Abstract
Propofol and remifentanil alter intracellular Ca2+ concentration ([Ca2+]i) in neural stem/progenitor cells by activating γ-aminobutyric acid type A receptors and by reducing testosterone levels. However, whether this process affects neural stem/progenitor cell proliferation and differentiation remains unknown. In the present study, we applied propofol and remifentanil, alone or in combination, at low, moderate or high concentrations (1, 2–2.5 and 4–5 times the clinically effective blood drug concentration), to neural stem/progenitor cells from the hippocampi of newborn rat pups. Low concentrations of propofol, remifentanil or both had no noticeable effect on cell proliferation or differentiation; however, moderate and high concentrations of propofol and/or remifentanil markedly suppressed neural stem/progenitor cell proliferation and differentiation, and induced a decrease in [Ca2+]i during the initial stage of neural stem/progenitor cell differentiation. We therefore propose that propofol and remifentanil interfere with the proliferation and differentiation of neural stem/progenitor cells by altering [Ca2+]i. Our findings suggest that propofol and/or remifentanil should be used with caution in pediatric anesthesia.
Keywords: nerve regeneration, propofol, remifentanil, neural stem cells, neural progenitor cells, proliferation, apoptosis, differentiation, [Ca2+]i, neural regeneration
Introduction
Propofol is a general anesthetic that acts on activating γ-aminobutyric acid type A (GABAA) receptors to increase chloride ion channel opening (Concas et al., 1991). Remifentanil is a potent, ultra-short-acting synthetic opioid analgesic drug and is used extensively in the clinic owing to its unique advantages (Lee et al., 2012; Rogliani et al., 2013). It is commonly used in combination with propofol for anesthesia. Remifentanil induces overexpression of the p53 gene and inhibits the proliferation of neural stem/progenitor cells, possibly by reducing testosterone levels (Shoae-Hassani et al., 2011). However, the safety of the application of remifentanil in pediatric anesthesia deserves further investigation (Jevtovic-Todorovic et al., 2003; Pelaez et al., 2004; Mellon et al., 2007; Bache et al., 2013; Chandler et al., 2013). In the present study, we observed the effects of propofol and remifentanil on the proliferation and differentiation of in vitro cultured neural stem/progenitor cells from neonatal rat hippocampi.
Previous studies suggest that propofol increases intracellular calcium ion concentration ([Ca2+]i) in neural stem/progenitor cells by activating GABAA receptors (Tozuka et al., 2005; Cheung et al., 2009). Ca2+ plays an important role as a signal transduction messenger during neural stem/progenitor cell proliferation and differentiation, and in the development of the central nervous system (Fiorio Pla et al., 2005; D’Ascenzo et al., 2006; Pan et al., 2013; Wang et al., 2013). Increased [Ca2+]i within the physiological range promotes cell proliferation, differentiation, synthesis and catabolism (Yuan et al., 2012; Rushton et al., 2013; Li et al., 2014). Here, we explore the effects of propofol and remifentanil on neural stem/progenitor cell proliferation and differentiation, and their relationship with [Ca2+]i.
Materials and Methods
Isolation and culture of neural stem/progenitor cells
One-day-old Sprague-Dawley rat pups were provided by the Laboratory Animal Center, Hubei University of Medicine, China (license No. SCXK (E) 2005-0008). The study was approved by the Animal Ethics Committee, Hubei University of Medicine and Affiliated Hospital of Taihe, China. Newborn rats were anesthetized on ice and killed by cervical dislocation.
Hippocampi from seven 1-day-old Sprague-Dawley rats were isolated in a biological safety cabinet and placed in a flask for primary cell culture, as described previously (Lu et al., 2012a, b). Penicillin (50 IU/mL) and streptomycin (50 μg/mL) were added to the primary culturing medium (Wen et al., 2002; Wen et al., 2007; Yu et al., 2007).
Cell intervention
The 5-bromo-2′-deoxyuridine (BrdU) incorporation assay was used to observe hippocampal neural stem/progenitor cell proliferation. Third-passage cells were seeded onto a 24-well plate coated with polylysine at a density of 5 × 104/mL for immunofluorescence staining. Living cells at 1 × 106/mL were seeded onto a 48-well plate without a coverslip or polylysine, for the detection of cell proliferation and apoptosis. After 30 minutes of drug exposure (described below), several wells in each group were used for [Ca2+]i measurement (Muskhelishvili et al., 2003; Lu et al., 2012a, b).
Each well contained 0.5–1 mL medium. Samples were incubated at 5% CO2, 37°C and saturated humidity for 6–8 hours until cells reached at least 80% confluence. Each well in the 24- or 48-well plates was treated with either propofol (AstraZeneca, London, UK) or remifentanil (Yichang Humanwell Pharmaceutical Co., Ltd., Hubei Province, China). For the cell proliferation assay, each well of the 48-well plate was treated with BrdU for 20 minutes before drug exposure.
There were 11 treatment groups of five wells each: (1) P1: 1.0 μg/mL propofol; (2) P2: 2.5 μg/mL propofol; (3) P3: 5.0 μg/mL propofol; (4) R1: 5 ng/mL remifentanil; (5) R2: 10 ng/mL remifentanil; (6) R3: 20 ng/mL remifentanil; (7) R1 + P1: 5 ng/mL remifentanil + 1.0 μg/mL propofol; (8) R2 + P2: 10 ng/mL remifentanil + 2.5 μg/mL propofol; (9) R3 + P3: 20 ng/mL remifentanil + 5.0 μg/mL propofol; (10) Intralipid (Huarui Pharmacy Ltd., Wuxi, Jiangsu Province, China) control group; (11) blank control group. The low, moderate and high doses of propofol and remifentanil were, respectively, 1, 2–2.5 and 4–5 times that of the clinically effective blood concentration (Ludbrook et al., 2002).
The neural stem/progenitor cells were regenerated using 10 ng/mL basic fibroblast growth factor (Promega, Madison, WI, USA) and 10 ng/mL epidermal growth factor in basic culture medium, which consisted of Dulbecco's modified Eagle's medium/F12 (1:1, v/v), B-27 (2%) and N2 (1%) supplements, 0.5 mmol/L L-glutamine and 0.5 mmol/L non-essential amino acid.
Our neural stem/progenitor cell differentiation culture medium contained 1% fetal bovine serum and 1% serum replacement based on the basic culture medium, and basic fibroblast growth factor and epidermal growth factor were not further constituted (Wen et al., 2002; Wen et al., 2007; Yu et al., 2007). One third of the volume was replaced by fresh medium every 3 days.
Determination of [Ca2+]i
Samples were incubated with the drugs for 30 minutes at 37°C, 5% CO2 and saturated humidity. Samples from several wells in each group were collected and the medium discarded. The samples were washed twice with Ca2+-free PBS and digested with 0.125% trypsin for 5 minutes. Ca2+-free PBS was added to terminate the digestion. The samples were lightly triturated into a cell suspension, washed three times with Ca2+-free PBS, and resuspended with Ca2+-free PBS to 1 mL (5 × 105/mL). Fluo 3-AM (AAT Bioquest, Inc., Sunnyvale, CA, USA) was added at a final concentration of 10 μmol/L and the samples were incubated for 1 hour in the dark at 37°C, 5% CO2 conditional incubator. The samples were shaken gently, washed three times with Ca2+-free PBS, and the cells were resuspended in 1 mL Ca2+-free PBS. Absorbance was measured under a laser confocal microscope (Carl Zeiss GmbH, Jena, Germany) at a wavelength of 526 nm.
BrdU labeling and immunofluorescence staining
BrdU is the specific protein probe for evaluation of proliferation of neural stem/progenitor cells in our experiment (Lu et al., 2012a, b). When the cells were completely adherent, 6–8 hours after incubation, 5 μmol/L BrdU (Sigma, St. Louis, MO, USA) was added to each well for 20 minutes. Then all samples were incubated for 8 hours. The medium was removed and the samples were fixed in 0.01 mol/L PBS containing 4% paraformaldehyde at room temperature for 15 minutes, and washed three times with 0.01 mol/L PBS. Samples in each well were treated with 200 μL HCl (2 mol/L) at room temperature for 30 minutes, washed three times with 0.01 mol/L PBS, and then blocked with 0.01 mol/L PBS (pH 7.2) containing 200 μL 10% goat serum at room temperature for 30 minutes. Mouse anti-rat BrdU monoclonal antibody (200 μL, 1:400; Sigma) was added and the samples were gently shaken for 1 hour at room temperature, then overnight at 4°C, and washed three times with PBS. Fluorescein isothiocyanate (FITC) goat anti-mouse IgG (supplied ready to use; Thermo Fisher Scientific Inc., Rockford, IL, USA) was added in the dark at room temperature and shaken gently for 2 hours. After three further washes with PBS, all samples were mounted with glycerol phosphate buffer. BrdU-immunopositive cells were examined under a fluorescence microscope (Olympus, Tokyo, Japan) (Poncelet and Carayon, 1985; Brando et al., 2000).
Flow cytometry for cell apoptosis after Annexin V-FITC/propidium iodide double-staining
After 48 hours of culture, the cell medium was removed to a centrifuge tube. The samples were washed twice with PBS, and digested with 0.125% trypsin for 5 minutes. After the adherent cells had loosened, they were gently pipetted under a microscope, then removed and added to the centrifuge tube, centrifuged at 2,000 × g for 5 minutes. Suspended cells were collected and washed twice with cold PBS and resuspended with 400 μL 1 × binding buffer at a density of 1 × 106/mL. The cell suspension was treated with 5 μL of Annexin V-FITC at 2–8°C in the dark for 15 minutes and 10 μL of propidium iodide at 2–8°C in the dark for 5 minutes. Optical density values were measured using a flow cytometer (Beckman-Coulter, Miami, FL, USA), at an excitation wavelength of 488 nm. All experiments were performed in triplicate.
Immunofluorescence staining for β-tubulin and glial fibrillary acidic protein expression in cells
After 72 hours of culture in the presence of the test drugs, the detection was conducted as described previously (Lu et al., 2012a, b). The neuron-specific protein was β-tubulin and the astrocyte-specific protein was glial fibrillary acidic protein. The medium was removed from the well, then each well was washed three times with 0.01 mol/L PBS (pH 7.4) for 5 minutes each time, and the samples were fixed with 4% paraformaldehyde at room temperature for 15 minutes, before washing again three times with 0.01 mol/L PBS. Nonspecific binding was blocked with 200 μL 0.01 mol/L PBS containing 10% goat serum at room temperature for 30 minutes. The samples were incubated with PBS supplemented with 0.4% Triton X-100 (Sigma) and 0.1% bovine serum albumin at 37°C for 15 minutes, and washed three times with 0.01 mol/L PBS, before being treated with mouse anti-rat β-tubulin monoclonal primary antibody (150 μL, 1:400; Millipore, Billerica, MA, USA) and rabbit anti-rat glial fibrillary acidic protein monoclonal antibody (Sigma) at room temperature for 1 hour in a wet box at 4°C overnight. After three washes with PBS, the samples were incubated in the dark with FITC goat anti-rabbit IgG (1:100; Thermo Fisher Scientific Inc., Rockford, IL, USA) and TRITC goat anti-mouse IgG (1:100; Thermo Fisher Scientific Inc.) in the wet box at room temperature for 2 hours, followed by three washes with 0.1 mol/L PBS (pH 7.5). 4′,6-Diamidino-2-phenylindole (DAPI) staining solution (0.3 mL) was added to each well at room temperature for 15 minutes. All samples were washed three times with PBS, and mounted with 50% glycerol phosphate buffer. For a negative control, 0.01 mol/L PBS was used instead of primary antibody. Cells were then observed under a confocal microscope (Carl Zeiss GmbH).
Detection of the proportion of differentiated neural stem/progenitor cells
Neural stem/progenitor cell differentiation was detected at 12 days in each group. Cells were immunostained for neuron-specific enolase and glial fibrillary acidic protein, and then evaluated by flow cytometry (Beckman-Coulter, Miami, FL, USA) (Poncelet and Carayon, 1985; Brando et al., 2000). Rabbit anti-nestin polyclonal antibody (1:400; Boster, Wuhan, Hubei Province, China) was the primary antibody; the remaining procedures were identical to those described for BrdU immunofluorescence.
Statistical analysis
Data were expressed as the mean ± SD and processed using SPSS 15.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance and Bonferroni post hoc tests were used. P < 0.05 was considered statistically significant.
Results
Effects of different concentrations of propofol and remifentanil on neural stem/progenitor cell morphology
Cell morphology was normal in the blank control and Intralipid groups, and no obvious morphological changes were observed in the groups exposed to low concentrations of propofol and/or remifentanil (P1, R1, and R1 + P1 groups). However, the morphology and viability of differentiated cells, and the development of synapses or axons, were impaired in the groups exposed to higher concentrations of the drugs (P2, P3, R2 and R3 groups). Non-apoptotic astrocyte hypertrophy was visible in the R2 and R3 groups. The number of differentiated cells was lower in the R2+P2 and R3+P3 groups (high concentrations of propofol + remifentanil), and a large number of cells had died, showing poor cell morphology and viability, and poor development of synapses or axons. Moreover, non-apoptotic astrocyte hypertrophy was visible (Figure 1).
Figure 1.

Effects of different concentrations of propofol and remifentanil on neural stem/ progenitor cell morphology (immunofluorescence staining, × 200).
The morphology of cells treated with low concentrations of propofol and/or remifentanil was not notably different from that of control cells. However, after treatment with high concentrations, differentiation was suppressed. Green: Astrocyte-specific glial fibrillary acidic protein (GFAP; FITC staining); red: neuron-specific β-tubulin (TRITC staining); blue: nuclei (DAPI staining). P1: 1.0 μg/mL propofol; P2: 2.5 μg/mL propofol; P3: 5.0 μg/mL propofol; R1: 5 ng/mL remifentanil; R2: 10 ng/mL remifentanil; R3: 20 ng/mL remifentanil; R1 + P1: 5 ng/mL remifentanil + 1.0 μg/mL propofol; R2 + P2: 10 ng/mL remifentanil + 2.5 μg/mL propofol; R3 + P3: 20 ng/mL remifentanil + 5.0 μg/mL propofol; FITC: fluorescein isothiocyanate; DAPI: 4′,6-diamidino-2-phenylindole.
Effects of propofol and remifentanil on proliferation and differentiation of neural stem/progenitor cells
Compared with the blank control and Intralipid groups, no significant differences in cell proliferation or apoptosis were detected in the R1, P1, or R1 + P1 groups (P > 0.05). However, treatment with high concentrations of propofol and/or remifentanil inhibited cell proliferation and significantly increased the apoptosis ratio (P < 0.05). The apoptosis ratio increased with increasing concentrations of propofol and/or remifentanil (Figure 2).
Figure 2.
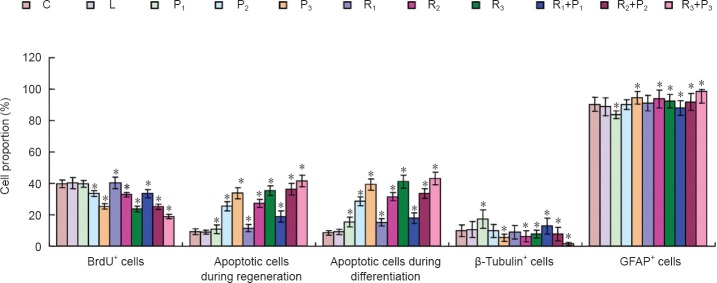
Propofol and remifentanil impair proliferation and differentiation of neural stem/progenitor cells.
Specific protein and self-renewal ability of neural stem/progenitor cells were examined using BrdU. Cell apoptosis was detected by flow cytometry using Annexin V-FITC/PI double-staining. β-Tubulin (neuron-specific) and glial fibrillary acidic protein (GFAP; astrocyte-specific) expression was detected using immunofluorescence staining. C: Blank control group; L: intralipid; P1: 1.0 μg/mL propofol; P2: 2.5 μg/mL propofol; P3: 5.0 μg/mL propofol; R1: 5 ng/mL remifentanil; R2: 10 ng/mL remifentanil; R3: 20 ng/mL remifentanil; R1 + P1: 5 ng/mL remifentanil + 1.0 μg/mL propofol; R2 + P2: 10 ng/mL remifentanil + 2.5 μg/mL propofol; R3 + P3: 20 ng/mL remifentanil + 5.0 μg/mL propofol. Data were expressed as the mean ± SD. One-way analysis of variance was used to compare multiple groups; Bonferroni post-hoc test was used for pairwise comparisons between groups. *P < 0.05, vs. blank control and Intralipid groups.
After 12 days of differentiation, the proportion of β-tubulin immunopositive neurons was significantly higher in the P1 and R1+P1 groups than in the blank control group (P < 0.05). There was no significant difference in the proportion of β-tubulin immunopositive neurons between the P2 and R1 groups and between the blank control and Intralipid groups (P > 0.05). The proportion of β-tubulin positive neurons was lower in the R2, R3, R2 + P2 and R3+P3 groups (P < 0.05, vs. blank control group; Figure 2).
Effects of different concentrations of propofol and remifentanil on [Ca2+]i at the initial stage of neural stem/progenitor cell differentiation
Compared with the blank control and Intralipid groups, [Ca2+]i was significantly lower in all groups (P < 0.05) except the P1 group, in which [Ca2+]i was significantly higher than in the control groups (P < 0.05; Figure 3).
Figure 3.
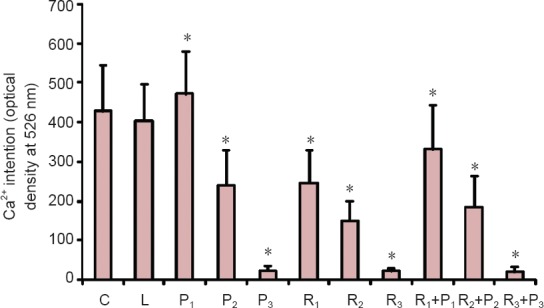
Effects of different concentrations of propofol and remifentanil on [Ca2+]i at the initial stage of differentiation in neural stem/progenitor cells.
C: Blank control group; L: intralipid; P1: 1.0 μg/mL propofol; P2: 2.5 μg/mL propofol; P3: 5.0 μg/mL propofol; R1: 5 ng/mL remifentanil; R2: 10 ng/mL remifentanil; R3: 20 ng/mL remifentanil; R1 + P1: 5 ng/mL remifentanil + 1.0 μg/mL propofol; R2 + P2: 10 ng/mL remifentanil + 2.5 μg/mL propofol; R3 + P3: 20 ng/mL remifentanil + 5.0 μg/mL propofol. Data were expressed as the mean ± SD. One-way analysis of variance was used to compare multiple groups; Bonferroni post-hoc test was used for pairwise comparisons between groups. *P < 0.05, vs. blank control and Intralipid groups.
Discussion
Previous studies have shown that during synapse formation (the key period of rapid development of the immature brain), an increase in neuronal differentiation and a reduction in proliferation of other nerve cells can affect the structure and physiological function of the hippocampus, and induce cognitive impairment (Sall et al., 2009; Stratmann et al., 2009). Our study supports these findings: propofol caused an increase in differentiation of neural stem/progenitor cells into neuronal cells but a decrease in the number of astrocytes. This indicates that propofol exposure during development would also affect the normal structure and physiological function of the hippocampus, and may result in later cognitive impairment. This may explain the effects of propofol on the rapid development of the immature brain in juvenile mammals (Karen et al., 2013; Kargaran et al., 2014; Sharma et al., 2014; Yu and Sun, 2014). Intralipid, the solvent of propofol, did not affect cell differentiation, illustrating that the effects observed are due to the pharmacological action of propofol rather than its solvent.
The results from this study demonstrate that clinically effective concentrations of propofol, remifentanil or their combination had no significant effect on the proliferation, differentiation, or apoptosis of rat neural stem/progenitor cells. However, moderate and high concentrations of propofol, remifentanil or their combination significantly inhibited the proliferation and differentiation of neural stem/progenitor cells, induced extensive apoptosis of differentiated cells, and disrupted the development of neuronal dendrites and axons. Our results suggest that propofol, remifentanil or their combination would produce noticeable effects on brain development in vivo, by altering the proliferation and differentiation of neural stem/progenitor cells in neonatal brain. This hypothesis warrants further investigation in vivo.
Furthermore, at a clinically effective concentration, propofol caused an increase of neural stem/progenitor cell differentiation into neuronal cells but a decrease in astrocyte differentiation.
We also explored [Ca2+]i as a possible mechanism for the observed effects. At the initial stage of neural stem/progenitor cell differentiation, compared with [Ca2+]i in control cells, [Ca2+]i was significantly higher only in the presence of low (clinically effective) concentrations of propofol, whereas at all other concentrations of propofol, remifentanil or their combination, initial [Ca2+]i was lower than in controls. The higher the drug concentration, the lower the initial [Ca2+]i.
Our study confirmed that the anesthetics propofol and remifentanil affect the proliferation and differentiation of neural stem/progenitor cells by altering [Ca2+]i. Moderate and high concentrations of remifentanil and propofol, alone or in combination, significantly diminished [Ca2+]i in the initial stage of neural stem/progenitor cell differentiation.
In summary, propofol and/or remifentanil affect neural stem/progenitor cell proliferation and differentiation, possibly by altering [Ca2+]i in these cells.
Footnotes
Funding: This study was supported by the Natural Science Foundation of Hubei Province of China, No. 2012FFC060; the Natural Science Foundation of Hubei University of Medicine in China, No. 2011QDZR-2; the Provincial Key Disciplines Foundation of Hubei Province of China, No. 2014XKJSSJ04.
Conflicts of interest: None declared.
Copyedited by Slone-Murphy J, Stow A, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Bache S, Stendell L, Olsen NV, Olsen KS. Problems in obtaining sufficient anaesthesia with propofol and remifentanil: three cases, a test infusion, and a review. Br J Anaesth. 2013;110:741–746. doi: 10.1093/bja/aes474. [DOI] [PubMed] [Google Scholar]
- 2.Brando B, Barnett D, Janossy G, Mandy F, Autran B, Rothe G, Scarpati B, D’Avanzo G, D’Hautcourt JL, Lenkei R, Schmitz G, Kunkl A, Chianese R, Papa S, Gratama JW. Cytofluorometric methods for assessing absolute numbers of cell subsets in blood. European Working Group on Clinical Cell Analysis. Cytometry. 2000;42:327–346. doi: 10.1002/1097-0320(20001215)42:6<327::aid-cyto1000>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Chandler JR, Myers D, Mehta D, Whyte E, Groberman MK, Montgomery CJ, Ansermino JM. Emergence delirium in children: a randomized trial to compare total intravenous anesthesia with propofol and remifentanil to inhalational sevoflurane anesthesia. Paediatr Anaesth. 2013;23:309–315. doi: 10.1111/pan.12090. [DOI] [PubMed] [Google Scholar]
- 4.Cheung G, Kann O, Kohsaka S, Faerber K, Kettenmann H. GABAergic activities enhance macrophage inflammatory protein-1alpha release from microglia (brain macrophages) in postnatal mouse brain. J Physiol. 2009;587:753–768. doi: 10.1113/jphysiol.2008.163923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Concas A, Santoro G, Serra M, Sanna E, Biggio G. Neurochemical action of the general anaesthetic propofol on the chloride ion channel coupled with GABAA receptors. Brain Res. 1991;542:225–232. doi: 10.1016/0006-8993(91)91571-h. [DOI] [PubMed] [Google Scholar]
- 6.D’Ascenzo M, Piacentini R, Casalbore P, Budoni M, Pallini R, Azzena GB, Grassi C. Role of L-type Ca 2+ channels in neural stem/progenitor cell differentiation. Eur J Neurosci. 2006;23:935–944. doi: 10.1111/j.1460-9568.2006.04628.x. [DOI] [PubMed] [Google Scholar]
- 7.Fiorio Pla A, Maric D, Brazer SC, Giacobini P, Liu X, Chang YH, Ambudkar IS, Barker JL. Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1- induced Ca 2+ entry and embryonic rat neural stem cell proliferation. J Neurosci. 2005;25:2687–2701. doi: 10.1523/JNEUROSCI.0951-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karen T, Schlager GW, Bendix I, Sifringer M, Herrmann R, Pantazis C, Enot D, Keller M, Kerner T, Felderhoff-Mueser U. Effect of propofol in the immature rat brain on short- and long-term neurodevelopmental outcome. PLoS One. 2013;8:e64480. doi: 10.1371/journal.pone.0064480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kargaran P, Lenglet S, Montecucco F, Mach F, Copin JC, Vutskits L. Impact of propofol anaesthesia on cytokine expression profiles in the developing rat brain: an experimental study. Eur J Anaesthesiol. 2014 doi: 10.1097/EJA.0000000000000128. doi: 10.1097/EJA. 0000000000000128. [DOI] [PubMed] [Google Scholar]
- 11.Lee JY, Yang H, Choi SH, Shin DW, Hong SK, Chun DH. The optimal effect-site concentration of remifentanil to attenuate the pain caused by propofol. Korean J Anesthesiol. 2012;63:108–112. doi: 10.4097/kjae.2012.63.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Cheng H, Tomaselli GF, Li RA. Mechanistic basis of excitation-contraction coupling in human pluripotent stem cell-derived ventricular cardiomyocytes revealed by Ca2+ spark characteristics: direct evidence of functional Ca2+ -induced Ca2+ release. Heart Rhythm. 2014;11:133–140. doi: 10.1016/j.hrthm.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Lu KH, Li DS. Distribution and localization of fibroblast growth factor-8 in rat brain and nerve cells during neural stem/progenitor cell differentiation. Neural Regen Res. 2012a;7:1455–1462. doi: 10.3969/j.issn.1673-5374.2012.19.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu J, Lu KH, Li DS. Changes in expression and secretion patterns of fibroblast growth factor 8 and Sonic Hedgehog signaling pathway moleculars during murine neural stem/progenitor cell differentiation in vitro. Neural Regen Res. 2012b;7:1688–1694. doi: 10.3969/j.issn.1673-5374.2012.22.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludbrook GL, Visco E, Lam AM. Propofol: relation between brain concentrations, electroencephalogram, middle cerebral artery blood flow velocity and cerebral oxygen extraction during induction of anesthesia. Anesthesiology. 2002;97:1363–1370. doi: 10.1097/00000542-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007;104:509–520. doi: 10.1213/01.ane.0000255729.96438.b0. [DOI] [PubMed] [Google Scholar]
- 17.Muskhelishvili L, Latendresse JR, Kodell RL, Henderson EB. Evaluation of cell proliferation in rat tissues with BrdU, PCNA, Ki-67 (MIB-5) immunohistochemistry and in situ hybridization for histone mRNA. J Histochem Cytochem. 2003;51:1681–1688. doi: 10.1177/002215540305101212. [DOI] [PubMed] [Google Scholar]
- 18.Pan GQ, Qi FP, Xu H, Wang XL. Gene expression and calcium ion concentration variation in bone marrow mesenchymal stem cells differentiating into neuron-like cells. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:5080–5086. [Google Scholar]
- 19.Pelaez R, Hortal FJ, Bastida E, Barrio JM, Riesgo MJ. Narcolepsy and cardiac surgery: can anesthesia with propofol and remifentanil be safe? J Cardiothorac Vasc Anesth. 2004;18:201–203. doi: 10.1053/j.jvca.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Poncelet P, Carayon P. Cytofluorometric quantification of cell-surface antigens by indirect immunofluorescence using monoclonal antibodies. J Immunol Methods. 1985;85:65–74. doi: 10.1016/0022-1759(85)90274-1. [DOI] [PubMed] [Google Scholar]
- 21.Rogliani P, Calzetta L, Rendina EA, Massullo D, Dauri M, Rinaldi B, Capuano A, Matera MG. The influence of propofol, remifentanil and lidocaine on the tone of human bronchial smooth muscle. Pulm Pharmacol Ther. 2013;26:325–331. doi: 10.1016/j.pupt.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Rushton DJ, Mattis VB, Svendsen CN, Allen ND, Kemp PJ. Stimulation of GABA-induced Ca2+ influx enhances maturation of human induced pluripotent stem cell-derived neurons. PLoS One. 2013;8:e81031. doi: 10.1371/journal.pone.0081031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sall JW, Stratmann G, Leong J, McKleroy W, Mason D, Shenoy S, Pleasure SJ, Bickler PE. Isoflurane inhibits growth but does not cause cell death in hippocampal neural precursor cells grown in culture. Anesthesiology. 2009;110:826–833. doi: 10.1097/ALN.0b013e31819b62e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma HS, Ponten E, Gordh T, Eriksson P, Fredriksson A, Sharma A. Propofol promotes blood-brain barrier breakdown and heat shock protein (HSP 72 kD) activation in the developing mouse brain. CNS Neurol Disord Drug Targets. 2014 doi: 10.2174/1871527313666140806122906. doi: 10.2174/1871527313666140806122906. [DOI] [PubMed] [Google Scholar]
- 25.Shoae-Hassani A, Sharif S, Tabatabaei SA, Verdi J. Could the endogenous opioid, morphine, prevent neural stem cell proliferation? Med Hypotheses. 2011;76:225–229. doi: 10.1016/j.mehy.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–848. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 27.Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, He JS, Xiong CZ, Feng XM, Wang JC, Yan LQ, Chen PT, Cai J. Effects of the L-type calcium channels on chondrocytes in response to basic fibroblast growth factor. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:8654–8659. [Google Scholar]
- 29.Wen T, Bao K, Li H. Blocking BE301622 gene expression by RNAi initiates differentiation of neural stem cells in rat. Cell Biochem Funct. 2007;25:775–779. doi: 10.1002/cbf.1367. [DOI] [PubMed] [Google Scholar]
- 30.Wen T, Gu P, Minning TA, Wu Q, Liu M, Chen F, Liu H, Huang H. Microarray analysis of neural stem cell differentiation in the striatum of the fetal rat. Cell Mol Neurobiol. 2002;22:407–416. doi: 10.1023/a:1021059520618. [DOI] [PubMed] [Google Scholar]
- 31.Yu D, Sun G. Is propofol more neurotoxic in the developing brain? J Anesth. 2014 doi: 10.1007/s00540-014-1896-5. doi: 10.1007/s00540-014-1896-5. [DOI] [PubMed] [Google Scholar]
- 32.Yu Y, Gu S, Huang H, Wen T. Combination of bFGF, heparin and laminin induce the generation of dopaminergic neurons from rat neural stem cells both in vitro and in vivo. J Neurol Sci. 2007;255:81–86. doi: 10.1016/j.jns.2007.01.076. [DOI] [PubMed] [Google Scholar]
- 33.Yuan GY, Ren MX, Guo YW, Zhou L. Effect of ionotropic glutamate receptor antagonists MK-801 on the proliferation of endogenous neural stem cells in rats with global cerebral ischemia-reperfusion injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16:1003–1006. [Google Scholar]


