina and anterior segment of the developing mammalian eye. Invest Opthamol Vis Sci. 1999;40:2996–3005. [PubMed] [Google Scholar]
- Beuerman RW, Schimmelpfennig B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp Neurol. 1980;69:196–201. doi: 10.1016/0014-4886(80)90154-5. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Chen X, Gallar J, Belmonte C. Reduction by antiinflammatory drugs of the response of corneal sensory nerve fibers to chemical irritation. Invest Ophthalmol Vis Sci. 1997;38:1944–1953. [PubMed] [Google Scholar]
- Chilton JK, Guthrie S. Cranial expression of class 3 secreted semaphorins and their neuropilin receptors. Dev Dyn. 2003;228:726–733. doi: 10.1002/dvdy.10396. [DOI] [PubMed] [Google Scholar]
- Conrad AH, Albrecht M, Pettit-Scott M, Conrad GW. Embryonic corneal Schwann cells express some Schwann cell marker mRNAs, but no mature Schwann cell marker proteins. Invest Ophthalmol Vis Sci. 2009;50:4173–4184. doi: 10.1167/iovs.08-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costagliola C, Cipollone U, Rinaldi M, della CM, Semeraro F, Romano MR. Intravitreal bevacizumab (Avastin) injection for neovascular glaucoma: a survey on 23 cases throughout 12-month follow-up. Br J Clin Pharmacol. 2008;66:667–673. doi: 10.1111/j.1365-2125.2008.03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro F, Silos-Santiago I, De Armentia ML, Barbacid M, Belmonte C. Corneal innervation and sensitivity to noxious stimuli in trkA knockout mice. European Journal of Neuroscience. 1998;10:146–152. doi: 10.1046/j.1460-9568.1998.00037.x. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Dillon TE, Saldanha J, Giger R, Verhaagen J, Rochlin MW. Sema3A regulates the timing of target contact by cranial sensory axons. Journal of Comparative Neurology. 2004;470:13–24. doi: 10.1002/cne.11029. [DOI] [PubMed] [Google Scholar]
- Ebendal T, Persson H. Detection of Nerve Growth-Factor Messenger-Rna in the Developing Chicken-Embryo. Development. 1988;102:101–106. doi: 10.1242/dev.102.1.101. [DOI] [PubMed] [Google Scholar]
- Fricke C, Lee JS, Geiger-Rudolph S, Bonhoeffer F, Chien CB. astray, a zebrafish roundabout homolog required for retinal axon guidance. Science. 2001;%20(292):507–510. doi: 10.1126/science.1059496. [DOI] [PubMed] [Google Scholar]
- Fu SY, Sharma K, Luo YL, Raper JA, Frank E. SEMA3A regulates developing sensory projections in the chicken spinal cord. Journal of Neurobiology. 2000;45:227–236. [PubMed] [Google Scholar]
- Garcia-Hirschfeld J, Lopez-Briones LG, Belmonte C. Neurotrophic influences on corneal epithelial cells. Exp Eye Res. 1994;59:597–605. doi: 10.1006/exer.1994.1145. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hao JC, Yu TW, Fujisawa K, Culotti JG, Gengyo-Ando K, Mitani S, Moulder G, Barstead R, Tessier-Lavigne M, Bargmann CI. C. elegans slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron. 2001;32:25–38. doi: 10.1016/s0896-6273(01)00448-2. [DOI] [PubMed] [Google Scholar]
- Holmes G, Niswander L. Expression of slit-2 and slit-3 during chick development. Dev Dyn. 2001;222:301–307. doi: 10.1002/dvdy.1182. [DOI] [PubMed] [Google Scholar]
- Kettunen P, Loes S, Furmanek T, Fjeld K, Kvinnsland IH, Behar O, Yagi T, Fujisawa H, Vainio S, Taniguchi M, Luukko K. Coordination of trigeminal axon navigation and patterning with tooth organ formation: epithelial-mesenchymal interactions, and epithelial Wnt4 and Tgfbeta1 regulate semaphorin 3a expression in the dental mesenchyme. Development. 2005;132:323–334. doi: 10.1242/dev.01541. [DOI] [PubMed] [Google Scholar]
- Keynes R, Tannahill D, Morgenstern DA, Johnson AR, Cook GM, Pini A. Surround repulsion of spinal sensory axons in higher vertebrate embryos. Neuron. 1997;18:889–897. doi: 10.1016/s0896-6273(00)80329-3. [DOI] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;%19(96):785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Kubilus JK, Linsenmayer TF. Developmental Corneal Innervation: Interactions between Nerves and Specialized Apical Corneal Epithelial Cells. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.09-3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton. 1990;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- Linsenmayer TF, Eavey RD, Schmid TM. Type X collagen: a hypertrophic cartilage-specific molecule. Pathol Immunopathol Res. 1988;7:14–19. doi: 10.1159/000157085. [DOI] [PubMed] [Google Scholar]
- Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- Lwigale PY, Bronner-Fraser M. Lens-derived Semaphorin3A regulates sensory innervation of the cornea. Dev Biol. 2007;306:750–759. doi: 10.1016/j.ydbio.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Ma L, Tessier-Lavigne M. Dual branch-promoting and branch-repelling actions of Slit/Robo signaling on peripheral and central branches of developing sensory axons. J Neurosci. 2007;%20(27):6843–6851. doi: 10.1523/JNEUROSCI.1479-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver MB, Tanelian DL. Free nerve ending terminal morphology is fiber type specific for A delta and C fibers innervating rabbit corneal epithelium. J Neurophysiol. 1993;69:1779–1783. doi: 10.1152/jn.1993.69.5.1779. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Kingsley RE, Echtenkamp SE. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Invest Ophthalmol Vis Sci. 1989;30:461–472. [PubMed] [Google Scholar]
- Morgan CW, Nadelhaft I, de Groat WC. Anatomical localization of corneal afferent cells in the trigeminal ganglion. Neurosurgery. 1978;2:252–258. doi: 10.1227/00006123-197805000-00012. [DOI] [PubMed] [Google Scholar]
- Muller LJ, Marfurt CF, Kruse F, Tervo TMT. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Muller LJ, Pels L, Vrensen GF. Ultrastructural organization of human corneal nerves. Invest Ophthalmol Vis Sci. 1996;37:476–488. [PubMed] [Google Scholar]
- Nguyen-Ba-Charvet KT, Chedotal A. Role of Slit proteins in the vertebrate brain. J Physiol Paris. 2002;96:91–98. doi: 10.1016/s0928-4257(01)00084-5. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Bicknese AR, De Carlos JA, Heffner CD, Koester SE, Kutka LJ, Terashima T. Target selection by cortical axons: alternative mechanisms to establish axonal connections in the developing brain. Cold Spring Harb Symp Quant Biol. 1990;55:453–68. 453–468. doi: 10.1101/sqb.1990.055.01.045. [DOI] [PubMed] [Google Scholar]
- Ozdinler PH, Erzurumlu RS. Slit2, a branching-arborization factor for sensory axons in the mammalian CNS. Journal of Neuroscience. 2002;22:4540–4549. doi: 10.1523/JNEUROSCI.22-11-04540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump AS, Erskine L, Sabatier C, Brose K, Epstein CJ, Goodman CS, Mason CA, Tessier-Lavigne M. Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron. 2002;33:219–232. doi: 10.1016/s0896-6273(01)00586-4. [DOI] [PubMed] [Google Scholar]
- Pond A, Roche FK, Letourneau PC. Temporal regulation of neuropilin-1 expression and sensitivity to semaphorin 3A in NGF- and NT3-responsive chick sensory neurons. J Neurobiol. 2002;51:43–53. doi: 10.1002/neu.10041. [DOI] [PubMed] [Google Scholar]
- Puschel AW, Adams RH, Betz H. The sensory innervation of the mouse spinal cord may be patterned by differential expression of and differential responsiveness to semaphorins. Mol Cell Neurosci. 1996;7:419–431. doi: 10.1006/mcne.1996.0030. [DOI] [PubMed] [Google Scholar]
- Rozsa AJ, Beuerman RW. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain. 1982;14:105–120. doi: 10.1016/0304-3959(82)90092-6. [DOI] [PubMed] [Google Scholar]
- Shepherd IT, Luo Y, Lefcort F, Reichardt LF, Raper JA. A sensory axon repellent secreted from ventral spinal cord explants is neutralized by antibodies raised against collapsin-1. Development. 1997;124:1377–1385. doi: 10.1242/dev.124.7.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillitoe RV, Hawkes R. Whole-mount immunohistochemistry: a high-throughput screen for patterning defects in the mouse cerebellum. J Histochem Cytochem. 2002;50:235–244. doi: 10.1177/002215540205000211. [DOI] [PubMed] [Google Scholar]
- Spurr SJ, Gipson IK. Isolation of corneal epithelium with dispase II or EDTA: Effects on the basement membrane zone. Invest Ophthalmol Vis Sci. 1985;26:818–827. [PubMed] [Google Scholar]
- Tang XQ, Tanelian DL, Smith GM. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J Neurosci. 2004;24:819–827. doi: 10.1523/JNEUROSCI.1263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Yuasa S, Fujisawa H, Naruse I, Saga S, Mishina M, Yagi T. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron. 1997;19:519–530. doi: 10.1016/s0896-6273(00)80368-2. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [Review] [120 refs] [DOI] [PubMed] [Google Scholar]
- Tucker KL, Meyer M, Barde YA. Neurotrophins are required for nerve growth during development. Nat Neurosci. 2001;4:29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96:771–784. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Ambrosio R. Laser in situ keratomileusis-induced neurotrophic epitheliopathy. Am J Ophthalmol. 2001;132:405–406. doi: 10.1016/s0002-9394(01)00995-3. [DOI] [PubMed] [Google Scholar]
- Wright DE, White FA, Gerfen RW, Silos-Santiago I, Snider WD. The guidance molecule semaphorin III is expressed in regions of spinal cord and periphery avoided by growing sensory axons. J Comp Neurol. 1995;361:321–333. doi: 10.1002/cne.903610209. [DOI] [PubMed] [Google Scholar]
- Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo SY, Miyashita T, Fricke C, Little MH, Yamada T, Kuwada JY, Huh TL, Chien CB, Okamoto H. Involvement of Islet-2 in the Slit signaling for axonal branching and defasciculation of the sensory neurons in embryonic zebrafish. Mech Dev. 2004;121:315–324. doi: 10.1016/j.mod.2004.03.006. [DOI] [PubMed] [Google Scholar]
- You LT, Kruse FE, Volcker HE. Neurotrophic factors in the human cornea. Invest Opthamol Vis Sci. 2000;41:692–702. [PubMed] [Google Scholar]
- Zallen JA, Yi BA, Bargmann CI. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell. 1998;92:217–227. doi: 10.1016/s0092-8674(00)80916-2. [DOI] [PubMed] [Google Scholar]
- ZANDER E, WEDDELL G. Observations on the innervation of the cornea. J Anat. 1951;85:68–99. [PMC free article] [PubMed] [Google Scholar]