Abstract
Peripheral nerve injury is a serious problem affecting significantly patients’ life. Autografts are the “gold standard” used to repair the injury gap, however, only 50% of patients fully recover from the trauma. Artificial conduits are a valid alternative to repairing peripheral nerve. They aim at confining the nerve environment throughout the regeneration process, and providing guidance to axon outgrowth. Biocompatible materials have been carefully designed to reduce inflammation and scar tissue formation, but modifications of the inner lumen are still required in order to optimise the scaffolds. Biomicking the native neural tissue with extracellular matrix fillers or coatings showed great promises in repairing longer gaps and extending cell survival. In addition, extracellular matrix molecules provide a platform to further bind growth factors that can be released in the system over time. Alternatively, conduit fillers can be used for cell transplantation at the injury site, reducing the lag time required for endogenous Schwann cells to proliferate and take part in the regeneration process. This review provides an overview on the importance of extracellular matrix molecules in peripheral nerve repair.
Keywords: peripheral nerve repair, extracellular matrix, nerve conduits, surface modification, fillers, growth factors
Introduction
Recovery after nerve injury depends on the severity of the lesion and on the patient's conditions. A transient concussion (neuropraxia) stops temporarily the conduction of the nerve pulse (Burnett and Zager, 2004) and nerve function is usually restored within 6 to 8 weeks post-injury (Kaye, 2005). Axotomy involves instead Wallerian degeneration causing complete denervation at the injury site. In case of whole nerve disruption (neurotmesis), the continuity of the neural components is completely lost, thereby impedes functional recovery (Wiberg and Terenghi, 2003; Burnett and Zager, 2004).
Nerve autografts are considered the “gold standard” for peripheral nerve repair, yet only 50% of the patients experience functional recovery (Lee and Wolfe, 2000; Belkas et al., 2004). In addition, autograft harvesting causes permanent denervation at the donor site, and further affects patients’ quality of life (Lassner et al., 1995; Siemionow et al., 2010). To overcome these limitations, material scientists and engineers, together with surgeons, are developing artificial conduits for nerve repair. Four key factors are to be controlled and optimised when designing a nerve conduit: (1) materials and design of the substrate (from macro- to nano-scales), (2) incorporated trophic factors, (3) glial cells and (4) extracellular matrix (ECM) proteins/peptides (Bellamkonda, 2006). This perspective article focuses on the latter and reports on the effect of biological molecules on nerve regeneration after injury.
The main goal of an artificial nerve conduit is to bridge the nerve gap, joining the proximal and distal stumps. The regenerative process is confined within a closed environment that enables axons sprouting from the proximal to the distal nerve segments, and reduces the formation of scar tissue (Kingham and Terenghi, 2006). During the whole regeneration process, five phases ensue: 1) a fluid phase, rich in proteins and neurotrophic factors; 2) a matrix phase, involving the development of a fibrin-rich scaffold; 3) a cellular migration phase, involving perineural, endothelial and Schwann cells (SC); 4) an axonal phase, and 5) a myelination phase, involving SC action (Daly et al., 2012; Kehoe et al., 2012).
Natural polymers, including collagen, hyaluronic acid, fibrin gels, alginate, agarose and chitosan (Bell and Haycock, 2012; Geuna et al., 2014) are commonly used for nerve regeneration due to their high affinity with SC. These cells express specific integrins, i.e. α1β1, α2β1, α6β1, α6β4, α5β1, αVβ3, that bind to ECM proteins and promote myelination through their interaction with the basal lamina (Chernousov and Carey, 2000). The degradation rate of these polymers and their effect on neuronal cell response can be controlled by modifying their polymerisation procedure (Schmidt & Leach, 2003). However, natural polymers are characterized by poor mechanical properties, causing the conduit to kink or collapse, and undergo fast degradation in vivo, hence co-polymers are required to improve the scaffold stability (Xie et al., 2008; Wang and Cai, 2010).
Synthetic conduits can be classified as “inert” or “active”, according to their behaviour. The former only provide a suitable guidance environment for the regenerative process, whilst the latter provide controlled release of growth factors or other biological molecules to improve neuron regeneration (Aldini et al., 2000). Silicone and polytetrafluoroethylene (PTFE) conduits are frequently used in clinical applications for nerve repair. They are non resorbable polymers, which can constrain and compress the regenerating nerve fibers (Siemionow and Brzezicki, 2009). Biodegradable polymers, e.g. poly(lactic acid) PLA, poly(glycolic acid) (PGA), poly(lactic-co-glycolic acid) (PLGA) and poly(ε-caprolactone) (PCL), enable nerve regeneration, showing comparable results to autografts (Wang and Cai, 2010; Daly, et al. 2012; Kehoe et al., 2012; Nectow et al., 2012). Biodegradable polymers are usually preferred in nerve regeneration to avoid removal post-implantation in long-term applications, but cytotoxic reactions can occur because of degradation byproducts released in the confined environment (Belkas et al., 2004).
An additional challenge in nerve repair is overcoming long gaps (> 3 cm) (Deal et al. 2012). Strategies are being explored with the implementation of new functional groups of biomaterials designed to mimic the biological properties of the native tissue.
Influence of ECM molecules on cell behaviour
The ECM is the acellular component of all tissues and organs, and is composed of two main categories of biomolecules: proteoglycans and fibrous proteins (i.e., collagen, elastin, fibronectin and laminin). It provides a well-defined physical and chemical environment for cell survival and developmental path, determining tissue morphogenesis, differentiation and homeostasis (Frantz et al., 2010), which are highly tissue-specific and markedly heterogeneous.
Surface chemistry of a biomaterial is important for the formation of cell focal adhesion points, which are responsible for integrin binding between cells and substrate, as well as for cell signalling (Keselowsky et al., 2004). More specifically, cell integrins bind to the ECM proteins adsorbed on the substrate by changing the conformation of the α and β subunits, form clusters, and transmit signals to the actin filaments through several protein assemblies (Owen et al., 2005; Geiger et al., 2009). ECM-integrin interactions then trigger the activation of focal adhesion kinase (FAK) and a cascade of phosphorylation reactions follow, leading to the generation of mechanical forces and the transcription and regulation of specific genes, responsible for cell growth and differentiation (Owen et al., 2005; Geiger et al., 2009). In the specific field of peripheral nerve repair, as reviewed by Gardiner, the importance of ECM proteins is already visible at the developmental stage of the nervous system, during which fibronectin is mainly involved in the migration and differentiation of the neural crest cells. In constrast, laminin is the main protein involved in the maturation stage of the peripheral nervous system and appears to be crucial for the SC to successfully myelinate axons (Gardiner, 2011). Xie and Auld (2011) further demonstrated that integrin complexes play an important role in maintaining the ensheathing layer of glial cells around the axons, dictating and modulating the process of myelination.
Adding different biomolecules, i.e., proteins, saccharides, lipids and drugs, to the surface of a substrate is a very common strategy to improve the material biocompatibility and promote/inhibit specific cell responses. Due to their importance during the development and the regeneration of the sensory nervous system, laminin, fibronectin and collagen have been successfully used as coatings of tissue culture plastic and poly-3-hydroxybutyrate mats to enhance Schwann cell (SC) response (Armstrong et al., 2007). Initial attachment of cells was unaffected by neither of the ECM molecules, whilst a remarkable improvement in SC proliferation was observed compared to untreated surfaces, with laminin showing the best results. Cell number was remarkably increased and stimulated neurites sprout from dissociated dorsal root ganglia (DRG) neurons (Armstrong et al., 2007). Results were later confirmed by Gamez Sazo et al. (2012) by culturing DRG explants on gelatin membranes photo-co-immobilized with both fibronectin or laminin, and neurotrophins. Absence of these chemical stimuli limited axon outgrowth, single ECM protein coating increased neurite sprouting, but the best outcome was observed with a synergistic action fibronectin + laminin, resulting in the longest and most robust neurites among the experimental groups. Using a co-culture model with SC and NG108-15 cells, it was demonstrated that the presence of laminin stimulated the phosphorylation of different transcription factors in the SC, promoting growth of longer neurites by the neuronal cells (Armstrong et al., 2008). Recent evidences showed that these molecules not only have beneficial effects on glial and neuronal cells, but also protect stem cells differentiating into SC-phenotype from anoikis (di Summa et al., 2013), encouraging their viability and attachment to the surrounding ECM-simulated environment in both serum-free medium and PBS. Also, this study further confirmed the high DRG neurite sprouting in presence of laminin (as previously shown by Armstrong et al., 2007), which was necessary for the formation and long and branched neurites from the neuronal body. Therefore, ECM-modified scaffolds can be a valid approach in peripheral nerve repair to either improve endogenous cell survival and migration at the injury site or transplant exogenous cells by pre-loading them in a biomimetic conduit. A study involving aligned electrospun nanofibers seem to confirm the important role of fibronectin in regulating SC migration along the topographical cues, causing in turn the deposition of a very well-organised fibronectin matrix (Mukhatyar et al., 2011). Conversely, in vitro cultures in fibronectin-free conditions resulted in significantly reduced cell migration along the topographical cues. However, all these studies mainly focused at the early cell response in vitro. Further evidences showed that after the initial promotion of the tissue regeneration, fibronectin debris might actually hinder the completion of the repairing process (Stoffels et al., 2013). Therefore, fibronectin deposition should be controlled and optimised in order to define highly regenerative systems, reducing risks of tissue obstruction.
Growth factors, i.e., brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), fibroblast growth factor (FGF), glial cell-derived neurotrophic factor (GDNF), nerve growth factor (NGF), neurotrophin-3 (NT-3), have been found to have specific affinity with particular ECM components (Pabari et al., 2011). For example, NT-3 and BDNF have shown good affinity to fibrinogen, but not NGF (Martino et al., 2013). This selective binding property can be quite advantageous when designing drug release constructs. In fact, the specific factors bound to the fibrinogen will be gradually released in the surrounding environment due to gel degradation, stimulating specific functions of the autologous cells. Cao et al. (2011) studied the incorporation of CNTF in laminin-coated collagen scaffolds, recreating a functional drug delivery system for nerve repair. This was achieved by modifying the growth factor sequence with a laminin-binding domain, resulting in a comparable neurotrophic bioactivity, but stronger binding to the laminin coating, and slower and gradual release of the drug compared to the native molecule. When BDNF and NT-3 were supplemented to a gelatine system, their gradual release into the surrounding environment was also beneficial for the attachment and proliferation of SC, and for the neurite sprouting from DRG explants (Gamez Sazo et al., 2012).
Besides ECM components, moieties derived from ECM proteins are considered very selective in cell binding and can be packed at higher density on the substrate (Hersel et al., 2003), leading to more specific cell-material interactions (Figure 1). To date, there is considerable evidence that specific amino acid sequences, such as RGD (Arg-Gly-Asp) (de Luca et al., 2013a, b), IKVAV (Ile-Lys-Val-Ala-Val) (Wei et al., 2007; Hosseinkhani et al., 2013) and YIGSR (Tyr-Ile-Gly-Ser-Arg) (Masaeli et al., 2014), facilitate the interaction between ECM proteins and integrins on cell membranes, and enhance the adhesion and proliferation of neural cells. However, RGD has shown very good affinity with several cell types due to its presence in many ECM proteins and it has been widely investigated for different biomaterials and tissue engineering applications, as reviewed by Perlin et al. (2008). The two laminin-derived pentapeptides have been instead demonstrated to target specific responses of SC (Masaeli et al., 2014) and neurons (Wei et al., 2007; Hosseinkhani et al., 2013), as well as neuron-like differentiated stem cells (Xie et al., 2013). YIGSR-modified nanofibrous scaffolds positively affected SC metabolic activity and stimulated the secretion of neurotrophins in the surrounding environment, including GDNF, BDNF, CTNF and NGF (Masaeli et al., 2014). Hosseinkhani et al. (2013) observed instead that IKVAV-modified collagen matrices promoted DRG neuron responses even when cultured in absence of neurotrophic factors, commonly used for in vitro experiments. In addition, they compared the pentapepetide moiety with a similar but scrambled sequence, resulting in no differences of cell response compared to the untreated material, hence confirming the importance of the specific pentapeptide sequence in peripheral nerve repair. Finally, Xie et al. (2013) demonstrated that the viability of stem cells was remarkably enhanced when in contact with the IKVAV moiety, providing the best environment for their proliferation and differentiation. Remarkable improvements at the cell-biomaterial interface have been reported, defining the importance of the selected material and its physical and mechanical properties in order to achieve effective surface modifications that are positively sensed by cells.
Figure 1.
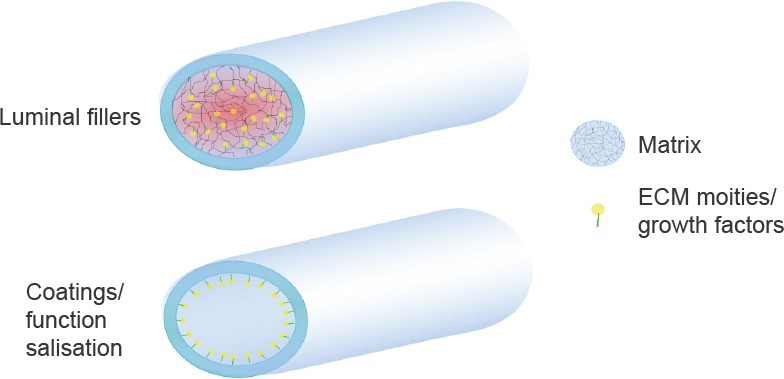
Modifications of the inner lumen of the nerve conduit.
The tube can be filled with a biological matrix, which in turn can incorporate extracellular matrix (ECM) moieties or growth factors to stimulate nerve regeneration. Alternatively, coatings with ECM-derived proteins or functionalization with specific moieties can (bio) mimic the biomaterial surface, enhancing cell response.
In vivo response of modified nerve guidance conduits
The influence of ECM on neural cells demonstrated in vitro was further translated in new approaches for peripheral nerve regeneration. Although peptide modifications of the culture substrates are widely investigated in vitro, not many studies on their effect in vivo are available in literature. An example of the potential application in vivo of functionalised conduits with RGD was shown by Yan et al. (2012). In their study, they bond the peptide moiety to the backbone of a polymer conduit and were successful in repairing a 10 mm injury gap in rats after 3 months. However, the most used approached in peripheral nerve repair can be divided in two categories: the fabrication of neural guidance using ECM components as bulk materials and the use of natural fillers/matrices in the inner lumen of the conduit.
Purified ECM proteins, such as collagen and fibrin, have been extensively reported in peripheral nerve repair due to their ability to support axonal outgrowth until nerve is completely regenerated (Wang and Cai, 2010; Gu et al., 2011; Bell and Haycock, 2012). In fact, as reviewed by Daly et al., (2012), the formation of a fibrin-rich scaffold can be usually observed between the proximal and distal stumps in order to support the following cell migration through an ECM bridge. The natural scaffold forms within 1 week after injury, guiding the migration of SC together with endothelial cells and fibroblasts, to form the bands of Büngner. By using ECM proteins is therefore possible to simulate and engineer the regenerative process as it would naturally occur in vivo.
To date, three collagen conduits are commercially available on the market: NeuraGen® and NeuroFlex® are both made of typeI collagen from bovine but they differ in the protein cross-linking which affects the degradation time of the scaffold, while RevolNerve® is made of typeI and typeIII collagen from porcine skin (Bell and Haycock, 2012; Kehoe, et al. 2012). A recent in vivo study using a rat model with a 10 mm defect confirmed the good interaction of these ECM-derived conduits with glial cells, as pre-loaded SC adhered and homogenously distributed onto the wall of the collagen scaffold (di Summa et al., 2014). No postoperative complications occurred and endogenous SC infiltration was stimulated for more than 2 weeks. However, the maximum length repaired with these conduits is 2–3 cm, with nerve functionality not always recovered. Fibrin devices, on the other hand, lack of consistency and handling capability make them not very suitable for surgery unless co-polymerised with a second material to guarantee valid properties for implantation.
Alternatively, ECM proteins can also be used as transplantation matrices in form of gels to fill artificial conduits, promoting axonal outgrowth and cell proliferation, as well as gradually releasing neurotrophic factors in the confined environment (Pabari et al., 2011; Daly et al., 2012). In this particular case the gel concentration and the conduit porosity are the main parameters that can influence the regenerative process, as they can obstruct the axon outgrowth (Labrador et al., 1998). The most common ECM proteins used for the production of luminal fillers are collagen, laminin, fibrin, heparin and heparin sulphate (Pabari et al., 2011). Nakayama et al. (2007) demonstrated that, conduits filled with fibrin gel allowed nerve regeneration and myelination after 4-week implantation in rats and promoted earlier axonal outgrowth compared to empty tubes. Fibrin was also combined with agarose to provide a better matrix performance in vivo and transplant exogenous cells in a 12 week experiment (Carriel et al., 2013). In particular, the property to pre-load cells in the conduit lumen with this approach appeared to be very advantageous, resulting in higher levels of axon regeneration and myelination, and better organisation of the cell-deposited ECM. Nevertheless, evidence show that fibrin maintains SC in a non-myelinating state (Akassoglou et al., 2002) and therefore the degradation time of the luminal gel should be optimized in order to trigger axon myelination in due time during the regeneration process. In addition, McGrath et al. (2010) demonstrated that pre-seeded SC into BD™PuraMatrix™ hydrogels were able to survive both in culture and after transplantation into the nerve defect. The cells remarkably stimulated the axonal outgrowth in the injury gap entering the proximal stump after 3 weeks in a highly aligned configuration. In fact, the encapsulated cells are able to remodel the matrix by secreting more ECM molecules and align in similar structures to the actual Bands of Büngner of autologous nerves (Suri and Schmidt, 2010).
However, further modification of the chemical composition of these fillers with additional ECM-derived components may be necessary to fasten regeneration of the transacted nerve. Labrador et al. (1998) demonstrated that the addition of laminin to collagen-based gels stimulates earlier regeneration at 2 weeks post-implantation in 4 mm gaps (Labrador et al., 1998). By changing matrix concentration and density, it is possible to modulate the degradation time of the filler, optimising the regenerating environment for better outcomes. It appears indeed that a combination of collagen, laminin and fibronectin as filler for a silicon tube induced high levels of regeneration after 6 weeks in a 10 mm gap compared to gels without ECM molecules (Chen et al., 2000). Functionalisation of the fillers with ECM molecules, such as laminin, also supported the regeneration of a sciatic nerve up to 80 mm in a dog model, confirming the important role played by these biomolecules in nerve regeneration (Matsumoto et al., 2000). However, tissue recovery was not entirely completed as demonstrated by histological and electrophysiological investigations, and future optimisation of the “conduit + filler + ECM molecules” construct is in high demand. More recently, a novel self-assembled peptide amphiphile (PA) gel further functionalised with the amino acid sequence RGDS was developed to fill the empty cavity of poly(lactic-co-glycolic acid) (PLGA) conduits, resulting in high motor function recovery comparable to the positive control of autografts (Li et al., 2014).
Further modification of the ECM proteins involves growth factors binding through specific functional groups and/or electrostatic interactions, which are then gradually released in the surrounding environment. Such composite tissue engineered constructs can induce faster axonal regeneration and higher compound muscle action potential (CMAP) (Jin et al., 2013). The type and the loading concentration of growth factors in the conduit filler, and the mechanism of drug delivery need to be optimised in order to guarantee a long-term effect on regeneration and functional recovery of the target organ.
Future perspectives
The latest results achieved in the field of peripheral nerve regeneration are promising, although long gaps are still hard to recover. New approaches currently under consideration include diverse modifications of the inner lumen of the artificial conduits with physical cues that could potentially guide the regenerative process in the confined environment. Although it was demonstrated that SC align in ECM fillers as it would happen in the natural endoneurial structure of an autologous nerve, pre-alignment of the ECM components with magnetic field would provide a guiding pattern to pre-orient the cells in the conduit and accelerate the regeneration process, as suggested by previous preliminary results (Rosner et al., 2005). A different approach may be the addition of micro-fibres or -filaments in the conduit lumen, which also enhance the scaffold permeability, reducing the risk of cell necrosis in the centre of the nerve guidance (Gu et al., 2011). These micro-filaments can be then further coated with ECM components in order to enhance cell-surface interactions, hence promoting higher regeneration of the injured tissue (Tong et al., 1994).
Footnotes
Funding: The project was supported by the Swiss National Fund (Fonds National Suisse de la Recherche Scientifique).
References
- 1.Akassoglou K, Yu WM, Akpinar P, Strickland S. Fibrin inhibits peripheral nerve remyelination by regulating Schwann cell differentiation. Neuron. 2002;33:861–875. doi: 10.1016/s0896-6273(02)00617-7. [DOI] [PubMed] [Google Scholar]
- 2.Aldini NN, Fini M, Rocca M, Giavaresi G, Giardino R, Nicoli Aldini N. Guided regeneration with resorbable conduits in experimental peripheral nerve injuries. Int Orthop. 2000;24:121–125. doi: 10.1007/s002640000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong SJ, Wiberg M, Terenghi G, Kingham PJ. ECM molecules mediate both Schwann cell proliferation and activation to enhance neurite outgrowth. Tissue Eng. 2007;13:2863–2870. doi: 10.1089/ten.2007.0055. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong SJ, Wiberg M, Terenghi G, Kingham PJ. Laminin activates NF-kappaB in Schwann cells to enhance neurite outgrowth. Neurosci Lett. 2008;439:42–46. doi: 10.1016/j.neulet.2008.04.091. [DOI] [PubMed] [Google Scholar]
- 5.Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance. Neurol Res. 2004;26:151–160. doi: 10.1179/016164104225013798. Neurol Res 26:151-160. [DOI] [PubMed] [Google Scholar]
- 6.Bell JH, Haycock JW. Next generation nerve guides: materials fabrication growth factors and cell delivery. Tissue Eng Part B. 2012;18:116–128. doi: 10.1089/ten.TEB.2011.0498. [DOI] [PubMed] [Google Scholar]
- 7.Bellamkonda RV. Peripheral nerve regeneration: an opinion on channels scaffolds and anisotropy. Biomaterials. 2006;27:3515–3518. doi: 10.1016/j.biomaterials.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16:1–7. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 9.Cao J, Sun C, Zhao H, Xiao Z, Chen B, Gao J, Zheng T, Wu W, Wu S, Wang J, Dai J. The use of laminin modified linear ordered collagen scaffolds loaded with laminin-binding ciliary neurotrophic factor for sciatic nerve regeneration in rats. Biomaterials. 2011;32:3939–3948. doi: 10.1016/j.biomaterials.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Carriel V, Garrido-Gómez J, Hernández-Cortés P, Garzón I, García-García S, Sáez-Moreno JA, Del Carmen Sánchez-Quevedo M, Campos A, Alaminos M. Combination of fibrin-agarose hydrogels and adipose-derived mesenchymal stem cells for peripheral nerve regeneration. J Neural Eng. 2013;10:026022. doi: 10.1088/1741-2560/10/2/026022. [DOI] [PubMed] [Google Scholar]
- 11.Chen YS, Hsieh CL, Tsai CC, Chen TH, Cheng WC, Hu CL, Yao CH. Peripheral nerve regeneration using silicone rubber chambers filled with collagen laminin and fibronectin. Biomaterials. 2000;21:1541–1547. doi: 10.1016/s0142-9612(00)00028-4. [DOI] [PubMed] [Google Scholar]
- 12.Chernousov MA, Carey DJ. Schwann cell extracellular matrix molecules and their receptors. Histol Histopathol. 2000;15:593–601. doi: 10.14670/HH-15.593. [DOI] [PubMed] [Google Scholar]
- 13.Daly W, Yao L, Zeugolis D, Windebank A, Pandit A. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. Interface. 2012;9:202–221. doi: 10.1098/rsif.2011.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deal DN, Griffin JW, Hogan MV. Nerve conduits for nerve repair or reconstruction. J Am Acad Orthop Surg. 2012;20:63–68. doi: 10.5435/JAAOS-20-02-063. [DOI] [PubMed] [Google Scholar]
- 15.de Luca AC, Faroni A, Downes S, Terenghi G. Differentiated adipose-derived stem cells act synergistically with RGD-modified surfaces to improve neurite outgrowth in a co-culture model. J Tissue Eng Regen Med. 2013a doi: 10.1002/term.1804. doi: 10.1002/term.1804. [DOI] [PubMed] [Google Scholar]
- 16.de Luca AC, Stevens JS, Schroeder SL, Guilbaud JB, Saiani A, Downes S, Terenghi G. Immobilization of cell-binding peptides on poly-epsilon-caprolactone film surface to biomimic the peripheral nervous system. J Biomed Mat Res Part A. 2013b;101:491–501. doi: 10.1002/jbm.a.34345. [DOI] [PubMed] [Google Scholar]
- 17.di Summa PG, Kalbermatten DF, Raffoul W, Terenghi G, Kingham PJ. Extracellular matrix molecules enhance the neurotrophic effect of Schwann cell-like differentiated adipose-derived stem cells and increase cell survival under stress conditions. (368-379).Tissue Eng Part A. 2013:3–4. doi: 10.1089/ten.tea.2012.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.di Summa PG, Kingham PJ, Campisi CC, Raffoul W, Kalbermatten DF. Collagen (NeuraGen®) nerve conduits and stem cells for peripheral nerve gap repair. Neurosci Lett. 2014;572:26–31. doi: 10.1016/j.neulet.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 19.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamez Sazo RE, Maenaka K, Gu W, Wood PM, Bartlett Bunge M. Fabrication of growth factor- and extracellular matrix-loaded gelatin-based scaffolds and their biocompetibility with Schwann cells and dorsal root ganglia. Biomaterials. 2012;33:8529–8539. doi: 10.1016/j.biomaterials.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardiner NJ. Integrins and the extracellular matrix: key mediators of development and regeneration of the sensory nervous system. Dev Neurobiol. 2011;71:1054–1072. doi: 10.1002/dneu.20950. [DOI] [PubMed] [Google Scholar]
- 22.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 23.Geuna S, Tos P, Titolo P, Ciclamini D, Beningo T, Battiston B. Update on nerve repair by biological tubulization. J Brachial Plex Peripher Nerve Inj. 2014;9:3. doi: 10.1186/1749-7221-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu X, Ding F, Yang Y, Liu J. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol. 2011;93:204–230. doi: 10.1016/j.pneurobio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 26.Hosseinkhani H, Hiraoka Y, Li CH, Chen YR, Yu DS, Hong PD, Qu KL. Engineering three-dimensional collagen-IKVAV matrix to mimic neural microenvironment. Chem Neurosci. 2013;4:1229–1235. doi: 10.1021/cn400075h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin J, Limburg S, Joshi SK, Landman R, Park M, Zhang Q, Kim HT, Kuo AC. Peripheral nerve repair in rats using composite hydrogel-filled aligned nanofiber conduits with incorporated nerve growth factor. Tissue Eng Dart A. 2013;19:2138–2146. doi: 10.1089/ten.tea.2012.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaye AH. 3rd edition. Wiley-Blackwell; 2005. Essential Neurosurgery; p. 312. [Google Scholar]
- 29.Kehoe S, Zhang XF, Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43:553–572. doi: 10.1016/j.injury.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 30.Keselowsky BG, Collard DM, García AJ. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials. 2004;25:5947–5954. doi: 10.1016/j.biomaterials.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 31.Kingham PJ, Terenghi G. Bioengineered nerve regeneration and muscle reinnervation. J Anat. 2006;209:511–526. doi: 10.1111/j.1469-7580.2006.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labrador RO, Butí M, Navarro X. Influence of collagen and laminin gels concentration on nerve regeneration after resection and tube repair. Exp Neurol. 1998;149:243–252. doi: 10.1006/exnr.1997.6650. [DOI] [PubMed] [Google Scholar]
- 33.Lassner F, Becker M, Berger A. Degeneration and regeneration in nerve autografts and allografts. Microsurgery. 1995;16:4–8. doi: 10.1002/micr.1920160104. [DOI] [PubMed] [Google Scholar]
- 34.Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243–252. doi: 10.5435/00124635-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Li A, Hokugo A, Yalom A, Berns EJ, Stephanopoulos N, McClendon MT, Segovia LA, Spigelman I, Stupp SI, Jarrahy R. A bioengineered peripheral nerve construct using aligned peptide amphiphile nanofibers. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2014.06.049. doi: 10.1016/jbiomaterials201406049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci U S A. 2013;110:4563–4568. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masaeli E, Wieringa PA, Morshed M, Nasr-Esfahani MH, Sadri S, van Blitterswijk CA, Moroni L. Peptide functionalised polyhydroxyalkanoate nanofibrous scaffolds enhance Schwann cells activity. Nanomed Nanotechn 2014. 2014;xx:1–11. doi: 10.1016/j.nano.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto K, Ohnishi K, Kiyotani T, Sekine T, Ueda H, Nakamura T, Veda H, Nakamura T, Endo K, Shimizu Y. Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)-collagen tube filled with laminin-coated collagen fibers: a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 2000;868:315–328. doi: 10.1016/s0006-8993(00)02207-1. [DOI] [PubMed] [Google Scholar]
- 39.McGrath AM, Novikova LN, Novikov LN, Wiberg M. BDTM PuraMatrixTM peptide hydrogel seeded with Schwann cells for peripheral nerve regeneration. Brain Res Bull. 2010;83:207–213. doi: 10.1016/j.brainresbull.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Mukhatyar VJ, Salmeron-Sanchez M, Rudra S, Mukhopadaya S, Barker TH, Garcia AJ, Bellamkonda RV. Role of fibronectin in topographical guidance of neurite extension on electrospun fibers. Biomaterials. 2011;32:3958–3968. doi: 10.1016/j.biomaterials.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakayama K, Takakuda K, Koyama Y, Itoh S, Wang W, Mukai T, Shirahama N. Enhancement of peripheral nerve regeneration using bioabsorbable polymer tubes packed with fibrin gel. Artif Organs. 2007;31:500–508. doi: 10.1111/j.1525-1594.2007.00418.x. [DOI] [PubMed] [Google Scholar]
- 42.Nectow AR, Marra KG, Kaplan DL. Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng Part B. 2012;18:40–50. doi: 10.1089/ten.teb.2011.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Owen GR, Meredith DO, ap Gwynn I, Richards RG. Focal adhesion quantification - a new assay of material biocompatibility? Review. Eur Cell Mater. 2005;9:85–96. doi: 10.22203/ecm.v009a10. [DOI] [PubMed] [Google Scholar]
- 44.Pabari A, Yang SY, Mosahebi A, Seifalian AM. Recent advances in artificial nerve conduit design: strategies for the delivery of luminal fillers. J Control Release. 2011;156:2–10. doi: 10.1016/j.jconrel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Perlin L, MacNeil S, Rimmer S. Production and performance of biomaterials containing RGD peptides. Soft Matter. 2008;4:2331. [Google Scholar]
- 46.Rosner BI, Hang T, Tranquillo RT. Schwann cell behavior in three-dimensional collagen gels: evidence for differential mechano-transduction and the influence of TGF-beta 1 in morphological polarization and differentiation. Exp Neurol. 2005;195:81–91. doi: 10.1016/j.expneurol.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 48.Siemionow M, Bozkurt M, Zor F. Regeneration and repair of peripheral nerves with different biomaterials: review. Microsurgery. 2010;30:574–588. doi: 10.1002/micr.20799. [DOI] [PubMed] [Google Scholar]
- 49.Siemionow M, Brzezicki G. Chapter 8: Current techniques and concepts in peripheral nerve repair. Int Rev Neurobiol. 2009;87:141–172. doi: 10.1016/S0074-7742(09)87008-6. [DOI] [PubMed] [Google Scholar]
- 50.Stoffels JM, Zhao C, Baron W. Fibronectin in tissue regeneration: timely disassembly of the scaffold is necessary to complete the build. Cell Mol Life Sci. 2013;70:4243–4253. doi: 10.1007/s00018-013-1350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suri S, Schmidt CE. Cell-laden hydrogel constructs of hyaluronic acid collagen and laminin for neural tissue engineering. Tissue Eng Part A. 2010;16:1703–1716. doi: 10.1089/ten.tea.2009.0381. [DOI] [PubMed] [Google Scholar]
- 52.Tong XJ, Hirai K, Shimada H, Mizutani Y, Izumi T, Toda N, Yu P. Sciatic nerve regeneration navigated by laminin-fibronectin double coated biodegradable collagen grafts in rats. Brain Res. 1994;663:155–162. doi: 10.1016/0006-8993(94)90473-1. [DOI] [PubMed] [Google Scholar]
- 53.Wang S, Cai L. Polymers for fabricating nerve conduits. Int J Polym Sci. 2010;2010:1–20. [Google Scholar]
- 54.Wei YT, Tian WM, Yu X, Cui FZ, Hou SP, Xu QY, Lee IS. Hyaluronic acid hydrogels with IKVAV peptides for tissue repair and axonal regeneration in an injured rat brain. Biomed Mater. 2007;2:S142–S146. doi: 10.1088/1748-6041/2/3/S11. [DOI] [PubMed] [Google Scholar]
- 55.Wiberg M, Terenghi G. Will it be possible to produce peripheral nerves? Surg Technol Int. 2003;11:303–310. [PubMed] [Google Scholar]
- 56.Xie F, Li QF, Gu B, Liu K, Shen GX. In vitro and in vivo evaluation of a biodegradable chitosan-PLA composite peripheral nerve guide conduit material. Microsurgery. 2008;28:471–479. doi: 10.1002/micr.20514. [DOI] [PubMed] [Google Scholar]
- 57.Xie H, Li J, Li L, Dong Y, Chen GQ, Chen KC. Enhanced proliferation and differentiation of neural stem cells grown on PHA films coated with recombinant fusion proteins. Acta Biomater. 2013;9:7845–7854. doi: 10.1016/j.actbio.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 58.Xie X, Auld VJ. Integrins are necessary for the development and maintenance of the glial layers in the Drosophila peripheral nerve. Development. 2011;138:3813–3822. doi: 10.1242/dev.064816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan Q, Yin Y, Li B. Use new PLGL-RGD-NGF nerve conduits for promoting peripheral nerve regeneration. Biomed Eng Online. 2012;11:36. doi: 10.1186/1475-925X-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]


