Abstract
Because there is no curative treatment for spinal cord injury, establishing an ideal animal model is important to identify injury mechanisms and develop therapies for individuals suffering from spinal cord injuries. In this article, we systematically review and analyze various kinds of animal models of spinal cord injury and assess their advantages and disadvantages for further studies.
Keywords: nerve regeneration, spinal cord injury, animal model, establishment, evaluation, reviews, neural regeneration
Introduction
As the economic development all over the world, spinal cord injury (SCI) occurs with an annual incidence of 12.1–57.8 cases per million (van den Berg et al., 2010). Spinal cord injury is associated with permanent disability and decreased life expectancy (Hartkopp et al., 1997). It results in varying degrees of paralysis, sensory loss, and bladder/bowel dysfunction. The effects of SCI are not limited to an individual's health—it also creates an enormous financial burden for families and society at large (Pickelsimer et al., 2010).
In most developing countries, the majority of patients are young adults (20–40 years of age), thereby inflicting a high burden to these countries (Ackery et al., 2004). Traumatic SCI is more prevalent in males than females (Sekhon and Fehlings, 2001). Motor vehicle collisions (MVCs) and falls are the leading causes of SCI, and a trend toward an increased prevalence of falls and MVC in developing countries is likely related to urbanization and increased use of motor vehicles. Falls carrying a heavy load is another common cause of SCI in agricultural economy countries (Hoque et al., 1999). Sports-related SCI has also been discussed for diving athletes. Because there is no curative treatment for SCI, establishing an ideal animal model is important to develop therapies for individuals suffering from SCI. Over the past 30 years, there has been substantial worldwide research for establishing animal models, and a large number of therapeutic strategies have been developed (Kwon et al., 2011; Tetzlaff et al., 2011). In this article, we systematically review and analyze the literature about animal models of SCI, and assess their advantages and disadvantages for future studies on SCI.
Experimental animals
The pathology of human SCI is not greatly different from that of experimental animals, although important specific differences exist. Like contusion injury in humans, rat contusion injury leads to the formation of both small and large cavities and fluid-filled cysts (Sroga et al., 2003; Norenberg et al., 2004). A similar contusion injury in mice results in the development of a dense connective tissue matrix that more closely resembles the long-term effects of some laceration and massive compression injuries in humans (Sroga et al., 2003; Norenberg et al., 2004). Over the last 10 years, numerous animals have been used for studying SCI, mainly including rats, mice, dogs, rabbits, pigs, and nonhuman primates (Table 1).
Table 1.
Spinal cord injury animal models used in the past 10 years
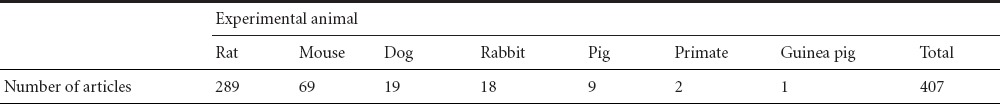
Some studies have predominately used rodents for in vivo SCI modeling and experimentation (Gonzalez-Lara et al., 2009; Basoglu et al., 2013). These studies have brought to light the pathophysiology of SCI and a growing number of novel treatments that promote behavioral recovery. There are numerous advantages of using rodents as models of SCI (Blight, 1992). For example, some animal studies benefit from the treatment of acute SCI. However, clinical trials have, thus far, been uniformly disappointing. There are some discrepancies between the promising animal studies and clinical trials, as well as the potential barriers in the translation of results from animal studies to humans (Akhtar et al., 2008). Methods for reproducible and controlled SCI models have been well described. These include contusion impactors (Krishna et al., 2013) and clip compression (Alluin et al., 2011). In addition, a multitude of behavioral outcome measures have become widely available. These include the Basso, Beattie, Bresnahan (BBB), the locomotor scale (Zeman et al., 2008), the cylinder rearing test (Lee et al., 2010), the horizontal ladder test (Lee et al., 2010), and the catwalk (Van Meeteren et al., 2003). These endpoint measurements produce interpretable and comparable results between studies. Histological, biochemical, and molecular techniques for assessing the outcome of SCI and/or its treatment have been well established. Furthermore, such small animal models are relatively inexpensive and require basic housing facilities readily available to most researchers. Despite these well recognized advantages, the SCI research community has also acknowledged the frustrating reality that even after the studies from these rodent models, clinical trials have failed to demonstrate convincing efficacy (Tator, 2006). Possible explanations for the discrepancy may be due to the heterogeneity of human SCI and the challenging nature of measuring neurological function in clinical trial settings (Kwon et al., 2011). However, at a more fundamental level, the differences in size, anatomy, and the pathophysiological responses to injury may quite possibly exist between SCI in rodents and in human patients. However, the direct translation of the results from rodents to clinical cases is difficult because of neurofunctional and anatomic differences between the two species. Prior to embarking on lengthy and expensive clinical trials, an animal SCI model that is an intermediary between both a rodent and human SCI may be a valuable translational research resource for pre-clinically evaluating novel therapies (Nout et al., 2012; Zurita et al., 2012; Lee et al., 2013).
Establishment of SCI models
For SCI research, it is essential to establish an ideal animal model of SCI. Ideal models should meet the following conditions (Akhtar et al., 2008): (1) simulate damage that is similar to clinical SCI; (2) be controlled, reproducible, and stable; (3) involve a simple technique that is easy to study; (4) the equipment used to make a model is straightforward and quick to produce.
Anatomical location
Differences in injury exist between experimental and clinical SCI. In both experimental and clinical SCI, contusion and compression are two of the most common injury types. However, in experimental animals, these injuries are most frequently induced dorsally and in the thoracic spine, whereas most clinical injuries occur anteriorly and in the cervical region (Akhtar et al., 2008). According to the National Spinal Cord Injury Statistical Center, in 2005, 51% of SCI cases in the U.S. occurred in the cervical region (Akhtar et al., 2008). Most SCI in humans affects the anterior spinal artery that supplies three quarters of cord tissue, in contrast to the dorsal arteries that are more commonly affected in experimental SCI (de la Torre, 1981).
Functional outcomes and assessment scales
Functional outcomes commonly used in experimental animal SCI include histopathological and electrophysiological measures, spinal cord blood flow, the Basso Mouse scale (Yu et al., 2014), and measurement of biomarkers such as lipid peroxidation. Evoked field potentials are commonly used to assess sensory and motor function in animals, however they mostly reflect the activity of large fibers and thus only a small part of axonal grouping is at the lesion site (Blight, 1992). Most methods of recording evoked potentials involve craniotomy and laminectomy for electrode placement, which allow for only single acute measurements (Sun et al., 2000). This lack of consistent correlation with functional outcome is another significant drawback with the use of surrogate markers. A great effort has been made to develop assessment scales and procedures to appropriately measure functional outcomes. Some of the most commonly used scales are the BBB scale (Basso et al., 1995), the Tarlov open field test (Tarlov and Klinger, 1954), and the inclined plane test (Rivlin and Tator, 1977). Both the Tarlov and inclined plane tests assess general locomotor ability and do not reflect specific changes in motor or sensory function (Kunkel-Bagden et al., 1993). Although the BBB scale is an improvement from the Tarlov scale, it is far from a comprehensive evaluation of motor function. This scale assesses hindlimb function only, and does not assess other movements which require coordinated spinal cord activity (Akhtar et al., 2008). Motor and sensory dysfunction has been periodically assessed using open field locomotion scoring, thermal/tactile pain/escape thresholds, and myogenic motor evoked potentials. Gait (CatWalk) and ladder climbing have also been assessed (van Gorp et al., 2013). Overall, we can conclude that accurate measurement of fine motor and sensory function in animals, especially in rodents, is quite difficult. Treatment effectiveness in animals with SCI is often measured simply by whether or not independent ambulation has occurred.
Animal models of SCI
There are numerous experimental animal models of SCI, including the spinal cord ischemia-reperfusion injury model (Lafci et al., 2013), spinal cord traumatic injury model (Koozekanani et al., 1976), photochemical-induced SCI model (Piao et al., 2009), spinal cord transection model (Min et al., 2011), and bidirectional distraction SCI model (Seifert et al., 2011). However, SCI caused by impact and compression is more common in clinical patients. Some models are used for investigating pathophysiological mechanisms (Table 2) and others for investigating the mechanisms underlying tissue engineering and spinal cord regeneration (Table 2).
Table 2.
Comparison of different methods used for spinal cord injury (SCI)
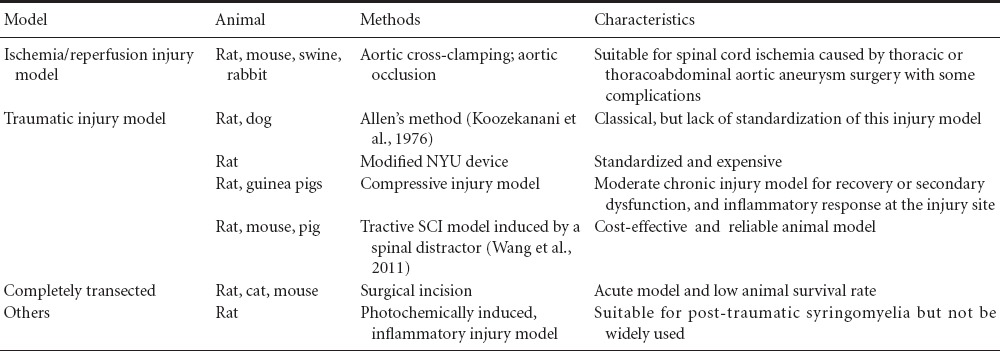
Traumatic SCI model
In 1911, Allen first created SCI models using a weight drop to affect the animal dorsal spinal cord, and this technique was later considered as a standard experimental spinal cord contusion injury model (Koozekanani et al., 1976). This method of inducing SCI may yield markedly different degrees of cord compression depending on the materials and apparatus. Some approaches to the standardization of this injury model have been suggested (Yeo et al., 2004). Experimental SCI is induced by dropping calibrated weights through a vented tube onto a small impounder resting on the surgically exposed cord (Koozekanani et al., 1976).
Contusive SCI models: To establish an experimental animal model of contusive SCI, a pneumatic impact device was made in Korea to induce a contusive injury on the dorsal spinal cord (Yeo et al., 2004). A NYU MASCIS (New York University, Multicenter Animal Spinal Cord Injury Study) impactor and an Ohio State University electromagnetic spinal cord injury device have been used to induce SCI in rodents, but these impact devices are expensive. A cost-effective approach for an experimental SCI rat model has been to create a customized impact device (Vijayaprakash and Sridharan, 2013), which is a customized blunt-force impact device designed to induce a standard animal model of contusive SCI at the thoracic level.
Compressive SCI model: A model of spinal cord trauma in guinea pigs is based on the concept of compression to a set thickness as an alternative to compression or contusion with a set force or displacement. This model is technically simple and reliable, and it was designed initially to produce moderate injuries, allowing significant recovery of function (Blight, 1991). Recently, some studies have adopted some experimental devices, designed in-house, to construct standardized ventral and dorsal rat SCI models (Zheng et al., 2012). They have used different weights falling from varied heights, leading to transversal compression on the spinal cord. To induce SCI, a 35-g circular rod is placed on the exposed L3 spinal segment and the spinal cord is compressed in the dorso-ventral direction for 15 minutes. This results in gradual increases in the degree of histopathological injury leading to decreased Tarlov and BBB scores for the behavioral test and increased Ashworth scores for the hind limb. Similar alterations have been observed in the ventral SCI rats proportional to the increase in compression weight (Zheng et al., 2012). These experimental findings indicate that the standardized experimental rat models of dorsal and ventral SCI may be stable, reliable, and reproducible.
Tractive SCI model: To establish an animal model of tractive SCI in rats, Liu et al. (2004) used spines that were tracted longitudinally with a special spinal retractor that was put on the processus transversus vertebrae of the rat after exposing the spinal cord to dual laminectomy. The tractive SCI rat models can also be successfully established with a spinal impactor, and monitoring the somatosensory evoked potential for several minutes is more suitable for studying tractive SCI (Wang et al., 2011). The tractive SCI model is a reliable animal model for studying pathological mechanisms and treatment of tractive SCI (Wang et al., 2011).
Complete SCI model: In some studies, a surgical incision has been used to sever the spinal cord to establish completely transected SCI models. The majority of studies for the recovery of locomotion from SCI have used rats (65%) and the remainder have used cats (23%) or mice (12%). The level of SCI in completely transected animals has ranged from T6 to T13 with 47% between T9 and T11 (Battistuzzo et al., 2012). Implantation of scaffolding biomaterials is applicable to this kind of SCI model, and these biomaterials provide effective bridging and stimulating effects on neural regeneration and prevent the formation of glial scars in the completely transected SCI rat models (Han et al., 2010).
Ischemia-reperfusion SCI injury model
Spinal blood flow plays a significant role in maintaining spinal function. Thoracic or thoracoabdominal aortic aneurysm surgery may cause spinal cord ischemia. Such animal models have been established via transient aortic occlusion by cross-clamping the descending aorta through a lateral thoracotomy (Awad et al., 2010). Lee et al. (2008) investigated whether ischemic tolerance could be induced by ischemic preconditioning of the spinal cord in a swine model. Furthermore, ischemia/reperfusion injury may directly or indirectly be responsible for aortic cross-clamping, and may result in severe postoperative complications caused by SCI (Lafci et al., 2013). Lozos et al. (2013) have reported that Aprikalim reduces the severity of ischemic SCI by 30 minutes of normothermic aortic cross-clamping in a rabbit model of spinal cord ischemia (Lozos et al., 2013). To accurately evaluate ischemic injury of the spinal cord, endoprosthesis implantation in the thoraco-abdominal aorta has been used (Vaquero et al., 2007).
Inflammatory SCI model
Inflammation is known to be detrimental to the neurologic outcome during the acute phase after an injury, and therefore provides a potential target for preventive or therapeutic approaches for spinal cord ischemia-reperfusion injury (Li et al., 2014). Spinal cord ischemia has been induced by balloon occlusion of the thoracic aorta in Sprague-Dawley rats (Fan et al., 2011).
Photochemical-induced SCI model
With the exposed spinal column intact, 560 nm light irradiation of the dorsal surface induces excitation of the systemically injected dye, rose bengal, in the spinal cord microvasculature (Watson et al., 1986). The resultant photochemical reaction leads to vascular stasis, and voluntary motor function is consistently lost in the subacute phase of injury (Watson et al., 1986). Hao et al. (1991) developed a rat model of photochemical-induced SCI, in which female rats were intravenously injected with Erythrosin B and the T10 vertebra was irradiated with a laser beam for 1, 5 or 10 minutes. These procedures initiated an intravascular photochemical reaction, resulting in ischemic SCI. Vascular thrombosis, resulting from an intravascular photochemical reaction induced by a rose Bengal-laser beam interaction, led to an extensive area of tissue deterioration, termed the “lesion cavity” within a few days (Bunge et al., 1994). This model technique may provide an appropriate milieu to better understand the aspects of the vexing problem of post-traumatic syringomyelia in the human.
Conclusions and perspectives
SCI animal models, including the contusive, compressive, tractive, photochemical-induced, inflammatory injury, and ischemia-reperfusion injury models have been mostly used for investigating the pathophysiology of SCI. However, the completely transected SCI models have been usually used for tissue engineering and spinal cord regeneration.
Several major problems still exist in understanding SCI in animal models and humans: (1) the lack of anatomical and pathophysiological correlation between experimental SCI and clinical SCI; (2) lack of congruent SCI pathology between different species and strains; (3) difficulties in interpreting outcomes measured in animals.
In the future, several points should be addressed. Species and strains of SCI need to be standardized. In these studies, environmental conditions may diminish some of the difficulties involved in performing and interpreting behavioral tests, and thus may serve to improve comparisons between studies. Standardizing laboratory procedures for different species and strains may also decrease the differences between normal and injured spinal cord biochemistry. However, there have been few such relatively direct studies.
Footnotes
Conflicts of interest: None declared.
Copyedited by Mark F, Robens J, Li CH, Song LP, Zhao M
References
- 1.Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004;21:1355–1370. doi: 10.1089/neu.2004.21.1355. [DOI] [PubMed] [Google Scholar]
- 2.Akhtar AZ, Pippin JJ, Sandusky CB. Animal models in spinal cord injury: a review. Rev Neurosci. 2008;19:47–60. doi: 10.1515/revneuro.2008.19.1.47. [DOI] [PubMed] [Google Scholar]
- 3.Alluin O, Karimi-Abdolrezaee S, Delivet-Mongrain H, Leblond H, Fehlings MG, Rossignol S. Kinematic study of locomotor recovery after spinal cord clip compression injury in rats. J Neurotrauma. 2011;28:1963–1981. doi: 10.1089/neu.2011.1840. [DOI] [PubMed] [Google Scholar]
- 4.Awad H, Ankeny DP, Guan Z, Wei P, McTigue DM, Popovich PG. A mouse model of ischemic spinal cord injury with delayed paralysis caused by aortic cross-clamping. Anesthesiology. 2010;113:880–891. doi: 10.1097/ALN.0b013e3181ec61ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basoglu H, Kurtoglu T, Cetin NK, Bilgin MD, Kiylioglu N. Assessment of in vivo spinal cord conduction velocity in rats in an experimental model of ischemic spinal cord injury. Spinal Cord. 2013;51:616–622. doi: 10.1038/sc.2013.40. [DOI] [PubMed] [Google Scholar]
- 6.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 7.Battistuzzo CR, Callister RJ, Callister R, Galea MP. A systematic review of exercise training to promote locomotor recovery in animal models of spinal cord injury. J Neurotrauma. 2012;29:1600–1613. doi: 10.1089/neu.2011.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blight AR. Morphometric analysis of a model of spinal cord injury in guinea pigs with behavioral evidence of delayed secondary pathology. J Neurol Sci. 1991;103:156–171. doi: 10.1016/0022-510x(91)90159-5. [DOI] [PubMed] [Google Scholar]
- 9.Blight AR. Spinal cord injury models: neurophysiology. J Neurotrauma. 1992;9:147–149. doi: 10.1089/neu.1992.9.147. [DOI] [PubMed] [Google Scholar]
- 10.Bunge MB, Holets VR, Bates ML, Clarke TS, Watson BD. Characterization of photochemically induced spinal cord injury in the rat by light and electron microscopy. Exp Neurol. 1994;127:76–93. doi: 10.1006/exnr.1994.1082. [DOI] [PubMed] [Google Scholar]
- 11.de la Torre JC. Spinal cord injury. Review of basic and applied research. Spine (Phila Pa 1976) 1981;6:315–335. [PubMed] [Google Scholar]
- 12.Fan L, Wang K, Shi Z, Die J, Wang C, Dang X. Tetramethylpyrazine protects spinal cord and reduces inflammation in a rat model of spinal cord ischemia-reperfusion injury. J Vasc Surg. 2011;54:192–200. doi: 10.1016/j.jvs.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Lara LE, Xu X, Hofstetrova K, Pniak A, Brown A, Foster PJ. In vivo magnetic resonance imaging of spinal cord injury in the mouse. J Neurotrauma. 2009;26:753–762. doi: 10.1089/neu.2008.0704. [DOI] [PubMed] [Google Scholar]
- 14.Han Q, Jin W, Xiao Z, Ni H, Wang J, Kong J, Wu J, Liang W, Chen L, Zhao Y, Chen B, Dai J. The promotion of neural regeneration in an extreme rat spinal cord injury model using a collagen scaffold containing a collagen binding neuroprotective protein and an EGFR neutralizing antibody. Biomaterials. 2010;31:9212–9220. doi: 10.1016/j.biomaterials.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 15.Hartkopp A, Bronnum-Hansen H, Seidenschnur AM, Biering-Sorensen F. Survival and cause of death after traumatic spinal cord injury. A long-term epidemiological survey from Denmark. Spinal Cord. 1997;35:76–85. doi: 10.1038/sj.sc.3100351. [DOI] [PubMed] [Google Scholar]
- 16.Hoque MF, Grangeon C, Reed K. Spinal cord lesions in Bangladesh: an epidemiological study 1994 - 1995. Spinal Cord. 1999;37:858–861. doi: 10.1038/sj.sc.3100938. [DOI] [PubMed] [Google Scholar]
- 17.Koozekanani SH, Vise WM, Hashemi RM, McGhee RB. Possible mechanisms for observed pathophysiological variability in experimental spinal cord injury by the method of Allen. J Neurosurg. 1976;44:429–434. doi: 10.3171/jns.1976.44.4.0429. [DOI] [PubMed] [Google Scholar]
- 18.Krishna V, Andrews H, Jin X, Yu J, Varma A, Wen X, Kindy M. A contusion model of severe spinal cord injury in rats. J Vis Exp. 2013 doi: 10.3791/50111. dio:10.3791/50111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunkel-Bagden E, Dai HN, Bregman BS. Methods to assess the development and recovery of locomotor function after spinal cord injury in rats. Exp Neurol. 1993;119:153–164. doi: 10.1006/exnr.1993.1017. [DOI] [PubMed] [Google Scholar]
- 20.Kwon BK, Okon EB, Plunet W, Baptiste D, Fouad K, Hillyer J, Weaver LC, Fehlings MG, Tetzlaff W. A systematic review of directly applied biologic therapies for acute spinal cord injury. J Neurotrauma. 2011;28:1589–1610. doi: 10.1089/neu.2009.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon BK, Okon EB, Tsai E, Beattie MS, Bresnahan JC, Magnuson DK, Reier PJ, McTigue DM, Popovich PG, Blight AR, Oudega M, Guest JD, Weaver LC, Fehlings MG, Tetzlaff W. A grading system to evaluate objectively the strength of pre-clinical data of acute neuroprotective therapies for clinical translation in spinal cord injury. J Neurotrauma. 2011;28:1525–1543. doi: 10.1089/neu.2010.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lafci G, Gedik HS, Korkmaz K, Erdem H, Cicek OF, Nacar OA, Yildirim L, Kaya E, Ankarali H. Efficacy of iloprost and montelukast combination on spinal cord ischemia/reperfusion injury in a rat model. J Cardiothorac Surg. 2013;8:64. doi: 10.1186/1749-8090-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JH, Tigchelaar S, Liu J, Stammers AM, Streijger F, Tetzlaff W, Kwon BK. Lack of neuroprotective effects of simvastatin and minocycline in a model of cervical spinal cord injury. Exp Neurol. 2010;225:219–230. doi: 10.1016/j.expneurol.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Jones CF, Okon EB, Anderson L, Tigchelaar S, Kooner P, Godbey T, Chua B, Gray G, Hildebrandt R, Cripton P, Tetzlaff W, Kwon BK. A novel porcine model of traumatic thoracic spinal cord injury. J Neurotrauma. 2013;30:142–159. doi: 10.1089/neu.2012.2386. [DOI] [PubMed] [Google Scholar]
- 25.Lee JS, Hong JM, Kim YJ. Ischemic preconditioning to prevent lethal ischemic spinal cord injury in a swine model. J Invest Surg. 2008;21:209–214. doi: 10.1080/08941930802262249. [DOI] [PubMed] [Google Scholar]
- 26.Li XQ, Wang J, Fang B, Tan WF, Ma H. Intrathecal antagonism of microglial TLR4 reduces inflammatory damage to blood-spinal cord barrier following ischemia/reperfusion injury in rats. Mol Brain. 2014;7:28. doi: 10.1186/1756-6606-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Chi LT, Tu ZQ, Sheng B, Zhou ZK, Pei FX. Observation and establishment of an animal model of tractive spinal cord injury in rats. Chin J Traumatol. 2004;7:372–377. [PubMed] [Google Scholar]
- 28.Lozos VA, Toumpoulis IK, Agrogiannis G, Giamarellos-Bourboulis EJ, Chamogeorgakis TP, Rizos IK, Patsouris ES, Anagnostopoulos CE, Rokkas CK. Aprikalim a potassium adenosine triphosphate channel opener reduces neurologic injury in a rabbit model of spinal cord ischemia. Int J Surg. 2013;11:354–359. doi: 10.1016/j.ijsu.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 29.Min SH, Lee SH, Shim H, Park JS, Lee YI, Kim HW, Hyun JK. Development of complete thoracic spinal cord transection model in rats for delayed transplantation of stem cells. Spine (Phila Pa 1976) 2011;36:E155–163. doi: 10.1097/BRS.0b013e3181d8b92a. [DOI] [PubMed] [Google Scholar]
- 30.Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 31.Nout YS, Rosenzweig ES, Brock JH, Strand SC, Moseanko R, Hawbecker S, Zdunowski S, Nielson JL, Roy RR, Courtine G, Ferguson AR, Edgerton VR, Beattie MS, Bresnahan JC, Tuszynski MH. Animal models of neurologic disorders: a nonhuman primate model of spinal cord injury. Neurotherapeutics. 2012;9:380–392. doi: 10.1007/s13311-012-0114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piao MS, Lee JK, Jang JW, Kim SH, Kim HS. A mouse model of photochemically induced spinal cord injury. J Korean Neurosurg Soc. 2009;46:479–483. doi: 10.3340/jkns.2009.46.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickelsimer E, Shiroma EJ, Wilson DA. Statewide investigation of medically attended adverse health conditions of persons with spinal cord injury. J Spinal Cord Med. 2010;33:221–231. doi: 10.1080/10790268.2010.11689699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivlin AS, Tator CH. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg. 1977;47:577–581. doi: 10.3171/jns.1977.47.4.0577. [DOI] [PubMed] [Google Scholar]
- 35.Seifert JL, Bell JE, Elmer BB, Sucato DJ, Romero MI. Characterization of a novel bidirectional distraction spinal cord injury animal model. J Neurosci Methods. 2011;197:97–103. doi: 10.1016/j.jneumeth.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Sekhon LH, Fehlings MG. Epidemiology demographics and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 2001;26:S2–12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- 37.Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;462:223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- 38.Sun T, Schlag MG, Hopf R, Shen Q, Redl H. Brainstem-evoked muscle potentials: their prognostic value in experimental spinal cord injury in the rat. Somatosens Mot Res. 2000;17:317–324. doi: 10.1080/08990220020002024. [DOI] [PubMed] [Google Scholar]
- 39.Tarlov IM, Klinger H. Spinal cord compression studies. II.Time limits for recovery after acute compression in dogs. AMA Arch Neurol Psychiatry. 1954;71:271–290. [PubMed] [Google Scholar]
- 40.Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties and recommendations. Neurosurgery. 2006;59:957–982. doi: 10.1227/01.NEU.0000245591.16087.89. [DOI] [PubMed] [Google Scholar]
- 41.Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG, Kwon BK. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611–1682. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Berg ME, Castellote JM, Mahillo-Fernandez I, de Pedro-Cuesta J. Incidence of spinal cord injury worldwide: a systematic review. Neuroepidemiology. 2010;34:184–192. doi: 10.1159/000279335. 192. [DOI] [PubMed] [Google Scholar]
- 43.van Gorp S, Leerink M, Kakinohana O, Platoshyn O, Santucci C, Galik J, Joosten EA, Hruska-Plochan M, Goldberg D, Marsala S, Johe K, Ciacci JD, Marsala M. Amelioration of motor/sensory dysfunction and spasticity in a rat model of acute lumbar spinal cord injury by human neural stem cell transplantation. Stem Cell Res Ther. 2013;4:57. doi: 10.1186/scrt209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Meeteren NL, Eggers R, Lankhorst AJ, Gispen WH, Hamers FP. Locomotor recovery after spinal cord contusion injury in rats is improved by spontaneous exercise. J Neurotrauma. 2003;20:1029–1037. doi: 10.1089/089771503770195876. [DOI] [PubMed] [Google Scholar]
- 45.Vaquero C, Arce N, Agudo J, Martinez R, Garjal C, Diago MV. Evaluation of ischemic injury of the spinal cord following endoprosthesis implantation in the thoraco-abdominal aorta on a rat model. Rev Port Cir Cardiotorac Vasc. 2007;14:33–37. [PubMed] [Google Scholar]
- 46.Vijayaprakash KM, Sridharan N. An experimental spinal cord injury rat model using customized impact device: A cost-effective approach. J Pharmacol Pharmacother. 2013;4:211–213. doi: 10.4103/0976-500X.114607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W, Yang T, Lei M, Pei F, Liu L. Establishment of tractive spinal cord injury model in rats with a novel spinal distractor. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011;25:705–710. [PubMed] [Google Scholar]
- 48.Watson BD, Prado R, Dietrich WD, Ginsberg MD, Green BA. Photochemically induced spinal cord injury in the rat. Brain Res. 1986;367:296–300. doi: 10.1016/0006-8993(86)91606-9. [DOI] [PubMed] [Google Scholar]
- 49.Yeo SJ, Hwang SN, Park SW, Kim YB, Min BK, Kwon JT, Suk JS. Development of a rat model of graded contusive spinal cord injury using a pneumatic impact device. J Korean Med Sci. 2004;19:574–580. doi: 10.3346/jkms.2004.19.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu CG, Singh R, Crowdus C, Raza K, Kincer J, Geddes JW. Fenbendazole improves pathological and functional recovery following traumatic spinal cord injury. Neuroscience. 2014;256:163–169. doi: 10.1016/j.neuroscience.2013.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeman RJ, Wen X, Ouyang N, Rocchio R, Shih L, Alfieri A, Moorthy C, Etlinger JD. Stereotactic radiosurgery improves locomotor recovery after spinal cord injury in rats. (987-988).Neurosurgery. 2008;63:981–987. doi: 10.1227/01.NEU.0000330404.37092.3E. [DOI] [PubMed] [Google Scholar]
- 52.Zheng YH, Fang Z, Cao P, Zheng T, Sun CH, Lu J, Shi RY. A model of acute compression spinal cord injury by a mini-invasive expandable balloon in goats. Zhonghua Yi Xue Za Zhi. 2012;92:1591–1595. [PubMed] [Google Scholar]
- 53.Zurita M, Aguayo C, Bonilla C, Otero L, Rico M, Rodriguez A, Vaquero J. The pig model of chronic paraplegia: a challenge for experimental studies in spinal cord injury. Prog Neurobiol. 2012;97:288–303. doi: 10.1016/j.pneurobio.2012.04.005. [DOI] [PubMed] [Google Scholar]


