Abstract
Epidermal tight junctions (TJ) have been well-described in human medicine and are involved in many skin diseases such as atopic dermatitis (AD). In dogs, there are no data regarding the implication of TJ in skin diseases including canine AD.
The aim of this study was to compare the expression and the distribution of ZO-1, occludin and claudin-1 in the epidermis of healthy and atopic dogs.
Skin biopsies from 6 high IgE-producing beagles sensitized to house dust mite (atopic group) were used. Skin specimens from nine healthy dogs without skin issues were sampled (healthy group).
Immunoperoxydase staining was used to study the staining pattern of zonula occludens-1 (ZO-1), occludin and claudin-1 in the epidermis of healthy and atopic dogs. Positive controls were healthy human skin samples.
Labeling patterns were assessed by 2 examiners blinded to the identities of the specimens. Comparisons between groups were performed using an exact Wilcoxon-Mann-Whitney test.
The mean total expression score of claudin-1 was lower in atopic dogs as compared to healthy subjects. Occludin and ZO-1 expression remained unchanged within each group.
These results suggest a defect in claudin-1 expression in the nonlesional epidermis of atopic dogs.
Résumé
Les jonctions serrées (JS) épidermiques sont bien décrites en médecine humaine et sont impliquées dans de nombreuses affections cutanées telles que la dermatite atopique (DA). Dans l’espèce canine, il n’existe aucune donnée concernant l’implication des JS dans la DA canine ou dans d’autres affections dermatologiques.
Le but de cette étude est de comparer l’expression et la distribution de ZO-1, de l’occludine et de la Claudine-1 dans l’épiderme de chiens atopiques et de chiens sains.
Les biopsies cutanées de six chiens sensibilisés dans leur jeune âge aux acariens de poussière et produisant de forts taux d’IgE (groupe atopique) on été utilisées. Des échantillons de peau exempte de lésions cutanées ont été prélevés avant tout challenge allergique. Des échantillons de peau saine provenant de neuf chiens sans problème dermatologique ont été recueillis (groupe sain).
Deux examinateurs ont évalué l’immunomarquage, en aveugle. Des comparaisons entre les différents groupes ont été réalisées à l’aide du test statistique de Wilcoxon-Mann-Whitney.
L’expression de la claudine-1 était plus faible dans l’épiderme de chiens atopiques par comparaison aux sujets sains. L’expression de ZO-1 et de l’occludine était identique dans chaque groupe.
Ces résultats suggèrent un défaut d’expression de la Claudine-1 dans l’épiderme non lésionnel des chiens atopiques.
(Traduit par les auteurs)
Introduction
The cutaneous barrier is composed of the stratum corneum layer, intercellular lipids, an immunological barrier, and tight junctions (TJ) (1,2). Tight junctions are intercellular junctions localized at the most apical part of the lateral cell membranes in a variety of polarized epithelia.
Tight junctions have been studied in simple epithelia for many years (3). However, the first description of TJ proteins in the epidermis of mice was in 1998 (4) and in humans in 2001 (5). Moreover, in suprabalasal layers of several stratified epithelia, TJ proteins have been observed in different junctional structures, including some that differ from typical TJ (6).
Tight junctions have a complex structure and are composed of transmembrane proteins [occludin (7), claudins (8), junctional adhesion molecules (JAM) (9), tricellulin (10), marvelD3 (11), scaffolding proteins (zonula occludens proteins [ZO-1, ZO-2, ZO-3]) (12), cingulin (13), and signaling and regulating proteins (14)]. With the discovery of this structural complexity, the understanding of their roles has evolved from a paracellular barrier to a complex structure involved in signaling cascades that control cell growth and differentiation (14). Tight junctions allow the selective passage of water, ions, and solutes between cells and play an important role in the cellular polarity. Moreover, they are involved in the control of paracellular migration of inflammatory cells through epithelia (15).
Over the last 15 y, more than 50 human diseases related to TJ have been discovered. Intestinal TJ defects have been implicated in the pathogenesis of several intestinal pathologies, such as intestinal inflammatory bowel diseases (IBD, Crohn’s disease, and ulcerative colitis) (16) and celiac disease (17). Tight junction disruption leads to an inadequate epithelial barrier to water and electrolyte loss, and to inflammation at the intestinal surface. A leaky intestinal barrier has also been implicated in extraintestinal diseases, such as food allergy (18) and type I diabetes (19). Additionally, permeability of TJ in respiratory epithelia is an important factor in several pulmonary diseases (20).
The importance of TJ in the cutaneous barrier has been illustrated in a model of claudin-1 deficient mice. These mice died within 1 d of birth, with wrinkled skin and increased transepidermal water loss (TEWL) (21). The investigations into skin conditions associated with TJ defects are in the early stages. Tight junctions have been implicated in human medicine in the pathogenesis of psoriasis (22,23), lichen planus (5), neonatal ichthyosis and cholangitis syndrome (24,25), and atopic dermatitis (AD) (26). It has been demonstrated that claudin-1 expression was markedly decreased in nonlesional skin from human patients suffering from AD, supporting a defect in TJ expression in human patients with AD (26).
In veterinary dermatology, one study evaluated the expression of claudin-5 in hyperplastic and neoplastic lesions of canine hepatoid glands (27). Nevertheless, the authors are not aware of data regarding the implication of TJ in canine AD (CAD). In a preliminary study (7), we defined the best immunoenzymatic technique to label TJ proteins in the epidermis of dogs and determined the distribution of TJ proteins in the epidermis of healthy dogs without any skin issues. The aim of this study was to compare the expression and the distribution of ZO-1, occludin, and claudin-1 in the epidermis of healthy and atopic dogs. We hypothesized that alterations of TJ could be involved in the pathogenesis of CAD, similar to that in humans and mice.
Materials and methods
Samples of canine and human epidermis
Three groups of skin samples were compared: normal skin from clinically normal dogs, skin from sensitized dogs, and human skin as a positive control. Skin samples from 6 high-IgE producing beagles (atopic group) were used. These research dogs had been epicutaneously sensitized to Dermatophagoides farinae at a young age and known to develop AD upon environmental exposure to D. farinae. Before sampling, dogs had not been challenged and nonlesional skin was collected.
Normal canine skin samples were obtained from 5 clinically normal dogs (2 beagles, 1 Labrador retriever, 1 mixed breed dog, and 1 Lakeland terrier) without any history of skin disease undergoing an elective surgical. Punch biopsy (8 mm) samples of the inguinal skin were collected. All samples were obtained with the consent of the owners and all procedures were reviewed and approved by the animal care and use committee of ONIRIS (Nantes-Atlantic National College of Veterinary Medicine, Food Science and Engineering, Nantes, France).
Positive controls were healthy human skin samples for ZO-1, occludin, and claudin-1 (28). The human skin samples were kindly provided by the Immunology and Dermatology Laboratory of Nantes University Hospital. Normal human skin specimens were obtained from surgical samples of healthy skin donors undergoing abdominoplasty, gynecomastia surgery, or thigh lifts from the Plastic Surgery Departments of Nantes University Hospital and Nantes Jules Verne Private Hospital (France). All patients provided their written informed consent. The study was conducted according to the principles of the Declaration of Helsinki, and the Medical Ethical Committee of Nantes University Hospital approved all procedures concerning human samples.
Selection of the antibodies
Primary antibodies were selected based on available knowledge of involvement of the corresponding TJ protein in a human or animal dermatological condition (21,23,26). The antibodies target human proteins, but have been shown to cross-react with canine TJ proteins (29,30). Moreover, sequence homologies determined with the basic local alignment search tool (BLAST) are high between canine and human proteins and vary between 93% (claudin-1, ZO-1) and 91% (occludin).
Immunohistochemistry
The choice of the dilution used for each antibody was described in a previous work (7). The primary antibodies selected are indicated in Table I. Sections (3 μm) were cut onto slides (Superfrost PlusTM slides; VWR, Fontenay-sous-Bois, France). Sections were dewaxed in 2 changes of xylene and then rehydrated in 2 changes of absolute ethanol for 10 min each and finally rinsed in distilled water.
Table I.
Mean labelling score for ZO-1, occludin, and claudin-1
| Mean score/dog (SD) | Stratum basale | Stratum spinosum | Stratum granulosum | Stratum corneum | Total |
|---|---|---|---|---|---|
| ZO-1 | |||||
| Atopic | 2.5 (0.5) | 3.8 (0.5) | 6.7 (0.5) | 0.0 (0) | 13.0 |
| Clinically normal | 3.0 (0.6) | 3.8 (0.4) | 6.0 (0.4) | 0.0 (0) | 12.8 |
| Occludin | |||||
| Atopic | 1.7 (0.1) | 0.7 (0.4) | 4.2 (0.4) | 0.0 (0) | 6.5 |
| Clinically normal | 1.3 (0.3) | 0.5 (0.1) | 3.8 (0.3) | 0.0 (0) | 5.5 |
| Claudin-1 | |||||
| Atopic | 0.5 (0.1) | 2.0 (0.5) | 3.3 (0.5) | 0.0 (0) | 5.8* |
| Clinically normal | 1.8 (0.5) | 3.2 (0.5) | 5.0 (0.5) | 0.0 (0) | 10.0 |
Statistically significant when P < 0.05.
A microwavable vessel containing Tris-EDTA buffer pH 9.0 (Diagnostic Biosystems, Pleasanton, California, USA) was preheated in an 850 watt domestic microwave oven until the temperature of the buffer reached 95°C to 100°C. Slides were then immersed in the buffer and the vessel was returned to the microwave for a further 20 min period at low power (250 watts). The temperature of the buffer solution was maintained between 95°C to 100°C. The vessel was then placed into cool tap water and allowed to cool for 20 min. Slides were finally rinsed in distilled water for 10 min.
Following antigen retrieval, sections were saturated in Tris buffered saline (TBS) (Sigma-Aldrich, Lyon, France) containing tween and 0.1% bovine serum albumin (BSA, Sigma) for 10 min. The slides were then incubated for 10 min with H2O2 solution (Dako, Trappes, France) in order to block endogenous peroxidase activity and rinsed in TBS with 0.1% BSA. Primary antibodies (Table I) were diluted in phosphate buffered saline (PBS, pH 7.4; Sigma) containing 1.5% normal goat serum (Dako). Sections were incubated with primary antibodies for 1 h at room temperature and then rinsed in TBS with 0.1% BSA. Rabbit immunoglobulin fraction (Dako) from the serum of healthy non-immunized rabbits was used instead of the primary antibody as a negative control for rabbit polyclonal antibodies. Monoclonal mouse antibody (Dako) clone DAK-GO1 was used as a negative control for murine monoclonal antibodies.
Visualization of labelling was done using a horseradish peroxidase (HRP)–streptavidin–biotin procedure. Sections were incubated with biotinylated secondary antibody (Dako REALTM Detection System; Dako) for 30 min at room temperature and rinsed in TBS with 0.1% BSA for 10 min. To detect the complex formed by the primary and secondary antibodies, slides were incubated with streptavidin– (Dako REALTM Detection System; Dako) for 30 min and rinsed in TBS with 0.1% BSA. Sections were incubated with the chromogenic substrate (3-amino-9-ethylcarbazole; AEC; Dako REALTM Detection System; Dako). The reaction was stopped with distilled water as soon as red colouration appeared or after 5 min. Slides were rinsed for 10 min in distilled water. Mayer’s hemalum (Dako) was used for counterstaining. Slides were mounted with a permanent aqueous medium (CC/Mount, Diagnostic Biosystems).
Immunostaining evaluation
A total of 47 slides were examined: 1 slide for each antibody and for each dog (33 slides), 1 positive control for each antibody (3 slides), and 1 negative control for each dog and for each concentration of antibody (11 slides). After a careful examination of the slides, the most representative fields were chosen and a digital image of each slide was taken at low (40×) and high (1000×) magnification with a microscope (Olympus CX31; Olympus, Rungis, France) equipped with a digital camera (5.6 megapixel; Olympus). For each slide, 2 ECVD diplomates (P.J.B. and V.B.) blinded to the identity of the specimen assessed the intensity and the distribution of labeling. The intensity of the labeling was scored with a 6-point grading scale (from 0 = absence to 5 = very intense red-brown labeling). Localization of the labelling was evaluated for each epidermal layer.
Statistical analysis
Computer software (XLSTAT 2011®; Addinsoft, Paris, France) was used for the statistical analysis. Comparisons between total score of atopic and clinically normal dogs were done using an exact Wilcoxon-Mann-Whitney test (P < 0.05).
Results
Positive and negative controls
Staining for ZO-1, occludin, and claudin-1 in human epidermis yielded an intense positive staining. Negative controls did not show any immunolabelling.
ZO-1
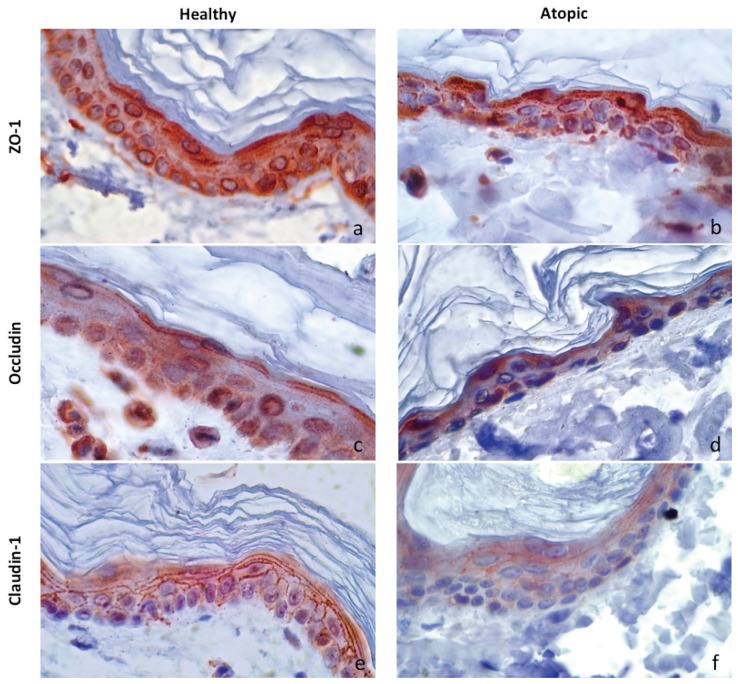
In clinically normal canine skin specimens, ZO-1 was expressed from the stratum basale to the stratum granulosum, with more intense staining in the stratum granulosum. No difference was observed between atopic dogs and clinically normal dogs regarding ZO-1, mean global expression (P = 0.956; Table I, Figures 1A, 1B).
Figure 1.
Immunolabelling for ZO-1, occludin, and claudin-1 in skin sections of atopic and clinically normal dogs (magnification 1000×). a,c,e — clinically normal group; b,d,f — atopic group; a,b — ZO-1 immunolabelling; c,d — occludin immunolabelling; e,f — claudin-1 immunolabelling.
Occludin
The expression of occludin in clinically normal skin samples was localized in the entire epidermis except in the stratum corneum (SC). Staining for occludin revealed no statistical difference between atopic dogs and clinically normal dogs (P = 0.666; Table I, Figures 1C, 1D).
Claudin-1
In clinically normal dogs, claudin-1 was expressed in all layers of the epidermis except in the SC. Staining for this protein yielded a honeycomb-staining pattern. The mean global expression of claudin- 1 in the atopic dogs was decreased compared to clinically normal control dogs (P = 0.05; Table I, Figures 1E, 1F).
Discussion
This study suggests a change in the expression of claudin-1 in the epidermis of atopic dogs. The decrease in claudin-1 expression observed in these atopic dogs is consistent with observations made in human patients with AD (26). Research on TJ and pathologies associated with the skin are more recent compared to research on the intestine. Tight junction alterations have been described as an early event in psoriasis (23). In the early stages of psoriasis, ZO-1, occludin, and claudin-4 exhibit a broader localization and claudin-1 and -7 are down-regulated (23). In plaque-type psoriasis, the staining patterns of occludin and ZO-1 do not change, whereas the claudins are further down-regulated (23). Interleukin-1β and tumor necrosis factor-α, 2 major cytokines involved in the pathogenesis of psoriasis, influence the expression and the functionality of TJ (23).
A syndrome called neonatal ichthyosis-sclerosing cholangitis is an autosomal recessive disorder caused by homozygous mutations in claudin-1 gene (31). This disease shares clinical features with AD such as dry and ichthyotic skin (31). Additionally, abnormalities in the bile duct system are found such as sclerosing cholangitis or a paucity of bile ducts (25). The deficiency in claudin-1 in cholangiocytes induces paracellular bile leakage and severe liver and gallbladder injuries (25).
Similar to the pathogenesis of food allergy and asthma, it has been hypothesized that a defect in TJ could be involved in AD. The importance of claudin-1 in the pathogenesis of AD in humans has been demonstrated (26). The expression of claudin-1 is significantly reduced at the mRNA and protein levels in the non-lesional epidermis of human patients with AD compared with non-atopic subjects (26). Additionally, the reduction in claudin-1 expression is associated in vitro with the increase of paracellular permeability and keratinocyte proliferation, as observed in AD (26). The results of this study are consistent with observations made in human medicine. In 2001, Furuse et al (21) produced genetically mutant claudin-1 knockout mice born with a wrinkled skin. The epidermal barrier of these mice is severely altered and they die within 1 d (21). These mice express no other abnormalities in epidermal lipids or proteins (21). Therefore, claudin-1 appeared to play a major role for a functional epidermal barrier in humans as well as in mice.
No statistically significant difference was observed regarding occludin expression and distribution between clinically normal and atopic dogs. Some studies have shown that occludin is not essential in TJ strands formation (32). However, its role in barrier function remains unclear. For example, filaggrin and loricrin deficiencies in flaky tail mice decrease occludin expression (33). Interestingly, the atopic dogs studied here had decreased filaggrin expression (34). Additional data regarding occludin expression are needed in atopic dogs with and without abnormalities in filaggrin expression.
The results obtained herein are in favor of a primary defect of TJ protein in the pathogenesis of CAD. Indeed, in this study, skin samples came from atopic dogs that had not been challenged and were not in an atopic flare. Nevertheless, even in clinical non-lesional skin of atopic dogs there could be a low grade inflammatory process that could affect TJ proteins. Moreover, TJ proteins can also be altered secondarily by other factors, such as pollen proteases (35), cysteine protease of Der p 1, and serine protease from mite fecal pellets (36), thus facilitating the transepidermal migration of allergens. Inflammatory cytokines can disrupt TJ proteins in airways (37) and in intestinal cells (38). Moreover, the addition of histamine in human keratinocyte culture down-regulates the expression of TJ proteins ZO-1, occludin, claudin-1, and claudin-4, as well as that of desmosomal junction proteins corneodesmosin and desmoglein-1 (39). Therefore, a primary defect in TJ could be further amplified by inflammation and environmental proteases.
Additional studies are needed at the mRNA and protein levels to further characterize TJ proteins and their potential implication in CAD. Western blot analysis could be used to determine the expression levels of TJ proteins per the same unit quantity of the whole proteins in the epidermal extracts and real-time RT-PCR could be performed to determine and compare the transcription level of the corresponding genes. Further research could also determine whether TJ could provide promising therapeutic options in CAD (40).
This study is the first report on the modified expression and distribution of TJ proteins in the epidermis of atopic dogs. Claudin-1 global expression was lower in atopic dogs. Occludin and ZO-1 expression remained unchanged between atopic and clinically normal dogs. These results suggest that a defect in TJ expression could be involved in the pathogenesis of CAD. Further studies are needed in order to specify their implication in CAD.
Acknowledgment
The authors thank the Immunology Dermatology Laboratory of Nantes University Hospital for hosting them and for technical support during preliminary studies.
References
- 1.Madison KC. Barrier function of the skin: “La raison d’être” of the epidermis. J Invest Dermatol. 2003;121:231–241. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- 2.Elias PM, Steinhoff M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis”. J Invest Dermatol. 2008;128:1067–1070. doi: 10.1038/jid.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morita K, Itoh M, Saitou M, et al. Subcellular distribution of tight junction-associated proteins (occludin, ZO-1, ZO-2) in rodent skin. J Invest Dermatol. 1998;110:862–866. doi: 10.1046/j.1523-1747.1998.00209.x. [DOI] [PubMed] [Google Scholar]
- 5.Pummi K, Malminen M, Aho H, Karvonen SL, Peltonen J, Peltonen S. Epidermal tight junctions: ZO-1 and occludin are expressed in mature, developing, and affected skin and in vitro differentiating keratinocytes. J Invest Dermatol. 2001;117:1050–1058. doi: 10.1046/j.0022-202x.2001.01493.x. [DOI] [PubMed] [Google Scholar]
- 6.Langbein L, Grund C, Kuhn C, et al. Tight junctions and compositionally related junctional structures in mammalian stratified epithelia and cell cultures derived therefrom. Eur J Cell Biol. 2002;81:419–435. doi: 10.1078/0171-9335-00270. [DOI] [PubMed] [Google Scholar]
- 7.Roussel AJ, Knol AC, Bourdeau PJ, Bruet V. Optimization of an immunohistochemical method to assess distribution of tight junction proteins in canine epidermis and adnexae. J Comp Pathol. 2014;150:35–46. doi: 10.1016/j.jcpa.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): More molecules with dual functions? J Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 10.Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steed E, Rodrigues NT, Balda MS, Matter K. Identification of MarvelD3 as a tight junction-associated transmembrane protein of the occludin family. BMC Cell Biol. 2009;10:95. doi: 10.1186/1471-2121-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordenonsi M, D’Atri F, Hammar E, et al. Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin. J Cell Biol. 1999;147:1569–1582. doi: 10.1083/jcb.147.7.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol. 2003;160:423–432. doi: 10.1083/jcb.200210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmerli SC, Hauser C. Langerhans cells and lymph node dendritic cells express the tight junction component claudin-1. J Invest Dermatol. 2007;127:2381–2390. doi: 10.1038/sj.jid.5700882. [DOI] [PubMed] [Google Scholar]
- 16.Gassler N, Rohr C, Schneider A, et al. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216–228. doi: 10.1152/ajpgi.2001.281.1.G216. [DOI] [PubMed] [Google Scholar]
- 17.Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:71–78. doi: 10.1007/s12016-011-8291-x. [DOI] [PubMed] [Google Scholar]
- 18.Perrier C, Corthesy B. Gut permeability and food allergies. Clin Exp Allergy. 2011;41:20–28. doi: 10.1111/j.1365-2222.2010.03639.x. [DOI] [PubMed] [Google Scholar]
- 19.Vaarala O. Leaking gut in type 1 diabetes. Curr Opin Gastroenterol. 2008;24:701–706. doi: 10.1097/MOG.0b013e32830e6d98. [DOI] [PubMed] [Google Scholar]
- 20.Soini Y. Claudins in lung diseases. Respir Res. 2011;12:70. doi: 10.1186/1465-9921-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuse M, Hata M, Furuse K, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peltonen S, Riehokainen J, Pummi K, Peltonen J. Tight junction components occludin, ZO-1, and claudin-1, -4 and -5 in active and healing psoriasis. Br J Dermatol. 2007;156:466–472. doi: 10.1111/j.1365-2133.2006.07642.x. [DOI] [PubMed] [Google Scholar]
- 23.Kirschner N, Poetzl C, von den Driesch P, et al. Alteration of tight junction proteins is an early event in psoriasis: Putative involvement of proinflammatory cytokines. Am J Pathol. 2009;175:1095–1106. doi: 10.2353/ajpath.2009.080973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldmeyer L, Huber M, Fellmann F, Beckmann JS, Frenk E, Hohl D. Confirmation of the origin of NISCH syndrome. Hum Mutat. 2006;27:408–410. doi: 10.1002/humu.20333. [DOI] [PubMed] [Google Scholar]
- 25.Grosse B, Cassio D, Yousef N, Bernardo C, Jacquemin E, Gonzales E. Claudin-1 involved in neonatal ichthyosis sclerosing cholangitis syndrome regulates hepatic paracellular permeability. Hepatology. 2012;55:1249–1259. doi: 10.1002/hep.24761. [DOI] [PubMed] [Google Scholar]
- 26.De Benedetto A, Rafaels NM, McGirt LY, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127:773–786. e771–777. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jakab C, Rusvai M, Galfi P, et al. Expression of claudin-5 in hepatoid gland biopsies. Vet Dermatol. 2010;21:276–281. doi: 10.1111/j.1365-3164.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 28.Kirschner N, Houdek P, Fromm M, Moll I, Brandner JM. Tight junctions form a barrier in human epidermis. Eur J Cell Biol. 2010;89:839–842. doi: 10.1016/j.ejcb.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Jakab C, Halasz J, Szasz AM, et al. Expression of claudin-1, -2, -3, -4, -5 and -7 proteins in benign and malignant canine mammary gland epithelial tumours. J Comp Pathol. 2008;139:238–245. doi: 10.1016/j.jcpa.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Bizikova P, Linder KE, Olivry T. Immunomapping of desmosomal and nondesmosomal adhesion molecules in healthy canine footpad, haired skin and buccal mucosal epithelia: Comparison with canine pemphigus foliaceus serum immunoglobulin G staining patterns. Vet Dermatol. 2011;22:132–142. doi: 10.1111/j.1365-3164.2010.00924.x. [DOI] [PubMed] [Google Scholar]
- 31.Hadj-Rabia S, Baala L, Vabres P, et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: A tight junction disease. Gastroenterol. 2004;127:1386–1390. doi: 10.1053/j.gastro.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 32.Saitou M, Fujimoto K, Doi Y, et al. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakai K, Yoneda K, Hosokawa Y, et al. Reduced expression of epidermal growth factor receptor, E-cadherin, and occludin in the skin of flaky tail mice is due to filaggrin and loricrin deficiencies. Am J Pathol. 2012;181:969–977. doi: 10.1016/j.ajpath.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Marsella R, Samuelson D, Harrington L. Immunohistochemical evaluation of filaggrin polyclonal antibody in atopic and normal beagles. Vet Dermatol. 2009;20:547–554. doi: 10.1111/j.1365-3164.2009.00844.x. [DOI] [PubMed] [Google Scholar]
- 35.Runswick S, Mitchell T, Davies P, Robinson C, Garrod DR. Pollen proteolytic enzymes degrade tight junctions. Respirology. 2007;12:834–842. doi: 10.1111/j.1440-1843.2007.01175.x. [DOI] [PubMed] [Google Scholar]
- 36.Wan H, Winton HL, Soeller C, et al. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clin Exp Allergy. 2001;31:279–294. doi: 10.1046/j.1365-2222.2001.00970.x. [DOI] [PubMed] [Google Scholar]
- 37.Petecchia L, Sabatini F, Usai C, Caci E, Varesio L, Rossi GA. Cytokines induce tight junction disassembly in airway cells via an EGFR-dependent MAPK/ERK1/2-pathway. Lab Invest. 2012;92:1140–1148. doi: 10.1038/labinvest.2012.67. [DOI] [PubMed] [Google Scholar]
- 38.He F, Peng J, Deng XL, et al. Mechanisms of tumor necrosis factor- alpha-induced leaks in intestine epithelial barrier. Cytokine. 2012;59:264–272. doi: 10.1016/j.cyto.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Gschwandtner M, Mildner M, Mlitz V, et al. Histamine suppresses epidermal keratinocyte differentiation and impairs skin barrier function in a human skin model. Allergy. 2013;68:37–47. doi: 10.1111/all.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ideta R, Sakuta T, Nakano Y, Uchiyama T. Orally administered glucosylceramide improves the skin barrier function by upregulating genes associated with the tight junction and cornified envelope formation. Biosci Biotechnol Biochem. 2011;75:1516–1523. doi: 10.1271/bbb.110215. [DOI] [PubMed] [Google Scholar]