Abstract
Background:
Oral recurrent aphthous stomatitis (RAS) is a chronic inflammatory disease affected oral mucosa which has afflicted about 20% of the society. Etiology of this disease is unknown. Three main factors influencing aphthous stomatitis are genetic, hematologic, and immunologic factors. Another proposed factor that may be considered in etiology of aphthous ulcer is oxidant, anti-oxidant imbalance in the body. The purpose of this study was the evaluation of lipid peroxide enzyme as an important oxidant agent and anti-oxidant vitamins in the saliva and serum of the patients suffering from RAS and compare it to healthy people.
Materials and Methods:
In this cross-sectional, descriptive – analytical study, 25 patients suffering from RAS were compared to 24 healthy individuals. Applying enzyme-linked immunosorbent assay method, the amount of malondialdehyde (MDA) as well as Vitamins A, E, and C were measured in the saliva and serum of two groups. The results were analyzed by Statistical Package for the Social Sciences (SPSS) software version (11.5), t-test and χ2.
Results:
No significant difference was detected between salivary and serum levels of antioxidant vitamins (A, E, and C) and MDA in both experimental groups (P > 0.05).
Conclusion:
According to the results of this study, RAS is not associated with oxidant–antioxidant imbalance in the body. However, it seems quite necessary to carry out more studies in this area.
Keywords: Lipid peroxidase, malondialdehyde, recurrent aphthous stomatitis, saliva, vitamin
INTRODUCTION
Recurrent aphthous stomatitis (RAS) is a chronic inflammatory disease of oral mucosa characterized by single or multiple painful ulcers and often in non-keratinized mucosa.[1,2] The major factors presently linked to RAS include genetic factor, hematologic deficiencies, immunologic abnormalities and minor factors such as trauma, stress, and food allergy.[3,4]
All of the above mentioned conditions can disturb oxidant–anti-oxidant balance of organism and can accelerate the formation of free radicals. The cytotoxic effects of free radicals are harmful For mammation cells and lead to disease such as Behcet disease, atherosclerosis, diabetes, inflammatory condition, cancer, aging.[5,6,7,8] In normal conditions, mucosa membranes are protected from damage by natural free radical scavengers which include the antioxidant vitamins, superoxide dismutase (SOD), glutathione peroxidase (GSHPX), reductase, and catalase (CAT).[8]
Previous studies have been shown that non-enzymatic antioxidant system which consist of vitamins A, E, and C could react with organic free radicals and protected bio membranes from damage of free radicals.[8,9]
Malondialdehyde (MDA) is a stable end product of peroxidation of membrane lipids by free radicals and it is an indicator of increased lipid peroxidation, MDA caused the disturbance of structure and function of cell membrane.[10]
There have been several studies about impaired oxidant–antioxidant balance in RAS patient.[11,12,13,14,15]
Cimen et al. in 2003 reported the increased MDA level and decreased anti-oxidant enzymatic system (SOD, GSHPX, and CAT) in RAS patients.[11]
Arikan et al. in 2009 reported significant elevation of plasma MDA and decreased GSHPX activities, vitamin E levels in patients with RAS.[12]
Although, in the study of Beitollohi there have not been any significant differences of SOD, GSHPX, and CAT in patient group in comparison to healthy controls.[13]
Regarding the controversy results to this issue, the aim of the present study was to measure the level of salivary and serum oxidant/antioxidant status in RAS patient and compare it with healthy subjects.
MATERIALS AND METHODS
This cross-sectional study was conducted in 25 patients with RAS who admitted to the clinic of Oral Medicine Department of Isfahan University of Medical Sciences. For comparison, 24 healthy individuals included as controls whose age and sex were matched.
The protocol of this study was approved by the institutional review board and Ethics Committee of Isfahan University of Medical Sciences and each person signed consent form.
The diagnostic criteria of aphthous ulcer included the round, shallow, and symmetric ulcers with prodromal burning sensation 2-48 h before an ulcer appearance. The diameter of these ulcers was 3-10 mm and healed without scaring in 7-14 days.[1]
The RAS patients had at least three recurrences of aphthous ulcer per year and had active aphthous lesions during this studyfgr [Figure 1]. They were not under a therapeutic regimen for the past 3 months and had not received medicine containing vitamins. History of smoking habits, alcohol consumption, Behcets disease, periodontal problem, chronic diarrhea, trauma history, and any systemic disease were among to the exclusion criteria.
Figure 1.
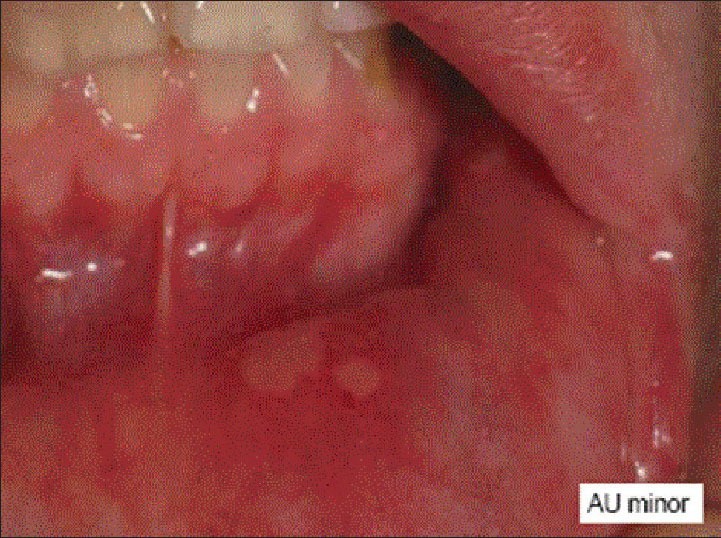
Minor aphthous ulcer
The control group consisted of 24 self-admitted healthy individuals that were either medical students or clinic staff, who do not have any history of RAS or vitamins consumption.
In this study, MDA, vitamins A, C, and E were measured in both samples of saliva and plasma.
Fasting blood samples (5 ml) of the patients and controls were taken between 8 and 9 in the morning. Samples were centrifuged at 3000 rpm at 4°C for 5 min and aspirated into tubes and stored at − 20°C until analyzed for vitamins A, E, C, and MDA. Saliva samples were gained in the morning following an overnight fasting. At first the patients rinsed their mouth using distilled water.
After 5 min, unstimulated saliva samples were gained. The patients sat comfortably and spitted into the plastic tubes five times per min for 5 min. Samples were centrifuged 4000 rpm for 10 min at 4°C and stored at − 20°C.
The level of vitamins A, E, C, and MDA was determined by enzyme-linked immunosorbent assay (ELISA) method.
The salivary and serum level of vitamin A is determined by Human Vit A ELISA kit Catalog No. CSBE07889h America.
At first, all the reagents brought to room temperature for at least 30 min before use. If crystals had formed in the concentrate mixed gently until, the crystals had completely dissolved. In order to dilution of the serum and plasma samples, 200 μl of biotin-antibody was added, incubated for 1 h at 37°C. After that 90 μl of TMB substrate was added and incubated for 10-30 min. Finally, the optical density of each well was determined within 30 min using a micro plate reader set to 450 nm. The method of ELISA for measurement of vitamin E, vitamin C, and MDA are nearly similar to this mentioned method (Human Vit C ELISA kit Catalog No. ABIN777846 America, Human Vit E ELISA kit Catalog No. CSBE7893h America, Human Peroxidase ELISA kit Catalog No. CSBA082431 America)
Human peroxidase kit ELISA Catalog No. CSBA082431 America was used for the measurement of vitamin C, vitamin E, and MDA, respectively.
Data are expressed as mean ± standard deviation. t-Test and χ2 were applied for statistical data analysis.
RESULTS
The patient group included 20 females and 5 males (mean age 29.16 ± 9.74 years) while the control group consisted of 19 females and 4 males (mean age 31.87 ± 9.34).
The values of the serum antioxidant vitamins and MDA are presented in Table 1.
Table 1.
Serum level of vitamins A, E, and C and malondialdehyde in the control and patient group
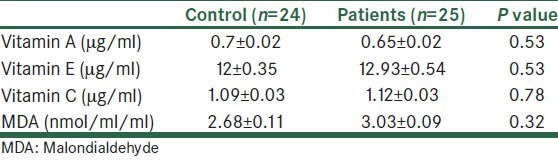
There was no significant difference between two groups (P > 0.05).
The level of salivary antioxidant vitamins and MDA are given in Table 2.
Table 2.
Salivary level of vitamins A, E, and malondialdehyde in the control and patient group
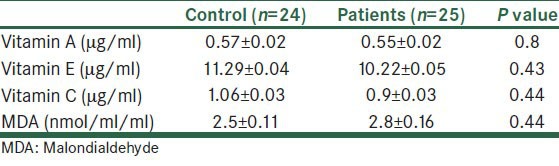
Furthermore, no statistically significant difference in saliva was found for levels of vitamins and MDA between patient and control group (P > 0.05).
DISCUSSION
In this study, we found that the serum and salivary level of some non-enzymatic indicators of the antioxidant protective system (vitamins A, E, and C) and level of MDA (and indicator of lipid peroxidation) do not have a significant difference in RAS patients compared to the control group (P > 0.05).
Moreover, it is widely accepted that imbalance between free radicals and antioxidant system causes many inflammatory oral pathologies.[16,17,18]
Cimen et al. in 2003 observed a relative reduction in enzymatic antioxidant capacity and increase of oxidant factors in patients with RAS.[11]
Caglayan in 2008 reported unchanged antioxidant enzymatic values and MDA level in RAS patients in comparison with the control group.[15]
Momen-Beitolahi et al. in 2010 showed that there is not any alteration in total antioxidant status activity in RAS when compared to the control group.[13]
In these mentioned studies, they examined enzymatic antioxidant capacity but in this study non-enzymatic antioxidant capacity was determined. Salar study was only similar to the present study.
They measured enzymatic antioxidant factors (vitamins A, E, and C) and MDA levels in saliva and serum of the RAS patients. They observed that serum and saliva levels of vitamins A, E, and C were significantly higher and the level of MDA was significantly lower in patients with RAS than in a matched group of the healthy control group,[13] but the present study obtained exactly opposite results. These relative controversies may be associated with several factors such as: Sample size variations, method of saliva, sampling, and genetic divergence of each population.
The method of saliva sampling was different in two studies. In Salar study, stimulated saliva was tested, but in this study we used whole and unstimulated saliva to detect oxidant–anti-oxidant parameters, whole saliva is the most relevant. It contains gingival cervicular fluid, tissue matabolites and reflects more closely the predominant intra-oral condition. Stimulation may increase the flow of gingival cervicular fluid and this may lead to false result in the concentration of these parameters in the saliva.[19] It seems that some acquired and environmental situation rather than genetic and inheritance factors influence the level of oxidant and antioxidants status in the body and these factors may explain the results.
CONCULSION
The results of this study indicated that there is no significant difference in the saliva and serum levels of oxidant/antioxidant parameters in both patient and control group and may be, oxidant–antioxidant imbalance acts as an accessory factor in the etiology of RAS.
We propose that future studies should be controlled, multicenter prospective studies which shall include large case series and this issue should be conducted.
ACKNOWLEDGMENT
We would like to appreciate the financial support of vice chancellor for research of Isfahan university of Medical Sciences and Health Services.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Woo SB, Greenberg MS. Ulcerative, Vesicular and bullous lesions. In: Green Berg MS, Glick M, Ship J., editors. Burket's Oral Medical, Diagnosis and Treatment. 10th ed. Hamilton: BC Decker; 2008. pp. 57–62. [Google Scholar]
- 2.Ship JA, Chavez EM, Doerr PA, Henson BS, Sarmadi M. Recurrent aphthous stomatitis. Quintessence Int. 2000;31:95–112. [PubMed] [Google Scholar]
- 3.Porter SR, Scully C, Pedersen A. Recurrent aphthous stomatitis. Crit Rev Oral Biol Med. 1998;9:306–21. doi: 10.1177/10454411980090030401. [DOI] [PubMed] [Google Scholar]
- 4.Scully C, Gorsky M, Lozada-Nur F. The diagnosis and management of recurrent aphthous stomatitis: A consensus approach. J Am Dent Assoc. 2003;134:200–7. doi: 10.14219/jada.archive.2003.0134. [DOI] [PubMed] [Google Scholar]
- 5.Hoelzl C, Bichler J, Ferk F, Simic T, Nersesyan A, Elbling L, et al. Methods for the detection of antioxidants which prevent age related diseases: A critical review with particular emphasis on human intervention studies. J Physiol Pharmacol. 2005;56:49–64. [PubMed] [Google Scholar]
- 6.Beevi SS, Rasheed AM, Geetha A. Evaluation of oxidative stress and nitric oxide levels in patients with oral cavity cancer. Jpn J Clin Oncol. 2004;34:379–85. doi: 10.1093/jjco/hyh058. [DOI] [PubMed] [Google Scholar]
- 7.Gunduz K, Ozturk G, Sozmen EY. Erythrocyte superoxide dismutase, catalase activities and plasma nitrite and nitrate levels in patients with Behçet disease and recurrent aphthous stomatitis. Clin Exp Dermatol. 2004;29:176–9. doi: 10.1111/j.1365-2230.2004.01488.x. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet. 1994;344:721–4. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 9.Cohen G. Enzymatic/nonenzymatic sources of oxyradicals and regulation of antioxidant defenses. Ann N Y Acad Sci. 1994;738:8–14. doi: 10.1111/j.1749-6632.1994.tb21784.x. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin Chem. 1997;43:1209–14. [PubMed] [Google Scholar]
- 11.Cimen MY, Kaya TI, Eskandari G, Tursen U, Ikizoglu G, Atik U. Oxidant/antioxidant status in patients with recurrent aphthous stomatitis. Clin Exp Dermatol. 2003;28:647–50. doi: 10.1046/j.1365-2230.2003.01415.x. [DOI] [PubMed] [Google Scholar]
- 12.Arikan S, Durusoy C, Akalin N, Haberal A, Seckin D. Oxidant/antioxidant status in recurrent aphthous stomatitis. Oral Dis. 2009;15:512–5. doi: 10.1111/j.1601-0825.2009.01580.x. [DOI] [PubMed] [Google Scholar]
- 13.Momen-Beitollahi J, Mansourian A, Momen-Heravi F, Amanlou M, Obradov S, Sahebjamee M. Assessment of salivary and serum antioxidant status in patients with recurrent aphthous stomatitis. Med Oral Patol Oral Cir Bucal. 2010;15:e557–61. doi: 10.4317/medoral.15.e557. [DOI] [PubMed] [Google Scholar]
- 14.Saral Y, Coskun BK, Ozturk P, Karatas F, Ayar A. Assessment of salivary and serum antioxidant vitamins and lipid peroxidation in patients with recurrent aphthous ulceration. Tohoku J Exp Med. 2005;206:305–12. doi: 10.1620/tjem.206.305. [DOI] [PubMed] [Google Scholar]
- 15.Cağlayan F, Miloglu O, Altun O, Erel O, Yilmaz AB. Oxidative stress and myeloperoxidase levels in saliva of patients with recurrent aphthous stomatitis. Oral Dis. 2008;14:700–4. doi: 10.1111/j.1601-0825.2008.01466.x. [DOI] [PubMed] [Google Scholar]
- 16.Battino M, Ferreiro MS, Gallardo I, Newman HN, Bullon P. The antioxidant capacity of saliva. J Clin Periodontol. 2002;29:189–94. doi: 10.1034/j.1600-051x.2002.290301x.x. [DOI] [PubMed] [Google Scholar]
- 17.Sezer E, Ozugurlu F, Ozyurt H, Sahin S, Etikan I. Lipid peroxidation and antioxidant status in lichen planus. Clin Exp Dermatol. 2007;32:430–4. doi: 10.1111/j.1365-2230.2007.02436.x. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Lam EW, Hammad HM, Oberley TD, Oberley LW. Antioxidant enzyme levels in oral squamous cell carcinoma and normal human oral epithelium. J Oral Pathol Med. 2002;31:71–7. doi: 10.1034/j.1600-0714.2002.310202.x. [DOI] [PubMed] [Google Scholar]
- 19.Buduneli N, Kardeşler L, Işik H, Willis CS, 3rd, Hawkins SI, Kinane DF, et al. Effects of smoking and gingival inflammation on salivary antioxidant capacity. J Clin Periodontol. 2006;33:159–64. doi: 10.1111/j.1600-051X.2006.00892.x. [DOI] [PubMed] [Google Scholar]


