Abstract
The purpose of this study was to assess fetal bovine acellular dermal matrix as a scaffold for supporting the differentiation of bone marrow mesenchymal stem cells into neural cells following induction with neural differentiation medium. We performed long-term, continuous observation of cell morphology, growth, differentiation, and neuronal development using several microscopy techniques in conjunction with immunohistochemistry. We examined specific neuronal proteins and Nissl bodies involved in the differentiation process in order to determine the neuronal differentiation of bone marrow mesenchymal stem cells. The results show that bone marrow mesenchymal stem cells that differentiate on fetal bovine acellular dermal matrix display neuronal morphology with unipolar and bi/multipolar neurite elongations that express neuronal-specific proteins, including βIII tubulin. The bone marrow mesenchymal stem cells grown on fetal bovine acellular dermal matrix and induced for long periods of time with neural differentiation medium differentiated into a multilayered neural network-like structure with long nerve fibers that was composed of several parallel microfibers and neuronal cells, forming a complete neural circuit with dendrite-dendrite to axon-dendrite to dendrite-axon synapses. In addition, growth cones with filopodia were observed using scanning electron microscopy. Paraffin sectioning showed differentiated bone marrow mesenchymal stem cells with the typical features of neuronal phenotype, such as a large, round nucleus and a cytoplasm full of Nissl bodies. The data suggest that the biological scaffold fetal bovine acellular dermal matrix is capable of supporting human bone marrow mesenchymal stem cell differentiation into functional neurons and the subsequent formation of tissue engineered nerve.
Keywords: nerve regeneration, peripheral nerve defects, fetal bovine, acellular dermal matrix, biological scaffold, bone marrow mesenchymal stem cells, neuronal differentiation, neurons, tissue engineered nerve, neural regeneration
Introduction
The successful treatment of brain, spinal cord, and peripheral nerve damage remains a challenging problem. Current treatment approaches, including autologous nerve grafts, are seriously limited by the lack of suitable donors (Siemionow and Sonmez, 2007; Houle et al., 2009; Ibarra and Martinon, 2009). New research using pluripotent stem cells and the development of tissue engineering technologies have revitalized the treatment of nerve damage (Rossi and Keirstead, 2009a; Neubauer et al., 2010; Cullen et al., 2011; Wang et al., 2013). Alternative treatments are being actively investigated, such as the creation of graft substitutes through the techniques of tissue engineering (Welin et al., 2008; Subramanian et al., 2009).
Human bone marrow mesenchymal stem cells (BMSCs) have an extensive capacity for self-renewal and the potential to differentiate into neuronal cells (Rossi and Keirstead, 2009b; Wang et al., 2010). The in vitro cultivation of neural cells derived from the differentiation of BMSCs on suitable biomaterial scaffolds may prove to be clinically useful (Neubauer et al., 2009; Subramanian et al., 2009). Therefore, more physiological tissue engineered nerve alternatives may be created by culturing and differentiating a patient's own self-derived BMSCs into neural cells on compatible biomaterial scaffolds (Dezawa, 2002; Wang et al., 2008). Several studies have reported that BMSCs can be easily obtained from patients (Jiang et al., 2002; Gnecchi and Melo, 2009) and successfully differentiated into neural cells in vitro (Sanchez-Ramos et al., 2000; Prabhakaran et al., 2009). Many biomaterial scaffolds for use in nerve tissue engineering (Subramanian et al., 2009) have been reported (Hudson et al., 2004a, b; Hu et al., 2007). These materials have demonstrated chemical and physical stability, and are also biocompatible. However, many developmental challenges remain to be addressed before they are ready for clinical application. Based on the reported properties of these materials, the biocompatibility and safety of matrices of animal-origin are well established (Rennekampff, 2009). Biomaterials made from allogeneic and xenogeneic acellular dermal matrices have been widely used in the clinical treatment of burns (Rennekampff, 2009; Xiao et al., 2009a) and in other conditions where skin replacement is required (Xiao et al., 2009a, b; Burns et al., 2010). Similarly, bovine acellular dermal matrix has been developed into commercialized products and used in clinical applications for abdominal wall reconstruction (Wietfeldt et al., 2009), chronic diabetic foot ulcers (Kavros, 2012; Kavros et al., 2014), skin grafting (Neill et al., 2012), and breast reconstruction (Lullove, 2012). However, to our knowledge, no study has yet reported the use of fetal bovine acellular dermal matrix as a scaffold for the differentiation of BMSCs into neuronal cells in vitro. The research presented here provides important experimental evidence of neurogenesis caused by the differentiation of BMSCs into neuronal cells on fetal bovine acellular dermal matrix in vitro, in addition to a theoretical basis for the development of a biological scaffold from fetal bovine acellular dermal matrix for tissue engineered nerves.
Fetal bovine acellular dermal matrix, which is derived from the skin of bovine fetuses, is a native extracellular matrix with favorable biochemical properties. Bovine fetuses are a valuable tissue resource because a large number of them are discarded as waste during the preparation of fetal bovine serum. Therefore, the tissue resources of bovine fetuses were developed in the present study. Previously, our group was able to successfully cultivate infant skin cells and Vero cells on fetal bovine acellular dermal matrix (data not reported) and to show that fetal bovine acellular dermal matrix is remarkably compatible with those cell types. The aim of the present study was to assess fetal bovine acellular dermal matrix as a scaffold for the culture and induction of BMSCs, and their subsequent differentiation into neuronal cells during the generation of a tissue engineered nerve construct.
Materials and Methods
Materials
Human BMSCs were purchased from Thermo Fisher Scientific (HyClone CET Stem Cells, Waltham, MA, USA). They had been isolated from human red bone marrow that was collected during a bone marrow aspiration procedure by Cellular Engineering Technologies (CET, Coralville, IA, USA). The neural differentiation medium was HyClone Advance STEM neural differentiation medium containing 12% HyClone Advance STEM cell growth supplements and 1% of 100 × antibiotic/antimycotic solution. The basal medium was HyClone Advance STEM basal medium for undifferentiated human MSCs containing 1% of 100 × antibiotic/antimycotic solution and 12% HyClone Advance STEM cell growth supplement. These media, as well as Dulbecco's phosphate-buffered saline and HyQtase, were purchased from Thermo Scientific. The neurite outgrowth Hitkit (Cellomics, Pittsburgh, PA, USA) included the neurite outgrowth primary antibody (a mouse monoclonal IgG antibody against neurite outgrowth in a wide range of mammalian species) that specifically labels both neurites and neuronal cell bodies, a secondary antibody (Goat anti-mouse IgG conjugated to Alexa Fluor 488), neurite outgrowth buffer (10 ×), wash buffer (10 ×), and Hoechst dye. A mouse anti-neuron-specific βIII tubulin antibody was purchased from R&D Systems (Minneapolis, MN, USA). Buffered glutaraldehyde solution (2%) was prepared by bringing 23.83 g of hydroxyethyl piperazine ethanesulfonic acid in 40 mL of 50% glutaraldehyde up to a volume of 1 L with distilled water (pH 7.0). A 1–2% osmium tetroxide solution was prepared from 4 mL of 4% osmium tetroxide, 4 mL of 0.4 mol/L hydroxyethyl piperazine ethanesulfonic acid (pH 7.0), and 8 mL of distilled water. Cell culture plates and flasks were Nunc brand (Thermo Fisher).
Fetal bovine acellular dermal matrix was prepared at the Key Laboratory for Biotechnology at the Northwest University for Nationalities in China, from the skins of 3–5-month-old healthy bovine fetuses. The harvested skins were mechanically scraped and treated successively with acid, alkali, and enzymatic digestion to remove the epidermis, subcutaneous layers, and associated cells, using conventional methods. Next, the skins were tested to ensure that all DNA had been removed. Then, they were cut into small swatches and freeze-dried. These swatches were sterilized with γ-irradiation at a dose of 18 kGy. To ensure proper preparation, after eliminating cell fragments and testing the biocompatibility, sample swatches were embedded in paraffin, sectioned, stained with hematoxylin-eosin. Other samples were prepared for scanning electron microscopy and were co-cultured with Vero cells.
Culture and seeding of BMSCs
The BMSCs were cultured in basal medium at 37°C in a humidified atmosphere containing 5% CO2 for 6 days, and the culture medium was changed every 2 days. When the cell monolayers reached 80–90% confluence, they were detached using HyQTase (Thermo Scientific) and dispersed into a single cell suspension by gentle pipetting. The cells were then counted and their vitality was assessed using trypan blue in a Beckman hemocytometer (Beckman Coulter, Inc., Brea, CA, USA).
Prior to cell seeding, the 18 kGy γ-irradiated, freeze-dried fetal bovine acellular dermal matrix swatches were cut into 0.8 cm × 0.8 cm pieces and subjected to ultraviolet radiation for 15–20 minutes in a laminar flow hood. The pieces were then placed into the wells of 4- or 24-well cell culture plates and soaked in 1 mL of basal medium for 1 hour. After aspirating the media, approximately 20,000 cells were seeded into the wells directly on to the fetal bovine acellular dermal matrix or into control wells without fetal bovine acellular dermal matrix. The plates were incubated at 37°C for 1 hour to allow the cells to attach to the fetal bovine acellular dermal matrix, and then 1.5 mL of fresh basal medium was added to each well. Finally, the plates were placed in a humidified incubator (Thermo Fisher Scientific) at 37°C with 5% CO2. The media were replaced with 1.5 mL of fresh basal medium every other day.
Neuronal differentiation induction
BMSCs were cultured in basal medium for 2, 3, 4, or 17 days. After the wells were washed, the BMSCs were incubated in neural differentiation medium for 3–31 days. The 1.5 mL of medium was replaced every 2 days.
Monitoring and verifying neuronal differentiation
The growth and morphology of the cells from each group were continuously monitored using inverted phase contrast microscopy, and they were photographed with an Axio CamHR3/FinePixF40fd camera. The cell body width and length or neurite length were measured with Adobe Photoshop CS4 image analysis software (Adobe Systems Inc., San Jose, CA, USA).
To identify neuronal cells among the differentiated BMSCs after culture in neural differentiation medium for 3 to 12 days, samples, including control cells, were fixed in 1 mL of fixation solution containing 4% formaldehyde and 0.05% Hoechst dye at room temperature for 40 minutes. The cells were then stained using the neurite outgrowth HitKit (Thermo Fisher Scientific) and observed using confocal laser scanning microscopy at wavelengths of 494 nm for Alexa Fluor 488 (to show the neurite outgrowth primary antibody) and 350 nm for the Hoechst dye (to show the nuclei). Alexa Fluor 488-labeled secondary antibody was used without a primary antibody as a negative control.
To verify the neurite outgrowth primary antibody, samples were prepared in the same manner as for the neurite outgrowth HitKit, except the primary antibody was replaced with 300 μL of mouse neuron-specific βIII tubulin antibody.
Visualization of cell morphology by scanning electron microscopy
The morphology of BMSCs grown on fetal bovine acellular dermal matrix in basal medium and neural differentiation medium was examined using scanning electron microscopy after the cells were cultured for 12–34 days. The fetal bovine acellular dermal matrices with attached cells were rinsed three times with PBS and fixed in 2% buffered glutaraldehyde solution for 24 hours. Next, they were rinsed three times with 0.1 mol/L hydroxyethyl piperazine ethanesulfonic acid buffer for 5 minutes each, soaked in 1–2% osmium tetroxide for 1 hour, and rinsed three more times with 0.1 mol/L hydroxyethyl piperazine ethanesulfonic acid buffer. They were then dehydrated in increasing ethanol concentration baths (50%, 70%, 95%, 100%) twice for 10 minutes each. Finally, the scaffolds with attached cells were treated with hexamethyldisilazane and air-dried in a fume hood overnight. The dried samples were mounted on aluminum stubs, sputter-coated with 20 nm of gold-palladium, and examined using an S-4000 scanning electron microscope (Hitachi, Tokyo, Japan).
Cell morphology detected by hematoxylin-eosin and toluidine blue staining
The morphology of BMSCs grown on fetal bovine acellular dermal matrix in basal medium and neural differentiation medium was examined using horizontal and vertical paraffin sections stained with hematoxylin-eosin and toluidine blue after 34 days of culture. The tissue was fixed, dehydrated, and embedded in paraffin as previously described (Kucherenko et al., 2011). Histological sections were prepared and stained with hematoxylin-eosin or toluidine blue (0.12 %).
Statistical analysis
All data were expressed as the mean ± SD and were analyzed with paired t-tests using SPSS 11.0 software (SPSS, Chicago, IL, USA). P < 0.05 was considered statistically significant. Additional statistical analysis was performed using Graphpad PRISM Version 5.0 software (GraphPad Software Inc., La Jolla, CA, USA).
Results
Appearance and structure of fetal bovine acellular dermal matrix
The dehydrated fetal bovine acellular dermal matrix appeared similar to white paper, with a thickness of 60–200 μm depending on the gestational age of the source fetus (Figure 1A). After rehydration in water for 1 minute, it became thin, soft, and translucent. Fetal bovine acellular dermal matrix resists tearing, can be easily cut into desired shapes and sizes, and can be sutured onto wounds. Pores of 3–10 μm were observed by scanning electron microscopy in the intact basement membrane of the fetal bovine acellular dermal matrix (Figure 1B). A network structure of woven fibers where the basement membrane was damaged during the preparation process (Figure 1C) was also seen. The woven fibers were predominately collagen, as confirmed using paraffin sections and hematoxylin-eosin staining (Figure 2A). The Vero cells grew well, and their cell viability was more than 90% at 20 days after being seeded on the fetal bovine acellular dermal matrix (data not shown).
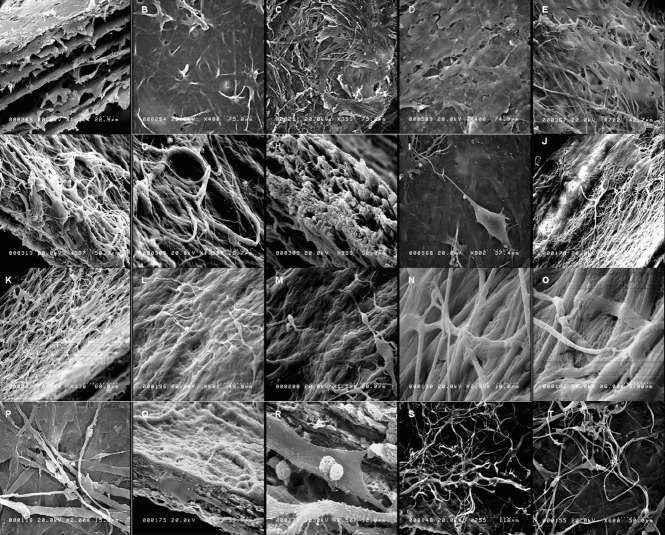
Figure 1.
Cell morphology and the network formed (scanning electron microscopy).
BMSCs were grown on FBADM either non-induced in basal medium for 12–34 days (spontaneous differentiation) or induced in basal medium for 3 days and then neural differentiation medium for 9 days, basal medium for 3 days and then for 31 days, or basal medium for 17 days and then neural differentiation medium for 17 days. (A–C) The structure of the FBADM. (A) A longitudinal section. (B) The surface of the FBADM with an intact basement membrane. (C) The surface of the FBADM with a damaged basement membrane. (D) Of the non-induced cells cultured in basal medium for 12 days on FBADM, most grew parallel to the flat surface. (I) The cells induced in basal medium for 3 days and then neural differentiation medium for 9 days on FBADM, showing the long processes of typical neurons, an uplifted cell body, and unipolarity. (E–H) The non-induced cells cultured in basal medium for 34 days on FBADM showing proliferation, spontaneous differentiation, and stratified growth (E); neuronal-like differentiation (F); vascular-like differentiation (G); and matrix morphology changes (H) compared with the structure of the original (A). (J–O) The BMSCs were induced in basal medium for 3 days and then neural differentiation medium for 31 days on FBADM, showing (J) the stratified growth of the differentiated cells with FBADM at lower magnification; (K) the differentiated cells connected to each other and into the mesh; (L, M) the differentiated cells formed a complete neural circuit with the formation of a growth cone and synaptic connections by dendrites and axons; and (N–O) nerve fibers formed by a parallel arrangement of microfibers at high magnification. (P–T) When the BMSCs were induced in basal medium for 17 days and then neural differentiation medium for 17 days on FBADM, the differentiated cells formed a crisscross neural network-like structure on the surface of the scaffold (P) and inside (longitudinal section, Q) a network connection. (R) A higher magnification image of Q displaying the synaptic connections and dendritic spines. (S, T) The long smooth axons, growth cone, and axon-dendritic and axon-body synaptic connections of the neural network-like structure. BMSCs: Bone marrow mesenchymal stem cells; FBADM: fetal bovine acellular dermal matrix.
Figure 2.
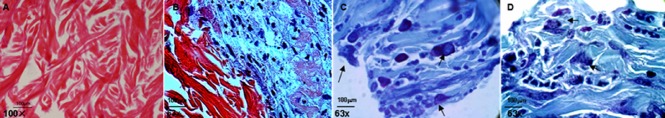
Structure of the FBADM and cell morphology of BMSCs cultured in basal medium or induced in neural differentiation medium on FBADM stained by hematoxylin-eosin or toluidine blue (optical microscopy).
(A) The structure of the FBADM without cells. The woven fibers were predominately collagen, as shown by the hematoxylin-eosin staining of paraffin sections. (B) Cells were cultured in basal medium for 34 days, and the BMSCs proliferated on the surface of the FBADM, assumed a different cell phenotype, and secreted extracellular matrix, predominately collagen, as shown by hematoxylin-eosin staining. The images suggest that BMSCs underwent self-renewal and differentiated along multiple lineages on the FBADM in nurturing long-term in basal medium. (C, D) BMSCs cultured for 3 days in basal medium and then induced for 31 days in neural differentiation medium on the surface of the FBADM. Arrows show visible Nissl bodies in the cell. Scale bars: 100 μm. BMSCs: Bone marrow mesenchymal stem cells; FBADM: fetal bovine acellular dermal matrix.
Morphology of BMSCs grown in basal medium
The morphologies of BMSCs grown in basal medium with or without fetal bovine acellular dermal matrix were different, as assessed by inverted phase contrast microscopy. The cells cultured in basal medium without fetal bovine acellular dermal matrix, a non-induced negative control, showed irregular diamond-shaped morphology (Figure 3A) until 10–25 days of culture, at which point they grew into a dense single layer (Figure 3B, C). The cells that were cultured in basal medium with fetal bovine acellular dermal matrix grew around (Figure 3D, 5E) and on the surface of the fetal bovine acellular dermal matrix (Figure 1D, 5A), and showed a regular elongated fibroblast-like morphology with a common growth orientation. However, because of the self-renewal and multiple differentiation potential of BMSCs, they proliferated and spontaneously differentiated after 34 days in culture (Li et al., 2005; Xin et al., 2007; Tondreau et al., 2008; Wang et al., 2010a Takeda et al., 2012). The fetal bovine acellular dermal matrix with attached cells displayed stratified growth (Figure 1E), with both neural-like cells (Figure 1F) and vascular-like differentiation (Figure 1G). Compared with the original fetal bovine acellular dermal matrix (Figure 1A), the structure of the fetal bovine acellular dermal matrix was changed during culture (Figure 1H).
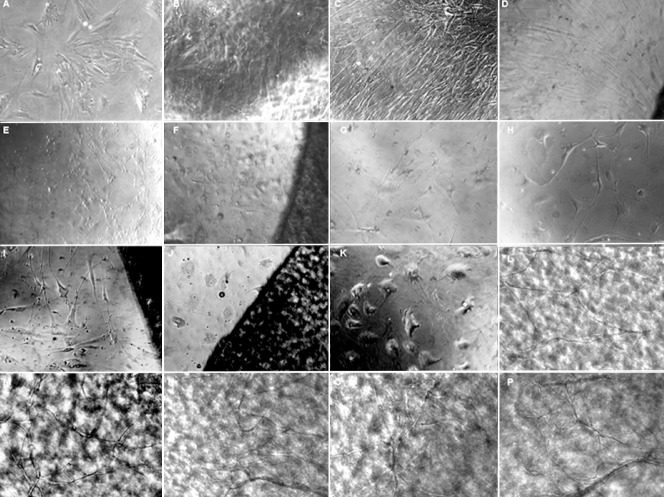
Figure 3.
Proliferation and differentiation of BMSCs in the induced and non-induced conditions with or without FBADM (inverted phase contrast microscopy, × 200).
(A–C) In the negative control without FBADM where the BMSCs were cultured in basal medium for 5, 10, and 25 days, the cells were diamond-shaped and became long fibroblasts-like cells over time. (D) Of the BMSCs cultured with the FBADM in basal medium for 10 days, most of the cells were spindle-shaped and arranged in parallel. Compared to the same culture conditions without the FBADM (B), the cell morphology was somewhat different. (E, F) The cells cultured in basal medium for 3 days and then induced in neural differentiation medium for 2 days. In the positive control (E) without FBADM, and (F) with FBADM, the cell morphology was changed after induction in neural differentiation medium for 2 days compared with (A). (G, H, K) Positive controls of the cells induced in basal medium for 3 days and then in neural differentiation medium for 7 days (compared to B and D), 11 days (compared to I), and 22 days (compared to C, J, L–P). After the same amount of time in culture, the cell morphology was changed in the different conditions, and there were clearly fewer cells in the induced groups than in the non-induced groups. These results indicate that the differentiated BMSCs did not proliferate or proliferated slowly. (I, J) In the cells with FBADM induced in basal medium for 3 days and then neural differentiation medium for 11 days or basal medium for 3 days and then neural differentiation medium for 22 days compared to (H) and (K), the cell morphologies after culture with and without the FBADM were different for the same induction conditions. These differences in BMSC differentiation between groups cultured with and without the FBADM suggest that FBADM has some effects on the induction of neuronal differentiation in BMSCs. (L–P) Compared to conditions without FBADM (K) and the round FBADM (J), the BMSCs induced in basal medium for 3 days and then neural differentiation medium for 22 days on the surface of FBADM showed longer neurites that were connected to each other, forming a network-like structure. However, the bodies of the cells without FBADM were larger and the neurites were shorter (K) then the positive controls (G, H). The neurites of the cells on round FBADM (J) were shorter than in (I), and even disappeared over time. The network-like structure on the FBADM indicated that the FBADM provides a platform for the maturation of the neurons, as well as neurite extension and network formation. In addition, if the cells are not removed, the BMSCs grown on the FBADM will proliferate and spontaneously differentiate in basal medium over long-term culture. BMSCs: Bone marrow mesenchymal stem cells; FBADM: fetal bovine acellular dermal matrix.
Figure 5.
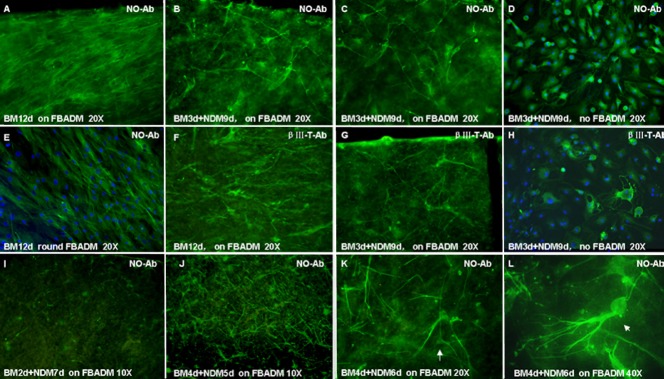
Cell morphology of BMSCs cultured with or without FBADM in induced or non-induced conditions (immunofluorescence and confocal microscopy).
(A–E, I–L) Neurite outgrowth primary antibody, marked as NO-Ab. (F–H) βIII tubulin primary antibody, marked as βIII-T Ab. (A, E, F) The BMSCs cultured on FBADM (A, F) and grown around FBADM (E) in basal medium (BM) for 12 days (non-induced) were parallel with long spindles. (B–D, G, H) The BMSCs cultured on FBADM (B, C, G) or without FBADM (D, H) in BM for 3 days and then induced in neural differentiation medium (NDM) for 9 days (induced, BM 3 d + NDM 9 d). (B, C) Some neurite outgrowth primary antibody-positive cells with bi/multipolar elongations formed a network-like structure on the surface of the FBADM, and they also appeared to express the neuron marker βIII tubulin (G). (I, J) In cells cultured for a total of 9 days, but in two different conditions, (BM 2 d + NDM 7d) or (BM 4 d + NDM 5 d), more neurite outgrowth-positive cells were found in the latter than in the former. (J) In the induced condition of (BM 4 d + NDM 5 d), the differentiated cells showed the morphology of unipolar and bipolar or multipolar neurons. (K, L) Induced in BM 4 d + NDM 6 d, the BMSCs differentiated into cells with the morphology of pyramidal cells (K, arrow, × 40) and cerebellum Purkinje's cells (L, arrow, × 40). These results suggest that BMSCs can differentiate into neuronal cells on FBADM after being induced in NDM for 5 to 6 days, and the differentiated neuronal cells can connect with each other via neurites to form a neural network-like structure. The FBADM can support the neuronal differentiation of BMSCs and the formation of a neural network-like structure. d: Days; NO-Ab: neurite outgrowth antibody; BMSCs: bone marrow mesenchymal stem cells; FBADM: fetal bovine acellular dermal matrix.
The average relative length of cell bodies (Figure 4A) of cells growing with fetal bovine acellular dermal matrix (CBtR) and the control cells without fetal bovine acellular dermal matrix (CCtrol) in basal medium for 11 days were significantly different (P < 0.0001). The average relative widths of the cell bodies (Figure 4B) were also slightly different between the cells growing with fetal bovine acellular dermal matrix (CBtR) and the control cells without fetal bovine acellular dermal matrix (CCtrol) in basal medium for 11 days (P < 0.05). Together, these results indicate that fetal bovine acellular dermal matrix has an effect on the elongation of cell bodies, perhaps by inducing cytoskeletal rearrangement.
Figure 4.
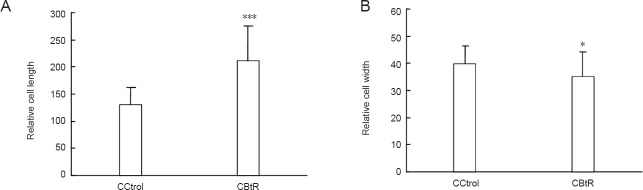
Average relative length and width of the cell body of BMSCs that were cultured in basal medium with or without FBADM.
(A) The difference in average relative length and (B) width of the cell bodies between BMSCs cultured with FBADM (CBtR) and without FBADM (CCtrol) in basal medium for 11 days. The lengths of the cell bodies of cells grown around the FBADM (CBtR) were longer and the widths (CBtR) were narrower than those without FBADM (CCtrol). These results suggest that the FBADM may induce the rearrangement of the cell skeleton. *P < 0.05, ***P < 0.0001, vs. CCtrol. Data are expressed as the mean ± SD (paired t-test). BMSCs: Bone marrow mesenchymal stem cells; FBADM: fetal bovine acellular dermal matrix.
Induction of neuronal morphology using neural differentiation medium
BMSCs were induced to differentiate into neuronal cells on fetal bovine acellular dermal matrix in neural differentiation medium. Following exposure to the induction medium for 24 hours, the morphology of the BMSCs changed. As shown in Figure 3E, G, H, and K, the cell morphology was transformed into that of neuronal cells over 2, 7, 11, and 22 days of exposure to neural differentiation medium for cells cultured without fetal bovine acellular dermal matrix as a positive control after being cultured in basal medium for 3 days. These findings indicated that the neural differentiation medium induced changes in the cell morphology of BMSCs, giving them the appearance of neuronal cells. Figure 3F, I, and J show the cell morphology over 2, 11, and 22 days of exposure to neural differentiation medium for cells cultured with fetal bovine acellular dermal matrix after being cultured in basal medium for 3 days. The cell morphologies of cells cultured with and without fetal bovine acellular dermal matrix were different. The cell morphology of BMSCs exposed to neural differentiation medium for 22 days on fetal bovine acellular dermal matrix after being cultured in basal medium for 3 days is shown in Figure 3L–P. The differentiated neuronal cells formed a nerve network on the surface of the fetal bovine acellular dermal matrix. After trypsinization and then re-culturing, these cells still showed a typical neuronal cell morphology (Figure 6), suggesting that the neuronal phenotype of differentiated cells was stable. Compared to the morphology of the induced differentiated cells that grew on the surface of the fetal bovine acellular dermal matrix (Figure 3L–P), the morphology of the cells that grew around the fetal bovine acellular dermal matrix (Figure 3I, J) and without the fetal bovine acellular dermal matrix (Figure 3H, K) were clearly different, even in the same experimental conditions: BMSCs cultured in basal medium for 3 days and then induced in neural differentiation medium for 11 to 22 days. The cells differentiated on fetal bovine acellular dermal matrix (Figure 3L–P) extended neurites and connected with each other, forming a neural network-like structure. However, the bodies of the cells that were induced to differentiate without fetal bovine acellular dermal matrix were larger and their neurites were shorter (Figure 3K) than those with the matrix (Figure 3H). The neurites of the cells grown around the fetal bovine acellular dermal matrix were shorter and even disappeared (Figure 3J) after induction for 22 days. These findings suggested that fetal bovine acellular dermal matrix was involved in the induction of neuronal differentiation. Further, the appearance of the network on the surface of the fetal bovine acellular dermal matrix indicated that it provides a surface for the maturation of neurons, the extension of neurites, and the formation of a nerve network.
The scanning electron microscopy images of cells cultured with fetal bovine acellular dermal matrix in basal medium and induced with neural differentiation medium are shown in Figure 1D–T. The BMSCs grown on fetal bovine acellular dermal matrix remained undifferentiated, showing a fibroblastic phenotype for 12 days (Figure 1D) in basal medium. The BMSCs in basal medium for 3 days and then neural differentiation medium for 9 days showed neuronal morphology similar to a unipolar neuron, as shown in Figure 1I. When induced in basal medium for 3 days and then neural differentiation medium for 31 days, the fetal bovine acellular dermal matrices with attached cells became a multilayered neural network-like structure, as shown in Figure 1J–O, with long nerve fibers composed of several parallel microfibers (Figure 1N, O), neuronal cells forming complete neural circuits comprised of dendrite-dendrite to axon-dendrite to dendrite-axon synapses, and dendrite spines and growth cones with filopodia (Figure 1L–M). When induced in basal medium for 17 days and then neural differentiation medium for 17 days on fetal bovine acellular dermal matrix, the differentiated BMSCs formed a crisscross neural network (Figure 1P, S, T) on the surface of the matrix and inside formed network connections (Figure 1Q, R; longitudinal section). Panel R is a higher amplification of panel Q that displays the synaptic connections and dendritic spines. The long smooth axons, growth cone, and the axon-dendrite and axon-body synaptic connections of the neural network formation are shown in Figure 1S, T.
The structure of the fetal bovine acellular dermal matrix using hematoxylin-eosin stained paraffin sections is shown in Figure 2A, which confirms that the woven fibers were predominately collagen. Although the BMSCs cultured in basal medium for 34 days on the surface of the fetal bovine acellular dermal matrix assumed different cell phenotypes, the secreted extracellular matrix they secreted was still predominately collagen (Figure 2B) as assessed by hematoxylin-eosin staining. This result suggests that the BMSCs self-renew and differentiate along multiple lineages when cultured on fetal bovine acellular dermal matrix in basal medium over a long period.
However, compared to the non-induced cells in basal medium for 34 days, the cells induced in basal medium for 3 days and then neural differentiation medium for 31 days showed typical neuronal phenotype. The toluidine blue stained paraffin sections clearly showed that such cells differentiated into a typical neuronal phenotype, both when cultured on and inside the fetal bovine acellular dermal matrix. The cells were large, showing a large round nucleus (light blue staining) located in the center of the cell, and a large round nucleolus located in the center of nucleus (dark blue staining). The cytoplasm was filled with blue basophilic Nissl bodies (Figure 2C, D), which supports the morphological characteristics of nerve cells with a measure of functional activity.
Identification of neuronal cells
The differentiation of BMSCs into neuronal cells on fetal bovine acellular dermal matrix was further evaluated by immunocytochemistry and confocal microscopy using a neuron-specific primary antibody against neurite outgrowth, which specifically labels both neurites and neuronal cell bodies from a wide range of mammalian species, as well as a neuron-specific βIII tubulin primary antibody labeled with a fluorescent secondary antibody. The morphological changes were observed following exposure of BMSCs to the neuronal induction media for 24 hours. However, the expression of neuronal markers was not detected until 3 to 12 days after induction.
The confocal microscopy images of BMSCs cultured on the surface of or around the fetal bovine acellular dermal matrix in basal medium for 12 days, which were induced in basal medium for 3 days and then neural differentiation medium for 9 days, are shown in Figure 5. The phenotypical features of neurons generated from the differentiated cells were distinguishable, including unipolar or bi/multipolar elongations and the formation of a neural network-like structure on the fetal bovine acellular dermal matrix. In addition, the cells expressed neuronal specific neurite outgrowth proteins and βIII tubulin.
Interestingly, the bi/multipolar elongations of most of the differentiated neuronal cells cultured on the surface of the fetal bovine acellular dermal matrix (Figure 5B, C, G) were longer than those of the cells cultured without the fetal bovine acellular dermal matrix (Figure 5D, H, positive control). In addition, the neural network-like structure on the surface of the fetal bovine acellular dermal matrix was more obvious than that assembled without the fetal bovine acellular dermal matrix, as shown in Figure 5B, C, G compared with Figure 5D, H. This comparison shows the same result as is shown in Figure 3, where the cells were induced in basal medium for 3 days and then neural differentiation medium for 22 days. These results suggest that BMSCs grown on fetal bovine acellular dermal matrix and induced using neural differentiation medium can differentiate into neuronal cells and that fetal bovine acellular dermal matrix promotes neurite outgrowth and supports the formation of a neural network-like structure. In contrast, minimal differences in the phenotype of BMSCs cultured on fetal bovine acellular dermal matrix or with fetal bovine acellular dermal matrix in basal medium and a weak positive reaction for both neurite outgrowth (Figure 5A, E) and neuron-specific βIII tubulin antibodies (Figure 5F) were observed. Moreover, there appeared to be much stronger staining with the neurite outgrowth primary antibody (No-Ab) than with the α-neuron-specific βIII tubulin antibody, suggesting that the neuronal specific neurite outgrowth proteins may be more highly expressed in differentiated neuronal cells than βIII tubulin at the time assayed. The images in Figure 5I, J show a higher number of differentiated No-Ab-positive cells after culture on fetal bovine acellular dermal matrix in basal medium for 4 days and then neural differentiation medium for 5 days than after culture in basal medium for 2 days and then neural differentiation medium for 7 days. This suggests that culturing for 2 additional days in basal medium on fetal bovine acellular dermal matrix was beneficial for BMSC proliferation. However, neuronally differentiated cells proliferate slowly or stop proliferating altogether.
In the induced condition (basal medium for 4 days and then neural differentiation medium for 5 days), the differentiated cells displayed the unipolar and bi/multipolar morphology of neurons as assessed by confocal microscopy (Figure 5J). The BMSCs induced in basal medium for 4 days and then neural differentiation medium for 6 days differentiated into pyramidal cells and cerebellum Purkinje's cells, which expressed neuronal-specific proteins (Figure 5K, L).
Discussion
A biocompatible scaffold, suitable neural cell source, and appropriate biochemical conditions are the basic requirements for the development of autologous nerve substitutes that may be able to restore, maintain, or improve nerve tissue function (Prabhakaran et al., 2009). In the present study, we proposed the use of fetal bovine acellular dermal matrix as a biocompatible scaffold, autologous BMSCs as the cell source, and a neural differentiation medium to induce the creation of three-dimensional autologous nerve substitutes in vitro.
Fetal bovine acellular dermal matrix, derived from the skin of bovine fetuses, is a native extracellular matrix with a collagen mesh structure as confirmed by hematoxylin-eosin staining (Figure 2A). BMSCs are capable of neuronal differentiation (Song et al., 2012; Hu et al., 2013; Liu et al., 2013). The results show that the BMSCs grown on fetal bovine acellular dermal matrix and induced for long periods of time with neural differentiation medium differentiated into cells with the typical neuron morphology, including a multilayered neural network-like structure with long nerve fibers.
Nissl bodies are a type of basophilic material within the cytoplasm with the main function of protein synthesis. Nissl bodies are closely related to the functional ability of neurons and can be used as status marker for neurons. The cytoplasm of the differentiated cells was filled with blue basophilic Nissl bodies, indicating that the differentiated cells were functionally active neuronal cells (Price and Porter, 1972). The cytoplasm of those differentiated cells, as seen in paraffin sections, was clearly filled with toluidine blue stained basophilic Nissl bodies, which supported the morphological classification of the nerve cells with an indicator of function.
The results suggest that fetal bovine acellular dermal matrix acts as a scaffold to support the proliferation and differentiation of BMSCs when cultured in basal medium and induced in neural differentiation medium, thereby suggesting the possibility of using fetal bovine acellular dermal matrix scaffolds with a patient's own BMSC-differentiated neuronal cells as a transplant to treat nerve injury. Moreover, the fetal bovine acellular dermal matrix provides both biological and mechanical support for the differentiation of BMSCs, enhancing neurogenesis (or nerve regeneration).
The data also suggest that fetal bovine acellular dermal matrix, when used as a biological scaffold, is capable of supporting human BMSC differentiation into functional neurons and the subsequent formation of a tissue engineered nerve. The number of differentiated cells derived from the BMSCs cultured on fetal bovine acellular dermal matrix, in basal medium for 4 days and then in neural differentiation medium for 5 days, was greater than when the cells were cultured in basal medium for 2 days and then neural differentiation medium for 7 days. In addition, the morphology of the cells differentiated from BMSCs on the surface of fetal bovine acellular dermal matrix was more obviously neuronal than that of those cultured without fetal bovine acellular dermal matrix under the same induction conditions, as seen by confocal microscopy. These data reinforce the idea that the collagen in the fetal bovine acellular dermal matrix regulates cell proliferation and function and supports the neuronal differentiation of BMSCs.
Here, we found that cells differentiated on fetal bovine acellular dermal matrix formed a neural network-like structure (Figure 3L–P, Figure 5B, C, G), but neural-like cells in the positive control group, those cultured without fetal bovine acellular dermal matrix, had shortened neurites or lost their neurites after induction for 9 to 22 days (Figure 3K, Figure 5D, H). These observations suggest that the presence of the fetal bovine acellular dermal matrix supported the growth and maturation of the neurons.
The morphological changes and neuronal protein expressions were further examined during the differentiation of the BMSCs into neuronal cells on the fetal bovine acellular dermal matrix scaffolds. BMSCs have a flattened, fibroblast-like morphology (Figure 3A). However, after culture in basal medium, the morphology of the cells grown on the surface of fetal bovine acellular dermal matrix (Figure 1D, 5A) or around the fetal bovine acellular dermal matrix (Figure 3D, 5E) became elongated and fibroblast-like. This change indicates that fetal bovine acellular dermal matrix has an effect on the induction of changes in cell morphology.
Because of their capacity for self-renewal and ability to differentiate along multiple lineages, BMSCs play an important role in tissue repair. Several reports have suggested that their differentiation into bone, cartilage, cardiac myocytes (Jiang et al., 2002; Li et al., 2005; Xin et al., 2007), and neuronal cells (Prabhakaran et al., 2009; Wang et al., 2010b). The results presented here of BMSCs cultured on fetal bovine acellular dermal matrix in basal medium for 34 days and imaged using scanning electron microscopy and histology show that the cells assumed several different phenotypes. This demonstrates that BMSCs have multi-directional differentiation potential when cultured on fetal bovine acellular dermal matrix in basal medium, and that fetal bovine acellular dermal matrix encourages spontaneous differentiation.
The neuronal differentiation of MSCs has been previously reported as a method for cell replacement therapy (Dezawa, 2002; Tang et al., 2012; Pan et al., 2013; Xu et al., 2013), and many reports are available on the conditions under which BMSCs differentiate into neural cells in vitro (Rossi and Keirstead, 2009b; Wang et al., 2010b; Cho et al., 2012; Tian et al., 2012). Morphological and immunocytochemical evaluations have been used to confirm the differentiation of MSCs into neuronal cells on electrospun PLCL/Coll nanofibers (Prabhakaran et al., 2009). However, to the best of our knowledge, neuronal cell maturation and the molecular mechanisms responsible for long-term neuronal differentiation remain to be clarified. In addition, we describe here, for the first time, the differentiation of MSCs into neuronal cells on fetal bovine acellular dermal matrix scaffolds.
In the present study, the basal medium was HyClone Advance STEM basal medium for undifferentiated human MSCs with 1% of 100 × antibiotic/antimycotic solution and 12% HyClone Advance STEM cell growth supplement. The neural differentiation medium contained HyClone Advance STEM neural differentiation medium with 12% HyClone Advance STEM cell growth supplement and 1% of 100 × antibiotic/antimycotic solution. Our results were reproducible and show stable proliferation and differentiation of BMSCs using these media.
The neuronal differentiation of BMSCs on fetal bovine acellular dermal matrix was assessed by immunocytochemistry and confocal microscopy using both a neuron-specific neurite outgrowth primary antibody, which specifically labels neurites and neuronal cell bodies from a wide range of mammalian species, and a mouse neuron-specific βIII tubulin primary antibody. The data presented here suggest that BMSCs grown on fetal bovine acellular dermal matrix and induced in neural differentiation medium differentiated into neuronal cells, and that the fetal bovine acellular dermal matrix itself promotes neurite outgrowth and the maturation of the neurons differentiated from BMSCs and supports the formation of a neural network-like structure.
BMSCs were grown on fetal bovine acellular dermal matrix first in basal medium for 17 days and then induced in neural differentiation medium for 17 days, and also in basal medium for 3 days and then induced in neural differentiation medium for 31 days. The images of these different regimens show that the fetal bovine acellular dermal matrices with attached cells became multilayered neural network-like structures with long nerve fibers, composed of several parallel micro-fibers, and neuronal cells, forming a complete neural circuit comprised of dendrite-dendrite to axon-dendrite to dendrite-axon synapses. The dendrite spines and growth cone with filopodia were also shown by scanning electron microscopy (Figure 1L–M, O–R). The immunohistochemistry of the paraffin sections showed neuron-like cells growing on and inside the fetal bovine acellular dermal matrix and the formation of nerve tissue within 31 days (Figure 2C, D). These results suggest that BMSCs differentiated into mature neuronal cells because they were able to transmit information between themselves.
A comparison of the scanning electron microscopy images of the original cells (Figure 1A) with the cells attached to the fetal bovine acellular dermal matrix and grown in basal medium shows that the cell morphology was changed. The structure of the fetal bovine acellular dermal matrix was also changed (Figure 1H). Taken together, these effects show that the fetal bovine acellular dermal matrix contributes to the growth, proliferation, and differentiation of BMSCs and, in turn, cell growth on the fetal bovine acellular dermal matrix also results in structural changes in the scaffold.
Based on the morphology results for long-term neuronal differentiation as assessed by scanning electron microscopy and immunocytochemical evaluation of paraffin sections, we conclude that BMSCs differentiate into neuronal cells on fetal bovine acellular dermal matrix and that the induction of neuronal differentiation depends on the niche microenvironment provided by the fetal bovine acellular dermal matrix, basal medium, and neural differentiation medium. The release of cytokines by BMSCs when grown on fetal bovine acellular dermal matrix in basal medium, including a complex set of growth factors and signaling molecules, also contributes. Fetal bovine acellular dermal matrix may facilitate the structural rearrangement of the cells, signal transmission between the cells, and neuronal differentiation of BMSCs once initiated by the neural differentiation medium. The techniques shown in the present study present a better approach for building useful autologous nerve substitutes than many other techniques.
The results of this study suggest that BMSCs can be differentiated on fetal bovine acellular dermal matrix scaffold into neuronal cells with active protein synthesis. Fetal bovine acellular dermal matrix supports the neuronal differentiation of BMSCs in the presence of the growth factors contained in the basal medium and neural differentiation medium, as well as the other cytokines and signaling molecules that are presumably released from BMSCs when being cultured in basal medium and induced in neural differentiation medium. This successful differentiation suggests the possibility of transplanting BMSCs-fetal bovine acellular dermal matrix scaffolds, after being cultured in basal medium for 3–4 days and induced in neural differentiation medium for 4–6 days, to the site of nerve injury. Moreover, fetal bovine acellular dermal matrix, similar to the PLCL/Coll scaffolds (Prabhakaran et al., 2009), provides both biological and mechanical support for the differentiation of BMSCs, enhancing the process of nerve regeneration (or neurogenesis). As a biological scaffold, fetal bovine acellular dermal matrix can support the differentiation of BMSCs into neuronal cells and the subsequent formation of a nerve network-like structure, thereby generating a tissue engineered nerve.
Here, we found that the proliferation of BMSCs on fetal bovine acellular dermal matrix in basal medium was higher than that of cell in neural differentiation medium. Therefore, when inducing BMSCs to differentiate into neuronal cells using this approach, we suggest that the BMSCs should first be cultured on fetal bovine acellular dermal matrix for 3 to 4 days in basal medium to ensure a better yield of cells before inducing differentiation into neuronal cells.
In addition, we found that when the BMSC-differentiated neuronal cells induced for 22 days on fetal bovine acellular dermal matrix were trypsinized and then subcultured in neural differentiation medium, they maintained their neuronal morphology. This finding suggests that these BMSC-derived differentiated neuronal cells are stable, and that it is possible to harvest those neuronal cells from the fetal bovine acellular dermal matrix.
Further, the results of this study show that the BMSCs induced towards neuronal differentiation on the fetal bovine acellular dermal matrix can not only provide a tissue engineered nerve to bridge peripheral nerve defects, but can also provide a source of neuronal cells for cell therapy for neurodegenerative diseases.
Fetal bovine acellular dermal matrix may be useful as a biological scaffold to support human BMSC growth, proliferation, and differentiation into neuronal cells. Long-term continuous induction in neural differentiation medium generated human BMSC-fetal bovine acellular dermal matrix scaffolds that looked like nerve tissue. The differentiated neuronal cells showed neuronal morphology, expressed neuronal-specific proteins, established neural signaling pathways, and formed nerve fibers in a multilayered neural network-like structure. We believe that fetal bovine acellular dermal matrix is a useful biological scaffold for nerve tissue engineering for the repair of nerve damage. Human BMSCs can attach to it and grow with excellent viability because of the biocompatibility of fetal bovine acellular dermal matrix. However, one disadvantage with the use of fetal bovine acellular dermal matrix is its potential immunogenicity; fetal bovine acellular dermal matrix from allogeneic or xenogeneic sources may be immunogenic to the host. Therefore, further research with the aim of reducing the immunogenicity of fetal bovine acellular dermal matrix should be pursued.
Acknowledgments:
We are very grateful to The Life Science & Engineering College of Northwest University for Nationalities of China, and Thermo Fisher HyClone R&D, USA. The majority of the experimental work was carried out in the Thermo Fisher HyClone R&D Lab. Scanning electron microscopy was performed by Professor Shen TC and Mrs. Kong FN the center for Surface Analysis & Applications of Utah State University, USA. Special thanks for the English corrections by Dr. Natalie Ward, Dr. Leland Foster from USU and Dr. Kjell Nilsson. Thanks for the help from the Center for Integrated Biosystems of Utah State University, USA.
Footnotes
Funding: This research was supported by a grant from Construction Project of Gansu Provincial Animal Cell Engineering Center, No. 0808NTGA013 and Program for Innovative Research Team in University of Ministry of Education of China, No. IRT13091.
Conflicts of interest: None declared.
Copyedited by McCarty W, Stow A, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Burns NK, Jaffari MV, Rios CN, Mathur AB, Butler CE. Non-cross-linked porcine acellular dermal matrices for abdominal wall reconstruction. Plast Reconstr Surg. 2010;125:167–176. doi: 10.1097/PRS.0b013e3181c2a6ed. [DOI] [PubMed] [Google Scholar]
- 2.Cho H, Seo YK, Yoon HH, Kim SC, Kim SM, Song KY, Park JK. Neural stimulation on human bone marrow-derived mesenchymal stem cells by extremely low frequency electromagnetic fields. Biotechnol Prog. 2012;28:1329–1335. doi: 10.1002/btpr.1607. [DOI] [PubMed] [Google Scholar]
- 3.Cullen DK, Wolf JA, Smith DH, Pfister BJ. Neural tissue engineering for neuroregeneration and biohybridized interface microsystems in vivo (Part 2) Crit Rev Biomed Eng. 2011;39:241–259. doi: 10.1615/critrevbiomedeng.v39.i3.40. [DOI] [PubMed] [Google Scholar]
- 4.Dezawa M. Central and peripheral nerve regeneration by transplantation of Schwann cells and transdifferentiated bone marrow stromal cells. Anat Sci Int. 2002;77:12–25. doi: 10.1046/j.0022-7722.2002.00012.x. [DOI] [PubMed] [Google Scholar]
- 5.Ding T, Luo ZJ, Zheng Y, Hu XY, Ye ZX. Rapid repair and regeneration of damaged rabbit sciatic nerves by tissue-engineered scaffold made from nano-silver and collagen type I. Injury. 2010;41:522–527. doi: 10.1016/j.injury.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol. 2009;482:281–294. doi: 10.1007/978-1-59745-060-7_18. [DOI] [PubMed] [Google Scholar]
- 7.Houle JD, Amin A, Cote MP, Lemay M, Miller K, Sandrow H, Santi L, Shumsky J, Tom V. Combining peripheral nerve grafting and matrix modulation to repair the injured rat spinal cord. J Vis Exp. 2009:1324. doi: 10.3791/1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu H, Zhang WC, Huang JF, Yan QJ, Yin YX, Li SP. Cytocompatibility between novel nerve conduit composite materials and bone marrow mesenchymal stem cells. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:2913–2920. [Google Scholar]
- 9.Hu J, Zhu QT, Liu XL, Xu YB, Zhu JK. Repair of extended peripheral nerve lesions in rhesus monkeys using acellular allogenic nerve grafts implanted with autologous mesenchymal stem cells. Exp Neurol. 2007;204:658–666. doi: 10.1016/j.expneurol.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Hudson TW, Liu SY, Schmidt CE. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004a;10:1346–1358. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 11.Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004b;10:1641–1651. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 12.Ibarra A, Martinon S. Pharmacological approaches to induce neuroregeneration in spinal cord injury: an overview. Curr Drug Discov Technol. 2009;6:82–90. doi: 10.2174/157016309788488320. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 14.Kavros SJ. Acellular fetal bovine dermal matrix for treatment of chronic ulcerations of the midfoot associated with Charcot neuroarthropathy. Foot Ankle Spec. 2012;5:230–234. doi: 10.1177/1938640012449037. [DOI] [PubMed] [Google Scholar]
- 15.Kavros SJ, Dutra T, Gonzalez-Cruz R, Liden B, Marcus B, McGuire J, Nazario-Guirau L. The use of PriMatrix, a fetal bovine acellular dermal matrix in healing chronic diabetic foot ulcers: a prospective multicenter study. Adv Skin Wound Care. 2014;27:356–362. doi: 10.1097/01.ASW.0000451891.87020.69. [DOI] [PubMed] [Google Scholar]
- 16.Li WJ, Tuli R, Huang X, Laquerriere P, Tuan RS. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26:5158–5166. doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Liu XG, Deng YB, Cai H. Glial cell line-derived neurotrophic factor promotes neuron-like cell differentiation of mesenchymal stem cells. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:1856–1861. [Google Scholar]
- 18.Lullove E. Acellular fetal bovine dermal matrix in the treatment of nonhealing wounds in patients with complex comorbidities. J Am Podiatr Med Assoc. 2012;102:233–239. doi: 10.7547/1020233. [DOI] [PubMed] [Google Scholar]
- 19.Masaeli E, Morshed M, Nasr-Esfahani MH, Sadri S, Hilderink J, van Apeldoorn A, van Blitterswijk CA, Moroni L. Fabrication, characterization and cellular compatibility of poly(hydroxy alkanoate) composite nanofibrous scaffolds for nerve tissue engineering. PLoS One. 2013;8:e57157. doi: 10.1371/journal.pone.0057157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neill J, James K, Lineaweaver W. Utilizing biologic assimilation of bovine fetal collagen in staged skin grafting. Ann Plast Surg. 2012;68:451–456. doi: 10.1097/SAP.0b013e31824189ed. [DOI] [PubMed] [Google Scholar]
- 21.Neubauer D, Graham JB, Muir D. Nerve grafts with various sensory and motor fiber compositions are equally effective for the repair of a mixed nerve defect. Exp Neurol. 2010;223:203–206. doi: 10.1016/j.expneurol.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Pan GQ, Qi FP, Xu H, Wang XL. Gene expression and calcium ion concentration variation in bone marrow mesenchymal stem cells differentiating into neuron-like cells. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:5080–5086. [Google Scholar]
- 23.Prabhakaran MP, Venugopal JR, Ramakrishna S. Mesenchymal stem cell differentiation to neuronal cells on electrospun nanofibrous substrates for nerve tissue engineering. Biomaterials. 2009;30:4996–5003. doi: 10.1016/j.biomaterials.2009.05.057. [DOI] [PubMed] [Google Scholar]
- 24.Price DL, Porter KR. The response of ventral horn neurons to axonal transection. J Cell Biol. 1972;53:24–37. doi: 10.1083/jcb.53.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rennekampff HO. Skin graft procedures in burn surgery. Unfallchirurg. 2009;112:543–549. doi: 10.1007/s00113-009-1655-5. [DOI] [PubMed] [Google Scholar]
- 26.Rennert RC, Sorkin M, Garg RK, Januszyk M, Gurtner GC. Cellular response to a novel fetal acellular collagen matrix: implications for tissue regeneration. Int J Biomater 2013. 2013:527957. doi: 10.1155/2013/527957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi SL, Keirstead HS. Stem cells and spinal cord regeneration. Curr Opin Biotech. 2009;20:552–562. doi: 10.1016/j.copbio.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 29.Siemionow M, Sonmez E. Nerve allograft transplantation: a review. J Reconstr Microsurg. 2007;23:511–520. doi: 10.1055/s-2007-1022694. [DOI] [PubMed] [Google Scholar]
- 30.Song Y, Wang Z, Wang Z, Zhang H, Li X, Chen B. Use of FK506 and bone marrow mesenchymal stem cells for rat hind limb allografts. Neural Regen Res. 2012;7:2681–2688. doi: 10.3969/j.issn.1673-5374.2012.34.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanian A, Krishnan UM, Sethuraman S. Development of biomaterial scaffold for nerve tissue engineering: biomaterial mediated neural regeneration. J Biomed Sci. 2009;16:108. doi: 10.1186/1423-0127-16-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeda A, Yamazaki Y, Baba K, Ishiguro M, Aoyagi K, Ikemoto S, Uchinuma E. Osteogenic potential of human bone marrow-derived mesenchymal stromal cells cultured in autologous serum: a preliminary study. 2012;70:e469–476. doi: 10.1016/j.joms.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Tang Y, Cui Y, Luo F, Liu X, Wang X, Wu A, Zhao J, Tian Z, Wu L. Cell viability and dopamine secretion of 6-hydroxydopamine-treated PC12 cells co-cultured with bone marrow-derived mesenchymal stem cells. Neural Regen Res. 2012;7:1101–1105. doi: 10.3969/j.issn.1673-5374.2012.14.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian X, Wang S, Zhang Z, Lv D. Rat bone marrow-derived Schwann-like cells differentiated by the optimal inducers combination on microfluidic chip and their functional performance. PLoS One. 2012;7:e42804. doi: 10.1371/journal.pone.0042804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tondreau T, Dejeneffe M, Meuleman N, Stamatopoulos B, Delforge A, Martiat P, Bron D, Lagneaux L. Gene expression pattern of functional neuronal cells derived from human bone marrow mesenchymal stromal cells. BMC Genomics. 2008;9:166. doi: 10.1186/1471-2164-9-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D, Wen Y, Lan X, Li H. Experimental study on bone marrow mesenchymal stem cells seeded in chitosan-alginate scaffolds for repairing spinal cord injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010a;24:190–196. [PubMed] [Google Scholar]
- 37.Wang D, Liu XL, Zhu JK, Jiang L, Hu J, Zhang Y, Yang LM, Wang HG, Yi JH. Bridging small-gap peripheral nerve defects using acellular nerve allograft implanted with autologous bone marrow stromal cells in primates. Brain Res. 2008;1188:44–53. doi: 10.1016/j.brainres.2007.09.098. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Cheng H, Li X, Lu W, Wang K, Wen TQ. Regulation of neural stem cell differentiation by transcription factors HNF4-1 and MAZ-1. Mol Neurobiol. 2013;47:228–240. doi: 10.1007/s12035-012-8335-0. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Wang ZH, Shen CY, You ML, Xiao JF, Chen GQ. Differentiation of human bone marrow mesenchymal stem cells grown in terpolyesters of 3-hydroxyalkanoates scaffolds into nerve cells. Biomaterials. 2010b;31:1691–1698. doi: 10.1016/j.biomaterials.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 40.Wanitphakdeedecha R, Chen TM, Nguyen TH. The use of acellular fetal bovine dermal matrix for acute full-thickness wounds. J Drugs Dermatol. 2008;7:781–784. [PubMed] [Google Scholar]
- 41.Welin D, Novikova LN, Wiberg M, Kellerth JO, Novikov LN. Survival and regeneration of cutaneous and muscular afferent neurons after peripheral nerve injury in adult rats. Exp Brain Res. 2008;186:315–323. doi: 10.1007/s00221-007-1232-5. [DOI] [PubMed] [Google Scholar]
- 42.Wietfeldt ED, Hassan I, Rakinic J. Utilization of bovine acellular dermal matrix for abdominal wall reconstruction: a retrospective case series. Ostomy/wound management. 2009;55:52–56. [PubMed] [Google Scholar]
- 43.Wislet-Gendebien S, Hans G, Leprince P, Rigo JM, Moonen G, Rogister B. Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem cells (Dayton, Ohio) 2005;23:392–402. doi: 10.1634/stemcells.2004-0149. [DOI] [PubMed] [Google Scholar]
- 44.Wu G, Dong C, Wang G, Gao W, Fan H, Xiao W, Zhang L. Preparation of three-dimensional porous scaffold of PLGA-silk fibroin-collagen nanofiber and its cytocompatibility study. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:1007–1011. [PubMed] [Google Scholar]
- 45.Xiao S, Zhu S, Li H, Yang J, Lv K, Xia Z. Feasibility study of composite skin reconstructed by mixing keratinocytes and acellular dermal matrix for wound repair. Swiss Med Wkly. 2009a;139:16–21. doi: 10.4414/smw.2009.12399. [DOI] [PubMed] [Google Scholar]
- 46.Xiao SC, Zhu SH, Li HY, Wang GY, Xia ZF. Repair of complex abdominal wall defects from high-voltage electric injury with two layers of acellular dermal matrix: a case report. J Burn Care Res. 2009b;30:352–354. doi: 10.1097/BCR.0b013e318198a6fa. [DOI] [PubMed] [Google Scholar]
- 47.Xin X, Hussain M, Mao JJ. Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials. 2007;28:316–325. doi: 10.1016/j.biomaterials.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu LL, Kuang T, Zhang J. In vitro neural-like cell differentiation from bone marrow mesenchymal stem cells: Co-culture versus chemical induction. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:7821–7821. [Google Scholar]