Bacteriophages (phages) are viruses that infect bacterial cells and hijack the metabolism of the host cells for replication and amplification of their genetic information. These bacterial viruses are known to be the most numerous life forms on earth. Recent metagenomic analyses have revealed the vast untapped diversity of bacteriophages reinforcing the idea that our current knowledge on the phage world indicates only the tip of the iceberg (Clark, 2012). The discovery of bacteriophages dramatically expanded the scope of applications of viruses in biotechnology and biomedicine.
Bacteriophages as viral nanomaterials
Filamentous phages including f1, fd and more importantly M13 are the most widely used phage systems for biomedical applications. M13 bacteriophages exist as filamentous rod-shaped nanofibers with a diameter of 6.5 nm. The phage capsid – a protein shell surrounding the phage genome – can be viewed as a nanocarrier able to deliver the phage genetic information from one cell to another. These rodlike naturally-occurring viruses represent potential to serve as promising biological building blocks for the development of a variety of functional nanomedicines and nanostructured materials. Several characteristics render filamentous bacteriophages ideal for nanotechnology applications. These phage virions have the capacity to self-assemble into nanoscale structures. Self-assembly (self-fabrication) means that major part of the structural information present in the phage capsid results from capsid proteins themselves and does not require the involvement of other proteins. Also, viral capsids are robust and monodisperse (Yang et al., 2013). However, the greatest advantage offered by filamentous bacteriophages for nanotechnological purposes is their huge genetic flexibility that paves the way for introducing a wide range of modifications on their surface. These manipulations allow for building nanomaterials with potential use in different areas of biotechnology and biomedicine.
The genetic engineering of phage nanoparticles is performed through phage display and provides the possibility for exerting enormous control on the phage particles for biomedical purposes. Phage display forms the cornerstone of phage nanotechnology. The emergence of phage display was the starting point for making use of bacteriophages as modern biotechnology tools and ushered these nanoparticles to previously unthought areas. George P. Smith is recognized as the patriarch of phage display who pioneered the use of this molecular technique for functional expression of foreign molecules on the phage surface (Smith, 1985). In phage display, foreign oligonucleotide sequences are cloned in-frame into a specific location in one of the phage coat protein genes. This leads to the expression of exogenous oligonucleotide-encoded amino acids as a segment of the corresponding coat protein while the physiological function and viability of the phage virion are maintained. The hybrid fusion protein, containing displayed foreign peptide, is ready for making interactions with external molecules. The fundamental concept underlying phage display technology is the direct physical linkage between the phenotype (e.g., a peptide) produced by a phage nanoparticle and its matching genotype (peptide-encoding DNA).
The generation of nanomaterial libraries provided new perspectives to the field of phage nanotechnology. These libraries present a highly diverse population of molecules (billions of peptides) with clinically relevant properties. The procedure of combinatorial search in these libraries is regarded as a type of biological selection - inspired by natural evolutionary screening processes - that occurs in the test tube. This in vitro screening is carried out with the ultimate goal of selecting peptides that can preferentially bind to a target agent. The library is screened through an affinity selection-based methodology called panning (Bakhshinejad et al., 2014). In this strategy, the engineered phage library is incubated with the target structure for a certain period of time. This target can be a biomolecule (protein, antigen, etc), a cell or tissue type (e.g., nerve cells/tissues), or an inorganic material. The target operates as a selector for rapid enrichment of target-specific clones in the library. Afterwards, the target undergoes a series of stringent washing steps to eliminate the unbound or weakly-bound phage particles. Tight binders are then eluted from the target and amplified by infecting host bacterial cells (Figure 1). These amplified target-binding phages enter the next round of screening. In this context, biopanning is a cyclic repetitive multistep procedure. After three to five rounds of affinity selection, a small number of phage clones displaying rare guest peptides with the highest binding affinity for the target are obtained. The primary structure of target-specific peptides is determined through DNA sequencing of the selected phages. Bacteriophages are antagonistic to bacteria and do not indicate tropism for mammalian cells. As a result, when panning is conducted on cellular targets the phages (obtained from the last round) either tightly bind to the cell surface or are internalized into the cytoplasm. Since the only distinguishing feature between different phages of the library is surface-displayed peptide, this peptide is the factor that determines which phages show preferential binding to a certain cell type.
Figure 1.
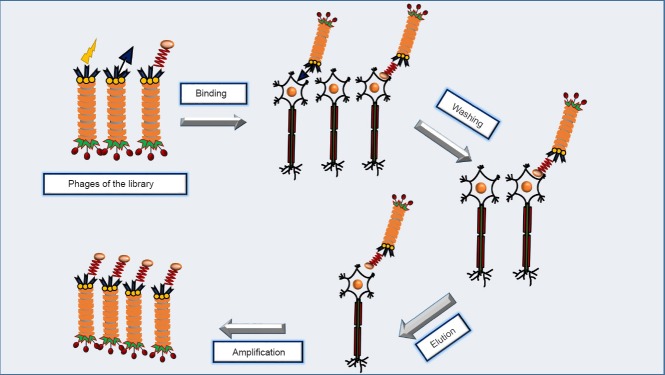
Screening of a phage peptide library on neuron as the target.
After binding of some phages of the library to neurons, multiple steps of stringent washing result in the removal of weakly-bound or unbound phages. Phages with tight binding to neurons are eluted and amplified for the next round. Several rounds of biopanning ultimately lead to the isolation of phages that show specific binding to neurons.
Nanofilamentous bacteriophages as neural tissue engineering scaffolds
One of the most important applications of bacteriophage nanoparticles in neural regeneration is development of biomimetic scaffolds for tissue engineering purposes. Neural tissue engineering is driven by the major goal of fabricating bioactive constructs with capability to offer an ideal substitute for the repair and regeneration of injured tissues in the nervous system. This strategy is conducted to achieve restoration of the complex functions of natural neural tissues. Due to intricacies in the structure and function of the nervous system, there is an obvious need to harness the power of multidisciplinary approaches for building ideally engineered neural tissue scaffolds. Keeping this in mind, the field of tissue engineering has undergone a remarkable metamorphosis within the last decades and this scientific territory has been taken to a new level of sophistication. Based on these dramatic advances, the focus has shifted from simple cultures of nerve cells to meticulously bioengineered constructs that mimic different structural and functional characteristics of natural tissues (Zhu et al., 2014).
Over the recent years, phage nanoparticles particularly M13 have received attention for the design and synthesis of nanomaterial scaffolds for neural tissue engineering. M13 nanofibers harbor several hallmark features that make them intriguing building blocks for use in tissue engineering. The ability of M13 virions to undergo genetic engineering and expression of peptide-based functional information on their surface leads to the display of high densities of signaling peptides along the surface of the nanofibrous phage. Also, it is possible to simultaneously display multiple signaling peptide motifs on different surface proteins of the phage. A large number of identical phage building blocks can be conveniently produced via amplification on a bacterial host. Phages do not have the intrinsic tropism for mammalian cells, thereby indicating a remarkable safety profile for clinical applications. The capacity of M13 phages to self-assemble into directionally ordered nanofilamentous structures provides a useful means for tuning physicochemical cues. Hence, directing the growth of nerve cells can be achieved. The long rod shape and monodispersity of M13 nanofibers result in the fabrication of different self-organized two-dimensional and three-dimensional structures at the nanoscale (Farr et al., 2014). The scaffolds, tailor made for regeneration of the injured neural tissues, provide an artificial microenvironment that is able to promote the growth and proliferation of neurons and control their cellular fate and function such as secreting inductive factors underlying axonal elongation. The establishment of these tissue scaffolds is fully inspired by the natural environment of nerve cells in the body. For the design of an idealized tissue engineering nanomaterial, properties of in vivo cellular environment - in which neuronal cells come into close contact with other cells - and extracellular matrix (ECM) of the nervous tissue are considered as fundamental factors. The ECM is made up of an interwoven reticulum of fibrillar proteins. These filamentary matrices form self-organized and self-arranged structures that create mechanical and chemical cues for controlling and guiding the behavior of nerve cells. The ECM component of bioengineered neural tissue constructs should precisely and closely be similar to the structure of natural ECM (Chung et al., 2010).
Phage peptide libraries and development of nanomaterials for neural regeneration
One of the most significant prospects of bacteriophage nanoparticles for neural regeneration is offered by phage peptide libraries. These libraries through providing access to an innumerable number of peptide ligands may contribute to the development of novel therapeutic approaches for the field of neural regeneration. In fact, the affinity screening of phage peptide libraries over the cells belonging to different parts of the nervous system can lead to the identification of peptides that are capable of specific binding to the desired cells. These nerve cell-binding peptides can be used for selective delivery of therapeutic cargoes into damaged cells of the nervous system. In line with this, biopanning of a 12mer phage library led to the isolation of a peptide with strong binding capacity to motor neurons and dorsal root ganglion (DRG) cells. This peptide has been suggested to be useful in cellular targeting of neurotherapeutic proteins and gene/drug delivery vectors for the treatment of motor neuron diseases (Liu et al., 2005). Also, a nonapeptide (nine amico acid-peptide) has been obtained from an intravenously administered phage display library that can selectively home to ischemic stroke tissue in a rat transient middle cerebral artery (MCA) occlusion model. This homing peptide can detect apoptotic neuronal cells (Hong et al., 2008). Such peptides that can bind to damaged ischemic tissues of the nervous system represent potential for both molecular imaging of ischemic tissues and targeted drug delivery to nerve cells and tissues injured by stroke.
But, one of the most impressive and exciting applications of bacteriophages and phage display technology in neural regeneration is screening of phage display libraries on neural stem cells (NSCs) in order to find peptide ligands with specific binding capacity to stem cells of the nervous system. NSCs are undifferentiated, self-renewing and multipotent progenitor cells that exist in the subventricular zone (SVZ) and the hippocampal subgranular zone (SGZ) of the adult brain of mammals. Due to some unique attributes, NSCs have attracted tremendous attention for the establishment of neuroregenerative therapies. These stem cells are able to differentiate into major cell types of the nervous system including neurons, astrocytes, and oligodendrocytes. Also, they are gifted with enormous expansion ability and do not indicate tumorigenicity when transplanted into different animal models of neurodegenerative diseases (Conti et al., 2006). Phage display-derived peptides showing preferential binding to NSCs can serve to selectively deliver therapeutic genes and drugs to stem cells of the nervous system. For this reason, NSC-targeting peptides are considered as valuable tools in the development of targeted platforms for gene/drug delivery into stem cells residing in the nervous system (Figure 2). Consistent with this, neural stem cell-binding peptides derived from phage display library have been shown to be able to selectively direct gene transfer to neural precursor cells in adult mice. When conjugated to an adenovirus vector harboring green fluorescent protein (GFP) as a reporter gene and injected into the mouse brain, these NSC-targeting peptides specifically transduced neural stem cells residing in the dentate gyrus of hippocampus. This was detected via observing fluorescence in the section of mouse brain tissue (Schmidt et al., 2007). In view of the capacity of NSCs in the repair and regeneration of injured tissues of the nervous system, targeted delivery of therapeutics into these cells holds major potential for regenerative treatment of the nervous system disorders. Of the important areas in which NSC-targeting peptides may present therapeutic promise is the issue of neurodegenerative diseases. Several disorders such as Huntington's, amyotrophic lateral sclerosis (ALS), Parkinson's and Alzheimer's occur as a result of neurodegeneration. Neurodegeneration involves the progressive structural and functional loss of neurons (Winner et al., 2011). Peptide ligands with specific binding to NSCs may find utility in the targeted delivery of therapeutics including genes and drugs to stem cells of the nervous system in order to stimulate their proliferation or control their differentiation. Therefore, it is possible to generate cells that are required for treating the neurodegenerative disorder of interest. Sometimes peptides isolated from screening of phage display libraries on NSCs can themselves possess bioactive properties. In this case, they are not only targeting agents but can exert functional effects on the target cell. These bioactive peptides attach to some receptors on the surface of NSCs, thereby affecting cell behavior in terms of different phenomena including proliferation, apoptosis, invasion, migration, angiogenesis, and viability. In this regard, a neural stem cell-specific peptide identified through in vitro panning on murine NSCs has been reported to enhance the proliferation of both cultured neural stem cells and neural progenitor cells freshly isolated from the brain of adult mouse (Staquicini et al., 2009). The capability of such peptides that induce neurogenesis might serve as a basis for the treatment of diseases concerned with loss of neurons. As neurodegenerative diseases have similarities at the sub-cellular level (e.g., abnormal protein assemblies and cell death), discovery of peptides that are capable of inducing neurogenesis or inhibiting neurodegeneration raises hopes to outline strategies for simultaneous treatment of multiple diseases.
Figure 2.
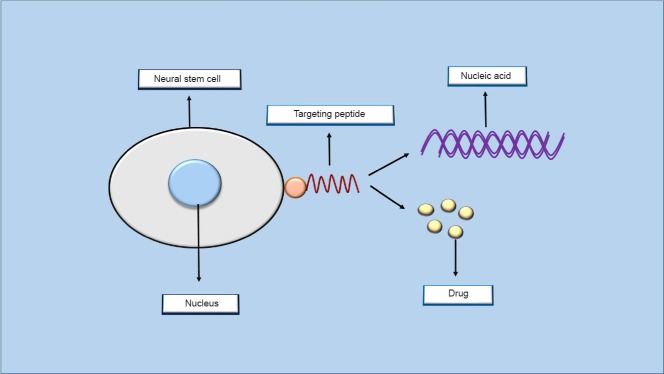
Targeted gene and drug delivery into neural stem cells.
Peptides that are obtained from screening of phage display libraries on neural stem cells can specifically attach to some receptors on the surface of these cells. These targeting peptides can then be used for targeted delivery of therapeutic nucleic acids and drug molecules into stem cells residing in the nervous system.
Altogether, recent findings reveal that bacteriophages are creating new opportunities in the area of neural regeneration. In the last several years, the use of bacteriophages in particular phage display technology for neural regeneration has experienced an exponential growth. Phage display has offered novel potential applications in elaborating desirable scaffolds for neural tissue engineering and developing efficient targeted platforms for gene and drug delivery into cells of the nervous system. The excellent biological safety of phage nanoparticles is a worthwhile trait for translation of phage-based nanomaterial products into the clinic. This safety is rooted in the fact that phages are an indispensable component of the body's natural ecosystem, thereby making a long-term and incessant evolutionary relationship with the cells of our body (Bakhshinejad and Sadeghizadeh, 2014). Beyond doubt, current advances have yielded new insights into the promises of bacteriophages for regenerative medicine of the nervous system. While still having a long way ahead, these ongoing breakthroughs offer introduction of more sophisticated treatment avenues for neurological disorders and pave the way for bacteriophages to play a more leading role in the future of neural regeneration.
Acknowledgments:
We are cordially grateful to Marzieh Karimi for her generous assistance in graphical works of the manuscript.
References
- 1.Bakhshinejad B, Sadeghizadeh M. Bacteriophages as vehicles for gene delivery into mammalian cells: prospects and problems. Expert Opin Drug Deliv. 2014;11:1561–1574. doi: 10.1517/17425247.2014.927437. [DOI] [PubMed] [Google Scholar]
- 2.Bakhshinejad B, Karimi M, Sadeghizadeh M. Bacteriophages and medical oncology: targeted gene therapy of cancer. Med Oncol. 2014;31:110. doi: 10.1007/s12032-014-0110-9. [DOI] [PubMed] [Google Scholar]
- 3.Chung WJ, Merzlyak A, Yoo SY, Lee SW. Genetically engineered liquid-crystalline viral films for directing neural cell growth. Langmuir. 2010;26:9885–9890. doi: 10.1021/la100226u. [DOI] [PubMed] [Google Scholar]
- 4.Clark JR. Bacteriophages: a biological library of Babel. Future Virol. 2012;7:333–335. [Google Scholar]
- 5.Conti L, Reitano E, Cattaneo E. Neural stem cell systems: diversities and properties after transplantation in animal models of diseases. Brain Pathol. 2006;16:143–154. doi: 10.1111/j.1750-3639.2006.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farr R, Choi DS, Lee SW. Phage-based nanomaterials for biomedical applications. Acta Biomater. 2014;10:1741–1750. doi: 10.1016/j.actbio.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 7.Hong HY, Choi JS, Kim YJ, Lee HY, Kwak W, Yoo J, Lee JT, Kwon TH, Kim IS, Han HS, Lee BH. Detection of apoptosis in a rat model of focal cerebral ischemia using a homing peptide selected from in vivo phage display. J Control Release. 2008;131:167–172. doi: 10.1016/j.jconrel.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Liu JK, Teng Q, Garrity-Moses M, Federici T, Tanase D, Imperiale MJ, Boulis NM. A novel peptide defined through phage display for therapeutic protein and vector neuronal targeting. Neurobiol Dis. 2005;19:407–418. doi: 10.1016/j.nbd.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt A, Haas SJ, Hildebrandt S, Scheibe J, Eckhoff B, Racek T, Kempermann G, Wree A, Putzer BM. Selective targeting of adenoviral vectors to neural precursor cells in the hippocampus of adult mice: new prospects for in situ gene therapy. Stem Cells. 2007;25:2910–2918. doi: 10.1634/stemcells.2007-0238. [DOI] [PubMed] [Google Scholar]
- 10.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 11.Staquicini FI, Dias-Neto E, Li J, Snyder EY, Sidman RL, Pasqualini R, Arap W. Discovery of a functional protein complex of netrin-4 laminin gamma1 chain and integrin alpha6beta1 in mouse neural stem cells. Proc Natl Acad Sci U S A. 2009;106:2903–2908. doi: 10.1073/pnas.0813286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winner B, Kohl Z, Gage FH. Neurodegenerative disease and adult neurogenesis. Eur J Neurosci. 2011;33:1139–1151. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- 13.Yang SH, Chung WJ, McFarland S, Lee SW. Assembly of bacteriophage into functional materials. Chem Rec. 2013;13:43–59. doi: 10.1002/tcr.201200012. [DOI] [PubMed] [Google Scholar]
- 14.Zhu W, O’Brien C, O’Brien JR, Zhang LG. 3D nano/microfabrication techniques and nanobiomaterials for neural tissue regeneration. Nanomedicine (Lond) 2014;9:859–875. doi: 10.2217/nnm.14.36. [DOI] [PubMed] [Google Scholar]


