Abstract
Based on postmortem brain studies, our overarching epigenetic hypothesis is that chronic schizophrenia (SZ) is a psychopathological condition involving dysregulation of the dynamic equilibrium among DNA-methylation/demethylation network components and the expression of SZ target genes, including GABAergic and glutamatergic genes.
SZ has a natural course, starting with a prodrome, a first episode that occurs in adolescence or in young adults, and later deterioration over the adult years. Hence, the epigenetic status at each neurodevelopmental stage of the disease cannot be studied just in postmortem brain of chronic SZ patients, but requires the use of a neurodevelopmental animal models. We have directed the focus of our research toward studying the epigenetic signature of SZ brain in the offspring of dams stressed during pregnancy (PRS mice). Adult PRS-mice have behavioral deficits reminiscent of behaviors observed in psychotic patients. The adult PRS brain, like that of postmortem chronic SZ patients, is characterized by a significant increase in DNA-methyltransferase 1 (DNMT1), Tet methylcytosine dioxygenase 1 (TET1), 5-methylcytosine (5MC) and 5-hydroxymethylcytosine (5HMC) at SZ candidate gene promoters, and a reduction in the expression of glutamatergic and GABAergic genes. In PRS mice, measurements of epigenetic biomarkers for SZ can be assessed at different stages of development with the goal of further elucidating the pathophysiology of this disease and predicting treatment responses at specific stages of the illness, with particular attention to early detection and possibly early intervention.
Keywords: DNA-methyltransferase, DNA-demethylase, GAD1, RELN, BDNF, Prenatally stressed mice, schizophrenia, Sadenosyl-methionine, valproic acid, clozapine
1) The epigenetic hypothesis of psychosis
There are several epidemiological, clinical, and molecular peculiarities associated with major psychosis [schizophrenia (SZ) and bipolar (BP) disorder] that are difficult to reconcile with a Mendelian genetic disorder, and in contrast correspond to features of an altered epigenetic homeostasis. Such features include: 1) incomplete phenotypic concordance between monozygotic twins (only about 50% concordance), 2) fluctuating disease course with periods of remission and relapse, 3) peaks of susceptibility to disease coinciding with major hormonal changes, and 4) parent-of-origin effects.1,2
1A) DNA-methyltransferase (DNMT)
In support of a role for aberrant epigenetic mechanisms in the pathogenesis of SZ and BP disorders, it recently has been reported that downregulation of glutamic acid decarboxylase 67 (gene symbol = GAD1) and reelin (RELN) in GABAergic neurons 3–8 and of brain derived nerve growth factor (BDNF) 9–11 and vesicular glutamate transporter (VGLUT1) in glutamatergic neurons12 is associated with an overexpression of DNMT 1 and 3a in cortical BA9, 10, 17 and in striatum of SZ and BP postmortem brain.13–17 DNMT belongs to a family of enzymes that catalyze the transfer of a methyl group from S-adenosyl methionine (SAM) to the 5 carbon of cytosine. DNMTs are highly expressed in telencephalic GABAergic interneurons in both humans and rodents.13, 18 In addition to the increased expression of DNMTs, the hypothesis that an epigenetic DNA methylation pathology operates in the transcriptional down-regulation of several target genes in SZ and BP disorder patients is supported by the following evidence: 1) increased SAM levels,19 2) enrichment of 5-methylcytosine (5MC) and 5-hydroxymethylcytosine (5HMC) at RELN,20–22 BDNF,11, 23 and GAD1 promoters,24–28 3) increased histone methylation at GABAergic gene promoters,29 4) an inverse correlation between DNA-methylation of the BDNF, RELN, and GAD1 genes and the level of their expression in the PFC,23,28 and 5) evidence of epigenetic dysregulation of several other GABAergic and glutamatergic genes.30
Support for the hypothesis that a chromatin methylation pathology is a major contributor to the down-regulation of GABAergic and glutamatergic genes in psychotic patients is sustained by clinical studies conducted in the early 1970s.31 In these studies, methionine, the precursor of SAM, when administered to SZ patients in large doses (10/20 g/day for 3–4 weeks), was reported to exacerbate psychotic symptoms. In both mouse frontal cortex (FC) and neuronal cultures, the administration of large doses of methionine induces an increase in SAM and hypermethylation of selective CpG rich promoters, including GAD1 and RELN, and facilitates down-regulation of their expression.24–27,32,33 Importantly, brain levels of GAD65 (GAD2) and the housekeeping genes are not affected.
These data are consistent with the epigenetic theory of major psychosis34 and suggest that DNA methylation and demethylation associated with GABAergic and glutamatergic gene regulatory domains are important casual events in the pathogenesis of SZ and BP disorders.
1B) DNA-demethylase
DNA methylation, potentially the longest lasting epigenetic mark, is uniquely able to account for the chronicity and often intractable nature of SZ and BP disorders. Recent evidence suggests that for inducible genes, steady state levels of DNA methylation are the result of a dynamic equilibrium between the counterbalancing actions of DNMTs, referred to as “DNA writers” because they modify DNA by adding methyl groups to cytosines, and an active DNA demethylation pathway (cytosine deaminase, base excision repair [BER] pathway), referred to as “DNA erasers” because they remove methyl groups from cytosines.35
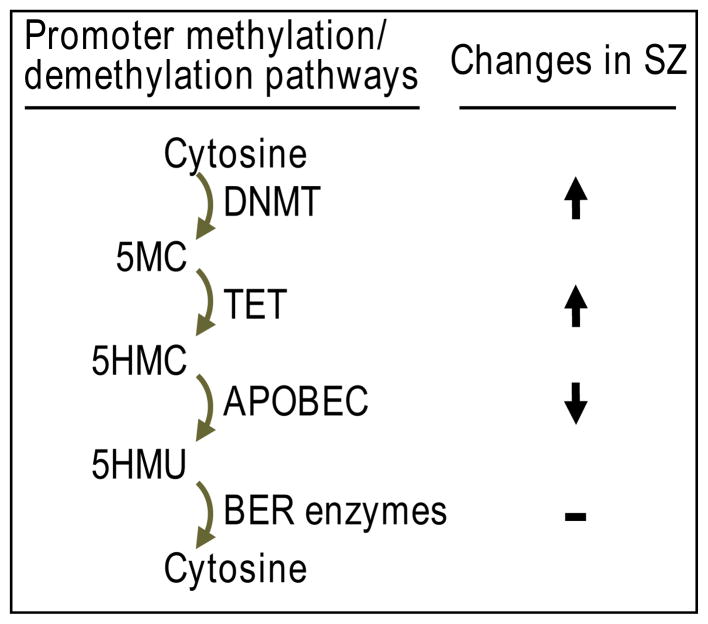
BER DNA demethylation is thought to occur as a result of the coordinated actions of ten-eleven translocation (TET) proteins, which hydroxylate 5-methyl cytosine (5MC) to form 5-hydroxymethylcytosine (5HMC). Then, through a growth arrest and DNA damage (GADD45) protein coordinated process, cytidine deaminases (Apobec) convert 5HMC to 5-hydroxymethyuracil (5HMU), which can be excised by thymine glycosylases leading to the restoration of the non-methylated state (FIG. 1).
FIG. 1.
Abbreviations: C, cytosine; DNMT, DNA methyltransferase; 5MC, 5-methylcytosine; TET, ten-eleven translocation protein; 5HMC, 5-hydroxymethyl cytosine; APOBEC, deaminase apolipoprotein B RNA editing; 5HMU, 5-hydroxymethyl uracil; BER, base excision repair
It is generally believed that the role of TET is to facilitate the removal of 5MC via formation of the intermediate 5HMC.35 Recent studies of postmortem brain by Dong et al. 28 indicate there is an almost two fold increase in TET1 mRNA and protein in the inferior parietal lobule of psychotic patients. Consistent with this increase in TET1, levels of 5HMC in total DNA are elevated. Moreover, higher 5HMC levels are detected at GAD1 and BDNF-IX promoters only in the psychotic group. The increase in TET1 in psychotic patients is inversely correlated with a decrease in GAD1 and BDNF-IX mRNA expression.23,28 In a recent study, early life maternal deprivation was found to be associated with changes in DNA-hydroxymethylation levels in the promoters of genes related to neurological or psychiatric disorders.36 Although TET-dependent active DNA demethylation and increased gene expression may be operative in the brain under normal physiological conditions,35 in the brain of psychotic patients, the increase in TET1 positively correlates with an increase in 5HMC at genomic DNA and at promoters including GAD1 and BDNF, and TET up-regulation has been associated with a down-regulation in the expression of these target genes.23,28 A possible explanation for the different actions of TET in normal physiology and in psychopathology is that the increase in TET in psychotic brain is associated with a down-regulation of the main Apobec-deaminating enzymes. Dong et al.28 showed that the most abundant Apobec isoforms (3A, 3C) are reduced significantly in psychosis (Table 1). This reduction in Apobec enzymes prevents active demethylation of critical promoters resulting in an enrichment (accumulation) of 5HMC, which by recruiting MeCP2 or MBD3-NURD complex (collectively referred to as “DNA readers”) acts to suppress transcription.2
Table 1.
Comparison of Molecular and Behavioral Abnormalities in SZ and BP Disorder Patients and PRS Mice
| SZ+BP disorder patients | PRS mice | |
|---|---|---|
| Molecular Changes: | ||
| • GABAergic gene expression 3–8 |
|
|
| • DNMT1, 3A and TET1 expression 13–17,28 |

|

|
| • 5MC and 5HMC levels at GAD1, RELN and BDNF promoters 11, 23, 28 |

|

|
| • APOBEC 3A/3C expression 28 |
|
N.D. |
|
| ||
| Behavioral Changes 43, 53, 54: | ||
| • Positive symptoms (stereotype behavior, NMDA receptor antagonist increased sensitivity) |

|

|
| • Negative symptoms (social interactions) |
|
|
| • Cognitive, information processing deficit (PPI, fear conditioning) |

|

|
Molecular changes refer to PFC and Hippocampus
Patients with SZ or BP disorders often receive antipsychotic medications. Since control subjects rarely receive these medications, the question is whether the altered expression of GABAergic and glutamatergic gene expression in the brain of these patients is the consequence of protracted antipsychotic treatment rather than an etiopathogenetic signature of SZ or BP disorder. Although, several post-mortem studies have reported no correlation between the levels of GAD1, RELN, or BDNF mRNA and proteins and life-time dosage of antipsychotic medications,4,37–39 the low statistical power of these postmortem studies may not be sufficient to definitively rule out medication as a confounding variable.
Therefore, we and others have turned to animal studies to address possible medication issues. Protracted haloperidol treatment of rats, at least in one study,4 fails to change RELN mRNA content in cortex and cerebellum. In another study, it was shown that protracted haloperidol treatment fails to change the expression of GAD1 mRNA in the PFC of nonhuman primates.37 Additionally, it has been reported that chronic (27 days) haloperidol or clozapine treatment increases, rather than decreases, the expression of GAD1 in cortico-limbic structures.38 Fatemi et al.39 also reported that chronic olanzapine administration facilitates the differential expression of genes involved in signal transduction, cell communication, metabolism, and immune responses, and leads to an up-regulation of RELN expression in frontal cortex of rats. Finally, Costa and his group,40 had previously reported that the turnover rate of GABA fails to change with haloperidol and increases with clozapine treatment in rats. Collectively, these data suggest that the down-regulation of RELN, BDNF, and GAD1 in the brains of SZ and BP disorder patients is not the consequence of antipsychotic treatment. Nevertheless, more extensive studies involving additional typical and atypical antipsychotic administration are needed.
2) Prenatal or early-life stress and impaired epigenetic profile
Clinical studies have shown that exposure of pregnant women to psychological stress, malnutrition, or viral infection exerts profound effects on neurodevelopment and the behavior of their children. Moreover, prenatal stress is associated with an increased incidence of SZ in these children later in life.41–46 It has been suggested that prenatal or early-life stress, through altered epigenetic mechanisms, is a predisposing factor for SZ and BP disorders by disrupting time- and spatial-dependent neurodevelopmental cues associated with neuronal differentiation and synaptic pruning.8, 47–50 Thus, prenatal stress is considered to be a contributing factor for several neurodevelopmental disorders, including psychotic disorders.50
Stevens and colleagues 51,52 demonstrated in mice born to stressed pregnant mothers (here defined as PRS mice) that the migration of inhibitory neuronal progenitors from the medial ganglionic eminence to the cortex is delayed and that this delay persists over time resulting in a reduction of GAD1 positive neurons in neonatal medial-frontal cortex of PRS mice. We have also found that the levels of GAD1 in GABAergic neurons in the cortex and hippocampus are altered by prenatal stress. 53–54, Dong, unpublished data
Uchida et al. (2014), by administering 5-bromo-2-deoxyuridine to label progenitor neurons, found that the number of GABAergic neurons, but not cortical plate cells, was significantly decreased in mouse fetal brain during maternal stress.55 Postnatally, the density of parvalbumin-positive GABAergic neurons was significantly decreased in the mPFC, hippocampus and sensorimotor cortex, suggesting that prenatal stress, in addition to down-regulating the expression of GAD1, results in a disruption in the proliferation of neurons destined to become GABAergic interneurons.
To explore whether the behavioral abnormalities observed in stressed animals are related to epigenetic mechanisms, Zhang et al. (2013) studied the effect of maternal behavior on GABA circuitry in the hippocampus of adult male Long-Evans rats.48 High maternal licking and grooming behavior of pup correlated with a decrease in DNMT1, a decrease in GAD1 promoter methylation and an increase in histone 3 lysine 9 acetylation (H3K9ac) at the GAD1 promoter followed by an up-regulation in the amount of GAD1 mRNA.48 In another study of adult male BL6/C57 mice, 56 chronic social defeat stress led to the persistent down-regulation of BDNF III and IV mRNA in the hippocampus which correlated with an increase in H3K27me2 at the BDNF promoter and an increase in avoidance behavior. Roth et al.57 reported that chronic stress in adult male Sprague-Dawly rats results in PTSD-like behavioral changes that are associated with an increase in hippocampal (CA1-DG) BDNF promoter methylation and a decrease in BDNF exon IV mRNA. As already discussed, in a study of the adult monkey cortex, early-life maternal deprivation was reported to be associated with changes in hydroxymethylation in the promoters of genes known to be related to neurological or psychiatric disorders.36
Thus, there is mounting evidence that early life stress results in a marked reduction in the potency of GABAergic inhibitory neurotransmission which has broad implications for information processing in the brain. In the cortex and hippocampus, GABA is released from fast-spiking GABAergic presynaptic terminals that impinge on post-synaptic GABAA receptors located on dendrites, somata, or initial axon segments of glutamatergic pyramidal neurons.8, 58–59 The release of GABA is efficient at synchronizing pyramidal neurons and monaminergic neuron firing rates and is likely crucial for optimizing cognitive and emotional function. It is therefore plausible that GABAergic neurotransmitter deficits measured in postmortem brains of SZ patients and in the brains of PRS mice leads to a disruption in intermittent synchronization patterns of pyramidal neuron firing thereby inducing cognitive and emotional impairment.58–59
3) The epigenetic modifications of GABAergic and glutamatergic genes induced by prenatal stress in mice are also detected in SZ and BP disorder patients
In a recent study, we tested whether in addition to the impaired migration of inhibitory neuronal progenitors from the median eminence to the cortex,51–52,55 the decreased expression of GABAergic genes in the cortex and hippocampus of adult prenatally stressed mice (PRS mice) is also associated with changes in the methylation/demethylation processes operative at these promoters.Dong et al., submitted; 53,54 We measured the expression of DNMT and TET in FC and hippocampus of adult PRS mice because both enzymes represent epigenetic biomarkers that are increased in postmortem brains of psychotic patients and because the altered expression of these enzymes predicts a dysfunction of DNA methylation and demethylation which impacts transcription of specific SZ candidate genes including GAD1, RELN, and BDNF.2 Our results show that the expression of DNMT 1 and TET 1 is elevated in FC and hippocampus of adult PRS offspring (like in the brain of SZ patients), and that this elevation is associated with increased binding of DNMT1 and TET1 to specific regulatory domains of the corresponding promoters along with elevated levels of 5MC and 5HMC. Consequently, the expression of critical target genes is down-regulated in SZ. Furthermore, the levels of DNMT1 and TET1 mRNA are much higher in PRS mice than the levels of the same enzymes in control mice whether measured at birth or at postnatal days 7, 14, 21 or 60–75. Hence, the offspring of mothers subjected to restraint stress during pregnancy are vulnerable to the development of behavioral abnormalities similar to those observed in psychotic patients including deficits in social interaction, prepulse inhibition of startle (PPI), and fear conditioning (Table 1).
4) PRS mice are a promising model for studies of the natural course of SZ and BP disorder
SZ and BP disorder have a natural course, starting with a prodromal phase, a first episode during adolescence or early adulthood with repeated episodes eventually leading to a deterioration that ensues over subsequent adult years. Hence, the epigenetic history of such complex neurodevelopmental disorders changing at each stage of the illness cannot be adequately studied only in postmortem brains of chronic SZ patients. In other words, each postmortem sample provides a unique window into a single time point of a chronic illness that is also progressive. To overcome these limitations, we have focused on studying the epigenetic signature and SZ-like behaviors in offspring of PRS mice as a function of postnatal maturation.
Brains of adult PRS mice and postmortem brains of SZ patients have increased levels of DNMT1 and TET1 in common (Table 1). PRS mice show higher levels of DNMT and TET in cortex and hippocampus from birth to adulthood than control mice. Hence, we can infer that the increase of DNMT and TET is likely to be the result of stress related changes that occur during embryonic life. The importance of stress during embryonic life is indicated by the finding that DNMT and TET are elevated in PRS mice even at birth before differences in maternal care could be a major factor. Further, it has been reported in Swiss albino mice that stressed mothers raising their own pups exhibit maternal care comparable to that of non-stressed mothers raising their pups.60
We cannot assume at the present time whether the time course of changes in DNMT and TET observed in the brains of PRS mice also occur in human brain, but it is conceivable that similar neurodevelopmental changes may occur in response to stressful stimulation either in utero or during early postnatal life and that such changes may prevent the normal decrease in DNMT and TET expression that progressively occurs from birth to adulthood. This hypothesis is supported by reports that the exposure of pregnant women to psychological stress, malnutrition, or viral infection during pregnancy is associated with an increased incidence of psychosis in their children later in life.41–47
It is plausible to theorize that in both in human and mouse brain, DNMT- and TET-induced GABAergic and glutamatergic stress related changes during embryonic life are the basis for a disturbance in the reciprocal interactions between GABAergic, glutamatergic, and monoaminergic neurons that is the likely source of the cognitive and emotional disruptions underlying psychotic symptoms. This theory is supported by the fact that psychotic symptoms can be exacerbated by the administration of NMDA receptor antagonists to SZ and BP disorder patients61 and to PRS mice. 53 To further investigate the hypothesis that prenatal stress, by increasing promoter methylation of GABAergic genes, may be responsible for the epigenetic alterations of GABA-glutamate neuron interactions in PRS mice, we administered valproic acid (VPA) and clozapine to adult PRS mice in doses that are known to act on chromatin remodeling involving RELN and GAD1 promoter demethylation.62–63 We observed that the combined VPA and clozapine treatment regimen abolished the hyperactivity, stereotypy, and deficits in social interaction and PPI displayed by PRS mice, whereas no effects were observed in vehicle-treated mice. The specificity of drug action in PRS mice is consistent with an epigenetic mechanism underlying PRS behavioral pathology. These preclinical studies support the concept that the PRS model has construct face validity and pharmacological utility as an experimental epigenetic model of SZ/ BP disorder. Furthermore, the PRS model theoretically has the potential for use in predicting the course of SZ-like behavioral pathology, and more importantly for predicting treatment response at different stages of the illness with particular attention to early detection of the disease.
Acknowledgments
Supported in part by RO1MH093348 and RO1MH101049 to A.G.
References
- 1.Ptak C, Petronis A. Epigenetics and complex disease: from etiology to new therapeutics. Ann Rev Pharmacol Toxicol. 2008;48:257–276. doi: 10.1146/annurev.pharmtox.48.113006.094731. [DOI] [PubMed] [Google Scholar]
- 2.Grayson DR, Guidotti A. The dynamics of DNA methylation in schizophrenia and related psychiatric disorders. Neuropsychopharmacology. 2013;38:138–166. doi: 10.1038/npp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 4.Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 5.Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fatemi SH, Earle JA, McMenomy T. Reduction in reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry. 2000;5:654–665. doi: 10.1038/sj.mp.4000783. [DOI] [PubMed] [Google Scholar]
- 7.Impagnatiello F, Guidotti A, Pesold C, Dwivedi Y, Caruncho H, Pisu M, Uzunov D, Smalheiser N, Davis J, Pandey G, Pappas G, Tueting P, Sharma R, Costa E. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci USA. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 9.Wong J, Hyde TM, Cassano HL, Deep-Soboslay A, Kleinman JE, Weickert CS. Promoter specific alterations of brain-derived neurotrophic factor mRNA in schizophrenia. Neuroscience. 2010;169:1071–1084. doi: 10.1016/j.neuroscience.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8:592–610. doi: 10.1038/sj.mp.4001308. [DOI] [PubMed] [Google Scholar]
- 11.Ikegame T, Bundo M, Sunaga F, Asai T, Nishimura F, Yoshikawa A, Kawamura Y, Hibino H, Tochigi M, Kakiuchi C, Sasaki T, Kato T, Kasai K, Iwamoto K. DNA methylation analysis of BDNF gene promoters in peripheral blood cells of schizophrenia patients. Neurosci Res. 2013;77:208–14. doi: 10.1016/j.neures.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L, Jia P, Assadzadeh A, Flanagan J, Schumacher A, Wang SC, Petronis A. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet. 2008;82:696–711.12. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veldic M, Caruncho JH, Liu WS, Davis J, Satta R, Grayson DR, Guidotti A, Costa E. DNA methyltransferase-1 (DNMT1) is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci USA. 2004;101:348–353. doi: 10.1073/pnas.2637013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci USA. 2005;102:2152–2157. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veldic M, Kadriu B, Maloku E, Agis-Balboa RC, Guidotti A, Davis JM, Costa E. Epigenetic mechanisms expressed in basal ganglia GABAergic neurons differentiate schizophrenia from bipolar disorder. Schizophr Res. 2007;91:51–61. doi: 10.1016/j.schres.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruzicka WB, Zhubi A, Veldic M, Grayson DR, Costa E, Guidotti A. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol Psychiatry. 2007;12:385–397. doi: 10.1038/sj.mp.4001954. [DOI] [PubMed] [Google Scholar]
- 17.Zhubi A, Veldic M, Puri NV, Kadriu B, Caruncho H, Loza I, Sershen H, Lajtah A, Smith RC, Guidotti A, Davis JM, Costa E. An upregulation of DNA-methyltransferase 1 and 3a expressed in telencephalic GABAergic neurons of schizophrenia patients is also detected in peripheral blood lymphocytes. Schiz Res. 2009;111:115–122. doi: 10.1016/j.schres.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadriu B, Guidotti A, Chen Y, Grayson DR. The DNA methyltransferases1 (DNMT1). and 3a (DNMT3a). co-localize with GAD67-positive neurons in the GAD67-GFP mouse brain. J Comp Neurol. 2012;9:1951–64. doi: 10.1002/cne.23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guidotti A, Ruzicka W, Grayson DR, Veldic M, Pinna G, Davis JM, Costa E. S-adenosyl methionine and DNA methyltransferase-1 mRNA overexpression in psychosis. Neuroreport. 2007;18:57–60. doi: 10.1097/WNR.0b013e32800fefd7. [DOI] [PubMed] [Google Scholar]
- 20.Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CO, Guidotti A, Costa E. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci USA. 2005;102:9341–9346. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, Wilcox M, Shafa R, Glatt SJ, Nguyen G, Ponte JF, Thiagalingam S, Tsuang MT. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am J Med Genet B Genet. 2005;134B:60–66. doi: 10.1002/ajmg.b.30140. [DOI] [PubMed] [Google Scholar]
- 22.Tochigi M, Iwamoto K, Bundo M, Komori A, Sasaki T, Kato N, Kato T. Methylation status of the reelin promoter region in the brain of schizophrenic patients. Biol Psychiatry. 2008;63:530–533. doi: 10.1016/j.biopsych.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Gavin DEP, Sharma RP, Chase KA, Matrisciano F, Dong E, Guidotti A. Growth Arrest and DNA-Damage-Inducible, Beta (GADD45b)-Mediated DNA Demethylation in Major Psychosis. Neuropsychopharmacology. 2012;2:531–542. doi: 10.1038/npp.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell CP, Chen Y, Kundakovic M, Costa E, Grayson DR. Histone deacetylase inhibitors decrease reelin promoter methylation in vitro. J Neurochem. 2005;93:483–492. doi: 10.1111/j.1471-4159.2005.03040.x. [DOI] [PubMed] [Google Scholar]
- 25.Noh JS, Sharma RP, Veldic M, Salvacion AA, Jia X, Chen Y, Costa E, Guidotti A. DNA methyltransferase 1 regulates reelin mRNA expression in mouse primary cortical cultures. Proc Natl Acad Sci USA. 2005;102:1749–1754. doi: 10.1073/pnas.0409648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Kundakovic M, Agis-Balboa RC, Pinna G, Grayson DR. Induction of the reelin promoter by retinoic acid is mediated by Sp1. J Neurochem. 2007;183:650–665. doi: 10.1111/j.1471-4159.2007.04797.x. [DOI] [PubMed] [Google Scholar]
- 27.Kundakovic M, Chen Y, Guidotti A, Grayson DR. The reelin and GAD67 promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes. Mol Pharmacol. 2009;75:342–354. doi: 10.1124/mol.108.051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong E, Gavin DP, Chen Y, Davis J. Upregulation of TET1 and down-regulation of APOBEC3A and APOBEC3C in the parietal cortex of psychotic patients. Transl Psychiatry. 2012;2:e159. doi: 10.1038/tp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houston I, Peter CJ, Mitchell A, Straubhaar J, Rogaev E, Akbarian S. Epigenetics in the human brain. Neuropsychopharmacology. 2013;38:183–197. doi: 10.1038/npp.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, Grayson DR, Matrisciano F, Pinna G, Satta R, Sharma RP, Tremolizzo L, Tueting P. Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology. 2011;60:1007–1016. doi: 10.1016/j.neuropharm.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyatt RJ, Termini BA, Davis J. Biochemical and sleep studies of schizophrenia. A review of the literature 1960–1970. Schiz Bull. 1971;4:10–44. [Google Scholar]
- 32.Tremolizzo L, Doueiri M-S, Dong E, Grayson DR, Davis J, Pinna G, Tueting P, Rodriguez-Menendez V, Costa E, Guidotti A. Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol Psychiatry. 2005;57:500–509. doi: 10.1016/j.biopsych.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 33.Dong E, Guidotti A, Grayson DR, Costa E. Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters. Proc Natl Acad Sci USA. 2007;104:4676–4681. doi: 10.1073/pnas.0700529104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costa E, Chen Y, Davis J, Dong E, Noh JS, Tremolizzo L, Veldic M, Grayson DR, Guidotti A. Reelin and schizophrenia: a disease at the interface of the genome and the epigenome. Mol Interv. 2002;2:47–57. doi: 10.1124/mi.2.1.47. [DOI] [PubMed] [Google Scholar]
- 35.Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell. 2011;145:423–434. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massart R, Suderman M, Provencal N, Yi C, Bennett AJ, Suomi S, Szyf M. Hydroxymethylation and DNA methylation profiles in the prefrontal cortex of the non-human primate rhesus macaque and the impact of maternal deprivation on hydroxymethylation. Neuroscience. 2014;268:139–48. doi: 10.1016/j.neuroscience.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 38.Lipska BK, Lerman DN, Khaing ZZ, Weickert CS, Weinberger DR. Gene expression in dopamine and GABA systems in an animal model of schizophrenia: effects of antipsychotic drugs. Eur J Neurosci. 2003;18:391–402. doi: 10.1046/j.1460-9568.2003.02738.x. [DOI] [PubMed] [Google Scholar]
- 39.Fatemi SH, Folsom TD, Reutiman TJ, Novak J, Engel RH. Comparative gene expression study of the chronic exposure to clozapine and haloperidol in rat frontal cortex. Schizophr Res. 2011;134:211–208. doi: 10.1016/j.schres.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Marco E, Mao CC, Cheney DL, Revuelta A, Costa E. The effects of antipsychotics on the turnover rate of GABA and acetylcholine in rat brain nuclei. Nature. 1976;264:363–365. doi: 10.1038/264363a0. [DOI] [PubMed] [Google Scholar]
- 41.Brown AS. The environment and susceptibility to schizophrenia. Prog Neurobiol. 2011;93:23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown AS, Patterson PH. Maternal infection and schizophrenia: implications for prevention. Schizophr Bull. 2011;37:284–290. doi: 10.1093/schbul/sbq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res. 2005;156:251–61. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 44.Mednick SA, Huttunen MO. Machón RAPrenatal influenza infections and adult schizophrenia. Schizophr Bull. 1994;20:263–7. doi: 10.1093/schbul/20.2.263. [DOI] [PubMed] [Google Scholar]
- 45.Izumoto Y, Inoue S, Yasuda N. Schizophrenia and the influenza epidemics of 1957 in Japan. Biol Psychiatry. 1999;46:119–24. doi: 10.1016/s0006-3223(98)00359-x. [DOI] [PubMed] [Google Scholar]
- 46.Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, Gorman JM. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- 47.Szyf M. Epigenetics, DNA methylation, and chromatin modifying drugs. Annu Rev Pharmacol Toxicol. 2009;49:243–263. doi: 10.1146/annurev-pharmtox-061008-103102. [DOI] [PubMed] [Google Scholar]
- 48.Zhang TY, 1, Labonté B, Wen XL, Turecki G, Meaney MJ. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology. 2013;38:111–23. doi: 10.1038/npp.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGowan PO, 1, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt MJ1, Mirnics K. Neurodevelopment, GABA System Dysfunction, and Schizophrenia. Neuropsychopharmacology. 2014 Apr 24; doi: 10.1038/npp.2014.95. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fine R, Zhang J, Stevens HE. Prenatal stress and inhibitory neuron systems: implications for neuropsychiatric disorders. Mol Psychiatry. 2014;19:641–51. doi: 10.1038/mp.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevens HE, 1, Su T, Yanagawa Y, Vaccarino FM. Prenatal stress delays inhibitory neuron progenitor migration in the developing neocortex. Psychoneuroendocrinology. 2013;38:509–21. doi: 10.1016/j.psyneuen.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matrisciano F, Tueting P, Dalal I, Kadriu B, Grayson DR, Davis JM, Nicoletti F, Guidotti A. Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology. 2013;68:184–94. doi: 10.1016/j.neuropharm.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matrisciano F, 1, Tueting P, Maccari S, Nicoletti F, Guidotti A. Pharmacological activation of group-II metabotropic glutamate receptors corrects a schizophrenia-like phenotype induced by prenatal stress in mice. Neuropsychopharmacology. 2012;37:929–938. doi: 10.1038/npp.2011.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uchida T, Furukawa T, Iwata S, Yanagawa Y, Fukuda A. Selective loss of parvalbumin-positive GABAergic interneurons in the cerebral cortex of maternally stressed Gad1-heterozygous mouse offspring. Transl Psychiatry. 2014;4:e371. doi: 10.1038/tp.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsankova NM, 1, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–25. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 57.Roth TL, Zoladz PR, Sweatt JD, Diamond DM. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. J Psychiatr Res. 2011;45:919–26. doi: 10.1016/j.jpsychires.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology. 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- 59.Lewis DA, González-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 60.Meek LR, 1, Dittel PL, Sheehan MC, Chan JY. Kjolhaug SREffects of stress during pregnancy on maternal behavior in mice. Physiol Behav. 2001;72:473–9. doi: 10.1016/s0031-9384(00)00431-5. [DOI] [PubMed] [Google Scholar]
- 61.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong E, Chen Y, Gavin DP, Grayson DR, Guidotti A. Valproate induces DNA demethylation in nuclear extracts from adult mouse brain. Epigenetics. 2010;5:730–735. doi: 10.4161/epi.5.8.13053. [DOI] [PubMed] [Google Scholar]
- 63.Guidotti A, Dong E, Kundakovic M, Satta R, Grayson DR, Costa E. Characterization of the action of antipsychotic subtypes on valproate-induced chromatin remodeling. Trends Pharmacol Sci. 2009;30:55–60. doi: 10.1016/j.tips.2008.10.010. [DOI] [PubMed] [Google Scholar]