Abstract
Objective
Pathologic evaluation of aortocaval nodes in patients with locally advanced cervical cancer in an effort to better tailor radiotherapy has gained popularity. We sought to determine which aortocaval nodes should be sampled during surgical staging procedures.
Methods
From 2004 to 2011, 246 patients with locally advanced cervical cancer underwent positron emission tomography (PET) before definitive chemoradiation. We reviewed the imaging studies to determine the location of PET-positive aortocaval nodes in relationship to the inferior mesenteric artery (IMA).
Results
Forty-two patients (17%) had PET images suggesting aortocaval metastasis. Ten patients had stage IB, 1 had stage IIA, 13 had stage IIB, 13 had stage IIIB, and 5 had stage IV disease. Of these 42 patients, 39 (93%) had FDG-avid pelvic nodes, 1 (2%) had PET-negative pelvic nodes but FDG-avid common iliac nodes, and 2 (5%) had direct spread to the aortocaval nodes. Three patients (7%) had FDG-avid aortocaval nodes above the IMA without FDG-avid nodes between the aortic bifurcation and IMA. All 3 of these patients also had FDG-avid nodes in the pelvis. Nineteen patients (45%) had FDG-avid nodes above and below the IMA, and 20 (48%) had FDG-avid nodes below the IMA only.
Conclusions
This hypothesis-generating study revealed that a small number of patients have PET-positive aortocaval nodes above the IMA only. For patients undergoing surgical staging for locally advanced cervical cancer, dissection to the renal vessels may be necessary. A future international, randomized study will prospectively evaluate the locations of pathologically positive aortocaval lymph nodes.
Keywords: cervical cancer, surgical staging, lymph nodes
Introduction
Metastasis to regional lymph nodes is the most important adverse prognostic factor for patients with cervical cancer. On pathologic examination, one-quarter of women with locally advanced cervical cancer (stages IB2-IVA) have metastatic disease in the aortocaval lymph nodes [1]. In addition to having implications for survival, the status of the aortocaval nodes determines whether a patient should receive only pelvic or extended-field radiation therapy as primary therapy. Unfortunately, current imaging modalities are poor at detecting metastatic disease in the aortocaval nodal basins. The most sensitive radiologic test commercially available today, positron emission tomography–computed tomography (PET-CT), reportedly has sensitivity of only 84% in detecting metastatic disease in aortocaval nodes [2].
For that reason, many investigators now support surgical staging for women with locally advanced cervical cancer. Although there is debate about the value of sampling pelvic lymph nodes in women with locally advanced cervical cancer [3, 4], most advocates of surgical staging maintain the importance of aortocaval nodal sampling in an effort to precisely define the size of the radiation field (pelvic vs. extended-field) [1, 3-6]. Universally these investigators describe a nodal dissection to the renal vessels. However, dissection to the renal vessels is technically more difficult than limiting the upper border of the dissection to the inferior mesenteric artery and adds operative risk. In addition, removing the nodal tissue above the inferior mesenteric artery (IMA) increases operative time and requires more pathologic specimen processing, both of which increase health care cost.
The lymphatic drainage of the cervix is thought to follow the uterine vessels through the parametrium to the obturator, internal iliac, and external iliac basins in the pelvic sidewall. Although there are reports of isolated aortocaval nodal metastases above the IMA [7], most hold that disease spreads first to the pelvic nodes and then cephalad up the nodal chains along the great vessels. Therefore, performing an infrarenal aortocaval lymphadenectomy as opposed to an inframesenteric dissection seems unjustified. In an effort to generate preliminary data about whether an infrarenal nodal dissection is warranted in women with locally advanced cervical cancer, we evaluated the location of radiologically positive (18-fluorine fluorodeoxyglucose [FDG]-avid) aortocaval nodes with respect to the IMA (i.e., below the IMA, above the IMA, or both).
Materials and Methods
This study was conducted with approval from the Institutional Review Board at The University of Texas MD Anderson Cancer Center. We reviewed the records of all patients who presented to MD Anderson with a new diagnosis of untreated advanced cervical cancer (1988 International Federation of Gynecology and Obstetrics stages IB2-IVB) from July 1, 2004, through February 28, 2011, and underwent PET-CT. The initial date of July 1, 2004, was chosen because this was when we began to routinely perform PET-CT in patients prior to initiation of definitive chemoradiation. From this group of patients, we selected the women who had PET-CT images suggestive of metastatic disease in the aortocaval region.
All PET-CT scans from patients with FDG-avid aortocaval nodes were re-reviewed by a single nuclear medicine radiologist (H.A.M.). On review of PET-CT scans, this radiologist evaluated the presence or absence of pelvic spread, presence or absence of non-nodal metastases, and location of FDG-avid aortocaval nodes with respect to the IMA (i.e., below the IMA, above the IMA, or both). Clinical records were then reviewed. Demographic and clinical information, including age at the time of diagnosis, race and ethnicity, and tumor characteristics, were obtained from medical records.
All PET-CT scans were performed on a dedicated PET-CT scanner that allowed fusion of PET and CT images. Standard imaging protocols for PET-CT were followed, and noncontrast CT was used for attenuation correction. The standard definition of abnormal lymph nodes in the abdomen and pelvis on cross-sectional imaging was lymph node size greater than 10 mm. PET-CT images were evaluated qualitatively for focal areas of abnormally increased FDG uptake. A positive finding was defined as moderately to markedly increased uptake of FDG relative to the uptake in comparable normal structures or surrounding tissues, with the exclusion of physiologic bowel and urinary activity. A negative finding was defined as no detectable FDG uptake.
Data are presented here in a descriptive manner. Missing data were coded as “unknown,” and those data points were excluded from the analysis. All data were collected and analyzed using SPSS 17 for Windows (SPSS, Inc., Chicago, IL).
Results
From July 1, 2004, through February 28, 2011, 246 patients with advanced cervical cancer underwent PET-CT. Of these, 42 (17%) had FDG-avid nodes in the aortocaval region. These 42 patients are the basis for this study. Demographic data for the entire cohort are listed in Table 1. The majority of patients were either Caucasian (43%) or Latina (43%) and had squamous lesions (81%) that were either moderately (45%) or poorly differentiated (48%).
Table 1. Patient characteristics (n=42).
| Age, years | |
| Median | 48.6 |
| Range | 27.4-72.6 |
| Race and ethnicity | |
| Caucasian | 18 (43%) |
| Latina | 18 (43%) |
| Black | 5 (12%) |
| Asian | 1 (2%) |
| Stage | |
| IB1 | 1 (2%) |
| IB2 | 9 (21%) |
| IIA | 1 (2%) |
| IIB | 13 (31%) |
| IIIA | 0 (0%) |
| IIIB | 13 (31%) |
| IVA | 3 (8%) |
| IVB | 2 (5%) |
| Histology | |
| Squamous | 34 (81%) |
| Adenocarcinoma | 5 (12%) |
| Undifferentiated | 2 (5%) |
| Serous | 1 (2%) |
| Grade | |
| Well differentiated | 2 (5%) |
| Moderately differentiated | 19 (45%) |
| Poorly differentiated | 20 (48%) |
| Unknown | 1 (2%) |
Of the 42 patients with FDG-avid aortocaval nodes, 39 (93%) had concurrent FDG-avid pelvic nodes (obturator, internal iliac, or external iliac), 1 (2%) had PET-negative pelvic nodes but concurrent FDG-avid common iliac nodes, and 2 (5%) had direct drainage to the aortocaval nodes below the IMA. Table 2 shows the complete distribution of FDG-avid nodes.
Table 2. Observed patterns of FDG-avid nodal basins and their frequency.
| FDG-avid nodal basins | Number of patients with this pattern (%) |
|---|---|
| Pelvic,a common iliac, and aortocaval | 30 (71) |
| Pelvica and aortocaval | 9 (21) |
| Common iliac and aortocaval | 1 (2) |
| Aortocaval only | 2 (5) |
Pelvic nodal basins include obturator, internal iliac, and external iliac basins.
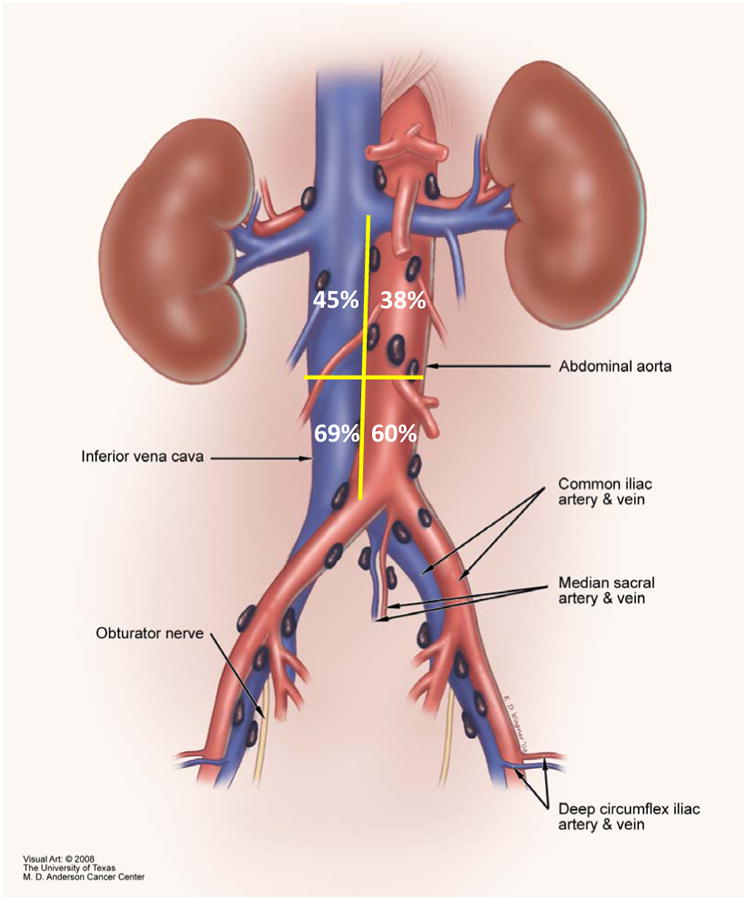
Of the 42 patients with FDG-avid aortocaval nodes, 20 (48%) had FDG-avid nodes below the IMA only, 19 (45%) had FDG-avid nodes both below and above the IMA, and 3 (7%) had isolated FDG-avid aortocaval nodes located above the IMA in the absence of FDG-avid aortocaval nodes located below the IMA. All 3 of the patients with isolated FDG-avid aortocaval nodes above the IMA also had FDG-avid nodes along the external iliac vessels. One of the 3 also had additional FDG-avid nodes along the common iliac vessels. In relationship to the great vessels, 10 patients (24%) had FDG-avid nodes along the aorta only, 15 (36%) had FDG-avid nodes along the vena cava only, and 17 (40%) had FDG-avid nodes along both the aorta and vena cava. Figure 1 shows the location of FDG-avid nodes by quadrant (above IMA along aorta, above IMA along vena cava, below IMA along aorta, and below IMA along vena cava). As 1 patient could have FDG-avid nodes in more than 1 location, the sum of all locations is greater than 100%.
Figure 1. Location of FDG-avid nodes along the great vessels in relationship to IMA.
Seven patients had PET-CT findings suggestive of non-nodal metastatic disease. These sites included peritoneal implants (n=2), ovarian implants (n=2), sigmoid disease (n=2), and boney metastasis (n=1). One of the patients with sigmoid disease also had FDG-avid inguinofemoral nodes. An eighth patient had FDG-avid disease in a retrocrural node.
Fifteen (36%) of the 42 patients had pathologic assessment of the FDG-avid aortocaval nodes. Eight had a nodal biopsy performed by an interventional radiologist, and the remaining 7 were enrolled in a phase II study evaluating the sensitivity of PET-CT for women with locally advanced cervical cancer [5]. As part of this protocol, patients with locally advanced cervical cancer were scheduled to undergo laparoscopic extraperitoneal lymph node dissection after PET-CT was complete. In total, 13 (87%) of the 15 patients who underwent pathologic assessment had pathologic confirmation of metastasis to the aortocaval region. The remaining 2 patients (13%) had no pathologic evidence of disease (i.e., false-positive PET-CT scan findings).
Discussion
This hypothesis-generating study shows that 7% of patients with locally advanced cervical cancer with FDG-avid aortocaval nodes will have metastasis to nodal basins above the IMA in the absence of metastasis to nodes along the aorta and/or vena cava below the IMA. These findings call into question what many have always believed about the lymphatic drainage of the cervix—namely, that it follows the uterine vessels through the parametrium to the pelvis, the common iliac nodal basin, and, rarely, directly to the presacral and low aortic nodal basins. These traditional routes have been established with lymphatic mapping and sentinel lymph node biopsy studies in patients with early cervical cancer [8]. In those studies, there does not appear to be direct drainage to nodal basins above the IMA, which means that for disease to implant there, it must first move through the nodal basins below the IMA.
This traditional conception of drainage from the uterine cervix is in stark contrast to the lymphatic drainage of the uterine fundus, which follows not only the uterine vessels via the cervix but also the ovarian vessels with direct drainage to nodal basins above the IMA. Mapping studies in patients with endometrial cancer show that sentinel nodes are often located above the IMA. In fact, in a sentinel node study performed in women with uterine cancer at The University of Texas MD Anderson Cancer Center, no patient had a sentinel node located between the bifurcation of the aorta and the IMA [9]. These mapping results have been correlated clinically. In a prospective study, Mariani and colleagues [10] found that in patients with uterine cancer, 60% of cases of aortocaval metastases were in nodes located above the IMA only, with no disease found in the area between the bifurcation of the aorta and the IMA. The findings presented in our current study combined with previous lymphatic mapping studies in cervical cancer would suggest that a similar occurrence in patients with cervical cancer is exceedingly rare.
Despite our finding that 3 (7%) of the 42 patients in our study had aortocaval metastases above the IMA without aortocaval metastases below the IMA, we remain skeptical that direct drainage from the cervix to nodes above the IMA exists. So how might we explain the radiologic findings in these 3 patients? One possibility is that metastases ascending up the nodal chain from the pelvis “skipped” the lower aorta to implant in nodal basins above the IMA. We find this answer highly unlikely as sentinel lymph node studies in virtually every solid tumor have shown that tumor implants do not pass through sentinel nodes to implant in upper-echelon, non-sentinel nodes [11].
We believe that the most likely explanation for FDG-avid aortocaval nodes above the IMA in the absence of FDG-avid aortocaval nodes below the IMA lies in the sensitivity and specificity of PET-CT in detecting metastatic disease in aortocaval nodes. We recently published results from a prospective phase II study comparing PET-CT characterization of aortocaval nodes with the characterization of those nodes on pathologic examination after surgical resection [5]. We found that 29% of patients with FDG-avid aortocaval nodes had no evidence of disease in those nodes on pathologic processing (false-positive PET-CT findings). Of the patients with no FDG-positive nodes along the aorta and vena cava, 17% had metastatic disease detected in those nodes on pathologic processing (false-negative PET-CT findings). Thus, we believe that for the 3 patients in our current study who had isolated disease above the IMA on PET-CT, the most likely explanation is either that the FDG-avid nodes above the IMA were falsely positive or that the FDG-negative nodes below the IMA were falsely negative.
Even with the high false-positive and false-negative rates of PET-CT in detecting aortocaval spread of disease, this modality has become the standard imaging technique for evaluation of lymph nodes in locally advanced cervical cancer. In 2005, Rockall et al. [12] described their experience with nanoparticle-enhanced magnetic resonance imaging for detection of metastatic nodes in women with both uterine and cervical cancers. Using ferumoxtran-10, a lymph node-specific contrast agent composed of ultrasmall particles of iron oxide (USPIO), the authors reported a sensitivity of 82-93% and a specificity of 97% for USPIO MRI. Although this technology appears promising, it is currently unavailable having been withdrawn by the manufacturer pending phase III evaluations.[13]
Although we remain unconvinced about the possibility of direct drainage from the cervix to the aortocaval nodes above the IMA, we will continue to perform surgical staging to the renal vessels in selected patients with locally advanced cervical cancer. Traditionally, we have used the laparoscopic extraperitoneal approach. Some advocate performing a laparoscopic transperitoneal lymphadenectomy as this minimally invasive approach allows for complete pelvic and aortocaval lymphadenectomy [3]. Although we do believe that debulking large pelvic nodes is of therapeutic benefit [14, 15], we do not typically remove small pelvic nodes as they lie well within a standard radiation field and therefore will be sterilized by radiation. Our philosophy is to perform surgical staging as a means to tailor the chemoradiation field to each individual patient.
In contrast, some have proposed that patients with PET-positive pelvic nodes and negative aortocaval nodes have the field extended routinely, even in the absence of pathologic confirmation of disease. Although this will “catch” the 22% of patients who have falsely-negative PET imaging of the aortocaval region, it will overtreat the 78% who do not [5]. There does not seem to be the same enthusiasm for prophylactically extending the radiation field for patients with PET-negative pelvic nodes and PET-negative aortocaval nodes even though 12% of them will have pathologically positive aortocaval nodes [5]. That is likely because prophylactically extending the radiation field is not without risk. The incidence of grade 4 or 5 toxic effects in patients undergoing extended-field radiation therapy is 8%, and the death rate is as high as 2% [16]. In contrast, the rate of serious complications from surgical staging using the laparoscopic extraperitoneal approach is <2% [1, 5].
This study is certainly not without its limitations. First, only 36% of patients had pathologic confirmation of PET-positive aortocaval nodes. This is a large proportion of patients but may not be a representative sample, and therefore the specificity of PET in detecting aortocaval disease cannot be estimated from this retrospective study. However, our prospective phase II study did find a false-positive rate of 29% [5]. Another potential problem with the retrospective design lies in the interpretation of PET-CT scans, which can be somewhat subjective and has been associated with reports of low interobserver reliability [17, 18]. We attempted to overcome this pitfall by having all PET-CT scans reread by an attending radiologist who specializes in nuclear medicine. One might argue that PET-CT scan reading in “real life” would not meet these high standards, which we see as another argument for why surgical staging is of such importance.
In an effort to determine whether surgical staging with tailored radiation therapy leads to better overall survival and/or decreased radiation complications compared to radiation field design based on PET-CT scans, we are set to open an international phase III study comparing the 2 approaches. In this study, 480 patients with locally advanced cervical cancer with PET-positive pelvic nodes and PET-negative aortocaval nodes will be randomized to either 1) surgical staging via the laparoscopic extraperitoneal approach followed by radiation therapy with fields based on pathologic findings or 2) radiation therapy planning based on PET scan only (1:1 randomization). The surgical staging group will have all lymph-bearing tissue from the high common iliacs to the renal vessels removed and examined pathologically. Specimens from below the IMA and above the IMA will be labeled and sent separately. Therefore, in addition to survival and morbidity endpoints, we will also be able to definitively answer the question of the extent of lymphadenectomy necessary for adequate surgical staging in women with locally advanced disease.
Table 3.
Anatomic location of FDG-avid nodal basins in relationship to inferior mesenteric artery.
| Anatomic location | Number of patients with nodes at location (%) |
|---|---|
| Below IMA only | 20 (48%) |
| Above and below IMA | 19 (45%) |
| Above IMA only | 3 (7%) |
Footnotes
Disclosure: The authors have no financial conflicts of interest to disclose.
Presented at the 17th International Meeting of the European Society of Gynaecological Oncology, Milan, Italy, September 11-14, 2011
References
- 1.Leblanc E, Narducci F, Frumovitz M, et al. Therapeutic value of pretherapeutic extraperitoneal laparoscopic staging of locally advanced cervical carcinoma. Gynecol Oncol. 2007;105:304–311. doi: 10.1016/j.ygyno.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Havrilesky LJ, Kulasingam SL, Matchar DB, et al. FDG-PET for management of cervical and ovarian cancer. Gynecol Oncol. 2005;97:183–191. doi: 10.1016/j.ygyno.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Lavoue V, Bats AS, Darai E. Pelvic and para-aortic lymphadenectomy are required to stage locally advanced cervical cancer. Gynecol Oncol. 2008;109:427–428. doi: 10.1016/j.ygyno.2007.09.005. author reply 428-429. [DOI] [PubMed] [Google Scholar]
- 4.Marnitz S, Kohler C, Roth C, et al. Is there a benefit of pretreatment laparoscopic transperitoneal surgical staging in patients with advanced cervical cancer? Gynecol Oncol. 2005;99:536–544. doi: 10.1016/j.ygyno.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez PT, Jhingran A, Macapinlac HA, et al. Laparoscopic extraperitoneal para-aortic lymphadenectomy in locally advanced cervical cancer: a prospective correlation of surgical findings with positron emission tomography/computed tomography findings. Cancer. 2011;117:1928–1934. doi: 10.1002/cncr.25739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uzan C, Souadka A, Gouy S, et al. Analysis of Morbidity and Clinical Implications of Laparoscopic Para-Aortic Lymphadenectomy in a Continuous Series of 98 Patients with Advanced-Stage Cervical Cancer and Negative PET-CT Imaging in the Para-Aortic Area. Oncologist. 2011 doi: 10.1634/theoncologist.2011-0007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michel G, Morice P, Castaigne D, et al. Lymphatic spread in stage Ib and II cervical carcinoma: anatomy and surgical implications. Obstet Gynecol. 1998;91:360–363. doi: 10.1016/s0029-7844(97)00696-0. [DOI] [PubMed] [Google Scholar]
- 8.Marnitz S, Kohler C, Bongardt S, et al. Topographic distribution of sentinel lymph nodes in patients with cervical cancer. Gynecol Oncol. 2006;103:35–44. doi: 10.1016/j.ygyno.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 9.Burke TW, Levenback C, Tornos C, et al. Intraabdominal lymphatic mapping to direct selective pelvic and paraaortic lymphadenectomy in women with high-risk endometrial cancer: results of a pilot study. Gynecol Oncol. 1996;62:169–173. doi: 10.1006/gyno.1996.0211. [DOI] [PubMed] [Google Scholar]
- 10.Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18. doi: 10.1016/j.ygyno.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gipponi M. Clinical applications of sentinel lymph-node biopsy for the staging and treatment of solid neoplasms. Minerva Chir. 2005;60:217–233. [PubMed] [Google Scholar]
- 12.Rockall AG, Sohaib SA, Harisinghani MG, et al. Diagnostic performance of nanoparticle-enhanced magnetic resonance imaging in the diagnosis of lymph node metastases in patients with endometrial and cervical cancer. J Clin Oncol. 2005;23:2813–2821. doi: 10.1200/JCO.2005.07.166. [DOI] [PubMed] [Google Scholar]
- 13.Haldorsen IS, Salvesen HB. Staging of endometrial carcinomas with MRI using traditional and novel MRI techniques. Clin Radiol. 2012;67:2–12. doi: 10.1016/j.crad.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Kupets R, Thomas GM, Covens A. Is there a role for pelvic lymph node debulking in advanced cervical cancer? Gynecol Oncol. 2002;87:163–170. doi: 10.1006/gyno.2002.6815. [DOI] [PubMed] [Google Scholar]
- 15.Wharton JT, Jones HW, 3rd, Day TG, Jr, et al. Preirradiation celiotomy and extended field irradiation for invasive carcinoma of the cervix. Obstet Gynecol. 1977;49:333–338. [PubMed] [Google Scholar]
- 16.Rotman M, Pajak TF, Choi K, et al. Prophylactic extended-field irradiation of para-aortic lymph nodes in stages IIB and bulky IB and IIA cervical carcinomas. Ten-year treatment results of RTOG 79-20. JAMA. 1995;274:387–393. [PubMed] [Google Scholar]
- 17.Marom EM, Munden RF, Truong MT, et al. Interobserver and intraobserver variability of standardized uptake value measurements in non-small-cell lung cancer. J Thorac Imaging. 2006;21:205–212. doi: 10.1097/01.rti.0000213643.49664.4d. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto Y, Tsujikawa T, Kondo C, et al. Accuracy of PET for diagnosis of solid pulmonary lesions with 18F-FDG uptake below the standardized uptake value of 2.5. J Nucl Med. 2006;47:426–431. [PubMed] [Google Scholar]


