Abstract
Background:
Nicotine as one of the potent psychostimulant drugs is characterized by its parasympathomimetic activity. Upon the abrupt discontinuation of nicotine intake, a number of symptoms such as anxiety, depression and cognition impairment develop. Kefir as a food supplement is rich in tryptophan. In this study, we have evaluated the effects of Kefir on nicotine cessation-induced anxiety, depression and cognition impairment.
Materials and Methods:
Forty adult male rats were divided into four groups. All the groups received 6 mg/kg/day of nicotine for 17 days and then the negative control groups got 5 mg/kg/day of normal saline. The positive control groups were given 40 mg/kg/day of Sertraline HCl for 7 days. The group treated with Cow Milk Kefir (CMK) and Soy Milk Kefir (SMK) received 5 mg/kg/day for 7 days. On the 25th day, Elevated Plus Maze (EPM), Open Field Test (OFT) and Forced Swim Test (FST) were used to investigate anxiety and depression. In addition, Moris Water Maze was applied to evaluate learning and memory in the animals between the 20th and 25th days.
Results:
The results showed that administration of CMK, SMK and Sertraline had higher anti-depression and anxiolytic effects on nicotine withdrawal-induced depression and anxiety in rats (P < 0.05). Moreover, CMK and SMK improved learning and memory impairment results in the nicotine withdrawal period (P < 0.05).
Conclusion:
This study revealed that Kefir had a potential effect on the treatment of nicotine cessation-induced depression, anxiety and cognition impairment in the animal model. Kefir may be useful for adjunct therapy for nicotine abandonment treatment protocols.
Keywords: Anxiety, cognition impairment, kefir, nicotine cessation-induced depression
INTRODUCTION
Nicotine as one of the potent psychostimulant drugs is characterized by its parasympathomimetic activity. Nicotine constant usage in the form of cigarettes leads to the problem of physical dependency identified as withdrawal syndrome.[1] Upon the abrupt discontinuation or decrease in nicotine intake, a number of symptoms such as anxiety, depression and cognition impairment develop.[2,3] Previous studies have demonstrated that nicotine activates the mesolimbic pathway (“reward system”) and arouses the feelings of pleasure and euphoria.[3,4] Other studies have also indicated that nicotine as a highly addictive chemical stimulates serotonin and dopamine reward circuits in the brain. Most research shows that nicotine withdrawal causes downregulation of the dopamine and serotonin levels.[5,6] Previous literature demonstrated that nicotine withdrawal after long-term administration and adaptations can result in increased anxiety and depression.[7,8] On the other hand, the anxiety and depression in nicotine cessation syndrome have been the main concerns in nicotine dependency treatment protocols.[9] In order to tackle this concern, some type of anxiolytic and antidepressant drugs such as selective serotonin reuptake inhibitors (SSRIs) was used alongside the nicotine detoxification program.[10] Recent studies have confirmed that downregulation of dopamine and serotonin in the hippocampus and mezolimbic regions is the major cause of this type of anxiety and depression.[11] Moreover, many previous studies showed that nicotine withdrawal is associated with a deficit in neurocognitive function, including sustained attention and working memory. Nicotine dependence treatment can be facilitated by a better understanding of the mechanisms underlying withdrawal-related cognitive deficits.[12] Kefir as a food supplement is a fermented dairy drink that has anti-depressant and anxiolytic effects.[13] Previous studies showed that tryptophan (serotonin precursor), as an essential amino acid abundant in kefir, is an initial substrate for the synthesis of 5-HT.[13,14] Recent reports have disclosed that serotonin (5-hydroxytryptamine) (5-HT) plays a crucial role in many aspects of the rewarding system and creating drug cessation-induced depression and anxiety. The present study strives to determine the effects of various kinds of kefir as the donor of serotonin in the treatment and management of nicotine cessation depression, anxiety and cognition in the animal model of nicotine dependency.
MATERIALS AND METHODS
Nicotine hydrogen tartrate salt was purchased from Sigma-Aldrich (St Louis, MO, USA) and sertraline HCl was purchased from Razak Pharmaceutical Company (Tehran, Iran).
In summer 2013, 48 adult male Wistar rats (180-210 g) were purchased from the Iran Razi Institute (Tehran, Iran). All the rats were maintained at standard conditions (22°C ± 2 and 12/12 h dark and light cycles), with free access to food and water. The protocol was approved as an undergraduate research and was projected by the Research Council of the University of Tehran, Faculty of Veterinary Medicine.
Kefir grain is added to UHT-treated cow or soy milk in a plastic container and left at 25°C for 24 h. Then, the beverage is kept in the refrigerator at 4°C in order to stop fermentation and keep the pH at 4.8. Both the cow and soy milk kefir were prepared by the researchers in the Laboratory of Department of Food Hygiene, Faculty of Veterinary Medicine, University of Tehran.
Group I as the negative control group received 6 mg/kg/day of nicotine for 17 days and then 5 mg/kg/day of normal saline for 7 days.
Group II as the positive control group received 6 mg/kg/day of nicotine for 17 days and then 40 mg/kg/day sertraline HCl dissolved in distilled water for 7 days.
Group III received 6 mg/kg/day of nicotine for 17 days and then 5 mg/kg/day of Cow Milk Kefir (CMK) for 7 days.
Group IV received 6 mg/kg/day of nicotine for 17 days and then 5 mg/kg/day of Soy Milk Kefir (SMK) for 7 days.
On the 25th day, some standard behavioral methods such as Elevated Plus Maze (EPM), Open Field Test (OFT) and Forced Swim Test (FST) were used to investigate the anxiety and depression level of animals being experimented. In addition, a standard behavioral protocol by Moris Water Maze was applied to evaluate learning and memory in the animals between the 20th and 25th days.
Elevated plus maze (EPM)
This test is designed to assess anxiety in the case of laboratory animals. The setting is made of a plus-shaped apparatus with two open arms and two closed arms and elevated 40 cm from the floor and the closed arm has 40 cm high walls. The length of each arm was considered 50 cm. Anxiety reduction is indicated by an increase in time spent in open arms.
Open field test (OFT)
This test is used to assess locomotor activity, exploratory and anxiety in the case of behaviors in rodents. The open field is a white table with walls commonly marked in grid crossings. The OFT task approaches the conflict between the innate fear that rodents have of the central area of a novel or brightly light open field against their desire to explore new environments. When anxious, the natural tendency of rodents is to prefer staying close to the walls. The animals are placed in the center of the field and locomotor activity is assessed for 5 min.
Forced swim test (FST)
This test is widely used to measure the effect of antidepressant drugs on the behavior of rodents. This test, consisting of a transparent plastic cylinder container (30 cm in diameter and 50 cm in height), is filled with water (25°C) to a depth of 13 cm. In order to evaluate the anti-depression effect of treatments in this study, the rats are exposed to water for 10 min and two parameters are considered: Duration of swimming and duration of immobility. When forced to swim, the rats become nearly immobile and refuse to escape. Immobility reduces when rats are treated with antidepressants.
Morris water maze (MWM)
The MWM is a dark circular pool, 145 cm in diameter and 80 cm high, filled with water at 22 ± 1°C 60 cm deep. A clear plexi glass platform (12 cm in diameter) was placed in the center of one of the designed orthogonal quadrants randomly and 1 cm below the surface of water. This platform was provided for the animals to escape from the water. The animal's position was recorded by a camera set up above the center of the pool. The distance to reach the platform was measured. The rats received four trials per day (two blocks separated by a 3-min interval) for five consecutive days and their memory was tested on the 25th day when the platform was removed.
The data were analyzed by Graph Pad PRISM v.6 software and averaged in every experimental group and expressed as means ± standard error of the means (SEM). Then, the differences between the control and treatment groups were evaluated by ANOVA. To evaluate the severity of behaviors, the differences among averages in each group were compared using the Tukey test at a significant level (P < 0.05).
RESULTS
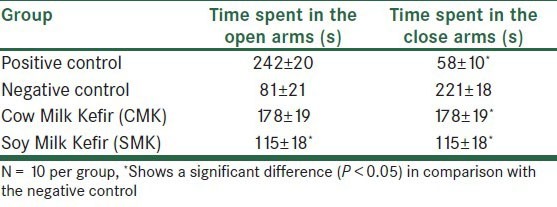
As shown in Table 1, the negative control group animals displayed profound anxiety in comparison with their controls (P < 0.05). This study also indicated that animals treated with CMK and SMK spent more time in the open arms of EPM, and this increased spent time was statistically significant in comparison with the negative control group (P < 0.05) [Table 1]. The positive control animals compared with the negative control group spent less time in the closed arms (s) (P < 0.05). Our data indicated that the animals that received CMK and SMK spent less time (s) in the closed arms of EPM (P < 0.05) [Table 1].
Table 1.
Duration of time spent in the open and closed arms (mean ± SEM) in the Elevated Plus Maze test
As shown in Table 2, the positive control group animals in comparison with the negative ones stayed immobile in the FST (P < 0.05). This research indicated that animals treated with CMK and SMK remained immobile in the FST, and this immobility was statistically significant in comparison with the negative control group (P < 0.05) [Table 2]. In the FST, the positive control animals in comparison with the negative ones spent more time swimming (P < 0.05), whereas the animals treated with CMK and SMK spent more time swimming in the FST (s), and this increased time was statistically significant compared with the negative control group (P < 0.05) [Table 2].
Table 2.
Duration of time spent swimming and immobility (s) (mean ± SEM) in the Forced Swim Test

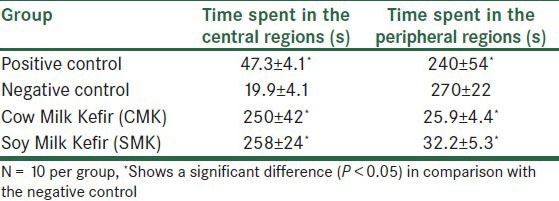
As shown in Table 3, the positive control animals in comparison with the negative ones spent more time in the central region of the OFT (P < 0.05). We found that the animals that received CMK and SMK spent more time (s) in the central region of the OFT, and this rise in time was statistically significant compared with the negative control group (P < 0.05) [Table 3]. The positive control animals versus the negative ones spent less time (s) in the peripheral region of the OFT (P < 0.05). The animals given CMK and SMK spent more time (s) in the peripheral region of the OFT, and this rise was statistically significant in comparison with the negative control group (P < 0.05) [Table 3].
Table 3.
Duration of time spent (s) (mean ± SEM) in the central and peripheral regions of the Open Field Test
Our data in the MWM showed that in the groups under treatment with CMK, SMK and sertraline learning increased significantly in comparison with the negative control group on the third, fourth and fifth training days (P < 0.05) [Figure 1]. There was a significant difference in their memory retention on the 6th day of training [Figure 2].
Figure 1.
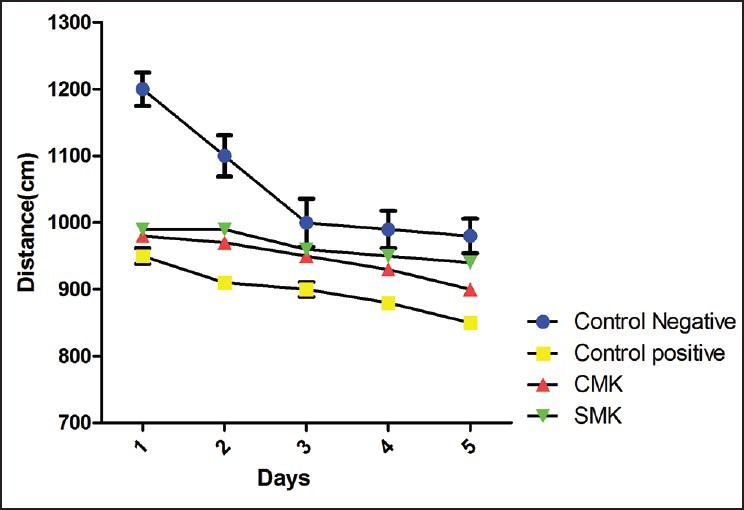
Comparison of distance to reach the platform on Days 1-5 between the controls groups and the groups under treatment with Cow Milk Kefir and Soy Milk Kefir in the Morris Water Maze task. In the negative control group, the distance to reach the platform was significantly higher than the other groups on the third, fourth and fifth training days, *P < 0.05. Ordinate shows the mean ± standard error of mean of distance (cm) to reach the platform region
Figure 2.
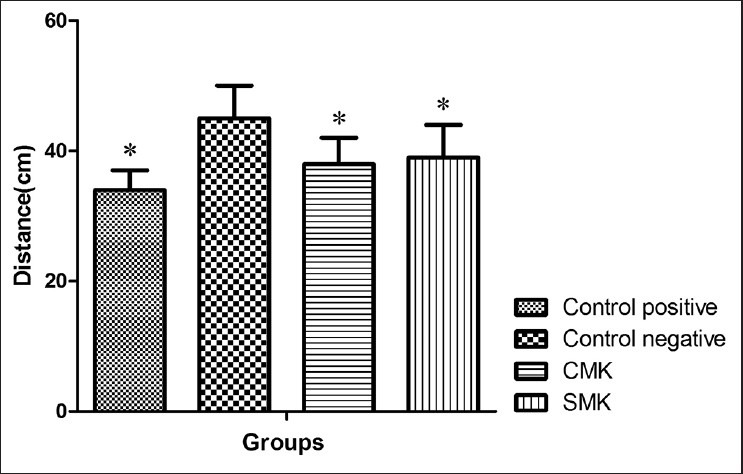
Comparison of memory retention on Day 6 as represented by the distance to reach the platform region between the control, sham and addicted groups in the Morris Water Maze task, *P < 0.05. Ordinate shows the mean ± standard error of mean of distance (cm) to reach the platform region
DISCUSSION
The present study indicates that kefir has the potential to treat nicotine cessation-induced depression, anxiety and cognition impairment in the animal model. Previous studies showed that food supplement and lifestyle could attenuate depression and anxiety after nicotine cessation.[15,16]
Many studies showed that dependency on nicotine and other drugs leads to a change in neurotransmitters functions like serotonin and dopamine in the brain regions such as the hippocampus and amygdala and the centers controlling the mood.[17]
Nicotine withdrawal is characterized by several symptoms, for instance depressed mood, anxiety, dysfunction in learning and memory and insomnia appearing within 24 h after the cessation or reduction in the amount of nicotine.[8] On the other hand, some practitioners use anti-depressant and anxiolytic drugs along with the specific nicotine cessation-related drugs to relieve depression and anxiety in these patients.[18] Previous studies show that some drugs, for example SSRIs, increase serotonin and dopamine levels by reuptake inhibition of these neurotransmitters in the brain. This function improves nicotine-induced mental disorders and decreases anxiety, stress and depression in the nicotine addict patients.[19] Also, there are many accurate studies about depression and anxiety induced by use and dependency on drugs like nicotine, morphine and alcohol that declare mental problems are associated with intervention in physiological function of tryptophan as a precursor of Serotonin (5-HT).[20] The present study showed that nicotine-induced dependency and its withdrawal cause an increase in depression, anxiety and cognition impairment in the FST, EPM, OFT and MWM tests (P < 0.05). Previous studies have demonstrated that this test is a standard method for evaluation of many kinds of anxiety and depression disorders such as nicotine cessation-induced anxiety and restlessness.[21,22,23,24] Our data also showed that nicotine cessation-induced anxiety and depression confirm the results of previous research. We can argue our findings with the basic concept that nicotine can alter and reduce brain neurotransmitters such as serotonin and dopamine responsible for anxiety and depression after the nicotine withdrawal period.[25]
It also seems that the treatment of the subjects by CMK and SMK in the nicotine withdrawal period reduces the severity of depression, anxiety and cognition impairment evaluated by the FST, EPM, OFT and MWM tests (P < 0.05). There are many researchers who believe that an imbalance in serotonin levels may influence mood in a way that it leads to depression and anxiety. Possible problems include low brain cell production of serotonin, a lack of receptor sites able to receive the serotonin made, inability of serotonin to reach the receptor sites or a shortage in tryptophan, the chemical from which serotonin is made.[26,27] If any of these biochemical glitches occur, it is believed it can result in depression, anxiety, panic and cognition impairment. Antidepressant medications that work on serotonin levels, such as SSRIs and serotonin and norepinephrine reuptake inhibitors (SNRIs) are thought to reduce the symptoms of depression.[28,29] We can argue our finding with the basic concept that kefir as a rich food supplement has a high level of tryptophan that can be converted to serotonin effective in relieving anxiety and depression in the nicotine withdrawal period.[30] This high level of serotonin can also protect the brain function and improve learning and memory.[31] Kefir has fractions and compounds that can activate NMDA receptors in the brain and alter some ions such as calcium and sodium that result in better learning and memory.[31,32,33] Tryptophan is an important amino acid involved in the production of melatonin and serotonin. This amino acid and its byproducts play an important role in regulating mood (anxiety and depression), sleep cycles and the perception of pain.[26,34] Many studies demonstrated that L-tryptophan administration is an augmentation strategy in the treatment of depression and anxiety. Based on this concept, we can conclude that kefir as a food supplement with a high level of tryptophan can alter the tryptophan and serotonin level of serum and brain and can be effective in anxiety and depression.[34,35] We can argue our study results with this knowledge that kefir as a food supplement with high tryptophan can increase the production of serotonin and melatonin in the brain and act as an antidepressant and anxiolytic agent against nicotine-induced depression and anxiety.[36,37] Also, previous studies demonstrated that serotonin 5-HT2A receptor and its constitutive activity were involved in cognitive function. Thus, it can be concluded that kefir with an increasing level of tryptophan and serotonin probably activated the 5-HT2A receptor and motivated the learning and memory.[38,39,40]
Recent studies report that the enhanced serotonergic neurotransmission in the hippocampus following tryptophan administration (50-100 mg/kg) improves learning acquisition and memory consolidation in Wistar rats. This study indicates that tryptophan protects the neurons responsible for memory.[40,41] A decrease in neurogenesis is thought to be related to the cognitive decline. On the other hand, serotonin as a neurotransmitter can promote hippocampus neurogenesis, especially neurons responsible for cognitive behavior, and can affect cognition. This report helps us argue our data considering the fact that kefir has a high level of tryptophan. Therefore, it can protect neurons responsible for memory and can diminish nicotine cessation-induced induce cognition alteration.[42,43] Previous studies also demonstrated that magnesium is an essential trace element in neurogenesis, and it has been reported that kefir is rich in this essential element. Thus, it can be effective in neurogenesis and the protection of neurons responsible for cognition.[43]
The present study shows that sertraline as a standard treatment reduces depression and anxiety induced by nicotine withdrawal (P < 0.05). The results of this study alongside previous ones suggest that kefir can elevate serotonin resources in the brain and its anti-inflammatory properties can control and relieve the inflammation of neurons destroyed and degenerated by nicotine and other similar drugs.[44] Furthermore, we can argue that kefir is rich in calcium and magnesium, which are important minerals for promoting brain physiologic functions such as learning and memory, and can act as a neuroprotective and neuromodulator compound.[44,45]
However, there seems to be no precise knowledge about the molecular pathway and target region of these supplementary compounds, and it is not clear whether these compounds can be effective in serotonergic and adrenergic neuron damage like the way TCAs and SSRI medication do.
It is suggested that the evaluation of these compounds’ effects should be carried out on the neuronal and glial cell cultures. The application of development histological and biochemical methods for the assessment of its efficacy for future studies is also recommended.
CONCLUSION
The results of the present study support the hypothesis that kefir may be used as a diet to prevent depression, anxiety and cognitive impairment and an available natural therapy for patients suffering from nicotine-induced anxiety and depression as seen in the animal model. It is believed that these results could be useful and referable in human smokers, but it needs to be explored by exclusive research on humans.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–17. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- 2.Dani JA, Ji D, Zhou FM. Synaptic plasticity and nicotine addiction. Neuron. 2001;31:349–52. doi: 10.1016/s0896-6273(01)00379-8. [DOI] [PubMed] [Google Scholar]
- 3.Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 4.Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–11. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- 5.Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, et al. Sex differences in [123I] beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse. 2001;41:275–84. doi: 10.1002/syn.1084. [DOI] [PubMed] [Google Scholar]
- 6.Rao TS, Correa LD, Adams P, Santori EM, Sacaan AI. Pharmacological characterization of dopamine, norepinephrine and serotonin release in the rat prefrontal cortex by neuronal nicotinic acetylcholine receptor agonists. Brain Res. 2003;990:203–8. doi: 10.1016/s0006-8993(03)03532-7. [DOI] [PubMed] [Google Scholar]
- 7.Chae Y, Yeom M, Han JH, Park HJ, Hahm DH, Shim I, et al. Effect of acupuncture on anxiety-like behavior during nicotine withdrawal and relevant mechanisms. Neurosci Lett. 2008;430:98–102. doi: 10.1016/j.neulet.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70:531–49. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 9.Benowitz NL. Neurobiology of nicotine addiction: Implications for smoking cessation treatment. Am J Med. 2008;121(Suppl 1):S3–10. doi: 10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 10.George TP, O’Malley SS. Current pharmacological treatments for nicotine dependence. Trends Pharmacol Sci. 2004;25:42–8. doi: 10.1016/j.tips.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Olausson P, Engel JA, Söderpalm B. Involvement of serotonin in nicotine dependence: Processes relevant to positive and negative regulation of drug intake. Pharmacol Biochem Behav. 2002;71:757–71. doi: 10.1016/s0091-3057(01)00673-6. [DOI] [PubMed] [Google Scholar]
- 12.Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: Involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry. 2005;62:649–59. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- 13.Garrote GL, Abraham AG, De Antoni GL. Chemical and microbiological characterisation of kefir grains. J Dairy Res. 2001;68:639–52. doi: 10.1017/s0022029901005210. [DOI] [PubMed] [Google Scholar]
- 14.Otles S, Cagindi Oe. Kefir: A probiotic dairy-composition, nutritional and therapeutic aspects. Pak J Nutr. 2003;2:54–9. [Google Scholar]
- 15.Eby GA, Eby KL. Rapid recovery from major depression using magnesium treatment. Med Hypotheses. 2006;67:362–70. doi: 10.1016/j.mehy.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 16.Kubow S, Fotouhinia M. US Patent; 2004. Kefir as a potent anti-oxidant composition. 20,040,028,696. [Google Scholar]
- 17.Singer S, Rossi S, Verzosa S, Hashim A, Lonow R, Cooper T, et al. Nicotine-induced changes in neurotransmitter levels in brain areas associated with cognitive function. Neurochem Res. 2004;29:1779–92. doi: 10.1023/b:nere.0000035814.45494.15. [DOI] [PubMed] [Google Scholar]
- 18.Cary DD. Nicotine addiction treatment. Google Patents. 2001 [Google Scholar]
- 19.Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- 20.Morissette SB, Tull MT, Gulliver SB, Kamholz BW, Zimering RT. Anxiety, anxiety disorders, tobacco use, and nicotine: A critical review of interrelationships. Psychol Bulletin. 2007;133:245–72. doi: 10.1037/0033-2909.133.2.245. [DOI] [PubMed] [Google Scholar]
- 21.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 22.Carobrez A, Bertoglio L. Ethological and temporal analyses of anxiety-like behavior: The elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004;7:387–90. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- 24.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 25.Tomkins DM, Sellers EM. Addiction and the brain: The role of neurotransmitters in the cause and treatment of drug dependence. CMAJ. 2001;164:817–21. [PMC free article] [PubMed] [Google Scholar]
- 26.Neumeister A. Tryptophan depletion, serotonin, and depression: Where do we stand? Psychopharmacol Bull. 2002;37:99–115. [PubMed] [Google Scholar]
- 27.Lacasse JR, Leo J. Serotonin and depression: A disconnect between the advertisements and the scientific literature. PLoS Med. 2005;2:e392. doi: 10.1371/journal.pmed.0020392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, et al. Antidepressant drug effects and depression severity: A patient-level meta-analysis. JAMA. 2010;303:47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sachs GS, Nierenberg AA, Calabrese JR, Marangell LB, Wisniewski SR, Gyulai L, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356:1711–22. doi: 10.1056/NEJMoa064135. [DOI] [PubMed] [Google Scholar]
- 30.Alberghina D, Giannetto C, Visser EK, Ellis AD. Effect of diet on plasma tryptophan and serotonin in trained mares and geldings. Vet Rec. 2010;166:133–6. doi: 10.1136/vr.c502. [DOI] [PubMed] [Google Scholar]
- 31.Meltzer HY, Horiguchi M, Massey BW. The role of serotonin in the NMDA receptor antagonist models of psychosis and cognitive impairment. Psychopharmacology (Berl) 2011;213:289–305. doi: 10.1007/s00213-010-2137-8. [DOI] [PubMed] [Google Scholar]
- 32.Fukami H, Tachimoto H, Kishi M, Kaga T, Tanaka Y. Acetic acid bacterial lipids improve cognitive function in dementia model rats. J Agric Food Chem. 2010;58:4084–9. doi: 10.1021/jf9045842. [DOI] [PubMed] [Google Scholar]
- 33.Holub I, Kozianowski G. Isomaltulose for use in enhancing mental performance. Google Patents. 2011 [Google Scholar]
- 34.Shaw KA, Turner J, Del Mar C. Tryptophan and 5-hydroxytryptophan for depression. Cochrane Database Syst Rev. 2002:1. doi: 10.1002/14651858.CD003198. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues KL, Caputo LR, Carvalho JC, Evangelista J, Schneedorf JM. Antimicrobial and healing activity of kefir and kefiran extract. Int J Antimicrob Agents. 2005;25:404–8. doi: 10.1016/j.ijantimicag.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Moreira ME, Dos Santos MH, Zolini GP, Wouters AT, Carvalho JC, Schneedorf JM. Anti-inflammatory and cicatrizing activities of a carbohydrate fraction isolated from sugary kefir. J Med Food. 2008;11:356–61. doi: 10.1089/jmf.2007.329. [DOI] [PubMed] [Google Scholar]
- 37.Doğan M. Rheological behaviour and physicochemical properties of kefir with honey. Journal für Verbraucherschutz und Lebensmittelsicherheit. 2011;6:327–32. [Google Scholar]
- 38.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–54. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 39.Hasselbalch SG, Madsen K, Svarer C, Pinborg LH, Holm S, Paulson OB, et al. Reduced 5-HT2A receptor binding in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1830–8. doi: 10.1016/j.neurobiolaging.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Harvey JA. Role of the serotonin 5-HT(2A) receptor in learning. Learn Mem. 2003;10:355–62. doi: 10.1101/lm.60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haider S, Khaliq S, Haleem DJ. Enhanced serotonergic neurotransmission in the hippocampus following tryptophan administration improves learning acquisition and memory consolidation in rats. Pharmacol Rep. 2007;59:53–7. [PubMed] [Google Scholar]
- 42.Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- 43.Lauder JM, Krebs H. Serotonin as a differentiation signal in early neurogenesis. Dev Neurosci. 1978;1:15–30. doi: 10.1159/000112549. [DOI] [PubMed] [Google Scholar]
- 44.Asemi Z, Jazayeri S, Najafi M, Samimi M, Mofid V, Shidfar F, et al. Effect of daily consumption of probiotic yogurt on oxidative stress in pregnant women: A randomized controlled clinical trial. Ann Nutr Metab. 2012;60:62–8. doi: 10.1159/000335468. [DOI] [PubMed] [Google Scholar]
- 45.Ghoneum M, Gimzewski J. Apoptotic effect of a novel kefir product, PFT, on multidrug-resistant myeloid leukemia cells via a hole-piercing mechanism. Int J Oncol. 2014;44:830–7. doi: 10.3892/ijo.2014.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]


