Abstract
Background
Ventilator‐associated pneumonia (VAP) is common in intensive care units (ICUs). Some evidence indicates that probiotics may reduce the incidence of VAP. Several additional published studies have demonstrated that probiotics are safe and efficacious in preventing VAP in ICUs. We aimed to systematically summarise the results of all available data to generate the best evidence for the prevention of VAP.
Objectives
To evaluate the effectiveness and safety of probiotics for preventing VAP.
Search methods
We searched CENTRAL (2014, Issue 8), MEDLINE (1948 to September week 1, 2014) and EMBASE (2010 to September 2014).
Selection criteria
Randomised controlled trials (RCTs) comparing probiotics with placebo or another control (excluding RCTs that use probiotics in both study groups) to prevent VAP.
Data collection and analysis
Two review authors independently assessed eligibility and the quality of trials, and extracted data.
Main results
We included eight RCTs, with 1083 participants. All studies compared a form of probiotic (Lactobacillus casei rhamnosus; Lactobacillus plantarum; Synbiotic 2000FORTE; Ergyphilus; combination Bifidobacterium longum + Lactobacillus bulgaricus + Streptococcus thermophilus) versus a control group (placebo; glutamine; fermentable fibre; peptide; chlorhexidine). The analysis of all RCTs showed that the use of probiotics decreased the incidence of VAP (odds ratio (OR) 0.70, 95% confidence interval (CI) 0.52 to 0.95, low quality evidence). However, the aggregated results were uncertain for ICU mortality (OR 0.84, 95% CI 0.58 to 1.22 very low quality evidence), in‐hospital mortality (OR 0.78, 95% CI 0.54 to 1.14, very low quality evidence), incidence of diarrhoea (OR 0.72, 95% CI 0.47 to 1.09, very low quality evidence), length of ICU stay (mean difference (MD) ‐1.60, 95% CI ‐6.53 to 3.33, very low quality evidence), duration of mechanical ventilation (MD ‐6.15, 95% CI ‐18.77 to 6.47, very low quality evidence) and antibiotic use (OR 1.23, 95% CI 0.51 to 2.96, low quality evidence). Antibiotics for VAP were used for a shorter duration (in days) when participants received probiotics in one small study (MD ‐3.00, 95% CI ‐6.04 to 0.04). However, the CI of the estimated effect was too wide to exclude no difference with probiotics. There were no reported events of nosocomial probiotic infections in any included study.
The overall methodological quality of the included studies, based on our 'Risk of bias' assessments, was moderate with half of the included studies rated as a 'low' risk of bias; however, we rated four included studies as a 'high' risk of bias across one or more of the domains. The study limitations, differences in probiotics administered and participants, and small sample sizes across the included studies mean that the power to detect a trend of overall effect may be limited and chance findings cannot be excluded.
To explore the influence of some potential confounding factors in the studies, we conducted an intention‐to‐treat (ITT) analysis, which did not change the inference of per‐protocol analysis. However, our sensitivity analysis did not indicate a significant difference between groups for instances of VAP.
Authors' conclusions
Evidence suggests that use of probiotics is associated with a reduction in the incidence of VAP. However, the quality of the evidence is low and the exclusion of the one study that did not provide a robust definition of VAP increased the uncertainty in this finding. The available evidence is not clear regarding a decrease in ICU or hospital mortality with probiotic use. Three trials reported on the incidence of diarrhoea and the pooled results indicate no clear evidence of a difference. The results of this meta‐analysis do not provide sufficient evidence to draw conclusions on the efficacy and safety of probiotics for the prevention of VAP in ICU patients.
Keywords: Humans; Lactobacillus; Pneumonia, Ventilator‐Associated; Pneumonia, Ventilator‐Associated/prevention & control; Probiotics; Probiotics/therapeutic use; Randomized Controlled Trials as Topic; Synbiotics
Plain language summary
Probiotics for preventing ventilator‐associated pneumonia
Review question
To critically assess the current evidence from published studies relating to the effect of probiotics for preventing ventilator‐associated pneumonia (VAP).
Background
VAP is a condition that can occur in patients who have been mechanically ventilated for more than 48 hours and can significantly increase the likelihood of death within intensive care unit (ICU) patients. Despite the use of preventive measures and advances in antimicrobial therapy, VAP is the second most common hospital‐related infection in the USA. It is associated with an increased chance of disease and death, and increased healthcare costs. It is believed that probiotics can reinforce the gut barrier function, which may result in clinical benefits. However, until now, there has been no clear evidence to determine whether probiotics are associated with better clinical outcomes.
Study characteristics
We identified eight studies with 1083 participants comparing probiotics versus placebo for preventing VAP. The studies were conducted between 2006 and 2011 in China, France, Greece, Slovenia, Sweden, the UK and the USA, with funding from various sources including hospital/National Health Service, pharmaceuticals and the National Institutes of Health. In the studies that stated the gender ratios, there were 611 males and 378 females. The evidence is current to September 2014.
Key results and quality of the evidence
The results from these trials show that probiotics are associated with a reduction in instances of VAP. However, the quality of the evidence is low and the exclusion of the one study that did not provide a robust definition of VAP increased the uncertainty in this finding. Results for all remaining reported outcomes (including mortality, incidence of diarrhoea, length of ICU stay, duration of mechanical ventilation and general antibiotic use) were uncertain between groups receiving either probiotics or placebo or standard treatment. Incidence of diarrhoea was reported in half of the included studies, which demonstrated no clear evidence of a difference between probiotics over standard care or placebo. The quality of the evidence was generally low to very low between studies. Due to the contradictions in the results from previously published systematic reviews and the uncertainty of these results, there is need for larger, well‐designed and robustly reported studies.
Summary of findings
Summary of findings for the main comparison. Per‐protocol analysis: probiotics versus control for preventing ventilator‐associated pneumonia.
| Per‐protocol analysis: probiotics versus control for preventing ventilator‐associated pneumonia | ||||||
| Patient or population: patients receiving mechanical ventilation Settings: inpatient: China, France, Greece, Slovenia, Sweden, UK and USA Intervention: per‐protocol analysis: probiotics versus control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Per‐protocol analysis: probiotics versus control | |||||
| Incidence of VAP Follow‐up: mean 37 days | Moderate1 | OR 0.7 (0.52 to 0.95) | 1018 (8 studies) | ⊕⊕⊝⊝ low2,3 | ||
| 309 per 1000 | 238 per 1000 (189 to 298) | |||||
| ICU mortality Follow‐up: mean 35 days | Moderate4 | OR 0.84 (0.58 to 1.22) | 703 (5 studies) | ⊕⊝⊝⊝ very low3,5,6 | ||
| 214 per 1000 | 186 per 1000 (136 to 249) | |||||
| Hospital mortality Follow‐up: median 37 days | Moderate7 | OR 0.78 (0.54 to 1.14) | 524 (4 studies) | ⊕⊝⊝⊝ very low3,6,8 | ||
| 306 per 1000 | 256 per 1000 (192 to 335) | |||||
| Diarrhoea Follow‐up: mean 40 days | Moderate9 | OR 0.72 (0.47 to 1.09) | 618 (4 studies) | ⊕⊝⊝⊝ very low3,6,8 | ||
| 435 per 1000 | 357 per 1000 (266 to 456) | |||||
| Length of ICU stay (days) Follow‐up: mean 44 days | The mean length of ICU stay (days) in the control groups was 20.9 days | The mean length of ICU stay (days) in the intervention groups was 1.6 lower (6.53 lower to 3.33 higher) | 396 (4 studies) | ⊕⊝⊝⊝ very low3,6,8,10 | ||
| Duration of mechanical ventilation (days) Follow‐up: mean 15 days | The mean duration of mechanical ventilation (days) in the control groups was 19.65 days | The mean duration of mechanical ventilation (days) in the intervention groups was 6.15 lower (18.77 lower to 6.47 higher) | 203 (2 studies) | ⊕⊝⊝⊝ very low3,6,11,12 | ||
| Antibiotic use Follow‐up: 28 days | Moderate13 | OR 1.23 (0.51 to 2.96) | 259 (1 study) | ⊕⊕⊝⊝ low14 | ||
| 907 per 1000 | 923 per 1000 (833 to 967) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;ICU: intensive care unit; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Assumed control risk: equates to median control group risk from the studies (28.8%). 2Risk of bias: 'serious' ‐ we rated 4 of the included studies as a high risk of bias across risk of bias domains of attrition bias and funding. 3Indirectness: 'serious' ‐ all included studies used different probiotic formulations and had differing control group interventions and differing patient populations, with only 3 included studies investigating incidence of VAP as primary outcome. 4Assumed control risk: equates to median control group risk from the studies (20.9%). 5Risk of bias: 'serious' ‐ we rated 3 of the included studies as a high risk of bias across risk of bias domains of attrition bias and funding. 6Imprecision: 'serious' ‐ 95% CI around the pooled or best estimate of effect includes both no effect and appreciable benefit or appreciable harm. 7Assumed control risk: equates to median control group risk from the studies (31.9%). 8Risk of bias: 'serious' ‐ we rated 2 of the included studies as a high risk of bias across risk of bias domains of attrition bias and funding. 9Assumed control risk: equates to median control group risk from the studies (38.6). 10Inconsistency: 'very serious' ‐ considerable degrees of heterogeneity (Tau² = 18.13; Chi² = 12.85, df = 3 (P value = 0.005); I² = 77%) present, attributable to one included study (Kotzampassi 2006). 11Risk of bias: 'serious' ‐ we rated 1 of the included studies as a high risk of bias across risk of bias domain of attrition bias. 12Inconsistency: 'very serious' ‐ considerable degrees of heterogeneity (Tau² = 76.72; Chi² = 12.82, df = 1 (P value = 0.0003); I² = 92%) present, attributable to one included study (Kotzampassi 2006). 13Assumed control risk: mean baseline risk used from single study. 14Risk of bias: 'serious' ‐ we rated the single included study as a high risk of bias across the risk of bias domains of attrition bias and funding; only one included study with n = 259 participants.
Background
Description of the condition
Ventilator‐associated pneumonia (VAP), a pneumonia that develops more than 48 hours after endotracheal intubation, is common in intensive care units (ICUs). It is estimated to be responsible for 27% to 47% of ICU‐acquired infections (ATS/IDSA 2005; Grap 2012). Despite the use of preventive measures and advances in antimicrobial therapy, VAP is the second most common nosocomial infection in the United States, associated with increased morbidity, mortality and healthcare costs (Kollef 2005; Richards 1999). It is estimated that VAP leads to an average additional USD 40,000 cost in hospital charges per patient (Rello 2002).
The pathogenesis of VAP is complex but typically involves colonisation of the aerodigestive tract with pathogenic bacteria, formation of biofilms and leakage of contaminated oropharyngeal secretions around the endotracheal tube cuff and into the lung (Bouza 2009). There are numerous studies assessing various means of VAP prevention that may be grouped into pharmacological and non‐pharmacological measures. Current efforts to prevent VAP focus on decreasing risk factors for colonisation and aspiration including elevation of the head of the bed, suctioning of subglottic secretions, use of silver‐coated endotracheal tubes, minimising the duration of mechanical ventilation through regular use of sedation vacations and weaning protocols (Morrow 2010; Rello 2010; Valencia 2009).
Description of the intervention
Probiotics are defined as living micro‐organisms able to colonise the host gastrointestinal (GI) environment (acid and bile) such that they ultimately exist transiently in the lower alimentary tract to confer health benefits to the host (Schrezenmeir 2001). Probiotics can be used alone or in combination with prebiotics, which are non‐digestible food ingredients that stimulate the growth or activity (or both) of one or a limited number of bacteria in the gut (Gibson 1995). The combination of pro‐ and prebiotics are known as synbiotics. Most bacterial probiotics are strains of Lactobacillus and Bifidobacterium (Klein 1998). In recent years, it has been suggested that orally administered probiotics might be used to prevent or treat various infections or inflammatory disorders of the intestinal tract (Gareau 2010).
How the intervention might work
Normal human gastrointestinal tract flora can promote the gut barrier function by normalising intestinal permeability. Normal flora of patients admitted to ICUs are often replaced with pathogens due to multiple factors. The use of broad‐spectrum antibiotics may also induce an imbalance of intestinal bacterial flora, which plays an important role in host health (Isakow 2007). It was thought that impaired host immunity caused by pathogens contributed to VAP in ICU patients receiving mechanical ventilation. Through creating an indigenous microflora with bacteria known to prevent the growth of non‐acid‐tolerant bacteria, probiotics may reinforce the gut barrier function, which may therefore confer clinical benefits at distant sites on an immunomodulatory basis. However, the exact mechanism by which probiotics prevent VAP is still not entirely understood. Some evidence indicates that probiotics may reduce the incidence of VAP by inhibiting pathogen adhesion, improving gut mucosal barrier function, reducing bacterial translocation and up‐regulating the immune system (Jain 2004; Morrow 2010). Due to its several advantages, such as ease of administration, low cost and minimal toxicity, administration of probiotics seems a promising strategy to prevent VAP in the ICU.
Why it is important to do this review
There have been several small randomised controlled trials (RCTs) evaluating the efficacy and safety of probiotics for preventing VAP (Forestier 2008; Klarin 2008; Knight 2009; Kotzampassi 2006; Spindler‐Vesel 2007). One previous meta‐analysis of these RCTs has shown that probiotic therapy can reduce the incidence of VAP in ICUs (Siempos 2010). However, the conclusion of the meta‐analysis was challenged because of the selection methodology (Van Silvestri 2010). More recently, three additional RCTs were published demonstrating that probiotics are safe and efficacious in preventing VAP in ICUs (Barraud 2010; Morrow 2010; Tan 2011). Therefore, we aim to summate the results of all available data systematically to generate the best evidence for the prevention of VAP.
Objectives
To evaluate the effectiveness and safety of probiotics for preventing VAP.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and excluded quasi‐RCTs, controlled clinical trials, controlled before and after studies, interrupted time series studies, cross‐over studies and cluster‐RCTs.
Types of participants
Adult ICU patients (≥ 18 years of age) receiving mechanical ventilation with a reported incidence of VAP.
Types of interventions
We included studies comparing probiotics (single or mixture of strains, any dosage regimen and any route of administration) with a placebo or other controls. We excluded RCTs using probiotics in both study groups.
Types of outcome measures
Primary outcomes
Incidence of VAP.
All‐cause mortality, including ICU mortality, 28/30‐day mortality, hospital mortality or mortality at an unspecified time.
Safety (including incidence of diarrhoea).
Secondary outcomes
Length of ICU stay.
Duration of mechanical ventilation.
Systematic antibiotic use.
Any adverse outcomes of the probiotics, i.e. toxicity, abdominal pain, occurrence of lactic acidosis or nosocomial probiotic infection.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 8) (accessed 17 September 2014), which includes the Cochrane ARI Group's Specialised Register, MEDLINE (1948 to September week 1, 2014) and EMBASE (January 2010 to September 2014).
We used the search strategy described in Appendix 1 to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted these terms to search EMBASE (Appendix 2).
Searching other resources
We searched Google Scholar with several keywords combined to identify grey literature. We identified one grey literature reference (i.e. one ongoing study), which we listed in the current review.
We searched a range of related information, including indexed published articles, grey literature, conference abstracts and unpublished data relevant to this topic. We consulted trial registers for registered eligible clinical trials. We sourced other references and eligible trials from the reference lists of identified trials. We contacted experts in the field and pharmaceutical companies for additional published or unpublished trials. Furthermore, we searched for conference abstracts from the following sources: CHEST, held by the American College of Chest Physicians (2001 to 2013), ATS International Conference (2001 to 2013), ERS International Congress (2001 to 2013) and SCCM Annual Congress (2001 to 2013). However, there were no new trials identified. We searched for completed and ongoing trials (latest search 15 March 2014) in the following registers:
ClinicalTrials.gov (http://clinicaltrial.gov/);
Chinese Clinical Trial Register (www.chictr.org);
Australian New Zealand Clinical Trials Registry (http://www.anzctr.org.au/);
ISRCTN (http://www.controlled‐trials.com/isrctn/); and
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/).
Data collection and analysis
Selection of studies
Two review authors (LB, JL) independently ran the literature searches in order to identify eligible articles for inclusion. VAP was based upon the definition used by the investigators of primary studies. We resolved disagreements by consensus or discussion with three other authors (XD, RH, MK) when necessary. We acquired the full text of any potentially relevant trials and examined them in detail to determine the eligibility of the paper.
Data extraction and management
Two review authors (LB, JL) independently and in duplicate extracted data from included studies using the Cochrane ARI Group's data extraction form. One review author (LB) entered all data into Review Manager (RevMan 2014), and another review author (JL) cross‐checked the printout against his own data abstraction form.
We extracted the following information from each study: author, year of publication, language, their institutions, source of funding, participants (age range, gender, socioeconomic status, inclusion and exclusion criteria), methodological design (methods of randomisation, allocation concealment, blinding and intention‐to‐treat (ITT) analysis), details of intervention (single or mixture of strains, dosage regimen, route of administration, duration), comparison treatment and results (that is, incidence of VAP, reasons for withdrawal, measures of compliance, adverse effects and loss to follow‐up, etc). We resolved disagreements by discussion and, when necessary, by consulting three other review authors (XD, RH, MK). We contacted the original trial authors or pharmaceutical companies (or both) when necessary, to clarify unclear data and to request additional information on methodological quality.
Assessment of risk of bias in included studies
Two review authors (LB, YB) initially assessed each included study according to the Cochrane Handbook for Systematic Reviews of Interventions criteria (Higgins 2011). These criteria emphasised the adequacy of the generation of the random sequence, allocation concealment, blinding, follow‐up and ITT. Based on these criteria, we broadly subdivided studies into the following three categories: low risk of bias; unclear risk of bias; or high risk of bias.
Measures of treatment effect
We measured the proportions of dichotomous outcome variables (such as the incidence of VAP, mortality). We used the means and the standard deviations of the means for continuous variables.
Unit of analysis issues
Individual participants in each clinical trial were the unit of analysis.
Dealing with missing data
We followed the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions regarding strategies for dealing with missing data (Higgins 2011). We analysed all data using 'per‐protocol' analysis, and also conducted an intention‐to‐treat (ITT) analysis for all primary and secondary outcomes where drop‐outs occurred in order to account for all originally randomised participants. For dichotomous outcomes, we assumed that missing participants did not experience the event outcome. For continuous outcomes we based our analysis on the total number of participants randomised and used 'last observation carried forward' (LOCF).
Assessment of heterogeneity
We assessed heterogeneity using the Cochran's Q statistic with a P value ≤ 0.1 interpreted as statistically significant. We obtained further information on the impact of statistical heterogeneity on the study results by calculating the I2 statistic (Higgins 2011). We used values of the I2 statistic above 50% as a cut‐off for considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
Had there been a sufficient amount of studies (i.e. at least 10 RCTs, as outlined in Chapter 10 theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)), we would have constructed funnel plots to assess for any publication bias.
Data synthesis
We expressed summary statistics for each study as an odds ratio (OR, with 95% confidence interval (CI)) for dichotomous outcomes such as incidence of VAP and mortality. We used mean differences (MD) for continuous outcomes such as duration of mechanical ventilation and ICU length of stay. We pooled data and expressed this as an OR with 95% CI. We used a fixed‐effect model if there was no considerable heterogeneity among studies. We used a random‐effects model if the I2 statistic was above 50% and Cochran's Q statistic has a P value ≤ 0.1.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis based on different placebo/control groups and different probiotic doses or duration of therapy. We interpreted the results of subgroup analyses cautiously.
Sensitivity analysis
We performed a sensitivity analysis to explore whether the heterogeneity was a result of a low quality trial. To determine whether the conclusions concerning the overall effects of probiotics were robust, we undertook the following sensitivity analyses: excluding trials with questionable diagnostic criteria for VAP; excluding studies with high risk of bias; changing from a fixed‐effect model to a random‐effects model (or vice versa). When sensitivity analyses identified particular factors that influenced the conclusions of the review, we explored the potential causes of the uncertainties and interpreted the results of the review with caution.
Summary of findings
We used the GRADE profiler to interpret findings and rate the quality of the evidence (GRADEPRO; Schünemann 2008). We imported data from RevMan 5.3 (RevMan 2014) to create a 'Summary of findings' table (Table 1). This table provides outcome‐specific information from our seven primary and secondary outcomes of interest and rates the overall quality of evidence from each included study in the comparison, as well as the magnitude of effect of the interventions examined and the sum of available data on all outcomes we considered important to patient care and decision‐making (see Differences between protocol and review).
Results
Description of studies
For more information relating to each individual study, please refer to Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search identified a total of 362 records prior to removal of duplicates, with 126 of the records retrieved from the search of MEDLINE, 101 records from EMBASE and 107 records from CENTRAL; 276 records were left after duplicates were removed (Figure 1). Handsearching of conference proceedings, contacting content experts and contacting authors produced no extra trials. We identified one ongoing trial and the results were not available for use in the current review (Thamlikitkul 2011). Information about the trial is provided in the Characteristics of ongoing studies table.
1.
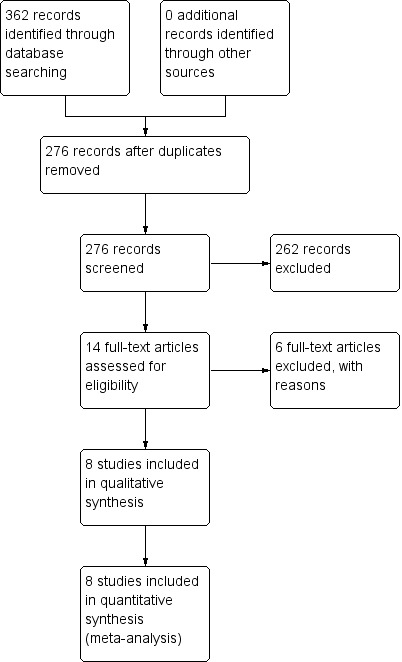
Study flow diagram: 2014 search
Included studies
We included eight RCTs. All were published between 2006 and 2011. The sample size of these studies ranged from 50 to 264 (total 1083). All included studies reported the occurrence of VAP; five reported ICU mortality and four reported hospital mortality; six reported length of ICU stay events; four reported duration of ventilation; two studies reported antibiotic use; and four reported instances of diarrhoea. Six studies found instances of probiotic‐related infection. Four studies used a probiotic formula (Barraud 2010; Forestier 2008; Klarin 2008; Morrow 2010; Tan 2011), and two studies used a synbiotic formula (Knight 2009; Kotzampassi 2006; Spindler‐Vesel 2007), i.e. Synbiotic 2000FORTE.
Excluded studies
We excluded six trials (see Characteristics of excluded studies table).
Risk of bias in included studies
Reporting of trial methodology was incomplete for many of the domains as summarised in Figure 2. However, we rated the majority of domains as a 'low' risk of bias across studies, as randomisation was generally well reported in most of the included studies (Barraud 2010; Forestier 2008; Knight 2009; Morrow 2010; Spindler‐Vesel 2007; Tan 2011) (for a visual representation of risk of bias, please view Figure 3).
2.
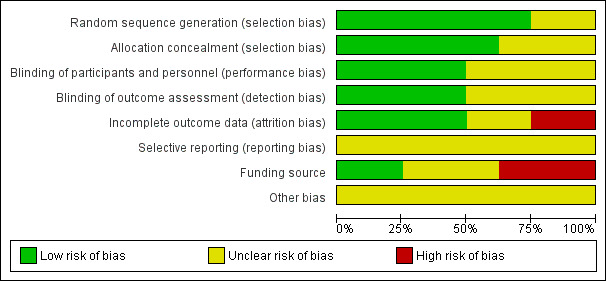
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
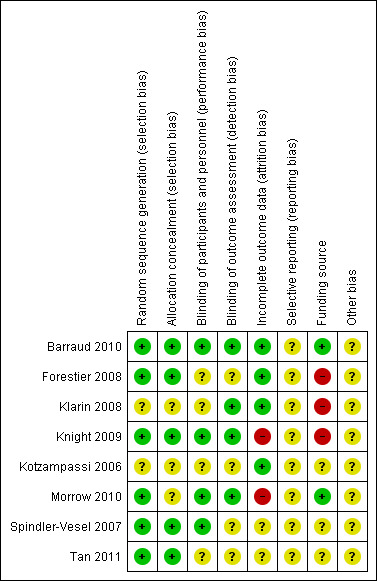
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was adequately performed in six trials (Barraud 2010; Forestier 2008; Knight 2009; Morrow 2010; Spindler‐Vesel 2007; Tan 2011), and unclear in two trials (Klarin 2008; Kotzampassi 2006). Five trials reported adequate allocation concealment (Barraud 2010; Forestier 2008; Knight 2009; Spindler‐Vesel 2007; Tan 2011). Three trials were unclear about their methods of allocation concealment (Klarin 2008; Kotzampassi 2006; Morrow 2010).
Blinding
Four trials adequately reported blinding of participants, outcome assessors and personnel (Barraud 2010; Knight 2009; Morrow 2010; Spindler‐Vesel 2007). Four trials adequately reported blinding of outcome assessors (Barraud 2010; Klarin 2008; Knight 2009; Morrow 2010). The remaining trials were unclear concerning the conduct of blinding.
Incomplete outcome data
Incomplete outcome data were adequately addressed in four trials (Barraud 2010; Forestier 2008; Kotzampassi 2006; Klarin 2008), and we considered two a 'high' risk (Knight 2009; Morrow 2010), with the remaining two studies rated as 'unclear'.
Selective reporting
We do not have access to the protocols for all the included studies, therefore there was not enough information to assess selective reporting bias and we rated all studies as 'unclear'.
Other potential sources of bias
Five trials declared their funding source (Barraud 2010; Forestier 2008; Klarin 2008; Knight 2009; Morrow 2010). Two of these trials were independently funded (Barraud 2010; Morrow 2010), three were industry funded (Forestier 2008; Klarin 2008; Knight 2009) (and subsequently rated as a 'high' risk of bias) and two were funded in whole or part by a grant (Spindler‐Vesel 2007; Tan 2011). One study did not disclose their funding source (Kotzampassi 2006).
Effects of interventions
See: Table 1
Probiotics versus control (per‐protocol analysis)
Primary outcomes
1. Incidence of ventilator‐associated pneumonia (VAP)
For this outcome we found eight relevant trials (n = 1018). There was a significant difference (P value = 0.02) between various probiotics and the control group for instance of VAP, with more instances in the control group (odds ratio (OR) 0.70, 95% confidence interval (CI) 0.52 to 0.95, Analysis 1.1).
1.1. Analysis.
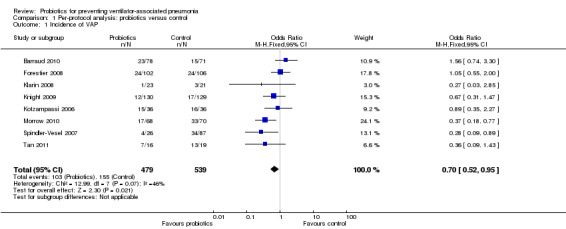
Comparison 1 Per‐protocol analysis: probiotics versus control, Outcome 1 Incidence of VAP.
2. All‐cause mortality
Intensive care unit (ICU) mortality
For this outcome we found five relevant trials (n = 703). There was no significant difference between various probiotics and the control group (OR 0.84, 95% CI 0.58 to 1.22, Analysis 1.2).
1.2. Analysis.
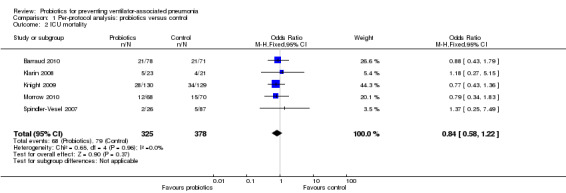
Comparison 1 Per‐protocol analysis: probiotics versus control, Outcome 2 ICU mortality.
Hospital mortality
For this outcome we found four relevant trials (n = 524). There was no significant difference between various probiotics and the control group (OR 0.78, 95% CI 0.54 to 1.14, Analysis 1.3).
1.3. Analysis.
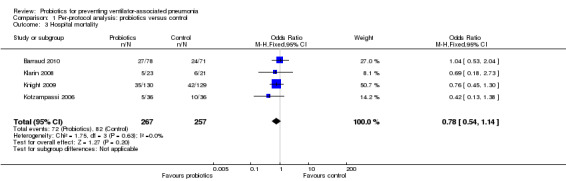
Comparison 1 Per‐protocol analysis: probiotics versus control, Outcome 3 Hospital mortality.
3. Safety, including incidence of diarrhoea
For this outcome we found four relevant trials (n = 618). There was no significant difference between various probiotics and the control group (OR 0.72, 95% CI 0.47 to 1.09, Analysis 1.4).
1.4. Analysis.
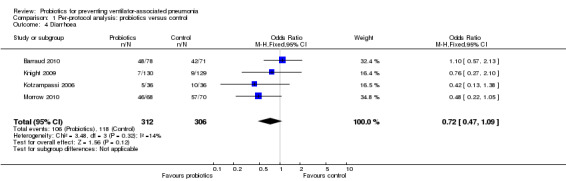
Comparison 1 Per‐protocol analysis: probiotics versus control, Outcome 4 Diarrhoea.
Secondary outcomes
1. Length of ICU stay (days)
For this outcome we found four relevant trials (n = 396). There was no significant difference between various probiotics and the control group (mean difference (MD) ‐1.60, 95% CI ‐6.53 to 3.33, Analysis 1.5). Results demonstrated considerable levels of heterogeneity (Tau² = 18.13; Chi² = 12.85, df = 3 (P value = 0.005); I² = 77%) and we carried out analysis using a random‐effects model. Also, two studies reported length of ICU stay in days with median and interquartile range, therefore these data could not be analysed in meta‐analysis and are presented separately (Analysis 1.6).
1.5. Analysis.
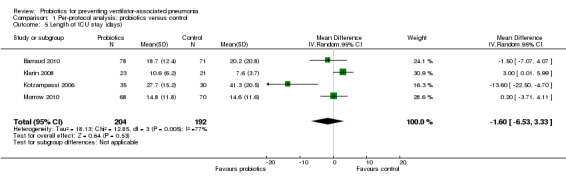
Comparison 1 Per‐protocol analysis: probiotics versus control, Outcome 5 Length of ICU stay (days).
1.6. Analysis.
Comparison 1 Per‐protocol analysis: probiotics versus control, Outcome 6 Length of ICU stay (days).
| Length of ICU stay (days) | ||||
|---|---|---|---|---|
| Study | Intervention | Median | Range | N |
| Knight 2009 | Synbiotic 2000FORTE | 6.0 | 3 to 11 | 130 |
| Knight 2009 | Placebo | 7.0 | 3 to 14 | 129 |
| Spindler‐Vesel 2007 | Nutricomp standard (B. Braun) + Synbiotic2000 | 12 | 8.5 to 21.3 | 26 |
| Spindler‐Vesel 2007 | 1. Alitraq 2. Nova Source 3. Nutricomp peptide |
14 16 11.5 |
8.3 to 23 10 to 21 6 to 20 |
32 29 26 |
2. Duration of mechanical ventilation (days)
For this outcome we found two relevant trials (n = 203). There was no significant difference between various probiotics and the control group (MD ‐6.15, 95% CI ‐18.77 to 6.47, Analysis 1.7). Results demonstrated considerable levels of heterogeneity (Tau² = 76.72; Chi² = 12.82, df = 1 (P value = 0.0003); I² = 92%) and we carried out analysis using a random‐effects model. Also, two studies reported durations of mechanical ventilation in days with median and interquartile range, therefore these data could not be analysed in meta‐analysis and are presented separately (Analysis 1.8).
1.7. Analysis.
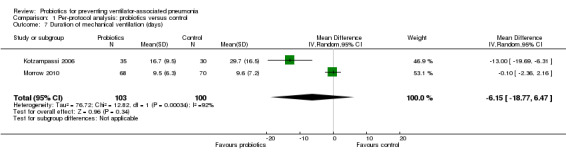
Comparison 1 Per‐protocol analysis: probiotics versus control, Outcome 7 Duration of mechanical ventilation (days).
1.8. Analysis.
Comparison 1 Per‐protocol analysis: probiotics versus control, Outcome 8 Duration of mechanical ventilation (days).
| Duration of mechanical ventilation (days) | ||||
|---|---|---|---|---|
| Study | Intervention | Median | Range | N |
| Knight 2009 | Synbiotic 2000FORTE | 5 | 2 to 9 | 130 |
| Knight 2009 | Placebo | 5 | 3 to 11 | 129 |
| Spindler‐Vesel 2007 | Nutricomp standard (B. Braun) + Synbiotic 2000 | 11 | 7 to 18.3 | 26 |
| Spindler‐Vesel 2007 | Alitraq Nova Source Nutricomp peptide |
10 12 8 |
6 to 16 8 to 15 4 to 15 |
32 29 26 |
3. Systematic antibiotic use
Antibiotic use for VAP (days)
For this outcome we found one relevant trial (Morrow 2010, n = 138). There was a significant difference (P value = 0.05) favouring probiotics over the control group (MD ‐3.00, 95% CI ‐6.04 to 0.04, Analysis 1.9).
1.9. Analysis.
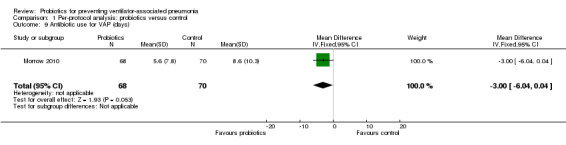
Comparison 1 Per‐protocol analysis: probiotics versus control, Outcome 9 Antibiotic use for VAP (days).
Antibiotic use
For this outcome we found one relevant trial (Knight 2009, n = 259). There was no significant difference between probiotics over the control group (OR 1.23, 95% CI 0.51 to 2.96, Analysis 1.10).
1.10. Analysis.
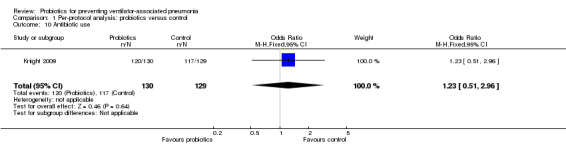
Comparison 1 Per‐protocol analysis: probiotics versus control, Outcome 10 Antibiotic use.
4. Any adverse outcomes of the probiotics: nosocomial probiotic infection
Six studies reported zero events of probiotic infection throughout the duration of the studies (Analysis 1.11).
1.11. Analysis.
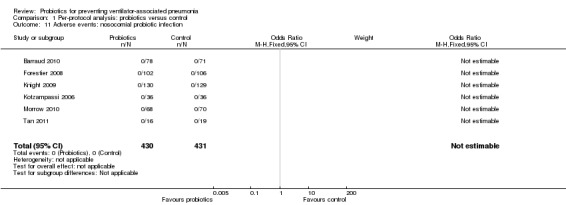
Comparison 1 Per‐protocol analysis: probiotics versus control, Outcome 11 Adverse events: nosocomial probiotic infection.
Probiotics versus control (ITT analysis)
Primary outcomes
1. Incidence of VAP
For this outcome we found eight relevant trials (n = 1058). There was a significant difference (P value = 0.02) between various probiotics and the control group for instance of VAP, with more instances in the control group (OR 0.70, 95% CI 0.52 to 0.95, Analysis 2.1).
2.1. Analysis.
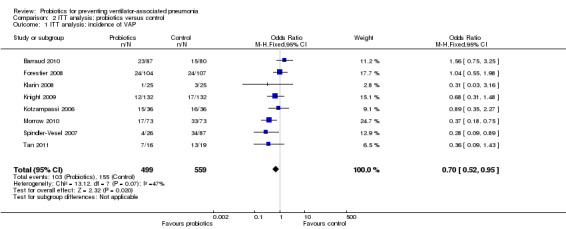
Comparison 2 ITT analysis: probiotics versus control, Outcome 1 ITT analysis: incidence of VAP.
2. All‐cause mortality
ICU mortality
For this outcome we found five relevant trials (n = 740). There was no significant difference between various probiotics and the control group (OR 0.85, 95% CI 0.59 to 1.23, Analysis 2.2).
2.2. Analysis.
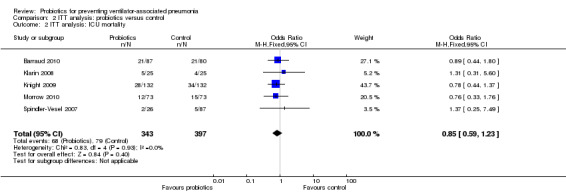
Comparison 2 ITT analysis: probiotics versus control, Outcome 2 ITT analysis: ICU mortality.
Hospital mortality
For this outcome we found four relevant trials (n = 553). There was no significant difference between various probiotics and the control group (OR 0.80, 95% CI 0.55 to 1.17, Analysis 2.3).
2.3. Analysis.
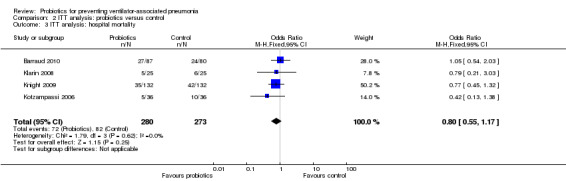
Comparison 2 ITT analysis: probiotics versus control, Outcome 3 ITT analysis: hospital mortality.
3. Safety, including incidence of diarrhoea
For this outcome we found four relevant trials (n = 649). There was no significant difference between various probiotics and the control group (OR 0.73, 95% CI 0.49 to 1.08, Analysis 2.4).
2.4. Analysis.
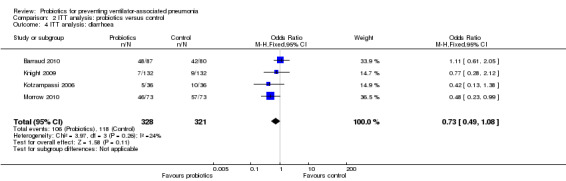
Comparison 2 ITT analysis: probiotics versus control, Outcome 4 ITT analysis: diarrhoea.
Secondary outcomes
1. Length of ICU stay (days)
For this outcome we found four relevant trials (n = 432). There was no significant difference between various probiotics and the control group (MD ‐1.77, 95% CI ‐6.77 to 3.24, Analysis 2.5). Results demonstrated considerable levels of heterogeneity (Tau² = 19.52; Chi² = 14.56, df = 3 (P value = 0.002); I² = 79%) and we carried out analysis using a random‐effects model.
2.5. Analysis.
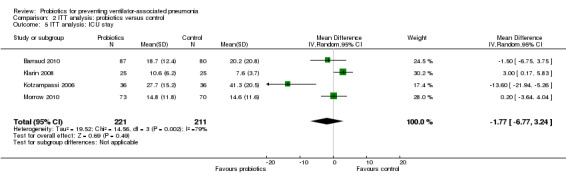
Comparison 2 ITT analysis: probiotics versus control, Outcome 5 ITT analysis: ICU stay.
2. Duration of mechanical ventilation (days)
For this outcome we found two relevant trials (n = 215). There was no significant difference between various probiotics and the control group (MD ‐6.21, 95% CI ‐18.83 to 6.41, Analysis 2.6). Results demonstrated considerable levels of heterogeneity (Chi² = 14.66, df = 1 (P value = 0.0001); I² = 93%) and we carried out analysis using a random‐effects model.
2.6. Analysis.
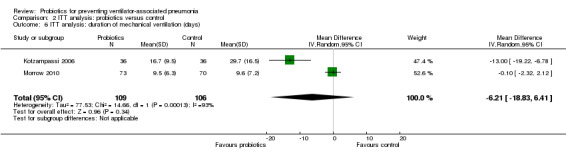
Comparison 2 ITT analysis: probiotics versus control, Outcome 6 ITT analysis: duration of mechanical ventilation (days).
3. Systematic antibiotic use
Antibiotic use for VAP (days)
For this outcome we found one relevant trial (Morrow 2010, n = 146). There was a significant difference (P value = 0.05) favouring probiotics over the control group (MD ‐3.00, 95% CI ‐5.96 to ‐0.04, Analysis 2.7).
2.7. Analysis.
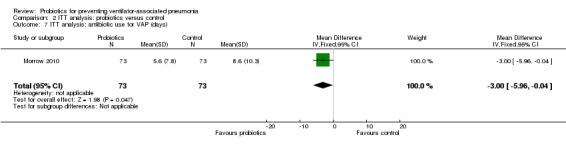
Comparison 2 ITT analysis: probiotics versus control, Outcome 7 ITT analysis: antibiotic use for VAP (days).
Antibiotic use
For this outcome we found one relevant trial (n = 264). There was no significant difference between various probiotics and the control group (OR 1.28, 95% CI 0.58 to 2.86, Analysis 2.8).
2.8. Analysis.
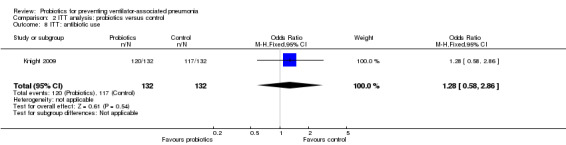
Comparison 2 ITT analysis: probiotics versus control, Outcome 8 ITT: antibiotic use.
4. Any adverse outcomes of the probiotics: nosocomial probiotic infection
Six studies reported zero events of probiotic infection throughout the duration of the studies (Analysis 2.9).
2.9. Analysis.
Comparison 2 ITT analysis: probiotics versus control, Outcome 9 ITT analysis: adverse events: nosocomial probiotic infection.
Subgroup analysis of primary outcomes: probiotics versus control
Primary outcome
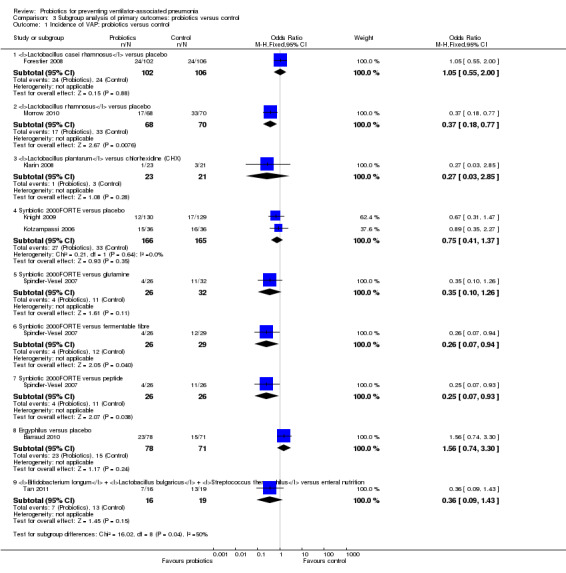
1. Incidence of VAP: probiotics versus control
Lactobacillus casei rhamnosus versus placebo
In this subgroup we only found one relevant trial (n = 208) (Forestier 2008). There was no significant difference between the probiotic Lactobacillus casei rhamnosus versus placebo (OR 1.05, 95% CI 0.55 to 2.0, Analysis 3.1).
3.1. Analysis.
Comparison 3 Subgroup analysis of primary outcomes: probiotics versus control, Outcome 1 Incidence of VAP: probiotics versus control.
Lactobacillus rhamnosus versus placebo
In this subgroup we only found one relevant trial (n = 138) (Morrow 2010). There was a statistically significant difference (P value = 0.008) favouring Lactobacillus rhamnosus over placebo (OR 0.37, 95% CI 0.18 to 0.77, Analysis 3.1).
Lactobacillus plantarum versus chlorhexidine
In this subgroup we only found one relevant trial (n = 44) (Klarin 2008). There was no significant difference between Lactobacillus plantarum versus chlorhexidine (OR 0.27, 95% CI 0.03 to 2.85, Analysis 3.1).
Synbiotic 2000FORTE versus placebo
In this subgroup we found two relevant trials (n = 331). There was no significant difference between Synbiotic 2000FORTE versus placebo (OR 0.75, 95% CI 0.41 to 1.37, Analysis 3.1).
Synbiotic 2000FORTE versus glutamine
In this subgroup we found one relevant trial (n = 57, Spindler‐Vesel 2007). There was no significant difference between Synbiotic 2000FORTE versus glutamine (OR 0.35, 95% CI 0.10 to 1.26, Analysis 3.1).
Synbiotic 2000FORTE versus fermentable fibre
In this subgroup we only found one relevant trial (n = 55, Spindler‐Vesel 2007). There was a statistically significant difference (P value = 0.04) favouring Synbiotic 2000FORTE over fermentable fibre (OR 0.26, 95% CI 0.07 to 0.94, Analysis 3.1).
Synbiotic 2000FORTE versus peptide
In this subgroup we only found one relevant trial (n = 52, Spindler‐Vesel 2007). There was a statistically significant difference (P value = 0.04) favouring Synbiotic 2000FORTE over peptide (OR 0.25, 95% CI 0.07 to 0.93, Analysis 3.1).
Ergyphilus versus placebo
In this subgroup we only found one relevant trial (n = 149) (Barraud 2010). There was no significant difference between Ergyphilus versus placebo (OR 1.56, 95% CI 0.74 to 3.3, Analysis 3.1).
Bifidobacterium longum + Lactobacillus bulgaricus + Streptococcus thermophilus versus enteral nutrition
In this subgroup we only found one relevant trial (n = 35) (Tan 2011). There was no significant difference between Bifidobacterium longum +Lactobacillus bulgaricus + Streptococcus thermophilus versus enteral nutrition (OR 0.36, 95% CI 0.09 to 1.43, Analysis 3.1).
2. Incidence of VAP: sensitivity analysis (probiotics applied solely to stomach)
In this subgroup we found seven trials (n = 975). There was no significant difference between groups when including studies that applied probiotics directly to the stomach (OR 0.68, 95% CI 0.43 to 1.08, Analysis 3.2). This outcome had considerable levels of heterogeneity (Tau² = 0.19; Chi² = 12.16, df = 6 (P value = 0.06); I² = 51%) and we used a random‐effects model for synthesis.
3.2. Analysis.
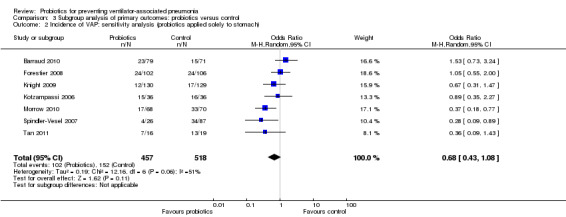
Comparison 3 Subgroup analysis of primary outcomes: probiotics versus control, Outcome 2 Incidence of VAP: sensitivity analysis (probiotics applied solely to stomach).
3. Incidence of VAP: sensitivity analysis (more than 1010 bacteria in one dose)
In this subgroup we found five trials (n = 638). There was no significant difference between groups when including studies that applied more than 1010 bacteria in one dose (OR 0.77, 95% CI 0.51 to 1.17, Analysis 3.3).
3.3. Analysis.
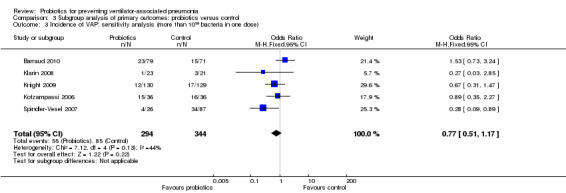
Comparison 3 Subgroup analysis of primary outcomes: probiotics versus control, Outcome 3 Incidence of VAP: sensitivity analysis (more than 1010 bacteria in one dose).
4. Incidence of VAP: sensitivity analysis (probiotics applied twice daily)
In this subgroup we found four trials (n = 649). There was a significant difference (P value = 0.03) favouring probiotics applied twice daily over the control group (OR 0.64, 95% CI 0.43 to 0.96, Analysis 3.4).
3.4. Analysis.
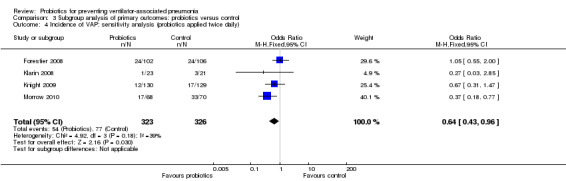
Comparison 3 Subgroup analysis of primary outcomes: probiotics versus control, Outcome 4 Incidence of VAP: sensitivity analysis (probiotics applied twice daily).
Sensitivity analysis
1. Study quality impact on heterogeneity
We investigated whether considerable levels of heterogeneity found in the results (Analysis 1.5; Analysis 1.7) were due to the inclusion of studies with a high risk of bias on one or more of the 'Risk of bias' domains. The exclusion of these studies made no difference to levels of heterogeneity. We identified one study as inducing the high levels of heterogeneity, particularly in the outcomes of length of ICU stay and duration of mechanical ventilation. Participants in Kotzampassi 2006 differed fundamentally to the participants in the other included studies, as they were critically ill trauma patients with severe multiple organ injuries. Results for this study alone were significantly in favour of Synbiotic 2000FORTE over placebo for both time spent in the ICU, as well as duration of mechanical ventilation. The removal of this study from meta‐analysis restores homogeneity, demonstrating no difference between groups.
2. Diagnostic criteria for VAP
Only one study did not provide a robust definition of VAP (Spindler‐Vesel 2007); after excluding this study from our primary outcome of incidence of VAP, data were no longer statistically significant (OR 0.76, 95% CI 0.56 to 1.05, P value = 0.10). There were no significant differences between groups for the remaining primary outcomes of mortality (hospital and ICU) or incidence of diarrhoea with the exclusion of this study.
3. Studies with a high risk of bias
After removing studies rated as a high risk of bias across one or more of the 'Risk of bias' domains, our primary outcome of incidence of VAP was no longer statistically significant (OR 0.79, 95% CI 0.50 to 1.25, P value = 0.31). There were no differences between groups for the remaining primary outcomes of mortality (hospital and ICU) or incidence of diarrhoea with the exclusion of these studies.
4. Fixed‐effect versus random‐effects
For our primary outcome of instances of VAP, results were no longer statistically significant when using a random‐effects model instead of a fixed‐effect model for data synthesis (OR 0.67, 95% CI 0.43 to 1.04, P value = 0.08). Again, there were no differences between groups for the remaining primary outcomes of mortality (hospital and ICU) or incidence of diarrhoea with the exclusion of these studies.
Discussion
Summary of main results
Overall, the results from this systematic review demonstrate that significantly fewer people receiving probiotics experienced ventilator‐associated pneumonia (VAP) than people in the control groups. The majority of studies reported this primary outcome of interest. However, other outcomes, specifically adverse events, duration of mechanical ventilation and antibiotic use, were underreported. The remaining included outcomes demonstrated no significant difference between groups. There was evidence from a medium‐sized study (n = 138) that probiotic used was associated with fewer days spent receiving antibiotics for VAP. However, further studies are needed to confirm this result.
To explore the influence of some potential confounding factors in the studies, we conducted subgroup analyses examining the effect of the probiotic or synbiotic formula administered, the dose and the method of administration on the overall estimate. We identified three statistically significant results from the subgroup analysis, demonstrating a significant difference between groups favouring the probiotic Lactobacillus rhamnosus when compared with placebo, or Synbiotic FORTE2000 when compared to either fermentable fibre or peptide. However, these significant findings should be interpreted with caution, due to the observational nature of the subgroups analysis and small sample size from the studies.
Sensitivity analyses found no significant reduction in the incidence of VAP based on any exclusion criteria; nor were there any significant differences between groups when we excluded studies rated as a 'high' risk of bias across one or more of the domains from the results. Synthesising data using a random‐effects model for incidence of VAP demonstrated no statistical significance in the results, as with a fixed‐effect model. With the remaining outcomes, when synthesising data using either a fixed‐effect or random‐effects model, there was no change in the estimate of effect where there were low to no levels of heterogeneity. We also performed an intention‐to‐treat (ITT) analysis and found that it did not change the inference of per‐protocol analysis.
Overall completeness and applicability of evidence
Although the mechanisms underpinning the protective effects of probiotics in critically ill patients remained to be elucidated, there is mounting evidence that probiotics could inhibit colonisation of pathogenic bacteria and enhance immunity. It is clear that there are multiple mechanisms by which different probiotic bacteria exert their effects and these effects vary with the strain and population studied. Our review found that probiotics significantly reduced the incidence of VAP. However, they did not provide any additional benefit in terms of the other outcomes measured.
Incidence of diarrhoea was one of the major concerns when using probiotics in critically ill patients. As this population is relatively immunocompromised, it was felt that these patients may be vulnerable to developing probiotic‐related diseases. Four trials reported on the incidence of diarrhoea and the pooled results indicated that probiotics were safe in light of this concern.
The main findings of our review were in agreement with the recent previous meta‐analyses by Siempos 2010 and Petrof 2012, but contradict the results of another meta‐analysis on the same topic (Gu 2012). The meta‐analysis by Siempos 2010 included five studies with a total of 689 participants. Their results showed that probiotics appeared to be associated with a lower incidence of VAP. The meta‐analysis by Petrof 2012 included 23 studies with a total of 2153 participants and indicated, again, that lower VAP incidence was associated with people receiving probiotics. The meta‐analysis by Gu 2012 included seven studies with a total of 1142 participants and indicated that probiotics show no benefit in VAP prophylaxis.
There are several important differences between these meta‐analyses that should be noted. The meta‐analysis by Gu 2012 excluded two studies that were presented in this review (Spindler‐Vesel 2007; Tan 2011), and included one study that was excluded from the present review (Oudhuis 2011). In this study the control group received selective decontamination of the digestive tract (SDD) using a four times daily regime comprising an oral paste (polymyxin E, gentamicin, amphotericin B), enteral solution (same antibiotics) and intravenous cefotaxime (first four days). The older review by Siempos 2010 did not include data from the more recent studies (Barraud 2010; Morrow 2010; Tan 2011), and the more recent review by Petrof 2012 did not include data from one study included in the present review (Spindler‐Vesel 2007).
Quality of the evidence
As shown in Figure 2 and Figure 3, most of the studies adequately reported their methodology. However, due to limited information presented, many of the 'Risk of bias' domains remain 'unclear'. Although we rated several studies as a 'low' risk of bias and they adequately reported methods of randomisation, attrition rates and funding sources, others did not and combined analysis showed that the studies included were generally of low to very low quality for our 'Summary of findings' (SoF) outcomes. In addition to risk of bias consideration, other domains of GRADE 'Summary of findings' assessment included inconsistencies between studies (i.e. heterogeneity), which was present in two SoF outcomes (length of intensive care unit (ICU) stay in days; and duration of mechanical ventilation in days). Indirectness of the evidence was agreed as being present in the majority of outcomes, since all included studies used different probiotic formulations and had differing control group interventions and differing patient populations, which led to downgrading of evidence on this factor. Finally, imprecision of the results led to downgrading of the quality of the evidence, since most data from pooled analysis of included studies found that the 95% confidence interval (CI) around the pooled or best estimate of effect included both no effect and appreciable benefit or appreciable harm (see Table 1).
Potential biases in the review process
Owing to the limited number of studies involved, it was impossible to detect publication bias. Only eight studies met the inclusion criteria in our analysis, thus the power to detect the trend of overall effect was limited and a chance finding can not be excluded.
Agreements and disagreements with other studies or reviews
A series of studies suggested that probiotics might reduce the incidence of VAP in patients undergoing mechanical ventilation. Two previously published meta‐analyses have found that administration of probiotics was associated with a significant reduction in the incidence of VAP. The beneficial effect was reinforced by a further systematic review that aimed to examine the overall effect of probiotics on patients undergoing mechanical ventilation, with VAP specifically defined. However, this review contradicts the previous conclusions. The discordant results may be due to following factors: firstly, our review included studies that provided any definition of VAP (see Sensitivity analysis). Therefore we included a study, Spindler‐Vesel 2007, that had been excluded in two previous meta‐analyses (Gu 2012; Petrof 2012); when we excluded this study from the primary outcome of instances of VAP, there was no significant difference between each group. Furthermore, extensive inclusion criteria made it difficult to draw precise conclusions for a heterogeneous group of critically ill patients, thus the overall effect of probiotics on ICU patients should be interpreted cautiously. Secondly, although we set strict inclusion criteria, potential heterogeneity remained in the present study. Dose, method of administration, probiotics formula and primary diagnosis varied among the studies included. Interestingly, there was a strong rationale for the use of probiotics in trauma patients. Studies showed that probiotics significantly decreased the incidence of VAP as well as improving survival in this group of patients. We would suggest that further studies should focus on this group of patients.
Authors' conclusions
Implications for practice.
The results from a previous meta‐analysis have yielded conflicting results regarding the prevention of ventilator‐associated pneumonia (VAP) by probiotics. In this meta‐analysis of 1083 participants strict inclusion criteria were adhered to and, based on pooled results, there is a suggestion that probiotics reduce incidence of VAP. However, due to differences in participants, probiotics administered and clinical and statistical heterogeneity, study limitations and small sample sizes across the included studies, there is not enough evidence to draw conclusions on the efficacy and safety of routine use of probiotics for the prevention of VAP in intensive care unit (ICU) patients. There was no clear evidence of a reduction in ICU or in‐hospital mortality. There is no evidence that use of probiotics was associated with an increased change in the incidence of diarrhoea, or nosocomial infections.
Other commonly applied VAP prevention strategies (ventilator care bundle) were not accounted for in the studies included and could potentially have a bearing on the overall results. However, the studies compared different patient populations and included six different probiotic or symbiotic treatments.
Implications for research.
There is still much to be learned about the probiotic‐host interaction. The human, animal and in vitro studies of probiotics carried out to date exhibit a high level of heterogeneity in the conditions targeted, models used and probiotics tested. Furthermore, it is likely that we are adopting a simplistic view of the mechanisms of action of probiotic species, bearing in mind that specific effects may be strain‐related.
In any further work that is undertaken, it may be worth concentrating on specific groups of patients, e.g. trauma patients. Even within these studies there is considerable heterogeneity with regard to probiotic species used and dosage, route and timing of administration. In future research careful consideration should be given to these factors, as well as greater measures taken to better assess the safety of probiotics.
Acknowledgements
The authors wish to thank Liz Dooley, Managing Editor of the Cochrane ARI Group. We also wish to thank the following people for commenting on the draft protocol and review: Ann Fonfa, Mohamed Alaa, Lee Morrow, Michael de Vrese, Ilias Siempos, Teresa Neeman and John Holden. We would like to thank Stephanie Sampson and Systematic Review Solutions Ltd. for their assistance with data extraction and analysis, as well as with copy edit and language problems.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
MEDLINE (Ovid)
1 Pneumonia/ 2 Pneumonia, Ventilator‐Associated/ 3 (pneumon* adj3 (ventilat* or respirator* or nosocomial*)).tw. 4 vap.tw. 5 1 or 2 or 3 or 4 6 Probiotics/ 7 Synbiotics/ 8 probiotic*.tw. 9 synbiotic*.tw. 10 exp Lactobacillus/ 11 Bifidobacterium/ 12 lactobacil*.tw,nm. 13 (bifidus or bifidobacter*).tw,nm. 14 streptococc*.tw,nm. 15 lactococc*.tw,nm. 16 leuconostoc.tw,nm. 17 pediococc*.tw,nm. 18 (beneficial adj3 bacter*).tw. 19 or/6‐18 20 5 and 19
Appendix 2. Embase.com search strategy
#17 #15 AND #16 #16 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim OR (random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR ((singl* OR doubl*) NEAR/1 blind*):ab,ti #15 #4 AND #14 #14 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 #13 streptococ*:ab,ti OR lactococ*:ab,ti OR leuconostoc*:ab,ti OR pediococ*:ab,ti #12 'streptococcus'/exp #11 bifidus:ab,ti OR bifidobacter*:ab,ti #10 'bifidobacterium'/exp #9 lactobacil*:ab,ti #8 'lactobacillus'/exp #7 probiotic*:ab,ti OR synbiotic*:ab,ti #6 'synbiotic agent'/de #5 'probiotic agent'/de A #4 #1 OR #2 OR #3 #3 vap:ab,ti #2 (pneumon* NEAR/3 (ventilator* OR respirator* OR nosocomial*)):ab,ti #1 'pneumonia'/de OR 'ventilator associated pneumonia'/de
Appendix 3. Secondary outcomes (from protocol)
Secondary outcomes
Length of ICU stay.
Duration of mechanical ventilation.
Systematic antibiotic use.
Any adverse outcomes of the probiotics, i.e. diarrhoea, toxicity, abdominal pain, occurrence of lactic acidosis or nosocomial probiotic infection.
Data and analyses
Comparison 1. Per‐protocol analysis: probiotics versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of VAP | 8 | 1018 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.52, 0.95] |
| 2 ICU mortality | 5 | 703 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.58, 1.22] |
| 3 Hospital mortality | 4 | 524 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.54, 1.14] |
| 4 Diarrhoea | 4 | 618 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.47, 1.09] |
| 5 Length of ICU stay (days) | 4 | 396 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐6.53, 3.33] |
| 6 Length of ICU stay (days) | Other data | No numeric data | ||
| 7 Duration of mechanical ventilation (days) | 2 | 203 | Mean Difference (IV, Random, 95% CI) | ‐6.15 [‐18.77, 6.47] |
| 8 Duration of mechanical ventilation (days) | Other data | No numeric data | ||
| 9 Antibiotic use for VAP (days) | 1 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐6.04, 0.04] |
| 10 Antibiotic use | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.51, 2.96] |
| 11 Adverse events: nosocomial probiotic infection | 6 | 861 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. ITT analysis: probiotics versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ITT analysis: incidence of VAP | 8 | 1058 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.52, 0.95] |
| 2 ITT analysis: ICU mortality | 5 | 740 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.59, 1.23] |
| 3 ITT analysis: hospital mortality | 4 | 553 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.55, 1.17] |
| 4 ITT analysis: diarrhoea | 4 | 649 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.49, 1.08] |
| 5 ITT analysis: ICU stay | 4 | 432 | Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐6.77, 3.24] |
| 6 ITT analysis: duration of mechanical ventilation (days) | 2 | 215 | Mean Difference (IV, Random, 95% CI) | ‐6.21 [‐18.83, 6.41] |
| 7 ITT analysis: antibiotic use for VAP (days) | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐5.96, ‐0.04] |
| 8 ITT: antibiotic use | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.58, 2.86] |
| 9 ITT analysis: adverse events: nosocomial probiotic infection | 5 | 860 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Subgroup analysis of primary outcomes: probiotics versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of VAP: probiotics versus control | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Lactobacillus casei rhamnosus versus placebo | 1 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.55, 2.00] |
| 1.2 Lactobacillus rhamnosus versus placebo | 1 | 138 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.77] |
| 1.3 Lactobacillus plantarum versus chlorhexidine (CHX) | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.85] |
| 1.4 Synbiotic 2000FORTE versus placebo | 2 | 331 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.41, 1.37] |
| 1.5 Synbiotic 2000FORTE versus glutamine | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.10, 1.26] |
| 1.6 Synbiotic 2000FORTE versus fermentable fibre | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.07, 0.94] |
| 1.7 Synbiotic 2000FORTE versus peptide | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.07, 0.93] |
| 1.8 Ergyphilus versus placebo | 1 | 149 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.74, 3.30] |
| 1.9 Bifidobacterium longum + Lactobacillus bulgaricus + Streptococcus thermophilus versus enteral nutrition | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.09, 1.43] |
| 2 Incidence of VAP: sensitivity analysis (probiotics applied solely to stomach) | 7 | 975 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.43, 1.08] |
| 3 Incidence of VAP: sensitivity analysis (more than 1010 bacteria in one dose) | 5 | 638 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.51, 1.17] |
| 4 Incidence of VAP: sensitivity analysis (probiotics applied twice daily) | 4 | 649 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.43, 0.96] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barraud 2010.
| Methods | Design: a double‐blind, concealed randomised, placebo‐controlled trial | |
| Participants | N = 167 Settings: a medical intensive care unit; 1 centre Country: France Study duration: 90 days; February 2006 to March 2008 Sex (M/F): 68/99 Inclusions: all intubated adult patients under mechanical ventilation for a predicted period of at least 2 days Exclusions: (1) a predicted a predicted duration of mechanical ventilation less than 2 days, (2) age less than 18 years, (3) pregnancy, (4) immunosuppression (AIDS, malignant haemopathy, neutrophil count less than 500 per mm3, cytostatic chemotherapy during past 3 months before ICU admission), (5) short bowel disease (a situation known to be a risk factor for development of laboratory‐associated infections) and (5) inclusion in another trial. Patients re‐admitted to the intensive care unit (ICU) and previously included in this study could not be randomised again |
|
| Interventions | Intervention group: 5 Ergyphilus® (Nutergia, Capdenac, France) capsules once a day. Ergyphilus® capsules consisted of a multi‐species probiotic preparation containing 2 x 1010 of revivable bacteria (mainly Lactobacillus rhamnosus GG but also Lactobacillus casei, Lactobacillus acidophilus and Bifidobacterium bifidum). Treatment was diluted in 20 ml of water and administered daily by the nurse through the enteral feeding tube for the entire period of mechanical ventilation (but for a duration not exceeding 28 days). After weaning from the ventilator, treatment was given for 2 additional days and then stopped in the case of successful extubation, or continued in the case of extubation failure, n = 87 Control group: placebo capsules only contained the excipient, n = 80 |
|
| Outcomes | Primary endpoint: 28‐day mortality Secondary endpoints: mortality at day 90, reversal of organ failure, occurrence of ICU‐acquired infections and colonisation by day 28 and ICU/hospital length of stay |
|
| Notes | Definition of ventilator‐associated pneumonia (VAP): a new and persistent infiltrate on chest radiograph associated with at least 1 of the following: purulent tracheal secretions, temperature 38.3 ℃ or higher and a leukocyte count of 10,000 μL‐1 or higher; and positive quantitative cultures of distal pulmonary secretions obtained from bronchoalveolar lavage (significant threshold more than 104 colony‐forming units (CFU)s/ml | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned by the pharmacist in a 1:1 ratio to the probiotics or the placebo group by using a concealed randomisation table. A block size of 4 was used without further stratification |
| Allocation concealment (selection bias) | Low risk | Randomly assigned by the pharmacist in a 1:1 ratio to the probiotics or the placebo group by using a concealed randomisation table. A block size of 4 was used without further stratification |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant, caregiver, investigator, outcomes assessor (obtained from http://clinicaltrials.gov/ct2/show/NCT00122408). Both treatment preparations were identical in appearance, weight, odour, taste, consistency and packaging |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A blinded interim analysis was planned and outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the treatment period and none were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unclear from the trial |
| Funding source | Low risk | CHU Nancy |
| Other bias | Unclear risk | None detected |
Forestier 2008.
| Methods | Design: a randomised, double‐blind, placebo‐controlled pilot study | |
| Participants | N = 236 Settings: a 17‐bed ICU in the teaching hospital of Clermont‐Ferand, France; 1 centre Country: France Study duration: 80 days; March 2003 to October 2004 Sex (M/F): 146/62 Inclusions: participants aged 18 years or older with a stay longer than 48 hours and a nasogastric feeding tube were eligible Exclusions: age under 18 years, immunosuppression, absolute neutrophile count under 500/mm3, gastrointestinal bleeding, contraindication to enteral feeding and isolation of P. aeruginosa from gastric aspirates or respiratory tract specimens during the first 4 days after admission |
|
| Interventions | Intervention group: L. casei rhamnosus (109 colony‐forming units) twice daily through a double‐lumen nasogastric suction tube or orally, after removal of the tube, from the third day after admission to the ICU until discharge or death, n = 118 Control group: placebo (growth medium without bacteria); the method of administration was the same as the treatment group, n = 118 |
|
| Outcomes | Primary outcome: the time of the first P. aeruginosa acquisition Secondary outcome: the times of P. aeruginosa respiratory tract infection or colonisation and P. aeruginosa gastric colonisation and the number of patients with persistent gastric colonisation with the L. casei rhamnosus |
|
| Notes | Definition of VAP: defined according mostly to the US Centers for Disease Control and Prevention's National Healthcare Safety Network criteria. These criteria require there to be at least 1 positive sample (protected specimen brush or plugged telescoping catheter for broncho‐alveolar minilavage (> 103 CFUs/ml) or endotracheal aspirate with (> 105 CFUs/ml and > 25 leucocytes/high‐power field)); also required is the presence of 1 or several new abnormal radio graphical and progressive parenchymatous infiltrates and 1 of the following signs: purulent sputum production, fever (temperature > 38.5 °C), pathogenic bacteria in blood culture without other infection source and bronchoalveolar minilavage with more than 5% cells with intracellular bacteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Equal randomisation to either group was done using a computer‐generated random allocation schedule. Envelopes numbered 1 to 400 contained the letter 'A' or 'B' |
| Allocation concealment (selection bias) | Low risk | Equal randomisation to either group was done using a computer‐generated random allocation schedule. Envelopes numbered 1 to 400 contained the letter 'A' or 'B' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Stated it was a double‐blinded trial but not for whom |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Stated it was a double‐blinded trial but not for whom |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 patients were excluded from analysis because of "P. aeruginosa within 4 days of admission" (p4) |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Funding source | High risk | The pharmaceutical company Lyocentre SA |
| Other bias | Unclear risk | None detected |
Klarin 2008.
| Methods | Design: a randomised, controlled, open pilot study | |
| Participants | N = 50 Settings: 1 ICU; 1 centre (Department of Anaesthesiology and Intensive Care, University Hospital, Lund, Sweden) Country: Sweden Study duration: 16 days; March 2003 to October 2004 Sex (M/F): 22/22 Inclusions and exclusions: 18 years of age or older; critically ill with ventilation and circulation had been stabilised; not moribund; not suffering from pneumonia at admission; no fractures in the facial skeleton or the base of the skull; no oral ulcers; not immune deficient; not a carrier of HIV or viral hepatitis |
|
| Interventions | Intervention group: initial mechanical steps were the same as in the control group but subsequent cleansing was instead performed with gauze swabs soaked in carbonated bottled water, after which Lp299 was applied to the mucosal surface of the oral cavity. 10 ml of a solution containing a total 1010 CFUs of Lp299 were used, n = 25 Control group: treated according to the department's standard protocol. Dental prostheses were removed; secretions were removed by suction; teeth were brushed using toothpaste; all mucosal surface were cleansed with swabs that had been moistened with a 1 mg/ml chlorhexidine (CHX) solution, n = 25 |
|
| Outcomes | Primary outcome: incidence of VAP Secondary outcome: ICU mortality; in‐hospital mortality; ICU stay and ventilator days |
|
| Notes | Definition of VAP: a new, persistent or progressive infiltrate on chest radiograph combined with at least 3 or the other 4 criteria; a purulent tracheal aspirate; positive culture of tracheal aspirates occurring after 48 hours of mechanical ventilation; rectal or urine bladder temperature higher than 38.0 °C or less than 35.5 °C; WBC count more than 12 or less than 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated it was a placebo‐controlled, randomised trial but not how it was performed |
| Allocation concealment (selection bias) | Unclear risk | Unclear from the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear from the trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cultures taken from the oropharynx and the trachea were sent blinded to the research laboratory |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "After screening, 50 patients were included in the study. Consent was withdrawn by two patients and another 3 were transferred to other ICUs shortly after inclusion. For 1 patient in the control group, samples were obtained only at inclusion. Altogether, 23 patients in the Lp group and 21 in the control group completed the study" (p3) |
| Selective reporting (reporting bias) | Unclear risk | Unclear from the trial |
| Funding source | High risk | Region Skane, Sweden; the Scandinavian Society for Antimicrobial Chemotherapy Foundation; Probi AB, Lund, Sweden (unconditional) |
| Other bias | Unclear risk | None detected |
Knight 2009.
| Methods | Design: a randomised, double‐blind, placebo‐controlled trial | |
| Participants | N = 264 Settings: a 14‐bedded general ICU of a 1400‐bedded UK tertiary care University Hospital; 1 centre Country: UK Study duration: 28 days, January 2004 and February 2005 Sex (M/F): 161/98 Inclusions: all intubated adult patients under mechanical ventilation for a minimum of 48 hours and with no contraindications to enteral nutrition Exclusions: age less than 16 years, active immunosuppression, pregnancy, transfer from other institution (if already intubated for more than 24 hours), intubation more than 24 hours after admission to ICU and participation in other pharmacological research within 30 days |
|
| Interventions | Intervention group: at least 2 days (4 doses in 48 hours) of Synbiotic 2000 FORTE® (Medipharm, Kågeröd, Sweden and Des Moines, IA), twice a day. Synbiotic 2000 FORTE® contains Pediococcus pentosaceus, Leuconostoc mesenteroides, Lactobacillus paracasei subsp paracasei and Lactobacillus plantarum (at a dose of 1010 bacteria per sachet) as probiotics and beta‐glucan, inulin, pectin and resistant starch (2.5 g of each) as prebiotics, n = 132 Control group: crystalline cellulose‐based placebo, n = 132 |
|
| Outcomes | Primary outcome: incidence of VAP Secondary outcome: oropharyngeal flora, ICU length of stay, ICU mortality and hospital mortality, number of ventilator days |
|
| Notes | Definition of VAP: VAP was suspected if there was new progressive, or persistent (24 hours), infiltration on chest radiograph plus at least 2 of the following: (1) temperature [38.0 °C, (2) leucocytosis (WBC count > 12 × 103 μL‐1) or leukopenia (WBC count < 4 × 103 μL‐1), (3) purulent tracheobronchial secretions. All suspected cases were reviewed with appropriate clinical, radiological and sequential microbiological data (tracheal aspirates and bronchoalveolar lavage). Diagnosis was made prospectively and only confirmed if a blinded microbiologist and intensive care physician agreed on the diagnosis. Pneumonia was classified as VAP when diagnosed 48 hours after intubation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assigned to either a synbiotic mixture or placebo according to the contents of randomly assorted, sequentially numbered, sealed, opaque envelopes |
| Allocation concealment (selection bias) | Low risk | Assigned to either a synbiotic mixture or placebo according to the contents of randomly assorted, sequentially numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The patient, study investigators, treating medical/nursing staff and microbiologists were all blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The patient, study investigators, treating medical/nursing staff and microbiologists were all blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 patients were excluded because of "consent withdrawn" (p857), N = 259 included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Unclear from the trial |
| Funding source | High risk | Special trustees fund, Nottingham NHS trust. Medipharm (free supply of synbiotic and placebo) |
| Other bias | Unclear risk | None detected |
Kotzampassi 2006.
| Methods | Design: a double‐blind, placebo‐controlled, multi‐centre, randomised clinical trial | |
| Participants | N = 72 Settings: 5 surgical ICUs of the Thessalomiki University's tertiary‐care AHEPA Hospitals and the affiliated 424th Military Hospital Country: Greece Study duration: 15 days administration Sex (M/F): not available Inclusions: trauma patients; severe multiple organ injuries necessitating emergency tracheal intubation and ventilation support and subsequent hospitalisation in ICU Exclusions: patients with any previous hospitalisation over the last 60 days |
|
| Interventions | Intervention group: formula Synbiotic 2000FORTE was diluted in 100 ml of tap water and administered by a nasogastric tube or through gastrostomy once daily for 15 consecutive days, n = 36 Control group: identical doses of placebo; the method of administration was the same as the treatment group, n = 36 |
|
| Outcomes | Primary outcome: sepsis in the field of bacteraemia Secondary outcomes: duration of ICU stay; days requiring mechanical ventilation; white blood cells (WBCs); C‐reactive protein (CRP); primary bacteraemia; VAP; hospital mortality; serum lipopolysaccharide (LPS); urine culture |
|
| Notes | VAP definition: new or persistent consolidation in lung X‐ray; purulent tracheobronchial secretion (TBS); and clinical pulmonary infection score of more than 6 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated that it was a placebo‐controlled, randomised trial but no details as to how it was performed |
| Allocation concealment (selection bias) | Unclear risk | Unclear from the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants in the placebo group received identical doses of powdered glucose polymer |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Stated it was a double‐blinded trial but not for whom |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None detected |
| Selective reporting (reporting bias) | Unclear risk | 2 separate reports of the same study indicate different enrolment numbers; N = 65 included participants and N = 72 |
| Funding source | Unclear risk | Unclear from the trial |
| Other bias | Unclear risk | None detected |
Morrow 2010.
| Methods | Design: a prospective, randomised, double‐blind, placebo‐controlled trial | |
| Participants | N = 146 Settings: a 325‐bed,university‐based hospital that provides level 1 trauma services Country: USA Study duration: July 2004 to January 2009 Sex (M/F): 86/60 Inclusions: adults at least 19 years old (the age of majority in Nebraska) were eligible for enrolment if the lead investigator and the treating physician agreed that there was a 95% likelihood that the patient would require mechanical ventilation with an endotracheal tube for at least 72 hours Exclusions: patient subsets previously described as being at risk for iatrogenic probiotic infection: pregnancy; immunosuppression; prosthetic cardiac valve or vascular graft; cardiac trauma; history of rheumatic fever, endocarditis, or congenital cardiac abnormality; gastroesophageal or intestinal injury or foregut surgery during the current admission; oropharyngeal mucosal injury; and placement of a tracheostomy. Patients were also excluded if the investigators were unable to obtain informed written consent and administer the first dose of the study drug within 24 hours of intubation |
|
| Interventions | Intervention group: 2 x 109 CFU of Lactobacillus rhamnosus GG on a twice‐daily basis. The contents of 1 capsule containing 109 CFU of Lactobacillus were suspended in sterile, water‐based surgical lubricant and administered as a slurry to the oropharynx; the contents of a second capsule containing 109 CFU of Lactobacillus were suspended in sterile water and given through the nasogastric tube, n = 73 Control group: the same methods were used to deliver the contents of identical appearing capsules containing the inert plant starch inulin to patients randomised to placebo, n = 73 |
|
| Outcomes | Primary outcome: microbiologically confirmed VAP incidence based on quantitative BAL culture with at least 104 CFU/ml in patients intubated for 48 hours or longer Secondary outcomes: mortality; time to occurrence of VAP; durations of mechanical ventilation, ICU stay and hospital stay; Clostridium difficile‐associated diarrhoea; other ICU‐associated diarrhoea; antibiotic consumption (total, VAP‐specific and C. difficile‐specific); and hospital charges |
|
| Notes | Definition of VAP: according to the American College of Chest Physicians (ACCP) clinical criteria, quantitative cultures of distal airways samples were obtained by non bronchoscopic bronchoalveolar lavage (BAL) using a protected catheter (Combicath; KOL Biomedical Instruments, Chantilly, VA). The ACCP clinical criteria require a new and persistent infiltrate on chest radiographs with 2 of 3 supporting findings: fever (> 38.5 ℃ or, < 35.0 ℃), leukocytosis (white blood cells < 10,000/mm3 or < 3000/mm3) and purulent sputum | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned in a 1:1 ratio to treatment groups using permutation blocks (n = 4 per block) |
| Allocation concealment (selection bias) | Unclear risk | Unclear from the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators, bedside nurses, primary care clinicians and microbiology laboratory personnel were blinded to group assignments |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators, bedside nurses, primary care clinicians and microbiology laboratory personnel were blinded to group assignments |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 patients were excluded because of "consent withdraw" or "exclusion criteria" (p1060), N = 138 included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Unclear from the trial |
| Funding source | Low risk | NIH grant K23 HL81491‐04 and the Creighton University Health Futures Foundation (L.E.M.) and by the Barnes‐Jewish Hospital Foundation (M.H.K.) |
| Other bias | Unclear risk | None detected |
Spindler‐Vesel 2007.
| Methods | Design: a prospective, randomised, single‐blind, multiple treatment arm study | |
| Participants | N = 113 Settings: a 20‐bed university surgical ICU, Ljubljana, Slovenia Country: Slovenia Study duration: not stated ‐ outcomes reported for 7 days Sex (M/F): 88/25 Inclusions: multiple injured patients with an Injury Severity Score (ISS) of > 18 and at least a 4‐day ICU stay Exclusions: not stated |
|
| Interventions | 4 treatment arms: 1. Nutricomp standard (B. Braun) 3.7 g protein, 13.7 g carbohydrate, 3.3 g fat per 100 ml. Osmolarity 240 mOsm/L combined with a supplement of a synbiotic consisting of 1010Pediococcus pentosaceus 5–33:3, 1010Lactococcus raffinolactis 32–77:1, 1010Lactobacillus paracasei subsp paracasei 19, 1010Lactobacillus plantarum 2362 and 2.5 g of each of the following 4 fibres: glucan, inulin, pectin and resistant starch per sachet (Synbiotic 2000; Medipharm Kågeröd, Sweden and Des Moines, IA). Dissolved in 100 ml of lukewarm sterile water, n = 26 2. Alitraq (Abbott‐Ross, Abbott Park, IL) 5.25 g protein, 16.5 g carbohydrate, 1.55 g fat and 1.55 g glutamine, 446 mg arginine, 154 mg linolenic acid per 100 ml. Osmolarity 480 mOsm/L, n = 32 3. Nova Source (Novartis Medical Nutrition, Basel, Switzerland) 4.1 g protein, 14.4 g carbohydrate, 3.5 g fat, 2.2 g fermentable fibres as fermentable guar gum per 100 ml. Osmolarity 228 mOsm/L, n = 29 4. Nutricomp peptide (B. Braun, Melsungen, Germany) 4.5 g hydrolysed protein, 16.8 g carbohydrate, 1.7 g fat per 100 ml. Osmolarity 400 mOsm/L, n = 26 |
|
| Outcomes | Primary outcome: intestinal permeability (IP) Secondary outcome: infection rate, mortality, intensive care unit (ICU) stay, days of mechanical ventilation and the occurrence of multiple organ failure |
|
| Notes | Quote: "Microbiological specimens were collected and nosocomial infections were recorded as recommended by the Centers for Disease Control and Prevention and consensus conferences on ventilator‐associated pneumonia" (p121). No definition of VAP | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised. Quote: "using closed envelopes, patients were randomly allocated into 4 groups at the beginning of the study" (p120) |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single‐blind (implied). Quote: "investigators were blinded to study groups" (p120) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "One hundred thirty‐two patients were initially considered for the study. Eight patients died before the fourth day, 9 were discharged from the ICU during the first 48 hours after admission, whereas informed consent was not obtained for 2 patients. Therefore, 113 patients (88 men and 25 women) were included in the final analysis" (p121). Unclear whether the N = 132 referred to were randomised |
| Selective reporting (reporting bias) | Unclear risk | None detected |
| Funding source | Unclear risk | Financial support provided by the Ministry of Science of Republic of Slovenia, grant J3–3508 |
| Other bias | Unclear risk | None detected |
Tan 2011.
| Methods | Design: a prospective, randomised, single‐blind, parallel‐arm pilot study | |
| Participants | N = 52 (only data from participants mechanically ventilated used, relevant n = 35) Settings: 6‐bed specialised ICU, Department of Neurosurgery, Affiliated Hospital of North Sichuan Medical College, Nanchong, China (October 2009 to January 2011) Country: China Study duration: 28 days Sex (M/F): 40/12 Inclusions: closed head injury alone; admission within 24 hours after trauma; a Glasgow Coma Scale score between 5 and 8; aged 18 to 60 years old; and able to be fed via nasogastric tube within 48 hours after admission Exclusions: previous significant digestive, haematological and endocrine diseases; immunosuppression; presence of pneumonia or other infectious diseases upon admission; HIV‐positive; other associated trauma such as extremity fractures and chest or abdominal trauma; cancer; pregnancy or lactation; and obesity (body mass index > 30) or malnutrition (body mass index < 18.5) |
|
| Interventions | All participants "received enteral nutrition (EN) (3.8 g protein, 13.8 g carbohydrate, 3.4 g fat/100 ml, osmolarity 250 mOsm/l, no fibers; Ruisu, Huarui Pharmaceutical Co., Ltd, Beijing, China) within 48 hours following hospital admission by nasogastric tube" Intervention: Golden Bifid (Shuangqi Pharmaceutical Co., Ltd, Inner Mongolia, China) 0.5 × 108Bifidobacterium longum, 0.5 × 107Lactobacillus bulgaricus and 0.5 × 107Streptococcus thermophilus, dissolved in 20 ml sterilised, distilled water and administered through a nasogastric tube for 21 consecutive days, 7 sachets administered BID at 7am, 3pm and 11pm (total 109), n = 26 (relevant n = 16) Control: continued to receive EN, n = 26 (relevant n = 19) |
|
| Outcomes | Primary outcome: Th1/Th2 balance Secondary outcome: infection rate (VAP), use of antibiotics, ICU length of stay, mortality rate (only data for VAP rate used ‐ no other data reported for participants receiving mechanical ventilation) |
|
| Notes | VAP was defined as "pneumonia occurring more than 48 hours after endotracheal intubation and was diagnosed by the presence of both a new or progressive radiographic infiltrate plus at least two clinical features ‐ fever > 38.0°C, leucocytosis (white blood cells count > 12 × 109/l), leucopenia (white blood cells count < 4 × 109/l), or purulent tracheobronchial secretions ‐ and positive semiquantitative cultures of tracheobronchial secretions" (p3) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised. Quote: "After inclusion (which was done within 48 hours after admission), patients were randomised with a ratio of 1:1 into the probiotic group or the control group. Randomization was done with a computer‐generated random number and sealed envelopes kept by a person not involved in the investigation" (p2) |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blind. Quote: "the enrolled patients, those who processed samples and the bedside nurses in the research unit were blind to the study design. The investigators as well as the physicians in charge knew whether or not the patient had received probiotics" (p2) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "three patients in the probiotic group died on days 4, 3 and 16, respectively; five of the controls died on days 24, 10, 10, 10 and 4, respectively; and two patients (one in each group) were predischarged from hospital on days 13 and 15 due to financial reasons. Except for the three patients who died within the first 4 days after the initiation of EN, all patients (100%) in the probiotic group and 80% of the patients in the control group were fed mainly via the enteral route by day 7 (P = 0.066). No patients stopped EN for more than 2 consecutive days. Thus, 43 patients successfully completed the 21‐day study and were included in the per‐protocol analysis" (p4) |
| Selective reporting (reporting bias) | Unclear risk | None detected |
| Funding source | Unclear risk | Funded in part by the 11th 5‐year research programme of the People's Liberation Army of China under Grant No. 09MA009 |
| Other bias | Unclear risk | None detected |
ACCP: American College of Chest Physicians BAL: bronchoscopic bronchoalveolar lavage BID: twice daily CFU: colony‐forming units CHX: chlorhexidine CRP: C‐reactive protein EN: enteral nutrition ICU: intensive care unit LPS: serum lipopolysaccharide N = participants NIH: National Institutes of Health TBS: purulent tracheobronchial secretion VAP: ventilator‐associated pneumonia WBC: white blood cell
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Besselink 2008 | Allocation: randomised Participants: severe acute pancreatitis (not on mechanical ventilation) |
| Cimperman 2011 | Allocation: randomised Participants: inpatients receiving antibiotics Intervention: Lactobacillus reuteri ATCC 55730 for the prevention of antibiotic‐associated diarrhoea (excluded) |
| Eren 2010 | Allocation: randomised Participants: children with acute non‐bloody diarrhoea Intervention: Saccharomyces boulardii and yogurt fluid (excluded) |
| Oudhuis 2011 | Allocation: randomised; multi‐centre (largest centre used open‐label cross‐over of units) (excluded) |
| Perez 2010 | Allocation: randomised Participants: children of low socio‐economic status Intervention: effect of probiotic supplementation on immunoglobulins, isoagglutinins and antibody response (excluded) |
| Sazawal 2010 | Allocation: randomised Participants: children Intervention: prebiotic and probiotic fortified milk in prevention of morbidities (excluded) |
Characteristics of ongoing studies [ordered by study ID]
Thamlikitkul 2011.
| Trial name or title | Probiotics for prevention of ventilator‐associated pneumonia (VAP) |
| Methods | Phase 4 study; the investigators will assess the effects of dairy product containing L. casei shirota for the prevention of VAP in hospitalised patients with ventilator |
| Participants | Hospitalised patients age ≥ 18 years who received ventilation and agreed to participate by signing informed consent form |
| Interventions | Intervention group: probiotics; 80 ml of fermented dairy product containing L. casei shirota via nasogastric tube once daily and 80 ml of fermented dairy product containing L. casei shirota oral rinse once daily Control group: control |
| Outcomes | Number of patients with pneumonia (time frame: up to 28 days) |
| Starting date | December 2011 |
| Contact information | Visanu Thamlikitkul, MD 662‐412‐59944; sivth@mahidol.ac.th |
| Notes | — |
Differences between protocol and review
Outcomes
We made changes to the consideration of 'incidence of diarrhoea' as an adverse event (see Appendix 3 for original secondary outcomes). The outcome of incidence of diarrhoea was not specified as an adverse event in any of the included studies, rather an indicator of 'safety'. The authors feel that by making this amendment readers would ascertain with greater clarity that incidence of diarrhoea was measured to determine any benefit of probiotics, not harm (see Types of outcome measures). We have also added detail to the protocol on dealing with missing data for clarification.
Data synthesis
In the protocol, it is stated that risk ratio (RR) and risk difference (RD) would be used for data synthesis. However, we used odds ratio (OR) for all dichotomous data.
Summary of findings
The published protocol did not consider GRADE in the rating of the quality of the evidence; in the full version of the review, we subsequently produced a 'Summary of findings' table, with all seven primary and secondary outcomes included and we amended the Data collection and analysis section to detail this change.
Contributions of authors
Lulong Bo drafted the protocol. Richard Hotchkiss and Marin Kollef revised the protocol. Xiaoming Deng and Lulong Bo suggested the title. Tianzhu Tao and Xiaofei Ye contributed to the statistical analysis of the review. Jinbao Li and Yu Bai contributed to the development of the methods of the protocol. Lulong Bo, Jinbao Li, Xiaoming Deng and Neil Crooks wrote the review. Lulong Bo and Jinbao Li contributed equally to the review.
Sources of support
Internal sources
Susan Fowler, MLIS, from Bernard Becker Medical Library, St. Louis, Missouri, USA.
External sources
Cochrane Acute Respiratory Infections Group, Australia.
Declarations of interest
Lulong Bo: none known. Jinbao Li: none known. Tianzhu Tao: none known. Yu Bai: none known. Xiaofei Ye: none known. Richard S Hotchkiss: none known. Marin H Kollef: Dr Kollef has done consulting work for Merck and Cubist and has provided lectures for Cubist and Hospira. Neil H Crooks: Dr Neil Crooks has been awarded internal and external funding for a pilot study on probiotics in the critical care population. The study is registered on the EudraCT database (No 2011‐002343‐99). Xiaoming Deng: none known.
New
References
References to studies included in this review
Barraud 2010 {published data only}
- Barraud D, Blard C, Hein F, Marcon O, Cravoisy A, Nace L, et al. Probiotics in the critically ill patient: a double blind, randomized, placebo‐controlled trial. Intensive Care Medicine 2010;36(9):1540‐7. [DOI] [PubMed] [Google Scholar]
Forestier 2008 {published data only}
- Forestier C, Guelon D, Cluytens V, Gillart T, Sirot J, Champs C. Oral probiotic and prevention of Pseudomonas aeruginosa infections: a randomized, double‐blind, placebo‐controlled pilot study in intensive care unit patients. Critical Care 2008;12(3):R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klarin 2008 {published data only}
- Klarin B, Molin G, Jeppsson B, Larsson A. Use of the probiotic Lactobacillus plantarum 299 to reduce pathogenic bacteria in the oropharynx of intubated patients: a randomised controlled open pilot study. Critical Care 2008;12(6):R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knight 2009 {published data only}
- Knight D, Gardiner D, Banks A, Snape SE, Weston VC, Bengmark S, et al. Effect of synbiotic therapy on the incidence of ventilator associated pneumonia in critically ill patients: a randomised, double‐blind, placebo‐controlled trial. Intensive Care Medicine 2009;35(5):854‐61. [DOI] [PubMed] [Google Scholar]
- Knight D, Girling K, Banks A, Snape S, Weston V, Bengmark S. The effect of enteral synbiotics on the incidence of ventilator‐associated pneumonia in mechanically ventilated critically ill patients. Critical Care 2006;10(Suppl 1):P213 (abstract number). [Google Scholar]
Kotzampassi 2006 {published data only}
- Giamarellos‐Bourboulis EJ, Bengmark S, Kanellakopoulou K, Kotzampassi K. Pro‐ and synbiotics to control inflammation and infection in patients with multiple injuries. Journal of Trauma 2009;67:815–21. [DOI] [PubMed] [Google Scholar]
- Kotzampassi K, Giamarellos‐Bourboulis EJ, Voudouris A, Kazamias P, Eleftheriadis E. Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically Ill trauma patients: early results of a randomized controlled trial. World Journal of Surgery 2006;30(10):1848‐55. [DOI] [PubMed] [Google Scholar]
Morrow 2010 {published data only}
- Morrow LE. Probiotics in the intensive care unit. Critical Care 2009;15(2):144‐8. [DOI] [PubMed] [Google Scholar]
- Morrow LE, Kollef MH, Bowers JB, Casale TB. Probiotic manipulation of the native flora in critically ill patients: an opportunity for ventilator‐associated pneumonia prophylaxis? [Abstract]. American Journal of Respiratory and Critical Care Medicine 2005;128(4):144s. [Google Scholar]
- Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator‐associated pneumonia: a blinded, randomized, controlled trial. American Journal of Respiratory and Critical Care Medicine 2010;182(8):1058‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spindler‐Vesel 2007 {published data only}
- Spindler‐Vesel A, Bengmark S, Vovk I, Cerovic O, Kompan L. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. Journal of Parenteral and Enteral Nutrition 2007;31(2):119‐26. [DOI] [PubMed] [Google Scholar]
Tan 2011 {published data only}
- Tan M, Zhu JC, Du J, Zhang LM, Yin HH. Effects of probiotics on serum levels of Th1/Th2cytokine and clinical outcomes in severe traumatic brain‐injured patients: a prospective randomized pilot study. Critical Care 2011;15(R290):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Besselink 2008 {published data only}
- Besselink GHM, Santvoort HC, Buskens E, Boermeester MA, Goor H, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double‐blind, placebo‐controlled trial. Lancet 2008;371(9613):651‐9. [DOI] [PubMed] [Google Scholar]
Cimperman 2011 {published data only}
- Cimperman L, Bayless G, Best K, Diligente A, Mordarski B, Oster M, et al. A randomized, double‐blind, placebo‐controlled pilot study of Lactobacillus reuteri ATCC 55730 for the prevention of antibiotic‐associated diarrhoea in hospitalized adults. Journal of Clinical Gastroenterology 2011;45(9):785‐9. [DOI] [PubMed] [Google Scholar]
Eren 2010 {published data only}
- Eren M, Dinleyici EC, Vandenplas Y. Clinical efficacy comparison of Saccharomyces boulardii and yogurt fluid in acute non‐bloody diarrhoea in children: a randomized, controlled, open label study. American Journal of Tropical Medicine and Hygiene 2010;82(3):488‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oudhuis 2011 {published data only}
- Oudhuis GJ, Bergmans DC, Dormans T, Zwaveling JH, Kessels A, Prins MH, et al. Probiotics versus antibiotic decontamination of the digestive tract: infection and mortality. Intensive Care Medicine 2011;37(1):110‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez 2010 {published data only}
- Perez N, Iannicelli JC, Girard‐Bosch C, Gonzalez S, Varea A, Disalvo L, et al. Effect of probiotic supplementation on immunoglobulins, isoagglutinins and antibody response in children of low socio‐economic status. European Journal of Nutrition 2010;49(3):173‐9. [DOI] [PubMed] [Google Scholar]
Sazawal 2010 {published data only}
- Sazawal S, Dhingra U, Hiremath G, Sarkar A, Dhingra P, Dutta A, et al. Prebiotic and probiotic fortified milk in prevention of morbidities among children: community‐based, randomized, double‐blind, controlled trial. PLoS One 2010;5(8):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Thamlikitkul 2011 {unpublished data only}
- Probiotics for prevention of ventilator‐associated pneumonia (VAP). Ongoing study December 2011.
Additional references
ATS/IDSA 2005
- American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. American Journal of Respiratory and Critical Care Medicine 2005;171:388‐416. [DOI] [PubMed] [Google Scholar]
Bouza 2009
- Bouza E, Burillo A. Advances in the prevention and management of ventilator‐associated pneumonia. Current Opinion in Infectious Diseases 2009;22(4):345‐51. [DOI] [PubMed] [Google Scholar]
Gareau 2010
- Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nature Reviews Gastroenterology and Hepatology 2010;7(9):503‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibson 1995
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. Journal of Nutrition 1995;125(6):1401‐12. [DOI] [PubMed] [Google Scholar]
Grap 2012
- Grap MJ, Munro CL, Unoki T, Hamilton VA, Ward KR. Ventilator‐associated pneumonia: the potential critical role of emergency medicine in prevention. Journal of Emergency Medicine 2012;42(3):353‐62. [DOI] [PubMed] [Google Scholar]
Gu 2012
- Gu WJ, Wei CY, Yin RX. Lack of efficacy of probiotics in preventing ventilator‐associated pneumonia: a systematic review and meta‐analysis of randomized controlled trials. Chest 2012;142(4):859‐68. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Isakow 2007
- Isakow W, Morrow LE, Kollef MH. Probiotics for preventing and treating nosocomial infections: review of current evidence and recommendations. Chest 2007;132(1):286‐94. [DOI] [PubMed] [Google Scholar]
Jain 2004
- Jain PK, McNaught CE, Anderson AD, MacFie J, Mitchell CJ. Influence of synbiotic containing Lactobacillus acidophilus La5, Bifidobacterium lactis Bb 12, Streptococcus thermophilus, Lactobacillus bulgaricus and oligofructose on gut barrier function and sepsis in critically ill patients: a randomised controlled trial. Clinical Nutrition 2004;23(4):467‐75. [DOI] [PubMed] [Google Scholar]
Klein 1998
- Klein G, Pack A, Bonaparte C, Reuter G. Taxonomy and physiology of probiotic lactic acid bacteria. International Journal of Food Microbiology 1998;41(2):103‐25. [DOI] [PubMed] [Google Scholar]
Kollef 2005
- Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health‐care‐associated pneumonia: results from a large US database of culture‐positive pneumonia. Chest 2005;128(6):3854‐62. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Petrof 2012
- Petrof EO, Dhaliwal R, Manzanares W, Johnstone J, Cook D, Heyland DK. Probiotics in the critically ill: a systematic review of the randomized trial evidence. Critical Care Medicine 2012;40(12):3290‐302. [DOI] [PubMed] [Google Scholar]
Rello 2002
- Rello J, Ollendorf DA, Oster G, Vera‐Llonch M, Bellm L, Redman R, et al. Epidemiology and outcomes of ventilator‐associated pneumonia in a large US database. Chest 2002;122(6):2115‐21. [DOI] [PubMed] [Google Scholar]
Rello 2010
- Rello J, Chastre J, Cornaglia G, Masterton R. A European care bundle for management of ventilator‐associated pneumonia. Journal of Critical Care 2010;26(1):3‐10. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richards 1999
- Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Critical Care Medicine 1999;27(5):887‐92. [DOI] [PubMed] [Google Scholar]
Schrezenmeir 2001
- Schrezenmeir J, Vrese M. Probiotics, prebiotics, and synbiotics ‐ approaching a definition. American Journal of Clinical Nutrition 2001;73(Suppl 2):361‐4. [DOI] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: Wiley‐Blackwell, 2008:359‐83. [Google Scholar]
Siempos 2010
- Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator‐associated pneumonia: a meta‐analysis of randomized controlled trials. Critical Care Medicine 2010;38(3):954‐62. [DOI] [PubMed] [Google Scholar]
Valencia 2009
- Valencia M, Torres A. Ventilator‐associated pneumonia. Current Opinion in Critical Care 2009;15(1):30‐5. [DOI] [PubMed] [Google Scholar]
Van Silvestri 2010
- Silvestri L, Saene HK, Gregori D, Agostini S, Francescon M, Taylor N. Probiotics to prevent ventilator‐associated pneumonia: no robust evidence from randomized controlled trials. Critical Care Medicine 2010;38(7):1616‐7. [DOI] [PubMed] [Google Scholar]


