Abstract
Background
Giant emphysamtous bulla (GEB) can negatively affect the pulmonary functions of chronic obstructive pulmonary diseases (COPD) patients, including decreased forced expiratory volume in 1 s (FEV1) and increased functional residual capacity (FRC). The aim of this study was to evaluate the efficacy of endobronchial valve (EBV) to treat bullae and to find efficacy predictors of successful treatment.
Methods
Five COPD patients with giant bulla were treated using EBVs. Before the EBV deployment, collateral ventilation (CV) between the targeted and adjacent lobes was evaluated with Chartis system.
Results
In the two patients with negative CV, the mean value of FEV1 increased from 27.1±11.4% of predicted value before EBV treatment to 32.8±12.0% (P>0.05) at 1 month after EBV treatment, than to 31.7±24.5% (P>0.05) at 6 months after EBV treatment. Only one patient, whose bulla occupied the whole right middle lung, displayed sustained improvement of FEV1 at 6 months after EBV treatment. In the three patients with positive CV, the mean value of FEV1 decreased from 28.8±19.0% of predicted value before EBV treatment to 24.8±12.6% (P>0.05) at 1 month after EBV treatment, than to 22.1±10.8% (P>0.05) at 6 months after EBV treatment.
Conclusions
EBV is an effective measure to treat highly selected COPD patients with giant bulla. Although, EBV treatment can achieve transient improvement of lung function at patients with CV negative bulla, long-term benefit was merely observed at the patient with a bulla at right middle lobe (RML).
Keywords: Endobronchial valve (EBV), bulla, chronic obstructive pulmonary diseases (COPD), collateral ventilation (CV)
Introduction
A bulla is an air-filled space in the lung with a diameter greater than one centimeter. While the volume of a bulla excesses one-third of a hemithorax, it is considered as a giant emphysematous bulla. Although bullae can appear in healthy persons or patients with relatively rare hereditary diseases, the most bullae are associated with COPD and emphysema. Giant emphysematous bulla can result in detrimental effects on lung function of COPD patients, such as decreased forced expiratory volume in 1 s (FEV1), increased total lung capacity (TLC) and functional residual capacity (FRC), and a reduction of diffusing capacity of the lung for carbon monoxide (DLCO) (1,2).
For the medical measures are not effective to treat giant emphysematous bulla, surgical intervention is recommended to eliminate the bullous lesions. The surgical approaches include bullectomy, lobectomy and pneumonectomy which may improve lung function and quality of life in selected patients (3,4). Although several less invasive approaches were attempted to treat giant emphysematous bulla, their efficacy is in debate at present (5-7). Recently, Dr. Santini and colleagues from Italy have reported successful endobronchial treatment of giant emphysematous bulla with one-way valves [Endobronchial valve (EBV), Zephyr®, Pulmonx, Inc., Palo Alto, Calif., USA] (8). In this study, we present our primary experiences of treatment of giant emphysematous bulla using EBV, and analyze the factors influencing the efficacy of the treatment.
Methods
Study design
This study is conducted by experienced pulmonologists in the Chinese PLA general hospital. It is a retrospective study to investigate the efficacy and safety of EBV treatment of giant emphysematous bulla. The research ethics committee of the hospital approved this study, and all the patients rendered informed consent.
Patient selection
The candidates of EBV treatment should have severe or worse airflow limitation according to the GOLD guideline. At these patients, medical therapy and rehabilitation have reached a platform to relieve the symptoms of dyspnea and to improve the exercise tolerance. The candidates should have giant emphysematous bulla displayed on the chest CT. The giant emphysematous bulla can be an isolated large bulla with relatively well conserved lung tissue in adjacent lobe(s) or multiply bullae in different lobes.
Lung function
Lung function tests were performed before and after EBV deployment. FEV1 and RV of all patients were measured using the spirometer and body plethysmography (MasterScreen Gold Standard; Jaeger, Wurzburg, Germany).
Imaging
The patients received HRCT in the department of radiology of our hospital using a multidetector system (Somatom Definition, Siemens Medical Solution, Forchheim, Germany). The reconstructions were performed by the radiologist (Dr. Nie YK), who has >20 years of experience in thoracic CT. The coronal reconstructions were made in one patient (patient 1) to evaluate the volume change of bullae after the EBV treatment.
Collateral ventilation (CV) evaluation
CV between target lobe and adjacent lobes was evaluated using Chartis® system (Pulmonx, Inc., Redwood, Calif., USA). The Chartis system consists of two parts. A balloon catheter was used to occlude the target lobe and transduce the airflow from the lobe to the console through the central lumen of the catheter. The one-way valve and sensors are integrated in the console, in which the airflow and pressure can be measured and displayed simultaneously and dynamically. The reduction of airflow from the target lobe occluded indicates that the CV between target lobe and adjacent lobe(s) was negative.
EBV deployment
The Zephyr® EBV prevents the airflow from entering the target lobe during inspiration and allows the airflow out of the lobe during expiration. The target bronchus was verified to be within Zephyr® EBV size range using sizing gauge located at the proximal end of the delivery housing. Before the placement of EBV, it would be confirmed that the minimum depth mark was distal to carina of target bronchus.
Statistical analysis
The mean value and standard deviation of FEV1 before the EBV treatment were compared with the data after the treatment using t-test. A P value <0.05 was regarded as statistically significant. SAS statistical software was used.
Results
Subject characteristics
From Oct 2011 to Jan 2013, we have treated five COPD patients with giant emphysematous bulla using EBV. The clinical data of these patients were retrieved retrospectively (Table 1). All the five EBV-treated patients were male, and the age ranged from 51 to 70 years (mean 63.0 years). All patients were at stage II or III of COPD. Figures 1,2,3,4,5, which displayed the CT images before and after the EBV treatment and Chartis images, are numbered sequentially according to the order of patients 1 to 5.
Table 1. Patient characteristics.
| Characteristics | Pt. 1 | Pt. 2 | Pt. 3 | Pt. 4 | Pt. 5 |
|---|---|---|---|---|---|
| Age | 66 | 70 | 65 | 51 | 63 |
| Sex | M | M | M | M | M |
| PaO2 (mmHg) | 67.9 | 66.4 | 71.2 | 63.1 | 55.5 |
| PaCO2 (mmHg) | 56.8 | 56.1 | 33.8 | 48.5 | 59.2 |
| FEV1 (L/s) | 0.51 | 1.15 | 1.07 | 0.37 | 0.87 |
| FEV1 (%) | 19.1 | 48.5 | 35.1 | 10.7 | 27.2 |
| FVC (L) | 1.60 | 1.79* | 2.63 | 0.83 | 2.77 |
| FVC (%) | 47.2 | 54.7* | 66.8 | 19.3 | 67.7 |
| TLC (L) | 8.09 | 3.94* | 8.38 | NA** | 7.11 |
| TLC (%) | 134.3 | 74.6* | 124.3 | NA** | 102.9 |
| RV (L) | 6.16 | 1.74* | 5.37 | NA** | 4.08 |
| RV (%) | 259.7 | 102.4* | 217.7 | NA** | 166.6 |
| DLCO (%) | 23.4 | 74.5 | 46.2 | NA** | 20.9 |
| 6MWT (m) | 120 | 370 | 330 | 50 | 180 |
| Location of bulla | RLL | RLL | RML | LUL | LLL |
*, Helium dilution method; **, the data was absent because of the patient 4 cannot tolerate the closed environment of body plethysmography. Pt., patient; M, male; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; TLC, total lung capacity; RV, residual volume; DLCO, diffusion capacity of carbon monoxide; 6MWT, 6-min walking testing; LLL, left lower lobe, RLL, right lower lobe; RUL, right upper lobe; RML, right middle lobe; LUL, left upper lobe.
Figure 1.
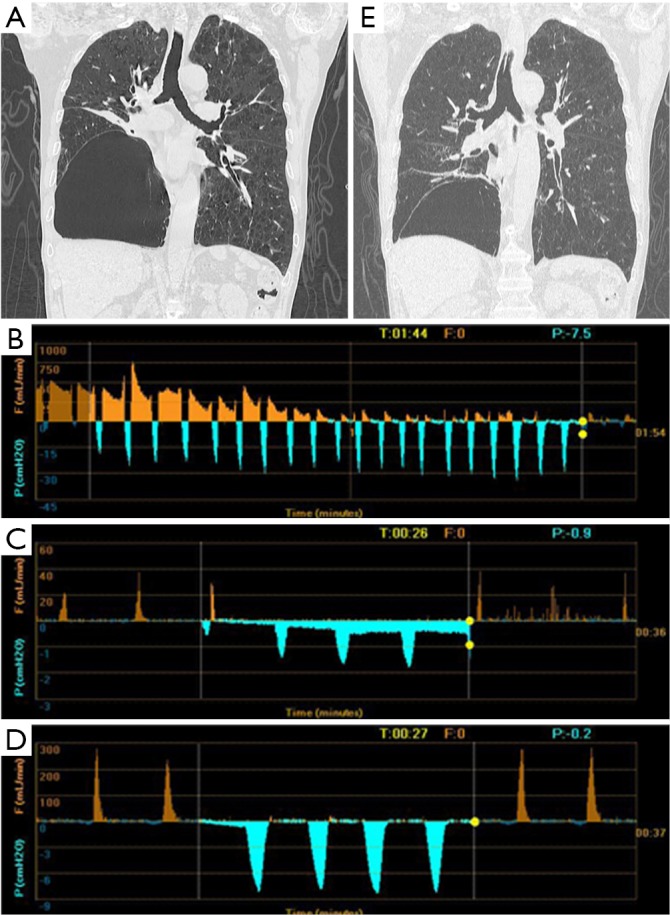
Chest CT and Chartis result of patient 1. (A) A bulla in the RLL and atelectasis of right RML; (B) drop of airflow to zero indicated CV is negative at RUL; (C) Chartis test showed low flow at RML; (D) Chartis test showed low flow at RLL; (E) the volume of bulla reduced and the atelectasis of RML disappeared. RLL, right lower lobe; RML, right middle lobe; CV, collateral ventilation; RUL, right upper lobe.
Figure 2.
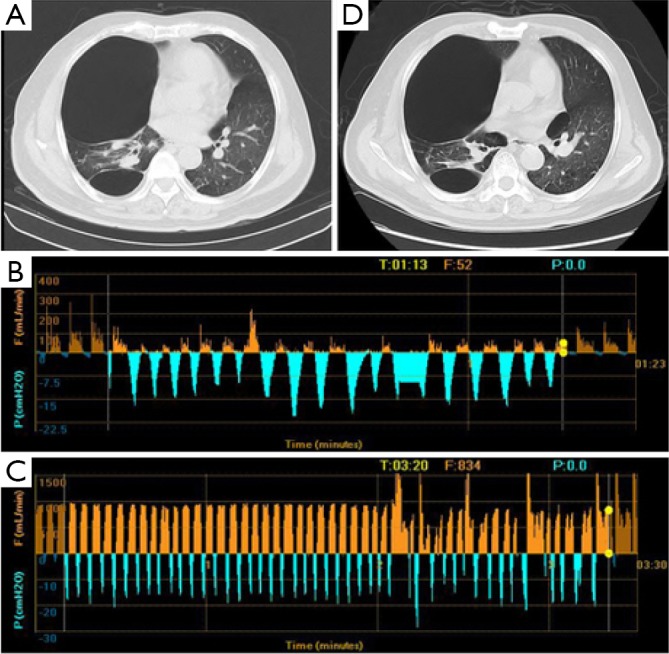
CT imaging and Chartis result of patient 2. (A) A bulla in RUL and severe destruction of lung tissue of RUL; (B) Chartis test showed low flow at RUL; (C) CV was positive as measured by Chartis system at RLL; (D) chest CT did not show reduction of bulla volume at RUL. RUL, right upper lobe; CV, collateral ventilation; RLL, right lower lobe.
Figure 3.
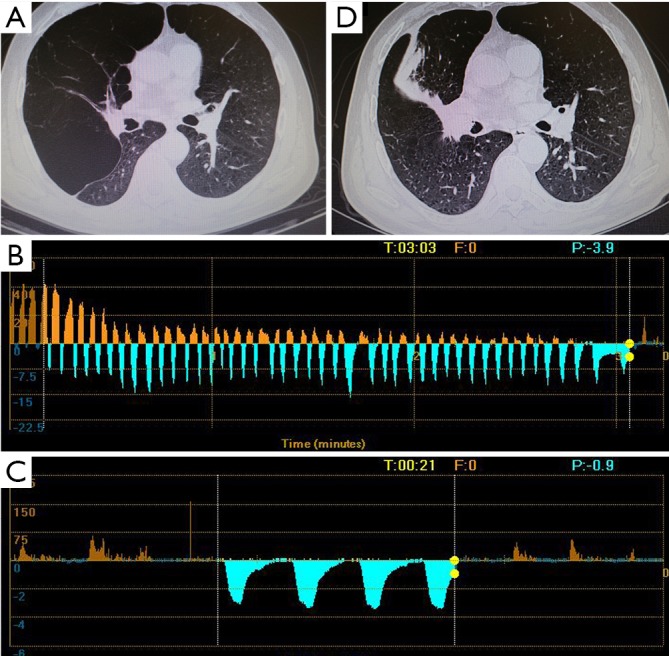
Chest CT and Chartis results of patient 3. (A) A bulla located in the RML; (B) CV is negative at RUL; (C) the Chartis assessment showed low flow at RML; (D) the bulla vanished after EBV treatment. RML, right middle lobe; CV, collateral ventilation; RUL, right upper lobe.
Figure 4.
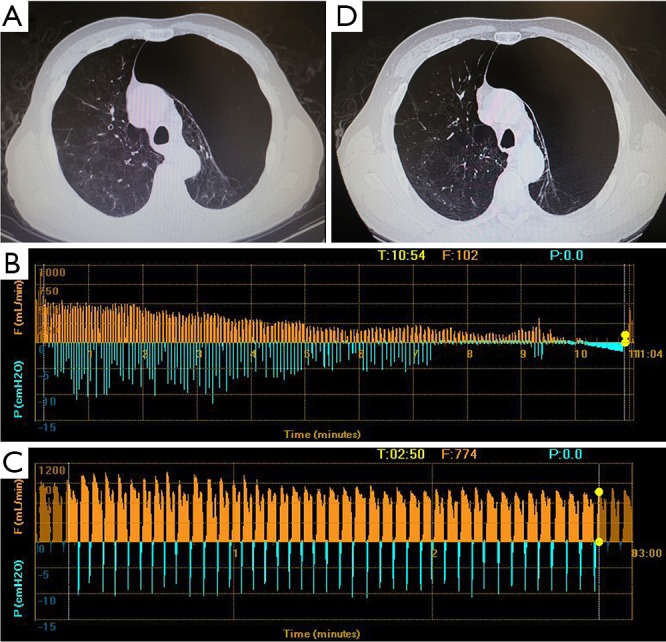
Chest CT and CV status of patient 4. (A) A bulla occupied the apical part of left semi-thoracic cave; (B) the airflow cannot drop to zero even after more than 10 minutes with LUL occluded; (C) the Chartis assessment showed sustained air flow while LLL was occluded; (D) the volume of LUL remained unchanged after EBV deployment. CV, collateral ventilation; LUL, left upper lobe; LLL, left lower lobe; EBV, endobronchial valve.
Figure 5.
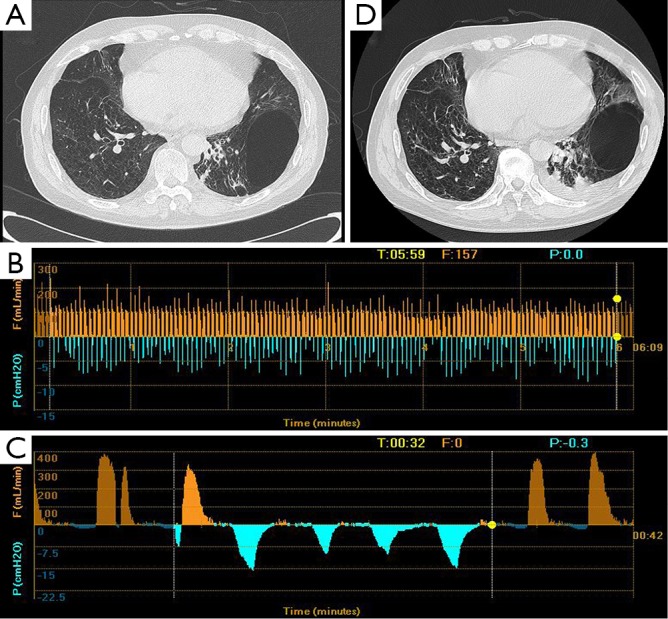
Clinical features of patient 5. (A) CT scan showed a bulla at the position of LLL; (B) the Chartis assessment showed the presence of CV at LUL; (C) low flow at LLL; (D) the volume of the bulla did not change after the EBV placement. CV, collateral ventilation; LLL, left lower lobe; LUL, left upper lobe; EBV, endobronchial valve.
CV status
The patient 1 had an isolated giant bulla in the right lower lobe (RLL) (Figure 1A). While assessing the CV with Chartis system, the patient 1 displayed decrease of flow at right upper lobe (RUL) (Figure 1B) and low flow at both right middle lobe (RML) (Figure 1C) and RLL (Figure 1D). The patient 2 had multiple bullae in the RLL and RUL (Figure 2A), and the Chartis test was positive both at RUL (Figure 2B) and RLL (Figure 2C) on the patient 2. An isolated giant bulla was located in the RML on the patient 3 (Figure 3A). For the RLL of patient number 3 cannot be occluded totally by the balloon of Chartis system, the Chartis test only showed decrease of flow at RUL (Figure 3B) and low flow at the targeted RML (Figure 3C) on the patient 3. On the patient 4, who has a bulla in the left upper lobe (LUL) (Figure 4A), although air flow of LUL showed continued decline, it cannot return to zero ever after 10 minutes (Figure 4B). So the Chartis test of LLL of patient 4 is typical positive (Figure 4C). On the patient 5 with a bulla in the left lower lobe (LLL) (Figure 5A), the Chartis test showed constant air flow at LUL (Figure 5B) and low flow at the targeted LLL (Figure 5C).
EBV treatment summary
The procedure was performed in all the seven patients under local anaesthetics (1% tetrracaine solution, <5 mL). Low dose of glucocorticoid was prescribed before EBV treatment. All the valves were delivered to the targeted lobes successfully. Five EBV 4.0 and five EBV 5.5 valves were used in these patients, and the median number of valves was 2 valves (range, 1 to 3 valves) per patient. In the patient 3, only one valve was inserted in the airway of RML corresponding to the bulla. No patient experienced respiratory failure during and after the procedure.
Follow-up
Lung function tests
In the two patients with negative CV, the mean value of FEV1 increased from 27.1±11.4% of predicted value before EBV treatment to 32.8±12.0% (P>0.05) at 1 month after EBV treatment, than to 31.7±24.5% (P>0.05) at 6 months after EBV treatment. Although the patient 1 experienced improvement of FEV1 1 month after EBV treatment, FEV1 dropped to the level even lower than it before the treatment. Only the FEV1 of patient 3 improved continuously after the procedure (Figure 6). However, in the three patients with positive CV, the mean value of FEV1 decreased from 28.8±19.0% of predicted value before EBV treatment to 24.8±12.6% (P>0.05) at 1 month after EBV treatment, than to 22.1±10.8% (P>0.05) at 6 months after EBV treatment. The FEV1 of two CV positive patients (patients 2 and 5) displayed continuous decrease at 1 and 6 months after EBV treatment. The FEV1 of patient 4 did not have significant change after the EBV treatment.
Figure 6.
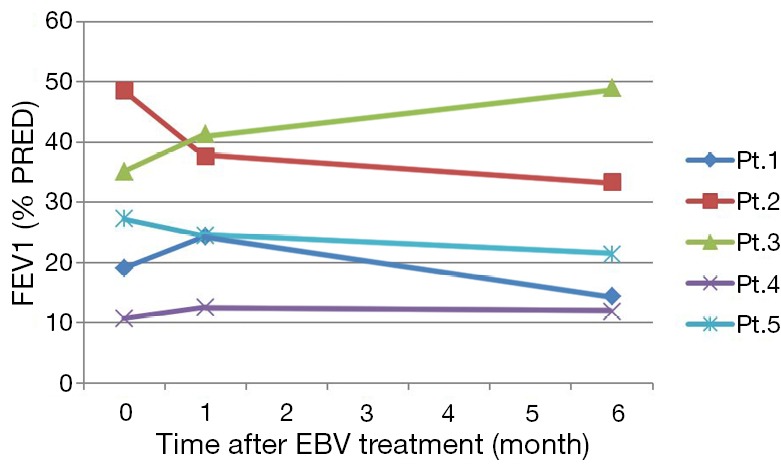
FEV1 before and after EBV treatment. At 6 months after the treatment, FEV1 went back to the level before the procedure in most of the patients, except in the patient 3, whose FEV1 increased from 35.1% to 49.0% of predicted value. FEV1, forced expiratory volume in 1 s; EBV, endobronchial valve.
Chest imaging
On patient 1, the volume of the bullae and the corresponding LLL decreased at 6 months after EBV treatment (Figure 1A,E). The volume of bullae did not change after EBV treatment on patient 2 (Figure 2A,D), 4 (Figure 4A,D) and 5 (Figure 5A,D). The bulla in the RML vanished, and atelectasis of RML happened at 6 months after EBV deployment on patient 3 (Figure 3A,D).
Discussion
At present, the limited data showed that the incidence of giant pulmonary bullae was not high (0.21 per 100,000 per year, Iceland) (9). Bullectomy was regarded as a safe and effective measure to improve pulmonary function of COPD patients. Persistent air leak is still a major complication of bullectomy. Although additional pleurodesis (10,11) and diverse reinforcement techniques (12,13) were used to prevent persistent air leak or recurrence of pneumothorax, these techniques were not available for most of patients who received bullectomy. The COPD patients with giant bulla were excluded from the largest clinical trial of EBV to treatment of COPD (EBV for emphysema palliation trail, VENT) (14), but one recent paper reported the efficacy of EBV treatment of giant bullae (8). Santini et al. have treated nine patients with giant emphysematous bullae using EBVs, and their preliminary data suggested the efficacy and feasibility of EBV to improve the lung function and quality of life. For that study, which has not rendered the detailed datum of lung function and chest CT of all the treated patients, did not perform subgroup analysis, the indicators of EBV treatment efficacy cannot be evaluated. At the same time, the Chartis system was not used to evaluate CV on those patients.
In our study, we have used Chartis system to measure CV of targeted lobes. And we compared the CV status and chest CT between patients with improvement of lung function and those without. As displayed in Table 2, the CV-negative patients (patients 1 and 3) showed improvement of lung function and reduced bulla volume at 1 month after the EBV treatment. However, the improved FEV1 of the patient 1 fell below the baseline before EBV treatment at 6 months after the treatment. Only FEV1 of patient 3 showed sustained improvement from 1 to 6 months after the EBV treatment. Two CV positive patients experienced deteriorated lung function, and one CV positive patient did not have significant change of lung function at six months after the EBV treatment.
Table 2. Clinical data of the treated patients.
| Patient number | FEV1 before EBV placement (%) | FEV1 1 month after EBV placement (%) | Change of FEV1 (%) | FEV1 6 months after EBV placement (%) | Change of FEV1 (%) | CV status | Ateclectasis | Volume reduction of bulla |
|---|---|---|---|---|---|---|---|---|
| 1 | 19.1 | 24.3 | 5 | 14.4 | −4.7 | − | + | + |
| 2 | 48.5 | 37.6 | −10.9 | 33.3 | −15.2 | + | − | − |
| 3 | 35.1 | 41.3 | 6.2 | 49 | 13.9 | − | + | + |
| 4 | 10.7 | 12.5 | 1.8 | 11.8 | 1.1 | + | − | − |
| 5 | 27.2 | 24.4 | −2.8 | 21.3 | −5.9 | + | − | − |
FEV1, forced expiratory volume in 1 s; EBV, endobronchial valve; CV, collateral ventilation.
Lung function of patient 1 experienced transient improvement at 1 month and deterioration at 6 months after EBV treatment. At patient 1, there is still some area of well-conserved lung parenchyma at treated RLL. But, at patient 3, the giant bulla occupied the whole RML, and only negligible lung parenchyma was conserved. The quantity of well-conserved lung parenchyma is an important factor to be considered before ELVR, for lung parenchyma is significantly valuable at severe COPD patients. At these patients, the improvement of lung function from any technique may be offset because of the sacrifice of the lung parenchyma.
While using the Chartis system to evaluate CV status of targeted lobe of EBV treatment, the CV status of patient with giant bulla displayed three patterns. For the bullae are under-ventilated as illustrated by previous studies (15,16), CV test will display low flow in the corresponding lobe of giant bulla. Low flow in the corresponding lobe of giant bulla and decrease of flow in the adjacent lobe(s) indicated CV is negative in the corresponding lobe of giant bulla. While low flow in the corresponding lobe of giant bulla and continuous flow in the adjacent lobe(s) suggested CV is positive. However, if the parenchyma of surrounding lung tissue and adjacent lobe are destructed severely by chronic inflammation, the bulla will communicate with adjacent lobe(s). Obviously, in this situation, continuous flow appears in both the corresponding lobe of giant bulla and adjacent lobe(s), CV is positive in the corresponding lobe of giant bulla.
Inevitably, there are several limitations in this study. Firstly, the patient population is too small to make statistics analysis. Secondly, at present, for the volume of bulla cannot be calculated precisely, it is difficult to set a cutoff value of ratio of bulla volume to conserved lung volume under which ELVR should be denied.
In conclusion, EBV is an effective measure to treat highly selected COPD patients with giant bulla. Although, EBV treatment can achieve transient improvement of lung function at patients with CV negative bulla, long-term benefit was merely observed at the patient with a bulla at RML. In the future, randomized clinical trials should be performed to verify the safety and effectiveness in larger COPD population with giant bulla. Despite the treatment of giant bulla at RML displayed good result in one patient, more patients with giant bulla at RML should be included in future investigation. Furthermore, efforts should be paid to develop novel mini-invasive ways to treat giant bulla, which have CV with neighbor lobes, via bronchoscopy. For example, if we can occlude the CV passage of one bulla, the COPD patient may benefit from EBV placement at the anatomical airway of the corresponding lobe of the bulla.
Acknowledgements
Authors’ contributions: TQ designed this research and finished this paper. QF collected clinical data of some patients. CL participated in the design of this research and performed most procedures of EBV deployment. AY collected and managed the data of patients. XB did follow-up work of treated patients.
Disclosure: The authors declare no conflict of interest.
References
- 1.Wade JF, 3rd, Mortenson R, Irvin CG. Physiologic evaluation of bullous emphysema. Chest 1991;100:1151-4. [DOI] [PubMed] [Google Scholar]
- 2.Nickoladze GD. Functional results of surgery for bullous emphysema. Chest 1992;101:119-22. [DOI] [PubMed] [Google Scholar]
- 3.Palla A, Desideri M, Rossi G, et al. Elective surgery for giant bullous emphysema: a 5-year clinical and functional follow-up. Chest 2005;128:2043-50. [DOI] [PubMed] [Google Scholar]
- 4.Schipper PH, Meyers BF, Battafarano RJ, et al. Outcomes after resection of giant emphysematous bullae. Ann Thorac Surg 2004;78:976-82. [DOI] [PubMed] [Google Scholar]
- 5.Venn GE, Williams PR, Goldstraw P. Intracavity drainage for bullous, emphysematous lung disease: experience with the Brompton technique. Thorax 1988;43:998-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takizawa H, Kondo K, Sakiyama S, et al. Computed tomography-guided drainage for large pulmonary bullae. Interact Cardiovasc Thorac Surg 2004;3:283-5. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya P, Sarkar D, Nag S, et al. Transbronchial decompression of emphysematous bullae: a new therapeutic approach. Eur Respir J 2007;29:1003-6. [DOI] [PubMed] [Google Scholar]
- 8.Santini M, Fiorelli A, Vicidomini G, et al. Endobronchial treatment of giant emphysematous bullae with one-way valves: a new approach for surgically unfit patients. Eur J Cardiothorac Surg 2011;40:1425-31. [DOI] [PubMed] [Google Scholar]
- 9.Gunnarsson SI, Johannesson KB, Gudjonsdottir M, et al. Incidence and outcomes of surgical resection for giant pulmonary bullae--a population-based study. Scand J Surg 2012;101:166-9. [DOI] [PubMed] [Google Scholar]
- 10.Dubois L, Malthaner RA. Video-assisted thoracoscopic bullectomy and talc poudrage for spontaneous pneumothoraces: effect on short-term lung function. J Thorac Cardiovasc Surg 2010;140:1272-5. [DOI] [PubMed] [Google Scholar]
- 11.Nakanishi K.Comparison of cryoprecipitate with commercial fibrinogen in bullectomy. Asian Cardiovasc Thorac Ann 2010;18:27-32. [DOI] [PubMed] [Google Scholar]
- 12.Saito T, Kaneda H, Konobu T, et al. The covering with forceps-assisted polymeric biodegradable sheet and endostapling method: a simplified technique for wide coverage and reinforcement of staple-line in video-assisted thoracoscopic bullectomy for spontaneous pneumothorax. Interact Cardiovasc Thorac Surg 2011;12:103-5. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto K, Takei H, Nishii T, et al. Staple line coverage with absorbable mesh after thoracoscopic bullectomy for spontaneous pneumothorax. Surg Endosc 2004;18:478-81. [DOI] [PubMed] [Google Scholar]
- 14.Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233-44. [DOI] [PubMed] [Google Scholar]
- 15.Morgan MD, Strickland B. Computed tomography in the assessment of bullous lung disease. Br J Dis Chest 1984;78:10-25. [PubMed] [Google Scholar]
- 16.Suga K, Iwanaga H, Tokuda O, et al. Intrabullous ventilation in pulmonary emphysema: assessment with dynamic xenon-133 gas SPECT. Nucl Med Commun 2012;33:371-8. [DOI] [PubMed] [Google Scholar]


