Abstract
Background
Alternate waiting list strategies expand listing criteria for patients awaiting heart transplantation (HTx). We retrospectively analyzed clinical events and outcome of patients listed as high-risk recipients for HTx.
Methods and Results
We analyzed 822 adult patients who underwent HTx of whom 111 patients met high-risk criteria. Clinical data were collected from medical records and outcome factors calculated for 61 characteristics. Significant factors were summarized in a prognostic score. Age >65 years (67%) and amyloidosis (19%) were the most common reasons for alternate listing. High-risk recipients were older (63.2±10.2 versus 51.4±11.8 years; P<0.001), had more renal dysfunction, prior cancer, and smoking. Survival analysis revealed lower post-HTx survival in high-risk recipients (82.2% versus 87.4% at 1-year; 59.8% versus 76.3% at 5-year post-HTx; P=0.0005). Prior cerebral vascular accident, albumin <3.5 mg/dL, re-HTx, renal dysfunction (glomerular filtration rate <40 mL/min), and >2 prior sternotomies were associated with poor survival after HTx. A prognostic risk score (CARRS [CVA, albumin, re-HTx, renal dysfunction, and sternotomies]) derived from these factors stratified survival post-HTx in high-risk (3+ points) versus low-risk (0–2 points) patients (87.9% versus 52.9% at 1-year; 65.9% versus 28.4% at 5-year post-HTx; P<0.001). Low-risk alternate patients had survival comparable with regular patients (87.9% versus 87.0% at 1-year and 65.9% versus 74.5% at 5-year post-HTx; P=0.46).
Conclusions
High-risk patients had reduced survival compared with regular patients post-HTx. Among patients previously accepted for alternate donor listing, application of the CARRS score identifies patients with unacceptably high mortality after HTx and those with a survival similar to regularly listed patients.
Keywords: heart failure, prognosis, transplantation
Heart transplantation (HTx) is the only curative treatment for patients with advanced heart failure (HF), but its use is limited because of organ donor shortage and the high number of patients on the HTx waiting list.1 Therefore, selection of transplant candidates requires constant reconsideration of objective assessments of mortality risk in patients with advanced HF, reflecting recent improvements of medical and surgical therapies for this patient population.2 Alternate waiting list strategies expand listing criteria for patients awaiting HTx, and matching high-risk recipients with marginal donor organs has been proposed and is being practiced to expand the donor pool.3–9
Survival after HTx for patients listed under alternate criteria has been reported to be comparable to that of regular listed patients.3 However, several studies have reported increased recipient-related complications, including prolonged ventilator support and intensive care unit time after transplant surgery, and an increased frequency of infection, in particular, sternal wound infections and lung infections, which were related to lower posttransplant survival in 1 study.3,10 No study has found differences in survival associated with donor-related factors, such as primary graft failure, transplant coronary artery disease, or higher rates of cellular or antibody-mediated rejection in high-risk HTx recipients.11–13 This is somewhat surprising, given the marginal nature of organs allocated to high-risk recipients with increased number of risk factors, such as higher donor age and left ventricular hypertrophy.
The aim of this study was the retrospective analysis of clinical events and outcome after HTx of patients listed as high-risk recipients in comparison to regular listed patients undergoing HTx at our institution. We here report the single-center experience on outcome after HTx of high-risk candidates at Columbia University Medical Center between 1999 and 2010.
Methods
Study Cohort
Information was collected retrospectively on 822 patients, who underwent HTx between 1999 and 2010 at Columbia University Medical Center. A total of 111 patients were classified as high risk and transplanted through an internal alternate listing system. All other transplanted patients were considered normal risk and listed under regular standards (regular list, n=711).
Collected data included preoperative baseline demographics, laboratory values, donor demographic data, and past medical histories and comorbidities. Intraoperative details collected included donor ischemic time, cardiopulmonary bypass time, and aortic cross-clamp time. Postoperative data included complications defined as complications occurring within the time period from operation to discharge from the hospital or within 30 days. All hospital course and medical history data were obtained by thorough chart review of physical and electronic medical records. The primary outcome of all-cause mortality after HTx was collected from the Social Security Death Index.
Additional data were collected from the United Network of Organ Sharing (UNOS) data set of deidentified patient data. We analyzed recipient characteristics of patients aged ≥18 years undergoing HTx between January 1, 1999, and December 31, 2008. Patients were followed from the date of transplant until death, retransplantation (cardiac), or date of last known follow-up, which was the last day of follow-up data provided by UNOS.
The data collection protocol was approved by the institutional review board of Columbia University. The protocol complied with the Health Insurance Portability and Accountability Act and all ethical guidelines outlined by the 1975 Declaration of Helsinki.
Surgical Procedures and Immunosuppression
All patients in this cohort underwent HTx using the bicaval technique. Heart transplant recipients received standard immunosuppressant therapy with calcineurin inhibitors, cyclosporine or tacrolimus, mycophenolate mofetil, and prednisone. Preoperatively, patients received 4 mg/kg of azathioprine and intraoperatively 500 mg of solumedrol. Postoperatively, patients received 125 mg solumedrol every 8 hours for 3 doses. Mycophenolate mofetil was started at a dose of 1500 mg twice daily. High-dose oral prednisone was started at 100 mg daily and tapered to 30 mg daily by 2 weeks. Induction therapy using interleukin-2 receptor antagonists was administered to eligible patients starting within 24 hours after HTx. Patients with active infections or undergoing retransplantation did not receive induction therapy.
Statistical Methods
Continuous variables are presented as a mean±SD, whereas categorical and binary data as frequency distributions. Comparison of continuous variables relied on 2-tailed, unpaired Student t tests, whereas categorical comparison occurred through χ2 and Fisher exact tests. All analysis assumed a P<0.05 level of significance. Receiver operating characteristic curves were drawn, and area under the curve were analyzed and compared for all proposed scoring systems.
Kaplan–Meier survival function curves were compared between subject sets by Mantel–Cox log-rank test and proportional hazards regression. All survival data were collected and censored after October 20, 2010. In an attempt to identify variables that independently predict post-HTx survival of patients on the alternate list, we conducted a multivariate Cox proportional hazards regression analysis. Univariable significant predictors were reduced backward from the model in a stepwise fashion. All data were analyzed using the Statistical Analysis Systems software JMP 7.0 (SAS Institute Inc, Cary, NC).
CARRS Score
The creation of the CARRS score was based on the results of the univariable and multivariable proportional hazards risk analysis.14,15 Because the multivariable analysis failed to identify multiple independent predictors, we could not score the covariables by relative hazard. Prior cerebral vascular accident, albumin <3.5 mg/dL, re-HTx, renal dysfunction (glomerular filtration rate [GFR] <40 mL/min), and >2 prior sternotomies were associated with poor survival after HTx. We relied on the univariable analysis and Kaplan–Meier analysis to score predictors with a hazards ratio >2 and a pronounced early survival effect with 2 points and GFR<40 mL/min with 1 point (attributable to lower impact compared with the other factors). Significant uni-variable predictors with >15% missing data or negligible hazards, as well as intraoperative and donor risk factors, were also not included. Stratification of high- and low-point values was varied according to survival predictive power before a final inflection point was set at 0 to 2 points for low risk and 3 to 9 points for high risk.
Results
Baseline Characteristics
Clinical characteristics of all patients at the time of HTx are summarized in Table 1. The primary reason for alternate listing included age >65 years (67% of all high-risk patients), cardiac amyloidosis (19%), HIV infection (4.5%), and other causes, such as severe peripheral vascular disease, diabetes mellitus with end-organ involvement, advanced renal dysfunction with GFR<40 mL/min, retransplantation at age>65 years, and prior stroke (together 9.5% of all high-risk patients). High-risk recipients were older (63.2±10.2 versus 51.4±11.8 years in regular patients; P<0.001), had more renal dysfunction, more frequent prior history of cancer, and smoking. High-risk recipients received organs from older donors (donor age: 40.9±13.6 years versus 33.2±13.0 years in regular listed patients; P<0.001), reflecting the selection of higher risk organs for this group.
Table 1.
Baseline Characteristics
| Regular List (n=711) | High-Risk List (n=111) | P Value | |
|---|---|---|---|
| Clinical characteristics at the time of cardiac transplantation | |||
| Age, y | 51.4±11.8 | 63.2±10.2 | <0.001 |
| Sex (male), % | 549 (77.2) | 86 (77) | 0.841 |
| Race (white), % | 434 (61.0) | 73 (66) | 0.892 |
| BMI, kg/m2 | 25.7±4.8 | 25.9±5.2 | 0.660 |
| Prior LVAD, % | 234 (32.9) | 27 (24.3) | 0.071 |
| Ejection fraction, % | 18±9 | 20±9 | 0.06 |
| Coronary artery disease, % | 305 (42.9) | 52 (46.9) | 0.438 |
| Prior myocardial infarction, % | 143 (20.1) | 32 (28.8) | 0.038 |
| Cerebral vascular accident, % | 82 (11.5) | 15 (13.5) | 0.546 |
| Peripheral vascular disease, % | 35 (4.9) | 8 (7.2) | 0.317 |
| Diabetes mellitus, % | 194 (27.3) | 38 (34.2) | 0.133 |
| Hypertension, % | 270 (38.0) | 50 (45.0) | 0.135 |
| Prior smoker, % | 239(34) | 41 (37.3) | 0.508 |
| Prior cancer, % | 39 (6.03) | 11 (11.6) | 0.044 |
| COPD, % | 21 (3.3) | 5 (4.9) | 0.410 |
| Preoperative laboratory values | |||
| Albumin, mg/dL | 4.0±2.2 | 3.9±0.6 | 0.459 |
| Sodium, mEq/L | 135±4 | 136±3 | 0.288 |
| Potassium, mEq/L | 4.3±0.5 | 4.5±0.5 | 0.149 |
| BUN, mg/dL | 28±15 | 34±18 | <0.001 |
| Creatinine, mg/dL | 1.4±0.8 | 1.5±0.5 | 0.407 |
| GFR–MDRD, mL/min | 68±30 | 57±23 | <0.001 |
| Total bilirubin, mg/dL | 1.2±1.1 | 1.1±0.9 | 0.520 |
| Direct bilirubin, mg/dL | 0.3±0.5 | 0.3±0.4 | 0.660 |
| AST, IU/L | 35±63 | 31±19 | 0.473 |
| ALT, IU/L | 36±76 | 27±22 | 0.227 |
| Total cholesterol, mg/dL | 151±46 | 153±45 | 0.567 |
| Triglyceride, mg/dL | 122±79 | 127±75 | 0.580 |
| Hematocrit, % | 36.1±6.2 | 37.2±5.7 | 0.080 |
| Hemoglobin, g/dL | 11.9±4.2 | 12.1±2.0 | 0.600 |
| Platelet count | 235±86 | 215±72 | 0.030 |
| Perioperative and donor-related information | |||
| Donor age, y | 33.0±13.0 | 40.9±13.6 | <0.001 |
| Donor ischemic time, min | 191±73 | 201±52 | 0.210 |
| Cardiopulmonary bypass time, min | 163±46 | 166±53 | 0.493 |
| Aortic cross-clamp time, min | 97±29 | 96±24 | 0.836 |
| Mean survival, d | 2937±60 | 2238±157 | 0.001 |
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; LVAD, left ventricular assist device; and MDRD, modification of diet in renal disease.
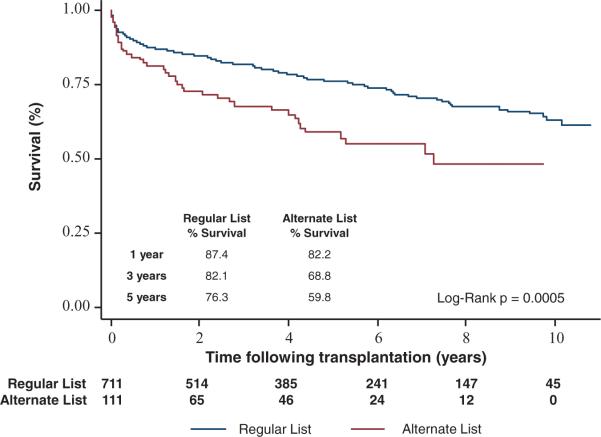
Survival analysis revealed lower posttransplant survival in high-risk recipients compared with regular listed recipients (82.2% versus 87.4% at 1-year; 59.8% versus 76.3% at 5-year post-HTx; P=0.0005) (Figure 1). At 1-year post-HTx, the number of deaths was 88 in regular listed patients and 19 in alternate listed patients. At 5 years, the number of deaths was 61 in the regular listed patients and 18 in alternate listed patients. Postoperative complications including atrial fibrillation, postoperative ventricular assist device placement, development of worsening renal function, as well as dialysis requiring renal failure, respiratory failure, reoperation for bleeding, sternal wound infections, and stroke were not significantly different between the 2 groups (Table 2).
Figure 1.
Kaplan–Meier survival curves after heart transplantation of alternate vs regular listed patients.
Table 2.
Frequency of Early Postoperative Complications
| Regular List (n=711) | High-Risk List (n=111) | P Value | |
|---|---|---|---|
| Postoperative atrial fibrillation, % | 72 (10.1) | 16 (14.4) | 0.140 |
| Postoperative VAD, % | 24 (3.4) | 4 (3.6) | 0.892 |
| Respiratory failure, % | 48 (6.8) | 12 (10.8) | 0.119 |
| Worsening renal function, % | 61 (8.6) | 12 (10.8) | 0.434 |
| Renal failure (hemodialysis) | 80 (11.3) | 16 (14.4) | 0.326 |
| Reoperation for bleeding, % | 84 (11.8) | 14 (12.6) | 0.789 |
| Sternal wound infection, % | 40 (5.6) | 8 (7.2) | 0.488 |
| Stroke, % | 11 (1.5) | 0 (0) | 0.189 |
| 30-Day mortality, % | 32 (4.5) | 4 (3.6) | 0.660 |
VAD indicates ventricular assist device.
Analysis of Factors Associated With Survival in High-Risk Patients
To determine donor- and recipient-related factors associated with survival after HTx, we performed uni- and multivariable proportional hazard analysis of outcome. Survival factors identified by univariable analysis are listed in Table 3. Multivariable analysis was limited by a high degree of colinearity of values within the group of high-risk listed patients. Retransplantation remained significant in the multivariable model (hazards ratio, 16.9; 95% confidence interval [CI], 2.26–126.8), and prior cerebral vascular accident showed a trend toward significance (hazards ratio, 2.55; 95% CI, 0.9–7.22), whereas all other factors showed colinearity and had to be removed from the analysis. Of note, factors known to be associated with outcome post-HTx, such as diabetes mellitus, cardiac amyloidosis, age >65 years, and prior cancer, were not identified as survival-associated factors likely attributable to a selection bias related to the listing criteria for this patient cohort on the alternate list. Of note, we did not identify any donor-related factors associated with survival in the high-risk recipient group.
Table 3.
Analysis of Univariable Predictors of Outcome in High-Risk Cardiac Transplantation
| Hazard Ratio (95% CI) | P Value | |
|---|---|---|
| Retransplantation | 6.735 (1.95–20.3) | <0.01 |
| >2 Prior sternotomies | 2.684 (1.15–2.46) | <0.01 |
| Prior cerebral vascular accident | 2.198 (1.01–4.62) | 0.04 |
| Pre-Tx albumin (<3.5 mg/dL) | 2.137 (1.27–3.70) | <0.01 |
| Preexisting renal dysfunction (GFR<40 mL/min) | 1.940 (1.00–3.74) | <0.05 |
| Sex (female) | 1.922 (1.01–3.66) | 0.05 |
| Pre-Tx total bilirubin (>1.3 mg/dL) | 1.869 (1.02–3.45) | 0.04 |
| Cardiopulmonary bypass time | 1.001 (1.00–1.01) | 0.02 |
| Pre-Tx LDL (>70 mg/dL) | 1.001 (1.00–1.02) | 0.04 |
CI indicates confidence interval; GFR, glomerular filtration rate; LDL, low-density lipoprotein; and TX, transplantation.
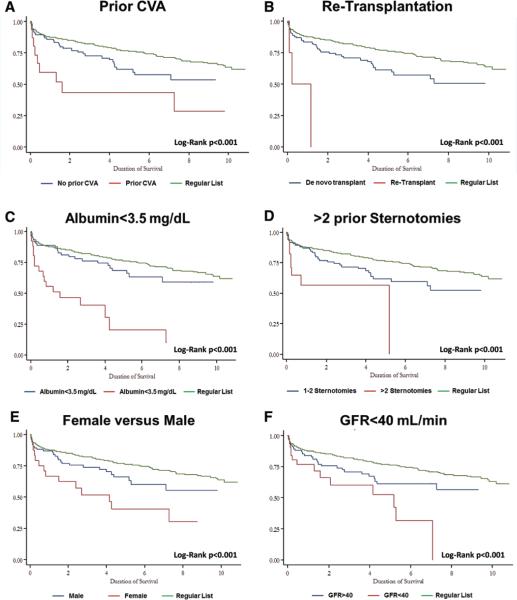
Subgroup analysis of patients of the high-risk cohort revealed the individual contribution of high-risk factors to survival. Kaplan–Meier survival curves describing outcome of patients within these subgroups are listed in Figure 2A–2F.
Figure 2.
Survival curves of alternate patients in various subgroups after heart transplantation.
CARRS Score
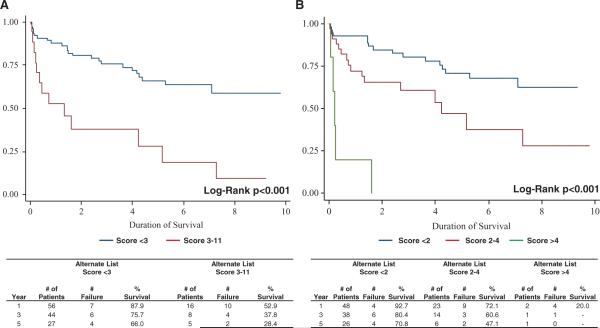
A prognostic risk score (CARRS based on cerebral vascular accident, albumin<3.5 mg/dL, re-HTx, renal dysfunction (GFR<40 mL/min), and >2 prior sternotomies) derived from the above identified factors (with each factor assigned 2 points except 1 point for GFR<40 mL/min) was created after multiple adjustments based on receiver operating characteristic curve analysis of various models (Table 4). The CARRS score effectively stratified high-risk (3+ points) versus low-risk (0–2 points) patients with regard to survival after HTx (87.9% low score versus 52.9% at 1-year; 65.9% low score versus 28.4% high score at 5-year post-HTx; P<0.001) (Figure 3A). Consistently, patients with a score of >4 had a 1-year survival of only 20% (Figure 3B). The low-risk alternate listed patients had survival post-HTx comparable to regular listed patients (87.9% in low-risk alternate patients versus 87.0% in regular listed patients at 1-year, and 65.9% in low-risk risk alternate patients versus 74.5% in regular listed patients at 5-year post-HTx; P=0.46). Receiver operating characteristic curve analysis of the CARRS score revealed an area under the curve of 0.77 with a sensitivity of 44% (95% CI, 21%–69%) and specificity of 87% (95% CI, 77%–94%) at a cutoff at 3 and a sensitivity of 72% (95% CI, 46.5%–90.3%) and specificity of 67% (95% CI, 54.3%–77.6%) at a cutoff at 2 for the prediction of survival in high-risk patients undergoing HTx.
Table 4.
CARRS Score
| Criteria | Score | |
|---|---|---|
| CVA (prior stroke) | Yes | 2 |
| Albumin, mg/dL | <3.5 | 2 |
| Retransplantation | Yes | 2 |
| GFR–MDRD (<40 mL/min) | <40 | 1 |
| Prior cardiothoracic surgeries | >2 | 2 |
| Total | 9 |
CARRS indicates CVA, albumin, re-HTx, renal dysfunction, and sternotomies; CVA, cerebral vascular accident; GFR, glomerular filtration rate; and MDRD, modification of diet in renal disease.
Figure 3.
Application of the CARRS score to alternate listed patients.
The CARRS score was also predictive of survival in patients undergoing cardiac transplantation on the regular list at our institution. One-year survival was 87.0% in low-risk patients (score 0–2) versus 76.1% in high-risk patients (score 3 and higher). Five-year survival was 74.5% in the low-risk group versus 62.4% in the high-risk group (P=0.0015).
To validate the CARRS score, we applied the scoring system to the UNOS data set. This multicenter analysis of patients after cardiac transplantation revealed an 1-year survival of 90% in low-risk patients (score 0–2) versus 85% in high-risk patients (score 3 and higher; P<0.001). Five-year survival was equally affected with survival of 76% in the low-risk group versus 71% in the high-risk group (P<0.001) (Figure 4).
Figure 4.
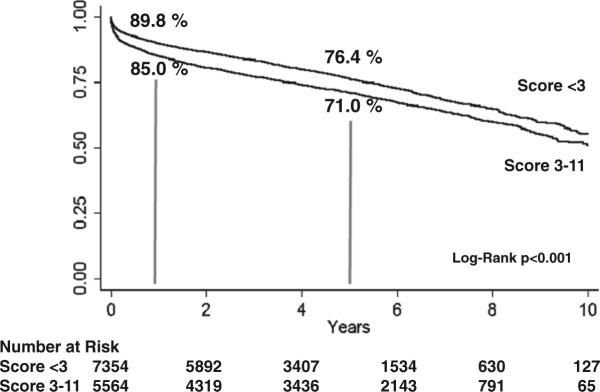
Application of the CARRS score to the United Network of Organ Sharing data set of patients undergoing heart transplantation.
Discussion
In the present study, we analyzed the survival of patients undergoing HTx at our institution after initiation of an alternate waiting list for patients with high-risk features. We compared the outcome of this patient cohort with outcome of patient on the regular list after HTx and identified significantly lower survival of alternate listed patients. Alternate listing was most common because of age>65 years and cardiac amyloid (86% of the high-risk patients). Significant preexisting comorbidities of patients listed on the alternate list included cerebral vascular accident, history of prior HTx, prior sternotomies, renal and hepatic dysfunction, HIV infection. Factors identified to be associated with worse survival were primarily factors of end-organ damage most significant for hepatic, renal, and neurological dysfunction. Of note, cardiac amyloidosis independent of end-organ damage does not predict poor outcome after cardiac transplantation in our high-risk cohort. Further, prior ventricular assist device implantation also does not affect post-HTx outcome in this patients cohort at our institution. The novel CARRS risk score was derived from outcome factors and predicts post-transplant survival of patients listed on the alternate list with high sensitivity and specificity. Further, the score performs well in patients on the regular list predicting survival after cardiac transplantation.
This analysis contrasts prior reports demonstrating no significant differences in survival after HTx between patients listed on the regular versus alternate list from our institution.3 We and others have previously reported an increased risk of infections, including ventilator-associated pneumonia and sternal wound infections, as well as a longer intensive care unit stay, after cardiac transplant in patients on the alternate list.3,6,10 Our current report did not find significant differences in early postoperative outcome and comparable early and inhospital mortality between the 2 cohorts but decreased long-term survival in patients of the alternate list. Although our analysis did not include the exact cause of death, we found that most of the deaths occurred within the first 6 months after transplantation. At our center, marginal donor hearts were classified as from patients with advanced age (up to 65 years), history of substance abuse, longstanding diabetes mellitus, with mild coronary artery disease on angiograms, evidence of left ventricular hypertrophy, and with significant inotropic requirements. Although donors for alternate listed recipients were older, no donor-related factors predicting outcome post-HTx could be identified in this analysis, which is well in line with previous reports by other groups.3,6,9,10,16
One of the main findings of this study is the determining impact of recipient end-organ function on outcome after HTx in patients on the alternate list. Hepatic, renal, and neurological dysfunction were identified as major factors contributing to morbidity and mortality of these patients. As shown in previous studies, these factors need to be carefully assessed when evaluating patients with age >65 years and potentially amyloid cardiomyopathy, the 2 most common reasons to list patients on the alternate list at our institution. It is, however, unclear whether dynamics in end-organ function are equally important for outcome analysis. Multiple studies have shown worsening end-organ function in patients with advanced HF before HTx or ventricular assist device placement and subsequent improvement after hemodynamic normalization.17,18 We have defined limits for laboratory values based on our clinical experience and institutional values based on laboratory analyses closest to the date of transplantation.
Our analysis included both patients with systemic light chain amyloidosis and amyloidosis secondary to transthyretin mutations, which are associated with different prognosis and complications and require different treatment approaches, including stem cell transplantation in patients with systemic light chain amyloidosis. We had to combine different types of cardiac amyloidosis because the subgroups were too small for meaningful analysis and conclusions. Cardiac amyloidosis, however, was a frequent cause (19% of all high-risk cases, second most common) for listing on the alternate list. In the subgroup of high-risk patients, cardiac amyloidosis itself was not significantly associated with poor prognosis in the uni- or multivariable survival analysis. Factors of end-organ damage (liver and renal dysfunction) were more significantly associated with survival compared with amyloidosis as a single factor in this subgroup, further emphasizing our concept that factors of end-organ damage are important outcome parameters independent of the underlying pathogenesis of HF. It is, however, important to note that cardiac amyloidosis was significantly associated with poor prognosis when we tested our entire cohort of patients undergoing cardiac transplantation (both regular and high-risk cardiac transplantation recipients).
In our analysis, we also identified female sex as a factor associated with higher mortality in the cohort of patients on the alternate list undergoing HTx. We, however, excluded sex as a factor from the CARRS score creation because it was identified only in the subgroup of high-risk patients and did not remain positive in any subsequent analysis. Further, we wanted to avoid any sex bias in our proposed algorithm. It is, however, important to note that several previous studies have shown that female sex is associated with lower survival in several studies of outcome after HTx.1
The main goal of this study was to identify factors associated with increased mortality in the high-risk cohort of patients listed for HTx on the alternate list. The alternate list established at several cardiac transplant centers was created to address organ donor shortage and match marginal hearts with more high-risk recipients. Our study has revealed several clinically relevant factors that together form the CARRS score and allow characterization of patients with high-risk features that have unacceptably low survival after HTx, which supports elimination of alternate listing. The current strategy of matching high-risk organs with high-risk recipients has, however, still a large survival benefit for patients with advanced HF and results in acceptable 5-year survival rates of 60% at our institution compared with <10% rates of 5-year survival of patients with class IV advanced HF.5 Clearly, this strategy will result in decreased survival for any given transplant center and will question the use of survival as the dominant criteria for judging transplant centers. Stratification of transplant outcome statistics for patient-associated clinical risk factors, as in the current study, center volume, and regional location within the and other prior studies.6 It is, however, important to note that current trends in the use of ventricular assist devices for destination therapy in patients with advanced HF have resulted in nearly identical outcomes of these patients when compared with high-risk cardiac transplantation recipients.
Our study has several limitations. First, the analysis is based on the single-center experience of cardiac transplantation between 1999 and 2010. The analysis is also limited by its retrospective nature. The selection of patients as high risk is determined by programmatic factors, such as age, amyloidosis, presence of HIV infection, and comorbidities with end-organ disease that had been established in our program and might differ from other programs that use an alternate list. The field of cardiac transplantation lacks an universal definition of high-risk or alternate list recipients, and our study did not validate the results independently in high-risk cohorts from other centers. There is also potential bias based on the use of data-derived thresholds. Further, we could not include PRA levels and the results of psychosocial evaluations into our analysis because of lack of data in a large number of patients. Differences in medical regimens were also not included in our analysis. Patients with amyloid disease were not subclassified with regard to the underlying cause of amyloidosis (example, light chain amyloidosis or transthyretin amyloid) because of the small number of subjects in these groups. The CARRS score will perform less well in unselected, lower risk cohorts, and this is reflected in the analysis of the UNOS data set. The development of the CARRS score was triggered by the high-risk cohort and its outcome at our center and is designed for this specific patient population. Finally, we did not include invasive hemodynamics into our analysis because of frequent changes of these values in response to changes in medical and surgical regimens.
In conclusion, risk stratification using the noninvasive CARRS score allows identification of patients with unacceptably high mortality after HTx. Among patients previously accepted for alternate donor listing, application of the CARRS score identifies patients with unacceptably high mortality after HTx and those with a survival similar to regularly listed patients.
Supplementary Material
CLINICAL PERSPECTIVE.
Heart transplantation (HTx) is the only curative treatment for patients with advanced heart failure, but its use is limited because of organ donor shortage and the high number of patients on the HTx waiting list. Selection of transplant candidates requires objective assessments of mortality risk in patients with advanced heart failure reflecting improvements of medical and surgical therapies. Alternate waiting list strategies expand listing criteria for patients awaiting HTx. Our study analyzed clinical events and outcome of 111 patients listed as high-risk recipients compared with 711 regularly listed patients at our institution. Age >65 years and amyloidosis were the most common reason for alternate listing. High-risk recipients were older, had more renal dysfunction, prior cancer, and smoking. Survival analysis revealed lower post-HTx survival in high-risk recipients (>5% lower at 1-year and >16% lower at 5-year post-HTx; P=0.0005). Statistical analysis on 61 clinical outcome characteristics revealed that prior cerebral vascular accident, albumin<3.5 mg/dL, re-HTx, renal dysfunction (glomerular filtration rate <40 mL/min), and >2 prior sternotomies were associated with poor survival after HTx. A prognostic risk score (CARRS) derived from these factors stratified survival post-HTx in high-risk (3+ points) versus low-risk (0–2 points) patients (87.9% versus 52.9% at 1-year post-HTx; 65.9% versus 28.4% at 5-year post-HTx; P<0.001). Low-risk alternate patients had post-HTx survival comparable with regular patients. Therefore, among patients previously accepted for alternate donor listing, application of the CARRS score identifies patients with unacceptably high mortality after HTx and those with a survival similar to regularly listed patients.
Acknowledgments
Sources of Funding
This work was supported by grants from the National Heart, Lung, and Blood Institute (K23 HL095742-01, P30 HL101272-01, UL1 RR 024156, HL073029) and the Herbert and Florence Irving Scholar Award.
Footnotes
The online-only Data Supplement is available at http://circheartfailure.ahajournals.org/lookup/suppl/doi:10.1161/CIRCHEARTFAILURE.112.000092/-/DC1.
Disclosures
None.
References
- 1.Stehlik J, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Kirk R, Dobbels F, Rahmel AO, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report–2010. J Heart Lung Transplant. 2010;29:1089–1103. doi: 10.1016/j.healun.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, Mohacsi P, Augustine S, Aaronson K, Barr M. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates–2006. J Heart Lung Transplant. 2006;25:1024–1042. doi: 10.1016/j.healun.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Chen JM, Russo MJ, Hammond KM, Mancini DM, Kherani AR, Fal JM, Mazzeo PA, Pinney SP, Edwards NM, Naka Y. Alternate waiting list strategies for heart transplantation maximize donor organ utilization. Ann Thorac Surg. 2005;80:224–228. doi: 10.1016/j.athoracsur.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Forni A, Luciani GB, Chiominto B, Pizzuti M, Mazzucco A, Faggian G. Results with expanded donor acceptance criteria in heart transplantation. Transplant Proc. 2011;43:953–959. doi: 10.1016/j.transproceed.2011.01.117. [DOI] [PubMed] [Google Scholar]
- 5.Lietz K, John R, Mancini DM, Edwards NM. Outcomes in cardiac transplant recipients using allografts from older donors versus mortality on the transplant waiting list; Implications for donor selection criteria. J Am Coll Cardiol. 2004;43:1553–1561. doi: 10.1016/j.jacc.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Russo MJ, Davies RR, Hong KN, Chen JM, Argenziano M, Moskowitz A, Ascheim DD, George I, Stewart AS, Williams M, Gelijns A, Naka Y. Matching high-risk recipients with marginal donor hearts is a clinically effective strategy. Ann Thorac Surg. 2009;87:1066–1070. doi: 10.1016/j.athoracsur.2008.12.020. discussion 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felker GM, Milano CA, Yager JE, Hernandez AF, Blue L, Higginbotham MB, Lodge AJ, Russell SD. Outcomes with an alternate list strategy for heart transplantation. J Heart Lung Transplant. 2005;24:1781–1786. doi: 10.1016/j.healun.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Laks H, Scholl FG, Drinkwater DC, Blitz A, Hamilton M, Moriguchi J, Fonarow G, Kobashigawa J. The alternate recipient list for heart transplantation: does it work? J Heart Lung Transplant. 1997;16:735–742. [PubMed] [Google Scholar]
- 9.Kobashigawa JA, Laks H, Wu G, Patel J, Moriguchi J, Hamilton M, Fonarow G, Fishbein M, Ardehali A. The University of California at Los Angeles heart transplantation experience. Clin Transpl. 2005:173–185. [PubMed] [Google Scholar]
- 10.Rajagopal K, Lima B, Petersen RP, Mesis RG, Daneshmand MA, Lemaire A, Felker GM, Hernandez AF, Rogers JG, Lodge AJ, Milano CA. Infectious complications in extended criteria heart transplantation. J Heart Lung Transplant. 2008;27:1217–1221. doi: 10.1016/j.healun.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Arnaoutakis GJ, George TJ, Allen JG, Russell SD, Shah AS, Conte JV, Weiss ES. Institutional volume and the effect of recipient risk on short-term mortality after orthotopic heart transplant. J Thorac Cardiovasc Surg. 2012;143:157–167. e151. doi: 10.1016/j.jtcvs.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilic A, Weiss ES, Arnaoutakis GJ, George TJ, Conte JV, Shah AS, Yuh DD. Identifying recipients at high risk for graft failure after heart retrans-plantation. Ann Thorac Surg. 2012;93:712–716. doi: 10.1016/j.athoracsur.2011.10.065. [DOI] [PubMed] [Google Scholar]
- 13.Lima B, Rajagopal K, Petersen RP, Shah AS, Soule B, Felker GM, Rogers JG, Lodge AJ, Milano CA. Marginal cardiac allografts do not have increased primary graft dysfunction in alternate list transplantation. Circulation. 2006;114(suppl 1):I27–I32. doi: 10.1161/CIRCULATIONAHA.105.000737. [DOI] [PubMed] [Google Scholar]
- 14.Hong KN, Iribarne A, Worku B, Takayama H, Gelijns AC, Naka Y, Jeevanandam V, Russo MJ. Who is the high-risk recipient? Predicting mortality after heart transplant using pretransplant donor and recipient risk factors. Ann Thorac Surg. 2011;92:520–527. doi: 10.1016/j.athoracsur.2011.02.086. discussion 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss ES, Allen JG, Arnaoutakis GJ, George TJ, Russell SD, Shah AS, Conte JV. Creation of a quantitative recipient risk index for mortality prediction after cardiac transplantation (IMPACT). Ann Thorac Surg. 2011;92:914–921. doi: 10.1016/j.athoracsur.2011.04.030. discussion 921. [DOI] [PubMed] [Google Scholar]
- 16.Laks H, Marelli D, Fonarow GC, Hamilton MA, Ardehali A, Moriguchi JD, Bresson J, Gjertson D, Kobashigawa JA, UCLA Heart Tranplant Group Use of two recipient lists for adults requiring heart transplantation. J Thorac Cardiovasc Surg. 2003;125:49–59. doi: 10.1067/mtc.2003.62. [DOI] [PubMed] [Google Scholar]
- 17.Daneshmand MA, Rajagopal K, Lima B, Khorram N, Blue LJ, Lodge AJ, Hernandez AF, Rogers JG, Milano CA. Left ventricular assist device destination therapy versus extended criteria cardiac transplant. Ann Thorac Surg. 2010;89:1205–1209. doi: 10.1016/j.athoracsur.2009.12.058. discussion 1210. [DOI] [PubMed] [Google Scholar]
- 18.Kato TS, Chokshi A, Singh P, Khawaja T, Cheema F, Akashi H, Shahzad K, Iwata S, Homma S, Takayama H, Naka Y, Jorde U, Farr M, Mancini DM, Schulze PC. Effects of continuous-flow versus pulsatile-flow left ventricular assist devices on myocardial unloading and remodeling. Circ Heart Fail. 2011;4:546–553. doi: 10.1161/CIRCHEARTFAILURE.111.962142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.