Abstract
Aim
Demonstrate that periadventitial delivery of adipose-derived mesenchymal stem cells (ADMSCs) slows aneurysm progression in an established murine elastase-perfusion model of abdominal aortic aneurysm (AAA).
Materials & methods
AAAs were induced in C57BL/6 mice using porcine elastase. During elastase perfusion, a delivery device consisting of a subcutaneous port, tubing and porous scaffold was implanted. Five days after elastase perfusion, 100,000 ADMSCs were delivered through the port to the aorta. After sacrifice at day 14, analyzed metrics included aortic diameter and structure of aortic elastin.
Results
ADMSC treated aneurysms had a smaller diameter and less fragmented elastin versus saline controls.
Conclusion
Periadventitial stem cell delivery prevented the expansion of an established aneurysm between days 5 and 14 after elastase perfusion.
Keywords: abdominal aortic aneurysm, adipose-derived stem cells, elastin, regeneration
Background
Abdominal aortic aneurysm (AAA) rupture was the cause of mortality in over 11,000 cases in 2008 in the USA [1]. Small AAAs grow slowly, and the disease can take years to reach a size when surgical intervention is recommended (>5.5 cm diameter) which is the placement of a synthetic graft to physically exclude the aneurysmal aorta from the pulsatile pressure blood flow. Surgical intervention does not benefit small AAAs [2], and management of these patients is limited to ‘watchful waiting’ (i.e., serial imaging of the AAA progression until the threshold for surgical treatment is met). Additionally, the use of pharmaceutical treatments that narrowly target specific pathways of the disease to alter the progression of small AAAs has not been proven effective [3–6].
The process of aneurysmal enlargement is believed to be the result of changes to the load-bearing medial and adventitial extracellular matrix – accelerated degeneration, remodeling and ineffective matrix maintenance/repair are all involved. While some of the matrix changes may be related to damage due to mechanical loading or systemic activities (e.g., oxidative damage), most of the changes to the aortic matrix are likely the result of abnormal local cellular processes. These processes include the elaboration of proteases, remodeling related to neovascularization, loss of normal matrix maintenance functions by smooth muscle cells (SMCs), and likely other undefined effects [7, 8].
The large numbers of inflammatory cells (both chronic and acute) in the media of aneurysmal, but not normal or atherosclerotic, aortic tissue has received much of the research focus. However, it remains unknown whether this is a primary or a secondary phenomenon of aneurysmal degeneration. Indirectly, the failure of powerful nonspecific anti-inflammatory medications (e.g., those used in association with transplantation) to prevent or slow aneurysm growth suggests that inflammation may not be the primary process causing the progressive matrix degeneration. Recent evidence directly evaluating human aortic tissue suggests that the SMC may be playing a major role in the matrix changes in the aortic wall [9]. Therefore, it may be necessary to alter or replace the function of the SMC in the aortic wall in order to successfully modify the aneurysmal process. For this reason, AAAs represent an optimal target for regenerative mesenchymal stem cell (MSC)-based therapy.
MSCs have the ability to provide functions that would promote aortic matrix stability. They can secrete growth factors [10,11] that could suppress inflammation and protease activity while stimulating elastin and collagen production. MSCs can also differentiate, thus providing a means to replace lost or dysfunctional SMCs. Furthermore, MSCs have already shown promise as a treatment for AAAs in elastase-perfused animal models when delivered systemically and by direct injection into the aortic wall immediately after an elastase insult. Systemic delivery showed a reduction in the inflammatory response suggesting a paracrine mechanism of action [12]. Direct injection displayed MSC engraftment into the aneurysm wall allowing for the possibility of MSC differentiation [13].
These approaches have provided proof-of-concept evidence, evaluating the effects of MSC treatment of AAA. However, prior studies employed therapeutic delivery methods and timings that are not ideal for clinical translation. Systemic delivery or direct wall injection of a stem cell-based AAA therapy would encounter practical resistance at the clinic. Essentially, all AAA patients present with an intraluminal thrombus (ILT) [14,15], a collection of clotting material and cells adherent to the luminal side of the aneurysm wall. The ILT presents a physical barrier to systemically delivered treatment cells as few cells are found deeper than 10% into the ILT [16]. Additionally, the ILT seems to entrap blood-borne cells [16], thus systemic delivery may result in low delivery efficiency to the load-bearing media and adventitia. Systemic delivery of stem cells also poses the threat of delivery to unintended locations. Direct injection of treatment cells avoids the problems encountered by systemic delivery. However, direct physical injury to the weakened and pressurized AAA wall may result in unintended consequences for aortic stability in the short-term. Additionally, therapeutic stem cells in the previous studies were administered immediately after initiation of the experimental aneurysms. The AAA is a complex disease that takes years to fully develop to the stage where clinical diagnosis can be made. In humans, the exact moment when critical amounts of elastin are lost and the disease will inevitably become clinically manifest is unknown. Therefore, any clinical stem cell-based therapy would be delivered only after the disease is clearly clinically identified, unlike the current experimental treatment models. The lapse between disease onset and diagnosis presents both the need and an opportunity for an alternate therapeutic model.
The objective of this study was to explore a possible therapeutic model using localized, periadventitial delivery of MSCs to an established and expanding aneurysm in a murine AAA model.
Methods
Cell source & culture
OriCell™ C57BL/6 green fluorescent protein (GFP)-labeled adipose-derived mouse MSCs (ADMSCs) were purchased commercially (Cyagen Biosciences Inc., CA, USA). The ADMSCs were prepared according to the manufacturer's protocols. Briefly, the ADMSCs were cultured at 37°C and 5.0% CO2 with OriCell™ adipose-derived stem cell growth medium (10% fetal bovine serum, 1% penicillin–streptomycin, 1% glutamine; Cyagen Biosciences Inc., CA, USA). The ADMSCs were used between passages 6 and 10. Media changes were performed every 2–3 days. Once the ADMSCs were approximately 80–90% confluent, the cells were washed three times in phosphate-buffered saline and then incubated with Trypsin-EDTA (Gibco, Life Technologies, NY, USA) solution for 5 min to remove them from the flasks.
Experimental animals
All mice used in the experiments were commercially obtained C57BL/6 inbred strain mice (Jackson Labs, ME, USA). Animals were housed in a controlled animal facility, and all mouse care and treatment occurred under protocols approved by the Washington University School of Medicine Animal Studies Committee.
Elastase perfusion model
Adult male mice, which consistently form larger AAAs compared with female mice [17], were subjected to transient elastase perfusion of the abdominal aorta as described previously [6,18 – 21]. Briefly, after sedation and sterile preparation, a midline laparotomy was made to expose the peritoneum. Once abdominal contents were displaced in moistened gauze, a small incision was made in the mouse's right retro-peritoneal muscle. Forceps created a subcutaneous space, and then a subcutaneous microport (Instech, PA, USA) connected to a polyurethane catheter tubing (Braintree Scientific, MA, USA) was attached and placed in the retroperitoneal space. The exposed tubing was set aside to proceed with dissection of the infrarenal aorta. The surrounding tissues were cleaned peri-aortically, and the aortic diameter was measured under magnification with a micrometer. A segment of infrarenal aorta was isolated, and a 5-min perfusion was performed through an arteriotomy at 100 mmHg with a solution containing type I porcine pancreatic elastase (0.16 U/ml; Sigma-Aldrich, MO, USA). All of the experiments were performed with a single porcine pancreatic elastase preparation derived from the same commercial source and lot. Following aortic perfusion the arteriotomy was repaired, an Ivalon sponge (5 × 8 mm) was connected to the end of the set aside tubing, and the sponge was tacked in place over the aorta (Figure 1). The incision was closed, and the animal was allowed to completely recover before returning to standard housing. The animals were maintained in standard housing with ad libitum access to standard food and water for 5 or 14 days. The described animal experiments have been approved by the Animal Studies Committee and the Institutional Animal Care and Use Committee at Washington University (MO, USA).
Figure 1. Elastase perfusion and localized adipose-derived mesenchymal stem cells treatment.
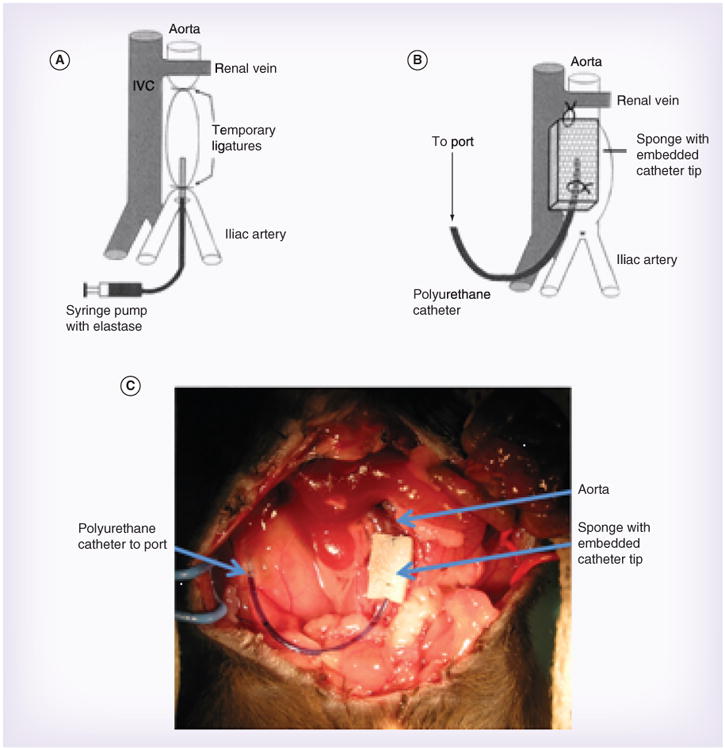
(A) Schematic representing elastase perfusion. (B) Schematic demonstrating our delayed, localized delivery system. (C) Photograph of delayed, localized delivery system in place after elastase perfusion.
(A & B) adapted with permission from [22].
The study included one experimental group and two control groups. See Figure 2 for descriptions and timings of experimental groups.
Figure 2. Experimental and control groups.
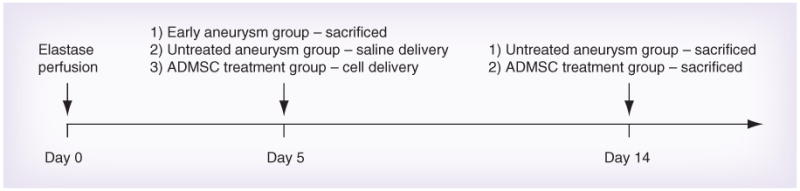
For experimental consistency, all animals had the local delivery sponge in place, and the elastase perfusion surgery (denoting day 0) was performed on all groups. On day 5, post-elastase perfusion (D5 post-EP) animals were sacrificed in order to demonstrate successful aneurysm induction (n = 3, early aneurysm group). On D5 post-EP, treatment group animals were given stem cell therapy (1 × 105 ADMSCs suspended in 400 μl of saline were delivered via port injection) and were sacrificed on D14 post-EP (n = 9, seven animals survived to D14 post-EP, local ADMSCs treatment group). On D5 post-EP, untreated control group animals were given saline (400 μl of saline was delivered via port injection) and were sacrificed on D14 post-EP (n = 6, untreated aneurysm group).
ADMSC: Adipose-derived mesenchymal stem cell.
Final aortic diameter measurement & specimen collection
Two weeks following elastase perfusion, the mice were again anesthetized and the laparotomy incision was reopened. Final aortic diameter was measured in vivo prior to sacrifice under magnification with a micro meter in the same manner as pre-elastase perfusion. Animals were euthanized, and the entire perfused segment of aorta was harvested for further analysis.
Histology
Aortic specimens were formalin fixed for 24 h before being preserved via paraffin embedding for future histological analysis. Paraffin-embedded tissue blocks were sectioned using a microtome at 5-μm thickness. Before staining, sections were deparaffinized and rehydrated by consecutive washes in xylene, alcohol and de-ionized water. Cross-sections of the aortic wall were stained with Verhoeff–Van Gieson (VVG) stain for elastin as well as hematoxylin and eosin to identify cellular composition.
Immunofluorescence
Rehydrated sections were blocked with 5% goat serum and incubated with primary mouse recombinant elastin antibody (polyclonal, 1:1000, generous gift from RP Mecham, Washington University [23]) overnight. Sections were then incubated with Alexa 647-conjugated goat anti-rabbit antibody (Molecular Probes, Life Technologies, NY, USA) followed by counterstaining with 4′,6-diamidino-2-phenylindole and imaged on a fluorescent microscope (Olympus, Provis 1, Center for Biological Imaging, University of Pittsburgh, PA, USA).
Multi-photon imaging
Unstained specimens were imaged using a multi-photon microscope (Olympus, Model FV10) to observe elastin fiber arrangement. Samples were excited at 790 nm wavelength, and elastin was detected according to intrinsic fluorescence wavelength (525 ± 25 nm).
Statistics
A two-way analysis of variance was conducted on aortic diameter measurement between animal groups. Statistical significance was assigned to p-values <0.05. Tukey tests were performed to determine which groups differed.
Results
Local periadventitial stem cell delivery halts AAA dilation
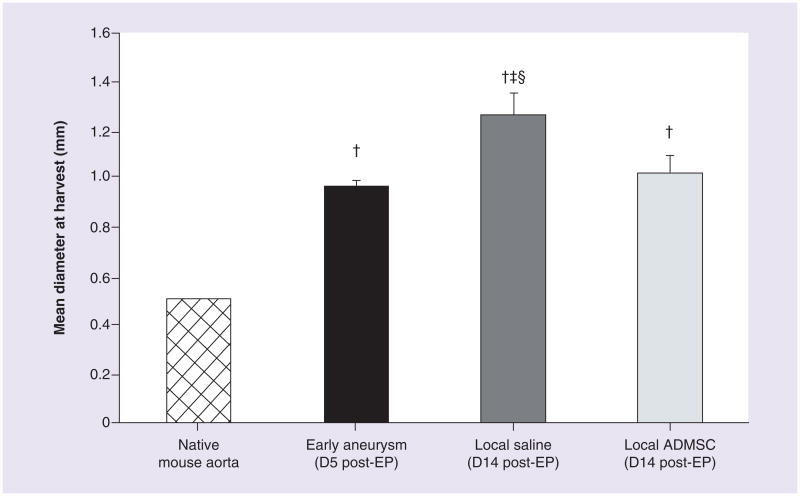
Five days after elastase perfusion, the artery dilates to double the original diameter. At this point, when the aneurysm has already been established, either saline or ADMSCs were delivered through the treatment port. The untreated (saline) aneurysm group had a larger diameter than the early aneurysm group indicating that the untreated aneurysm continued to enlarge (Figure 3). By contrast, the group treated with ADMSCs demonstrated an aortic diameter equivalent to the early aneurysm group (and smaller than the untreated group) indicating that the expansion of the AAA had essentially been halted at the time of ADMSC treatment.
Figure 3. Progression of aneurysm is halted with local adipose-derived mesenchymal stem cells treatment.
Aortic diameter measurements (mean ± standard deviation) for native aortas prior to EP (0.50 ± 0.00 mm, n = 18, hatched bar), early aneurysm group (0.97 ± 0.03 mm, n = 3, black bar), untreated aneurysm group (1.26 ± 0.09 mm, n = 6, dark grey bar), and local ADMSCs treatment group (1.02 ± 0.06 mm, n = 7, light grey bar). A two-way analysis of variance revealed unequal means (p < 0.001) between groups. Tukey tests revealed which groups differed.
†Group different from native aorta.
‡Group different from early aneurysm group.
§Group different from local ADMSC treatment group.
ADMSC: Adipose-derived mesenchymal stem cell; D: Day; EP: Elastase perfusion.
Qualitative assessment of elastin structure & monocyte infiltration
VVG staining is shown in Figure 4. Qualitative examination of the imaged sections revealed less disruption of the elastic lamella in the local ADMSC treatment group when compared with the untreated aneurysm group. This is most apparent with VVG staining where elastin fiber breaks are highlighted by red arrows. The elastic fibers look similar between the early aneurysm group and the local ADMSC treatment group indicating that the delivery of ADSMCs is associated with preserved elastin integrity at the time of ADMSC treatment. Elastin autofluorescence and immunofluorescent staining confirmed the VVG results (Supplementary Figure 1; see online at www.futuremedicine.com/doi/full/10.2217/rme.14.61).
Figure 4. Qualitative examination of elastin.
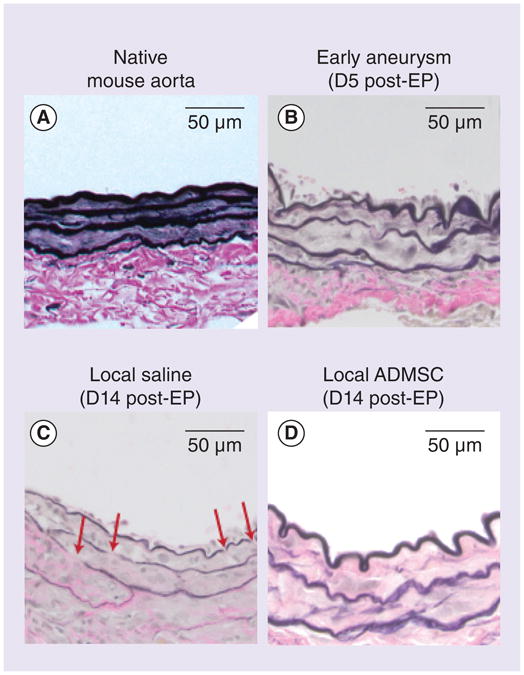
Images from native aorta, early aneurysm, untreated aneurysm and local ADMSC treatment groups are shown after Verhoeff–Van Gieson staining (n = 2 all groups).
ADMSC: Adipose-derived mesenchymal stem cell; D: Day; EP: Elastase perfusion.
Aneurysm progression in this model is mediated by inflammation – inflammatory cells are recruited by elastin degradation peptides and actively contribute to further matrix degradation. In our study, moderately severe inflammation was apparent at day 5 (note the presence of mononuclear cells in Figure 5). At day 14, the presence of mononuclear cells decreased, but no significant difference was seen between treatment groups.
Figure 5. Monocyte infiltration of the abdominal aortic aneurysm is not significantly reduced with adipose-derived mesenchymal stem cells delivery.
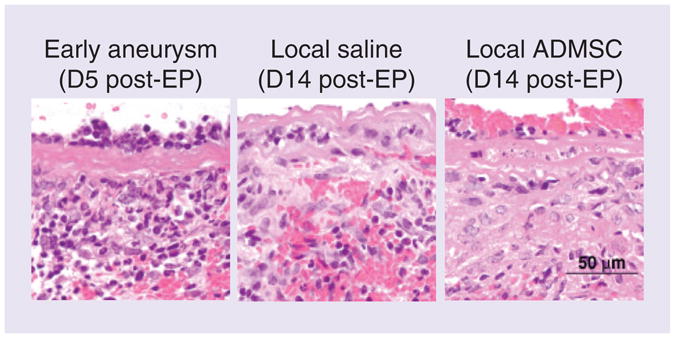
Images from early aneurysm, untreated aneurysm and local ADMSC treatment groups are shown after staining with hematoxylin and eosin (n = 2 all groups).
ADMSC: Adipose-derived mesenchymal stem cell; D: Day; EP: Elastase perfusion.
Discussion
Local ADMSC treatment halted aortic diameter enlargement at the time of cell delivery. It has been estimated that if enlargement rates of small aneurysms (<4.0 cm diameter in humans) could be reduced by even 50%, the need for surgical intervention could be delayed by 10 years, thus preventing the need for intervention in many patients [24].
From a qualitative perspective, ADMSC treatment preserved the structure of elastic lamellae at levels comparable to the time of cell delivery, although quantitative analysis has not been performed. This could indicate a role for ADMSC in preventing elastin degradation or promoting elastic fiber production. Elastin degradation is both a hallmark of a developed AAA and an active recruiter of inflammatory cells [25] that continue the AAA destructive cycle. Preserving elastin integrity, and thus decreasing inflammation, could halt AAA progression. In our study, we cannot conclusively state that ADMSC diminished the inflammatory response, but they could theoretically have offset any monocyte-derived elastase activity. Alternately, and not exclusive, to preventing degradation, therapeutic cells could stimulate elastin production. Elastic fibers can be produced by human vascular SMCs in vitro when stimulated with TGF-β1 [26]; this growth factor and others with the potential for stimulating elastogenesis are secreted by ADMSCs [11]. Therefore, ADMSCs could stimulate repair by native vascular SMCs.
The macroscopic results of periadventitial stem cell delivery are similar to the results shown by Sharma et al. [12] where the systemic delivery of MSCs reduced the rate of AAA progression and preserved elastin lamella integrity in elastase-perfused mice. Our study extends the work of Sharma et al. by demonstrating that the macroscopic benefits of stem cell therapy are accessible to established and expanding aneurysms and not limited to attenuating the inflammation response immediately following elastase perfusion. Our study also shows that periadventitial delivery of a stem cell therapy is effective and may avoid potential problems of systemic delivery such as unintended stem cell migration and engraftment. It also focuses the cells on the anatomically defined segment of the aorta affected by the disease and circumvents the physical barriers presented by endothelium, atherosclerotic plaque and ILT. This an important finding in the development of treatments for patients with an identified AAA, of which 90% are smaller than the size recommended for surgical repair (5.5 cm) [27].
We were unable to confirm treatment cell engraftment into the wall as demonstrated by Turnbull et al. [13] who used utilized direct injection of treatment cells into the aortic wall as well as intraluminal infusion. The differences in cellular delivery technique and total number of cells delivered (our study used 10- and 100-times less cells than Sharma et al. and Turnbull et al., respectively) may account for the differences in treatment cell engraftment between our study and the study performed by Turnbull et al.
Although this study yielded exciting results, it does have limitations. This proof-of-concept study was designed to be a short-term study. While the murine elastase-perfused aneurysm does not expand after our chosen end point of 14 days [22], the human aneurysm expands progressively. Longer studies will need to be completed in order to understand the long term effects of our treatment. Additionally, future studies will investigate the progression of the disease in real-time by sacrificing animals at more frequent intervals and utilizing noninvasive imaging, such as ultrasound or micro-CT/micro-MRI.
In this study, we had sought to develop and show proof-of-concept for an alternative therapeutic model for the treatment of AAAs. A localized, periadventitial route of stem cell therapy administration avoids the drawbacks of both systemic delivery (e.g., uncertain destination of cells and presence of ILT) and local delivery via direct injection into a weakened aneurysmal wall. This model also allows for initiation of therapy at any point in the development of the model aneurysm. A final advantage of our approach is the use of MSCs sourced from adipose tissue. ADMSCs are a very attractive clinical source of stem cells due to the ease of obtaining adipose tissue from donors seeking liposuction treatment and the high yield of MSCs from adipose tissue [28,29]. Our study revealed how ADMSCs can alter the progression of an already established and expanding aneurysm while others have shown the ADMSCs have immunomodulatory properties [30].
Conclusion
We have developed an animal model for delayed, periadventitial delivery of ADMSCs to ameliorate elastase-induced AAA. Delayed, periadventitial delivery of ADMSCs halted two aspects of aneurysm progression – expansion of the aortic diameter and fragmentation of the elastic lamella. This work represents an important step towards developing clinically realistic stem cell therapies for AAA patients.
Future perspective
Since current stem cell therapies have proven effective at modulating the inflammatory response (which should limit matrix degradation) the critical need in the next 10 years of research is to achieve elastin regeneration in situ [31]. Once this is accomplished, we envision a paradigm shift in AAA treatment methodology to a minimally invasive, localized delivery of stem cells embedded within a material capable of providing transient mechanical support to the weakened AAA tissue. With this new technology, patients with small AAAs will have a new chance at life free of AAA progression.
Supplementary Material
Executive summary.
A paradigm shift is needed for treating established, expanding, abdominal aortic aneurysm
Current surgical treatments for abdominal aortic aneurysm (AAA) do not address the cause of enlargement, and are only suitable for patients with large or rapidly expanding aneurysms. 90% of patients identified with AAAs do not meet the criteria for surgery.
Stem cell therapies for AAAs have shown promise in murine and porcine elastase perfusion AAA models, but the treatment strategies need to be further developed for clinical translation.
A proposed solution: periadventitial stem cell therapy
We developed a unique cellular delivery system that allowed for a durable effect after a single dose of local, periadventitial delivery of adipose-derived mesenchymal stem cells (ADMSCs) to an established and expanding murine elastase induced AAA.
The method could be translated to human use via retroperitoneoscopic or translumbar percutaneous techniques to the peri-aneurysmal retroperitoneum.
Our cellular delivery method also avoids problems associated with systemic delivery and allows for stem cell therapy to an established aneurysm.
Stem cell delivery preserves aortic diameter & elastin
Macroscopically, treating the AAAs with 1 × 105 ADMSCs 5 days after elastase perfusion halted the enlargement of the aortic diameter at the time of treatment. Untreated controls continued to enlarge by approximately 30%.
Microscopically, treating the AAAs with 1 × 105 ADMSCs 5 days after elastase perfusion preserved the integrity of the elastic lamella at the time of treatment. Untreated controls showed fragmented elastic lamella.
Acknowledgments
The authors would like to thank the Center for Biologic Imaging at the University of Pittsburgh for their technical assistance with acquiring multi-photon and immunofluorescence mages, and Jayashree Rao for her staining and imaging of mmunofluorescence samples. They would also like to thank Kacey Marra for her consultation about the use and sourcing of ADMSCs.
Footnotes
Financial & competing interests disclosure: The authors would like to disclose funding support by the National Institutes of Health (Cardiovascular Bioengineering Training Program T32 HL076124 and Biomechanics in Regenerative Medicine T32 EB000392 to KJ Blose, HL086418 to DA Vorp, AG037120 to JA Curci) and the Periphera Vascular Surgical Society (Academic Award 2012-2013 to JA Curci). This material is based upon work supported (or supported in part) by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (JA Curci). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research: The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
References
Papers of special note have been highlighted as:
••of considerable interest
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics – 2012 update a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenhalgh R, Forbes J, Fowkes F, et al. Early elective open surgical repair of small abdominal aortic aneurysms is not recommended: results of the UK Small Aneurysm Trial Steering Committee. Eur J Vasc Endovasc. 1998;16(6):462. doi: 10.1016/s1078-5884(98)80234-7. [DOI] [PubMed] [Google Scholar]
- 3.Baxter BT. Could medical intervention work for aortic aneurysms? Am J Surg. 2004;188(6):628–632. doi: 10.1016/j.amjsurg.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Baxter BT, Pearce WH, Waltke EA, et al. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (Phase II) multicenter study. J Vasc Surg. 2002;36(1):1–12. doi: 10.1067/mva.2002.125018. [DOI] [PubMed] [Google Scholar]
- 5.Mosorin M, Juvonen J, Biancari F, et al. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J Vasc Surg. 2001;34(4):606–610. doi: 10.1067/mva.2001.117891. [DOI] [PubMed] [Google Scholar]
- 6.Thompson R, Liao S, Curci J. Therapeutic potential of tetracycline derivatives to suppress the growth of abdominal aortic aneurysms. Adv Den Res. 1998;12(1):159–165. doi: 10.1177/08959374980120011301. [DOI] [PubMed] [Google Scholar]
- 7.Phillippi JA, Pasta S, Vorp DA. Biomechanics and pathobiology of aortic aneurysms. In: McGloughlin T, editor. Biomechanics and Mechanobiology of Aneurysms. Springer; 2011. pp. 67–118. [Google Scholar]
- 8.Vorp DA, Lee PC, Wang DH, et al. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg. 2001;34(2):291–299. doi: 10.1067/mva.2001.114813. [DOI] [PubMed] [Google Scholar]
- 9.Airhart N, Brownstein BH, Cobb JP, et al. Smooth muscle cells from abdominal aortic aneurysms are unique and can independently and synergistic ally degrade insoluble elastin. J Vasc Surg. 2013;60(4):1033–1041. doi: 10.1016/j.jvs.2013.07.097. discussion 1041–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinnaird T, Stabile E, Burnett M, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94(5):678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 11.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 12••.Sharma AK, Lu G, Jester A, et al. Experimental abdominal aortic aneurysm formation ismediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation. 2012;126(11 Suppl. 1):S38–S45. doi: 10.1161/CIRCULATIONAHA.111.083451. Mesenchymal stem cells were delivered systemically to a mouse immediately following elastase perfusion. The inflammatory response was attenuated, and the aortic diameter was reduced compared with untreated controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Turnbull IC, Hadri L, Rapti K, et al. Aortic implantation of mesenchymal stem cells after aneurysm injury in a porcine model. J Surg Res. 2011;170(1):e179. doi: 10.1016/j.jss.2011.05.042. Mesenchymal stem cells were directly injected into the wall of an elastase-induced abdominal aortic aneurysm (AAA) in a porcine model. Treatment cells remained engrafted into the wall for up to 1 week. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hans SS, Jareunpoon O, Balasubramaniam M, Zelenock GB. Size and location of thrombus in intact and ruptured abdominal aortic aneurysms. J Vasc Surg. 2005;41(4):584–588. doi: 10.1016/j.jvs.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Palazzuoli A, Gallotta M, Guerrieri G, et al. Prevalence of risk factors, coronary and systemic atherosclerosis in abdominal aortic aneurysm: comparison with high cardiovascular risk population. Vasc Health Risk Manag. 2008;4(4):877. doi: 10.2147/vhrm.s1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randall A, Vorp DA, Steed DL, Webster MW, Kameneva MV, Watkins SC. Cellular content and permeability of intraluminal thrombus in abdominal aortic aneurysm. J Vasc Surg. 1997;25(5):916–926. doi: 10.1016/s0741-5214(97)70223-4. [DOI] [PubMed] [Google Scholar]
- 17.Ailawadi G, Eliason JL, Roelofs KJ, et al. Gender differences in experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2004;24(11):2116–2122. doi: 10.1161/01.ATV.0000143386.26399.84. [DOI] [PubMed] [Google Scholar]
- 18.Bergoeing MP, Arif B, Hackmann AE, Ennis TL, Thompson RW, Curci JA. Cigarette smoking increases aortic dilatation without affecting matrix metalloproteinase-9 and-12 expression in a modified mouse model of aneurysm formation. J Vasc Surg. 2007;45(6):1217–1227. doi: 10.1016/j.jvs.2007.01.058. [DOI] [PubMed] [Google Scholar]
- 19.Colonnello JS, Hance KA, Shames ML, et al. Transient exposure to elastase induces mouse aortic wall smooth muscle cell production of MCP-1 and RANTES during development of experimental aortic aneurysm. J Vasc Surg. 2003;38(1):138–146. doi: 10.1016/s0741-5214(03)00125-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee JK, Borhani M, Ennis TL, Upchurch GR, Thompson RW. Experimental abdominal aortic aneurysms in mice lacking expression of inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2001;21(9):1393–1401. doi: 10.1161/hq0901.095750. [DOI] [PubMed] [Google Scholar]
- 21.Pyo R, Lee JK, Shipley JM, et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105(11):1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22••.Bartoli MA, Parodi FE, Chu J, et al. Localized administration of doxycycline suppresses aortic dilatation in an experimental mouse model of abdominal aortic aneurysm. Ann Vasc Surg. 2006;20(2):228–236. doi: 10.1007/s10016-006-9017-z. Doxycycline was administered to murine elastase perfused AAA through the use of a subcutaneous pump, polyurethane catheter, and sponge placed on the anterior of the aorta. [DOI] [PubMed] [Google Scholar]
- 23.Kozel BA, Ciliberto CH, Mecham RP. Deposition of tropoelastin into the extracellular matrix requires a competent elastic fiber scaffold but not live cells. Matrix Biol. 2004;23(1):23–34. doi: 10.1016/j.matbio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Klink A, Hyafil F, Rudd J, et al. Diagnostic and therapeutic strategies for small abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8(6):338–347. doi: 10.1038/nrcardio.2011.1. [DOI] [PubMed] [Google Scholar]
- 25.Cohen JR, Keegan L, Sarfati I, Danna D, Ilardi C, Wise L. Neutrophil chemotaxis and neutrophil elastase in the aortic wall in patients with abdominal aortic aneurysms. Invest Surg. 1991;4(4):423–430. doi: 10.3109/08941939109141172. [DOI] [PubMed] [Google Scholar]
- 26.Lin S, Sandig M, Mequanint K. Three-dimensional topography of synthetic scaffolds induces elastin synthesis by human coronary artery smooth muscle cells. Tissue Eng Part A. 2011;17(11–12):1561–1571. doi: 10.1089/ten.TEA.2010.0593. [DOI] [PubMed] [Google Scholar]
- 27.Lederle F, Johnson G, Wilson S, et al. Aneurysm detection and management (adam) veterans affairs cooperative study group Prevalence and associations of abdominal aortic aneurysm detected through screening. Ann Int Med. 1997;126(6):441–449. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
- 28.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189(1):54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 29.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 30.Hashemi SM, Hassan ZM, Pourfathollah AA, Soudi S, Shafiee A, Soleimani M. Comparative immunomodulatory properties of adipose-derived mesenchymal stem cells conditioned media from BALB/c, C57BL/6, and DBA mouse strains. J Cell Biochem. 2013;114(4):955–965. doi: 10.1002/jcb.24437. [DOI] [PubMed] [Google Scholar]
- 31••.Bashur CA, Rao RR, Ramamurthi A. Perspectives on stem cell-based elastic matrix regenerative therapies for abdominal aortic aneurysms. Stem Cells Transl Med. 2013;2(6):401–408. doi: 10.5966/sctm.2012-0185. The ideal treatment for AAA is discussed with regard to stem cell-based therapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.