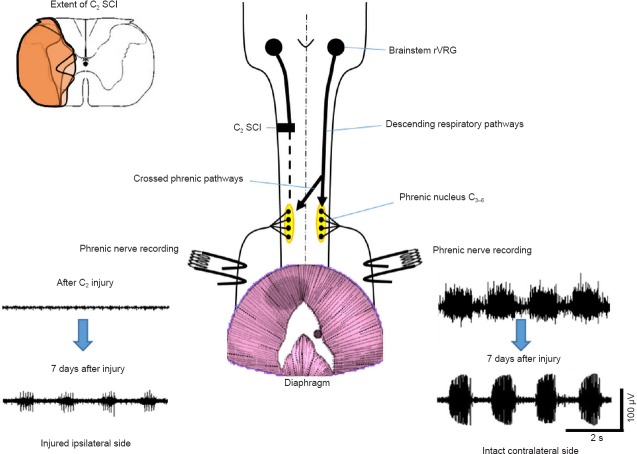
Traumatic cervical spinal cord injury (SCI), with an annual incidence of 12,000 new cases in USA (NSCISC 2013), causes devastating locomotor and respiratory paralysis and unfortunately compromises the human patient's lifespan. The severity of the injury depends on the degree and the extent of the initial trauma. In fact, respiratory failure is the leading cause of mortality following upper cervical SCI. However, 80% of the injuries are incomplete, allowing some modest spontaneous recovery. To date, no effective treatment is available in order to restore the loss of function. The only existing therapy is to place the patient under ventilatory assistance. Few patients can be weaned off the ventilatory assistance, mostly due to spontaneous recovery which occurs with post-lesional delay. Thus, enhancing spontaneous plasticity may be of great importance, or at least of a more immediate goal, than regeneration of spinal tracts since the majority of spinal injuries are incomplete. The necessity of having a preclinical model that combines respiratory insufficiency with spared non-functional respiratory pathways is crucial in order for scientists to study putative therapeutics. An incomplete cervical SCI allows partial recovery of the respiratory and somatic motor functions due to spontaneous (but limited) plasticity in spared spinal synaptic pathways. Keomani and colleagues propose a rodent model of cervical spinal cord injury to study post-lesional respiratory neuroplasticity (Keomani et al., 2014). This model consists in doing a surgical cervical dorso-ventral hemisection at the C2 level. The cervical hemisection induces a disruption of the main bulbospinal respiratory pathways, leading to a deafferentation of the phrenic motoneuron pool on the ipsilateral side of the injury. This induces an abolition of phrenic nerve activity and the subsequent diaphragm paralysis, resulting in a persistent impairment of breathing capacity (Dougherty et al., 2012). The contralateral side remains intact and allows the survival and recovery of the animal (Figure 1). Despite the fact that the main respiratory pathways are injured, the integrity of the phrenic motoneuron pools on both sides are preserved. Following the C2 hemisection, a slight spontaneous recovery can be recorded on the ipsilateral side (phrenic nerve and diaphragm activities), mainly due to activation of contralateral silent synaptic pathways that cross the spinal midline at the segmental level C3–6 (from contralateral spared side to ipsilateral injured side, Figure 1). This activation, known as Crossed Phrenic Phenomenon (CPP), is sustained by the CPP pathways and leads to a partial reactivation of the ipsilateral phrenic nerve and diaphragm activities; such activation can be observed from hours to weeks post-injury (Goshgarian, 2009; Vinit and Kastner, 2009). The real beneficial effect of these CPP pathways on the respiratory recovery is limited and further studies must be conducted to improve the magnitude of the observed spontaneous recovery (Dougherty et al., 2012). As written in (Keomani et al., 2014), this C2 hemisection model to study post-lesional respiratory neuroplasticity and the application of putative strategies to improve/restore the respiratory insufficiency induced by the injury itself represents numerous advantages: 1) rats are readily available from commercial breeders around the world; 2) because of their small size and short lifespan, environmental conditions can be carefully monitored and strictly controlled from birth to adulthood; 3) rats have become the premier model of respiratory neurobiology, replacing the more traditional model, cats. Accordingly, large amounts of information/extensive data are available in the literature concerning rat neuroanatomy, neurochemistry, neurophysiology and reflex ventilatory responses, providing context in which to perform and interpret the experimental results; 4) the (relatively) low genetic heterogeneity among commercially available rat strains allows the reduction in the number of animals required to achieve statistical difference, and facilitates the comparison of results between different laboratories; 5) rats have a very low mortality rate following cervical spinal cord injury which reduces the number of animals required for statistical difference; 6) rats have a very rapid motor recovery rate following cervical spinal cord injury when compared to cats, dogs or primates. Accordingly, the use of rats reduces the length of time the animal requires post-surgical intensive care (e.g., bladder expression, fluid administration, etc.), and minimizes animal subject distress; 7) The injury is restricted to projections on one side of the spinal cord, allowing investigation of an interesting population of spinal synaptic pathways that cross the spinal midline and innervate phrenic motoneurons. The key aspect of this rodent model is the CPP. This particular topic has an extensive published literature using rats as a model and many of the publications have come from members of our laboratory; 8) Rats and humans share many common features in their respiratory control system, which make the rat a good preclinical model to study respiratory insufficiency following cervical SCI. The last advantage of using the C2 SCI model to study respiratory neuroplasticity is that that function does not require animal motivation and is easily quantifiable. Unlike the locomotor function, which requires trained scientists to observe and report the animal's performance on a defined scale (BBB score or grid walking for example), the respiratory function is always evaluated with metrics (frequency, amplitude, volumes). The evaluation can be done in awakened and unrestrained animals (plethysmography, telemetry) in different conditions (respiratory challenges) to anesthetized preparation (electrophysiological recordings of phrenic nerve and diaphragm activities). The range of evaluation make the respiratory function one of the best system for studying post-lesional neuroplasticity. This C2 SCI model has been already used to study various types of plasticity and putative therapeutic strategies. For example, the deafferented phrenic motoneurons and identified pre-motoneurons located into the brainstem (rVRG nucleus) have been studied to elucidate the molecular and cellular changes in response to a C2 SCI (Vinit and Kastner, 2009). Moreover, a better comprehension of the subsequent inflammatory processes and cytoarchitectural changes (perineuronal net changes) can help in understanding and developing therapies in appropriate post-lesion windows (Alilain et al., 2011). The spinal structural changes (implication of substitutive pathways and the involvement of spinal interneurons (Lane, 2011)) and/or the ultrastructural changes at the diaphragm motor end plate (Mantilla and Sieck, 2009) also actively participate in the spontaneous restoration of the respiratory activity following a C2 SCI. The physiological consequences of the initial injury on the entire respiratory system (Tidal volume, frequency in non-anesthetized animals) and its subsequent spontaneous recovery (on anesthetized preparations i.e., phrenic nerve activity, diaphragm activity and more recently, the intercostal activity) sustained by the spared and CPP pathways are one of the most studied topics on this particular C2 SCI model. Recently, this C2 SCI rodent model has also been used to study respiratory and hindlimb impairment and the subsequent spontaneous recovery and induced recovery following a non-invasive strategy (Intermittent hypoxias), and a successful translational application has been conducted in spinal injured patients (Lovett-Barr et al., 2012). But the most impressive result obtained with this model is a total functional restoration of the respiratory activity by grafting a peripheral nerve into the spinal cord to bypass the injury site, combined with chondroitinase ABC treatment to further ameliorate nerve insertion and axonal regrowth (Alilain et al., 2011).
Figure 1.
Schematic view of the C2 spinal cord injury (SCI) pre-clinical model.
A C2 SCI (extent represented in orange for 3 representative animals, top left corner) induces a deafferentation of the descending respiratory pathways to the phrenic nucleus on the injured ipsilateral side, inducing an abolition of the rhythmic phrenic nerve activity. The contralateral side remains intact and allows the animal to survive. 7 days after the injury, a partial reactivation of the phrenic nerve activity is observed on the ipsilateral side, mainly due to the activation of the crossed phrenic pathways. Adapted from Keomani et al. (2014).
However, despite the extensive results published in the literature and the growing community of scientists using this model, some limitations must be discussed in this perspective article. The crucial point, when developing therapeutic strategies, is the functional outcome without adverse effects. The respiratory deficit induced by a C2 SCI in this rodent model is correlated with the size of the injury, and is experimenter dependent since this injury is surgically “handmade”. A meticulous reconstruction of the injury for each subject is needed to evaluate the putative effect of therapeutics on the functional outcome (Vinit and Kastner, 2009). Another point of discussion is the surgery process itself. Since each animal is unique, the vascularity at the site of injury, the anesthetics and even the animal's hydration could lead to more harm to the spinal cord by inducing more secondary damages (Warren and Alilain, 2014). Post-surgical recovery has also to be strict and appropriate since the animals have trouble accessing food and water in the first days post-surgery. Cautiousness should be used when comparing the results of different laboratories from the literature. Numerous factors can introduce variability (such as animal gender, age and substrain) and can lead to misinterpretation of the results and be problematic when assessing the effect of putative therapeutic strategies on this SCI model (Steward et al., 2012). Moreover, one laboratory has started to successfully develop a C2 hemisection on a mouse model (Minor et al., 2006). This approach provides great enthusiasm about the future use of transgenic animals, but more investigations are needed to show the full reproducibility of the results. A more clinically relevant animal model is a contusive injury at the cervical level (Golder et al., 2011). However, the reproducibility of the injury is unfortunately inconsistent, mainly due to the location of the descending respiratory pathways and the impracticality to do an extensive contusion (which will drastically reduce the survival rate of the animals). More in-depth work has to be done on the elaboration of contusive models in the cervical spinal cord to determine the appropriate way to induce an injury with permanent deficits.
To conclude, C2 SCI rodent model is a powerful and useful pre-clinical model to study respiratory and non-respiratory neuroplasticity. Nonetheless, this model requires to be thoroughly investigated by many more laboratories and further used in the study of various post-lesional neuroplasticity processes. This model may allow rapid advancement in developing new putative therapeutic strategies that will improve the respiration in SCI patients.
The laboratory is supported by funding from the European Union Seventh framework Programme (FP7/2007-2013) under grant agreement No. 246556 (European project RBUCE-UP), HandiMedEx allocated by the French Public Investment Board, the Chancellerie des Universités de Paris (Legs Poix), the « Centre d’Assistance Respiratoire à Domicile d’Ile de France (CARDIF) » and the « Fonds de Dotation de Recherche en Santé Respiratoire ».
References
- 1.Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475:196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dougherty BJ, Lee KZ, Lane MA, Reier PJ, Fuller DD. Contribution of the spontaneous crossed-phrenic phenomenon to inspiratory tidal volume in spontaneously breathing rats. J Appl Physiol. 2012;112:96–105. doi: 10.1152/japplphysiol.00690.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golder FJ, Fuller DD, Lovett-Barr MR, Vinit S, Resnick DK, Mitchell GS. Breathing patterns after mid-cervical spinal contusion in rats. Exp Neurol. 2011;231:97–103. doi: 10.1016/j.expneurol.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goshgarian HG. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir Physiol Neurobiol. 2009;169:85–93. doi: 10.1016/j.resp.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keomani E, Deramaudt TB, Petitjean M, Bonay M, Lofaso F, Vinit S. A murine model of cervical spinal cord injury to study post-lesional respiratory neuroplasticity. J Vis Exp. 2014 doi: 10.3791/51235. doi: 10.3791/51235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane MA. Spinal respiratory motoneurons and interneurons. Respir Physiol Neurobiol. 2011;179:3–13. doi: 10.1016/j.resp.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci. 2012;32:3591–3600. doi: 10.1523/JNEUROSCI.2908-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantilla CB, Sieck GC. Neuromuscular adaptations to respiratory muscle inactivity. Respir Physiol Neurobiol. 2009;169:133–140. doi: 10.1016/j.resp.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minor KH, Akison LK, Goshgarian HG, Seeds NW. Spinal cord injury-induced plasticity in the mouse--the crossed phrenic phenomenon. Exp Neurol. 2006;200:486–495. doi: 10.1016/j.expneurol.2006.02.125. [DOI] [PubMed] [Google Scholar]
- 10.Steward O, Popovich PG, Dietrich WD, Kleitman N. Replication and reproducibility in spinal cord injury research. Exp Neurol. 2012;233:597–605. doi: 10.1016/j.expneurol.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Vinit S, Kastner A. Descending bulbospinal pathways and recovery of respiratory motor function following spinal cord injury. Respir Physiol Neurobiol. 2009;169:115–122. doi: 10.1016/j.resp.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Warren PM, Alilain WJ. The challenges of respiratory motor system recovery following cervical spinal cord injury. Prog Brain Res. 2014;212:173–220. doi: 10.1016/B978-0-444-63488-7.00010-0. [DOI] [PubMed] [Google Scholar]