Abstract
In this study, a genetically engineered live attenuated Salmonella Enteritidis (SE) vaccine was evaluated for its ability to protect against Salmonella Typhimurium (ST) infection in chickens. The birds were orally primed with the vaccine on the 1st day of life and given an oral booster at 5 wk of age. Control birds were orally inoculated with phosphate-buffered saline. Both groups of birds were orally challenged with a virulent ST strain at 9 wk of age. Compared with the control chickens, the vaccinated chickens had significantly higher levels of systemic IgG and mucosal IgA against specific ST antigens and a significantly greater lymphoproliferative response to ST antigens. The excretion of ST into the feces was significantly lower in the vaccinated group than in the control group on days 9 and 13 d after challenge. In addition, the vaccinated group had significantly fewer pronounced gross lesions in the liver and spleen and lower bacterial counts in the internal organs than the control group after challenge. These data indicate that genetically engineered live attenuated SE may induce humoral and cellular immune responses against ST antigens and may confer protection against virulent ST challenge.
Résumé
Dans la présente étude on évalua la capacité d’un vaccin vivant atténué génétiquement modifié de Salmonella Enteritidis (SE) à protéger contre une infection par Salmonella Typhimurium (ST) chez le poulet. Les poulets furent inoculés oralement avec le vaccin à leur premier jour de vie et reçurent un rappel à 5 semaines d’âge. Les oiseaux témoins reçurent par voie orale de la saline tamponnée. Les deux groupes furent challengés par voie orale avec une souche virulente de ST à 9 sem d’âge. Comparativement aux oiseaux témoins, les poulets vaccinés avaient des taux significativement plus élevés d’IgG systémiques et d’IgA locaux contre des antigènes spécifiques de ST et une réponse lympho-proliférative significativement plus importante aux antigènes de ST. L’excrétion de ST dans les fèces était significativement moindre dans le groupe vacciné comparativement au groupe témoin aux jours 9 et 13 après le challenge. De plus, après le challenge le groupe vacciné avait significativement moins de lésions macroscopiques marquées dans le foie et la rate et des dénombrements bactériens moindres dans les organes internes que le groupe témoin. Ces résultats indiquent que le vaccin SE vivant génétiquement modifié peut induire une réponse immunitaire humorale et cellulaire contre des antigènes de ST et peut conférer une protection contre un challenge avec une souche de ST virulente.
(Traduit par Docteur Serge Messier)
Introduction
World wide, the bacterial pathogen Salmonella enterica is associated with approximately 93.8 million cases of nontyphoidal gastroenteritis in humans each year (1,2). Around 86% of these cases are the result of foodborne infection (2). The Salmonella Typhimurium (ST) and Salmonella Enteritidis (SE) serotypes are the most frequent causes of food contamination leading to salmonellosis in humans (3). Poultry products, including eggs and egg-derived products, are the most frequently identified food vehicles in outbreaks of foodborne salmonellosis (4). Both ST and SE infect chickens by the fecal–oral route, colonize the alimentary tract, invade internal organs such as the liver and spleen, and finally spread to the reproductive tract (5). Therefore, Salmonella control within farms that breed and rear poultry is a significant public health issue.
Vaccination is an ideal strategy for the prevention of salmonellosis. Oral live S. enterica vaccines can confer protection against salmonellosis by inducing both cell-mediated and mucosal immune responses (6–8). In certain circumstances, cross-protection can enhance the clearance of pathogens through the acquired immune response (9). Several live vaccine strains have conferred high levels of protection against infection with heterologous serovars (10–12). A vaccine constructed for a single serovar of Salmonella may induce immunity against other heterologous serovars of Salmonella (13,14).
In our previous study, a genetically engineered live attenuated SE, JOL919, was constructed and evaluated as a candidate vaccine against SE infection in chickens (6,15,16). In the present study, we examined the ability of JOL919 to induce humoral and cellular immune responses against ST antigens. In addition, we evaluated whether the vaccine provided protection against virulent ST challenge in chickens.
Materials and methods
Bacterial strains and growth conditions
The genetically engineered JOL919 was constructed by deletion of the lon and cpxR genes of a wild-type SE (16). The wild-type ST isolate JOL401 was used as a virulent challenge strain. All strains were grown overnight in Luria–Bertani (LB) broth (Becton, Dickinson and Company, Sparks, Maryland, USA) at 37°C, washed 3 times with phosphate-buffered saline (PBS), pH 7.4, and adjusted to appropriate concentrations for inoculation according to the optical density at 600 nm.
Vaccination and challenge
The experiment was conducted with approval (CBU 2012-0017) from the Animal Ethics Committee of Chonbuk National University, Jeonju, Republic of Korea, in accordance with the guidelines of the Korean Council on Animal Care. Female Brown Nick chickens were equally divided into a group to be vaccinated and a control group (n = 16 per group). In the vaccinated group the birds were orally vaccinated on day 1 of life and given an oral booster at 5 wk of age; they received 0.2 mL of a bacterial suspension containing 1 × 107 colony-forming units (CFU) of JOL919, the live attenuated SE. The control birds were orally inoculated on day 1 of life and at 5 wk of age with 0.2 mL of PBS. At 9 wk of age, all birds were challenged orally with 0.2 mL of a suspension containing 1 × 109 CFU of JOL401, the virulent ST strain. Throughout the experiment the chickens were given antibiotic-free food and water ad libitum.
Assay for plasma IgG and intestinal secretory IgA (sIgA)
Peripheral blood and intestinal lavage samples were collected from 5 birds in each group every week after the initial and booster vaccinations to examine immune responses. The plasma was separated by centrifugation. The lavage samples were collected as described previously (6,17). The concentrations of plasma IgG and intestinal sIgA specific for the outer membrane proteins (OMPs) of ST were determined by enzyme-linked immunosorbent assay (ELISA) with Chicken IgG and IgA ELISA Quantitation kits (Bethyl Laboratories, Montgomery, Texas, USA) according to the manufacturer’s protocols. The OMP fraction was prepared from the ST wild-type strain JOL401 as previously reported, with slight modification (18). Microlon ELISA plate wells (Greiner Bio-One GmbH, Frickenhausen, Germany) were coated with 100 μL of OMPs at a concentration of 0.2 mg/mL, and then the plasma and intestinal lavage samples were added at dilutions of 1:250 and 1:100, respectively. After 1 h, horseradish-peroxidase-conjugated goat IgG and IgA against chicken antigen were added at dilutions of 1:100 000 and 1:60 000, respectively. After another hour, absorbance of the developed samples was measured at 492 nm. The standard curve describing the relationship between the concentration of standards and their absorbance values was plotted, and the concentration of antibody for each sample was indicated in nanograms per milliliter.
Lymphocyte-proliferation assay
Soluble antigen was prepared from the ST wild-type strain JOL401 as previously described (19). After the 4 weeks of initial and booster vaccinations, peripheral lymphocytes were isolated from 5 chickens per group by the gentle-swirl technique (20). Their proliferation was then assayed as described previously (6) to determine cell viability. Adenosine triphosphate (ATP) bioluminescence was determined with the ViaLight Plus Kit (Lonza, Rockland, Maine, USA), according to the product information, the emitted light intensity being measured for 1 s by means of a luminometer (TriStarLB941; Berthold Technologies, Bad Wildbad, Germany) with an integrated program. As previously described, the blastogenic response against soluble antigen was expressed as the mean stimulation index (SI) (19).
Isolation of ST challenge strain from feces
The presence of the virulent challenge strain in feces was monitored by collecting samples from the same 5 birds in each group under aseptic condition 5, 9, and 13 d after challenge. The birds were placed individually into clean empty buckets and allowed to defecate. The feces were collected and weighed, diluted 1:10 by weight with buffered peptone water (BPW; Becton, Dickinson and Company), then homogenized by mechanical disruption with a sterile wooden stick. Next, 100 μL of homogenate was spread on brilliant green agar (BGA; Becton, Dickinson and Company) and incubated for 24 h at 37°C. Subsequently the bacterial cell number was counted and expressed as the mean ± standard deviation (SD) log10 CFU/g (21). Samples that yielded no colonies of Salmonella after direct plating were further processed for enrichment culture. The diluted fecal material was further incubated overnight at 37°C with continuous shaking, then cultured in Rappaport–Vassiliadis (RV) R10 broth (Becton, Dickinson and Company) at 42°C for 48 h. A loop of RV broth was then streaked onto BGA and the plate examined for Salmonella-type colonies after incubation at 37°C for 24 h. After enrichment, the positive samples were counted as 1 log10 CFU/g, whereas negative samples were indicated as 0 log10 CFU/g (21). The presence of the virulent challenge strain of ST was confirmed by polymerase chain reaction (PCR) with ST-specific forward primer 5′-TTGTTCACTTTTTACCCCTG-3′ and reverse primer 5′-CCCTGACAGCCGTTAGATAT-3′ (22).
Examination for gross lesions and bacterial recovery from infected organs
On days 6 and 14 after challenge, internal organs of 8 birds per group were studied to evaluate the efficacy of the vaccine. Individual gross lesions in the liver and spleen were given scores of 0, 1, 2, or 3. A score of 0 indicated no lesions, a score of 1 was assigned to necrotic foci, a score of 2 indicated enlarged and necrotic organs, and a score of 3 indicated more debilitated, necrotic, and distorted organs. A mean lesion score per group was determined. Samples of the liver, spleen, and ceca were minced and homogenized in 2 mL of BPW, then further processed for direct and enrichment culture as described above. The bacterial cell number was counted and expressed as the mean ± SD log10 CFU/g, as previously described (6,21). The positive samples were confirmed by PCR with ST-specific primers (22).
Statistical analysis
All data are expressed as mean ± SD unless otherwise specified. The analyses were done with SPSS 16.0 (SPSS Inc., Chicago, Illinois, USA). The Mann–Whitney U-test was used to evaluate the significance of differences in immune response between the vaccinated and control groups. In addition, the chi-square test was used to analyze the significance of differences in gross lesion score and bacterial recovery between the vaccinated and control groups.
Results
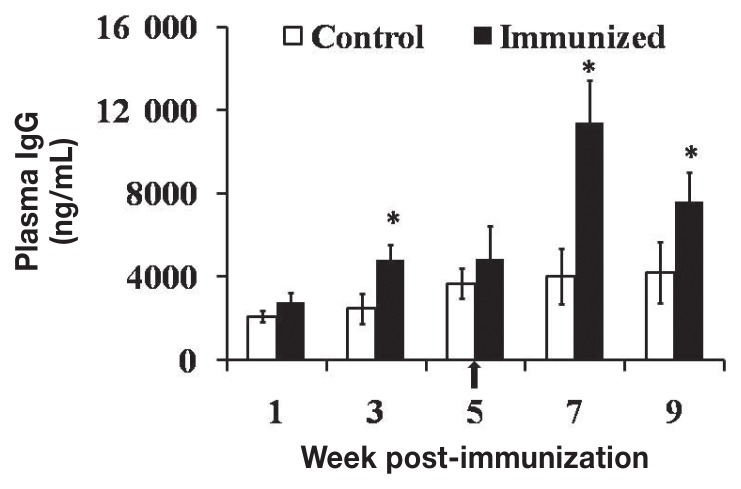
The plasma IgG titers were 1.9 and 2.8 times higher (P < 0.05) in the JOL919-inoculated chickens than in the unvaccinated control chickens at week 3 after primary vaccination and week 2 after booster vaccination, respectively (Figure 1). The intestinal sIgA titers were 1.6 and 2.3 times higher (P < 0.05), respectively, in the vaccinated chickens than in control chickens at these times (Figure 2). These findings suggest that specific plasma IgG and intestinal sIgA antibodies were produced at significant levels in the chickens vaccinated with JOL919.
Figure 1.
Mean levels [± standard deviation (SD)] of plasma IgG against antigens specific to Salmonella Typhimurium (ST) in chickens vaccinated on day 1 of life with the genetically engineered live attenuated Salmonella Enteritidis (SE) JOL919. The arrow indicates booster vaccination. The asterisks indicate significant differences (P < 0.05) between the values in the vaccinated group (black bars) and those in the control group (white bars).
Figure 2.
Mean levels (± SD) of secretory IgA (sIgA) against ST-specific antigens measured in intestinal lavage samples from chickens vaccinated with JOL919. The arrow, asterisks, and bars have the same meanings as in Figure 1.
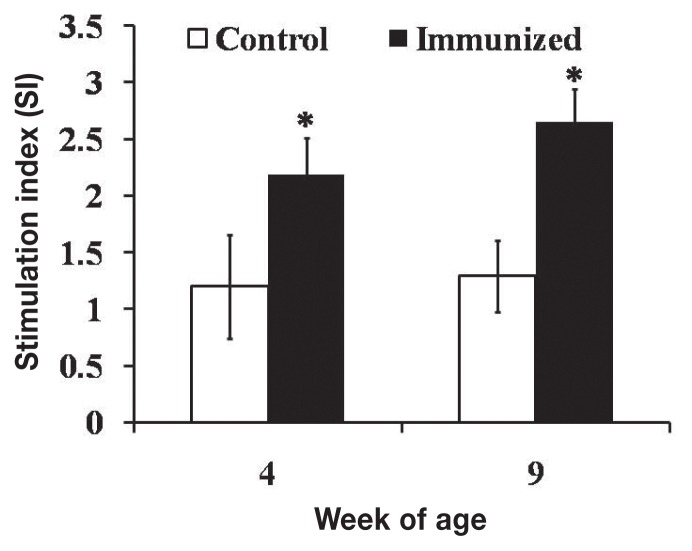
Stimulation of peripheral lymphocyte proliferation by ST antigens, measured to evaluate cellular immune responses, resulted in SI values 1.8 and 2.1 times higher (P < 0.05) in the JOL919-inoculated chickens than in the control chickens at week 4 after primary vaccination and week 4 after booster vaccination, respectively (Figure 3).
Figure 3.
Lymphocyte-stimulation responses against ST-specific antigens, expressed as the mean stimulation index (± SD). The asterisks and bars have the same meanings as in Figure 1.
As shown in Table I, the ST challenge strain was isolated from fecal samples collected on days 5, 9, and 13 after challenge. The bacterial counts and the numbers of positive samples were significantly lower for the vaccinated group than for the control group on days 9 and 13. However, there was no significant difference between the groups in bacterial count or number of positive samples on day 5 after challenge.
Table I.
Bacterial recovery from feces of the same 5 chickens per group 5, 9, and 13 d after challenge at 9 weeks of age with a virulent strain of Salmonella Typhimurium (ST), JOL401
| Bacterial recovery;a day after challenge | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Day 5 | Day 9 | Day 13 | ||||
|
|
|
|
||||
| Group | Number of positive samples | Count | Number of positive samples | Count | Number of positive samples | Count |
| Control | 4/5 | 4.1 ± 3.0 | 5/5 | 1.0 ± 0.0 | 4/5 | 0.8 ± 0.4 |
| Vaccinated | 3/5 | 2.8 ± 3.1 | 1/5b | 0.2 ± 0.4b | 0/5b | 0.0 ± 0.0b |
Observed after direct and enrichment culture and expressed as mean log10 number of colony-forming units (CFU) per gram [± standard deviation (SD)].
Significantly lower (P < 0.05) in the group vaccinated on day 1 of life with the genetically engineered live attenuated Salmonella Enteritidis (SE) JOL919 compared with the control group.
No deaths occurred in either group after ST challenge. As shown in Table II, the vaccinated group had consistently lower gross lesion scores for the liver and spleen 6 and 14 d after challenge compared with the control group. In addition, the vaccinated group had significantly lower bacterial counts in the liver, spleen, and ceca on those days compared with the control group. Furthermore, the number of birds carrying the ST challenge strain in the spleen and ceca was significantly lower in the vaccinated group than in the control group. Therefore, JOL919 vaccination significantly reduced both gross lesions and bacterial counts of ST in the internal organs after virulent ST challenge.
Table II.
Scores for gross lesions observed in 8 chickens per group 6 and 14 d after ST challenge
| Score,b mean ± SD | |||
|---|---|---|---|
|
|
|||
| Group | Number ST-infecteda | Liver | Spleen |
| Day 6 | |||
| Control | 8/8 | 0.9 ± 0.8 | 1.4 ± 0.5 |
| Vaccinated | 6/8 | 0.3 ± 0.4c | 0.6 ± 0.9c |
| Day 14 | |||
| Control | 7/8 | 0.3 ± 0.7 | 0.8 ± 0.8 |
| Vaccinated | 3/8c | 0.0 ± 0.0 | 0.1 ± 0.3c |
As determined by direct and enrichment culture.
0 — no lesions; 1 — necrotic foci; 2 — enlarged and necrotic organs; 3 — more debilitated, necrotic, and distorted organs.
Significantly lower (P < 0.1) than in the control group.
Discussion
Salmonella infection from the consumption of contaminated poultry meat and eggs still represents a major public health concern world wide. A vaccine capable of protecting chickens against a broad range of S. enterica serovars would be a valuable tool for producers of chicken meat and eggs (13). In our previous studies, we demonstrated that the live attenuated JOL919 SE vaccine protects against SE infection in chickens (6,15,16). In addition, JOL919 has not survived in internal organs beyond 3 wk after vaccination (6). In the present study, we evaluated the ability of JOL919 to confer cross-protection against ST infection.
Previously, live Salmonella vaccines have been reported to induce cross-immunity against related serovars (14,23,24). The success of vaccination against intracellular pathogens such as Salmonella is largely attributed to the induction of systemic and mucosal immune responses (25). In the present study, vaccination with an SE strain induced significantly higher levels of systemic IgG and mucosal sIgA antibodies specific for ST OMPs than the mock vaccination, and the levels were more markedly increased after booster vaccination. This is most likely due to immunologic memory, which is defined as a faster and stronger immune response after re-exposure to the same antigen (26). Systemic antibodies are essential to kill Salmonella bacteria, which escape from infected cells to reach distant tissue sites and form new foci of infection (27). Owing to the largely enteric existence of Salmonella, IgA is more likely to be effective in providing a protective immune response after secretion into the gut lumen (28–30). Gut mucosal sIgA prevents the bacterium from entering the intestinal epithelium (31).
Live vaccines confer better protection against intracellular pathogens than killed vaccines because of the ability of live vaccines to induce both humoral and cellular immune responses (32,33). The lymphocyte-proliferation response is commonly used as a measure of cell-mediated immunity. In this study, the lymphocyte-proliferation responses provided an important insight about the cross-reactivity of cell-mediated immune responses. The T-cell response confers protection by activating mononuclear cells to liberate intracellular Salmonella (34). Early-memory T-cells undergo vigorous secondary expansion in response to booster vaccination, which leads to increased numbers of effector and memory T-cells and enhanced protective immunity against intracellular bacterial infection (35). In this study, a lymphocyte-proliferation assay using ATP bioluminescence as a marker of cell viability detected significant responses at week 4 after primary and booster vaccination, suggesting that the JOL919 vaccine candidate can induce cellular immunity against ST-specific soluble antigens.
No deaths were observed in either the vaccinated or the control group after virulent ST challenge. The bacterial counts and the number of positive fecal samples in the vaccinated group were significantly lower than those in the control group at days 9 and 13 after challenge. In addition, the gross lesion scores of and bacterial recovery from the internal organs suggested that JOL919 protects against ST infection. The gross lesion scores were significantly lower in the vaccinated group than in the control group after ST challenge. In addition, the bacterial counts were significantly lower in the liver, spleen, and cecal tissues of the vaccinated group compared with the control group. There were also significantly fewer vaccinated than control birds carrying the challenge strain in the spleen and ceca. The lower number of ST-positive birds with fecal shedding and cecal colonization may be associated with enhanced intestinal mucosal immunity. In a previous study, a temperature-sensitive SE mutant induced significant cross-protection against virulent ST challenge (36). The data from the present study are consistent with these findings. Specifically, we believe that JOL919 can reduce the risk of ST infection in humans who consume ST-contaminated chicken.
Taken together, the data from the present study indicate that vaccination with JOL919 can induce acquired immunity by increasing the production of systemic IgG and mucosal sIgA and by increasing the proliferation of lymphocytes against ST antigens. In addition, subsequent booster vaccination may help to maintain a strong immune response that can provide cross-protection against ST infection in chickens. This may ultimately help reduce the incidence of human ST infections resulting from the consumption of contaminated chicken meat and eggs.
Table III.
Bacterial recovery from internal organs of the same 8 chickens per group 6 and 14 d after ST challenge
| Bacterial recoverya | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Liver | Spleen | Ceca | ||||
|
|
|
|
||||
| Group | Number of positive samples | Count | Number of positive samples | Count | Number of positive samples | Count |
| Day 6 | ||||||
| Control | 4/8 | 0.9 ± 1.0 | 7/8 | 2.5 ± 1.4 | 7/8 | 2.6 ± 1.8 |
| Vaccinated | 1/8 | 0.1 ± 0.3b | 6/8 | 1.5 ± 1.2b | 2/8c | 0.7 ± 1.4c |
| Day 14 | ||||||
| Control | 1/8 | 0.1 ± 0.3 | 7/8 | 2.0 ± 1.6 | 4/8 | 1.4 ± 1.6 |
| Vaccinated | 0/8 | 0.0 ± 0.0 | 3/8b | 0.4 ± 0.5c | 0/0c | 0.0 ± 0.0c |
Observed after direct and enrichment culture and expressed as mean log10 CFU/g ± SD.
Significantly lower at P < 0.1 than in the control group.
Significantly lower at P < 0.05 than in the control group.
Acknowledgments
This work was supported by grant 2013R1A4A1069486 from the National Research Foundation of Korea, funded by the Korean government, and by research funds of Chonbuk National University in 2013.
References
- 1.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 2.Majowicz SE, Musto J, Scallan E, et al. International Collaboration on Enteric Disease “Burden of Illness” Studies. The global burden of non-typhoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 3.Robert-Koch-Institut. Epidemiol Bull. 2006;13:99–107. [Google Scholar]
- 4.Braden CR. Salmonella enterica serotype Enteritidis and eggs: A national epidemic in the United States. Clin Infect Dis. 2006;43:512–517. doi: 10.1086/505973. [DOI] [PubMed] [Google Scholar]
- 5.Chappell L, Kaiser P, Barrow P, Jones MA, Johnston C, Wigley P. The immunobiology of avian systemic salmonellosis. Vet Immunol Immunopathol. 2009;128:53–59. doi: 10.1016/j.vetimm.2008.10.295. Epub 2008 Oct 17. [DOI] [PubMed] [Google Scholar]
- 6.Nandre RM, Chaudhari AA, Matsuda K, Lee JH. Immunogenicity of a Salmonella Enteritidis mutant as vaccine candidate and its protective efficacy against salmonellosis in chickens. Vet Immunol Immunopathol. 2011;144:299–311. doi: 10.1016/j.vetimm.2011.08.015. Epub 2011 Aug 26. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda K, Chaudhari AA, Lee JH. Safety and efficacy of a virulence gene-deleted live vaccine candidate for fowl typhoid in young chickens. Avian Pathol. 2011;40:309–314. doi: 10.1080/03079457.2011.566259. [DOI] [PubMed] [Google Scholar]
- 8.Hur J, Kim MY, Lee JH. Evaluation of efficacy of a new live Salmonella Typhimurium vaccine candidate in a murine model. Comp Immunol Microbiol Infect Dis. 2011;34:171–177. doi: 10.1016/j.cimid.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Nnalue NA. Mice vaccinated with a non-virulent, aromatic-dependent mutant of Salmonella Choleraesuis die from challenge with its virulent parent but survive challenge with Salmonella Typhimurium. J Med Microbiol. 1990;31:225–233. doi: 10.1099/00222615-31-4-225. [DOI] [PubMed] [Google Scholar]
- 10.Dueger EL, House JK, Heithoff DM, Mahan MJ. Salmonella DNA adenine methylase mutants elicit protective immune responses to homologous and heterologous serovars in chickens. Infect Immun. 2001;69:7950–7954. doi: 10.1128/IAI.69.12.7950-7954.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan JO, Curtiss R., III Development and evaluation of an experimental vaccination program using a live avirulent Salmonella Typhimurium strain to protect immunized chickens against challenge with homologous and heterologous Salmonella serotypes. Infect Immun. 1994;62:5519–5527. doi: 10.1128/iai.62.12.5519-5527.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Methner U, Berndt A, Steinbach G. Combination of competitive exclusion and immunization with an attenuated live Salmonella vaccine strain in chickens. Avian Dis. 2001;45:631–638. [PubMed] [Google Scholar]
- 13.Beal RK, Wigley P, Powers C, Barrow PA, Smith AL. Cross-reactive cellular and humoral immune responses to Salmonella enterica serovars Typhimurium and Enteritidis are associated with protection to heterologous re-challenge. Vet Immunol Immunopathol. 2006;114:84–93. doi: 10.1016/j.vetimm.2006.07.011. Epub 2006 Aug 28. [DOI] [PubMed] [Google Scholar]
- 14.Chacana PA, Terzolo HR. Protection conferred by a live Salmonella Enteritidis vaccine against fowl typhoid in laying hens. Avian Dis. 2006;50:280–283. doi: 10.1637/7463-102705R.1. [DOI] [PubMed] [Google Scholar]
- 15.Nandre R, Matsuda K, Lee JH. Efficacy for a new live attenuated Salmonella Enteritidis vaccine candidate to reduce internal egg contamination. Zoonoses Public Health. 2014;61:55–63. doi: 10.1111/zph.12042. Epub 2013 Feb 15. [DOI] [PubMed] [Google Scholar]
- 16.Nandre RM, Matsuda K, Chaudhari AA, Kim B, Lee JH. A genetically engineered derivative of Salmonella Enteritidis as a novel live vaccine candidate for salmonellosis in chickens. Res Vet Sci. 2012;93:596–603. doi: 10.1016/j.rvsc.2011.11.005. Epub 2011 Dec 20. [DOI] [PubMed] [Google Scholar]
- 17.Porter RE, Jr, Holt PS. Use of a pilocarpine-based lavage procedure to study secretory immunoglobulin concentration in the alimentary tract of White Leghorn chickens. Avian Dis. 1992;36:529–536. [PubMed] [Google Scholar]
- 18.Kang HY, Srinivasan J, Curtiss R., III Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar Typhimurium vaccine. Infect Immun. 2002;70:1739–1749. doi: 10.1128/IAI.70.4.1739-1749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rana N, Kulshreshtha RC. Cell-mediated and humoral immune responses to a virulent plasmid-cured mutant strain of Salmonella enterica serotype Gallinarum in broiler chickens. Vet Microbiol. 2006;115:156–162. doi: 10.1016/j.vetmic.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Gogal RM, Jr, Ahmed SA, Larsen CT. Analysis of avian lymphocyte proliferation by a new, simple, nonradioactive assay (lympho-pro) Avian Dis. 1997;41:714–725. [PubMed] [Google Scholar]
- 21.Betancor L, Schelotto F, Fernandez M, Pereira M, Rial A, Chabalgoity JA. An attenuated Salmonella Enteritidis strain derivative of the main genotype circulating in Uruguay is an effective vaccine for chickens. Vet Microbiol. 2005;107:81–89. doi: 10.1016/j.vetmic.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez J, Sota M, Vivanco AB, et al. Development of a multiplex PCR technique for detection and epidemiological typing of Salmonella in human clinical samples. J Clin Microbiol. 2004;42:1734–1738. doi: 10.1128/JCM.42.4.1734-1738.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matulova M, Havlickova H, Sisak F, Babak V, Rychlik I. SPI1 defective mutants of Salmonella enterica induce cross-protective immunity in chickens against challenge with serovars Typhimurium and Enteritidis. Vaccine. 2013;31:3156–3162. doi: 10.1016/j.vaccine.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Mohler VL, Heithoff DM, Mahan MJ, et al. Cross-protective immunity conferred by a DNA adenine methylase deficient Salmonella enterica serovar Typhimurium vaccine in calves challenged with Salmonella serovar Newport. Vaccine. 2008;26:1751–1758. doi: 10.1016/j.vaccine.2008.01.018. Epub 2008 Feb. 4. [DOI] [PubMed] [Google Scholar]
- 25.Titball RW. Vaccines against intracellular bacterial pathogens. Drug Discov Today. 2008;13:596–600. doi: 10.1016/j.drudis.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 27.Dougan G, John V, Palmer S, Mastroeni P. Immunity to salmonellosis. Immunol Rev. 2011;240:196–210. doi: 10.1111/j.1600-065X.2010.00999.x. [DOI] [PubMed] [Google Scholar]
- 28.Lebacq-Verheyden AM, Vaerman JP, Heremans JF. A possible homologue of mammalian IgA in chicken serum and secretions. Immunology. 1972;22:165–175. [PMC free article] [PubMed] [Google Scholar]
- 29.Leslie GA, Martin LN. Studies on the secretory immunologic system of fowl. Part 3. Serum and secretory IgA of the chicken. J Immunol. 1973;110:1–9. [PubMed] [Google Scholar]
- 30.Berthelot-Hérault F, Mompart F, Zygmunt MS, Dubray G, Duchet-Suchaux M. Antibody responses in the serum and gut of chicken lines differing in cecal carriage of Salmonella Enteritidis. Vet Immunol Immunopathol. 2003;96:43–52. doi: 10.1016/s0165-2427(03)00155-7. [DOI] [PubMed] [Google Scholar]
- 31.Lena S, Malin F, Ingvar S. Mucosal immunization with purified flagellin from Salmonella induces systemic and mucosal immune responses in C3H/HeJ mice. Vaccine. 2004;22:3797–3808. doi: 10.1016/j.vaccine.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 32.Chong C, Bost KL, Clements JD. Differential production of interleukin-12 mRNA by murine macrophages in response to viable or killed Salmonella spp. Infect Immun. 1996;64:1154–1160. doi: 10.1128/iai.64.4.1154-1160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mastroeni P, Chabalgoity JA, Dunstan SJ, Maskell DJ, Dougan G. Salmonella: Immune responses and vaccines. Vet J. 2001;161:132–164. doi: 10.1053/tvjl.2000.0502. [DOI] [PubMed] [Google Scholar]
- 34.Gautreaux MD, Deitch EA, Berg RD. T lymphocytes in host defense against bacterial translocation from the gastrointestinal tract. Infect Immun. 1994;62:2874–2884. doi: 10.1128/iai.62.7.2874-2884.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. Epub 2005 Jun 12. [DOI] [PubMed] [Google Scholar]
- 36.Gherardi MM, Gómez MI, García VE, Sordelli DO, Cerquetti MC. Salmonella Enteritidis temperature-sensitive mutants protect mice against challenge with virulent Salmonella strains of different serotypes. FEMS Immunol Med Microbiol. 2000;29:81–88. doi: 10.1111/j.1574-695X.2000.tb01508.x. [DOI] [PubMed] [Google Scholar]