Abstract
The primary purpose of this study was to examine motor physiology disturbances in a group of patients with untreated major depressive disorder using sensitive instrumental procedures. The secondary aim of the study was to examine the relationship of the affective symptom state to these motor assessments. The authors studied 40 individuals meeting DSM-IV criteria for unipolar major depressive disorder and 40 healthy comparison subjects. Electromechanical measures of force steadiness (FS), simple reaction time (RT), movement time (MT) and scaling of movement velocity to distance (velocity scaling, VS) were performed. The authors found that performance on the force steadiness, movement time, and velocity scaling measures was significantly poorer in the subjects with depression. There was no difference between the groups on the measure of reaction time. The force steadiness, reaction time, movement time, and velocity scaling scores were not associated with affective state. This study demonstrates that motor abnormalities suggestive of basal ganglia dysfunction occur in many patients with major depressive disorder, and that these abnormalities may exist in the absence of current psychotropic medication treatment. The finding of impaired movement time and velocity scaling in the presence of normal reaction time suggests a neuromotor or parkinsonian pathophysiology for slowness in depression.
Keywords: Depression, movement disorder, isochrony, basal ganglia
Introduction
Abnormalities in motor performance are common in depression and understanding the clinical importance of motor dysfunction in depression has been a focus of research for nearly 40 years. Motor manifestations, often called psychomotor abnormalities, can range from agitation to retardation depending on clinical state (Sobin and Sackeim, 1997). Previous studies have employed a wide variety of qualitative and quantitative procedures to understand the relationships between motor abnormalities and clinical state and treatment response. Traditional approaches to the study of psychomotor agitation and retardation in depression have included activity monitoring (Foster and Kupfer, 1975; Kupfer et al., 1974; Wolff et al., 1985), and assessments of gross body movement (Ulrich and Harms, 1985), speech production (Greden et al., 1981; Widlocher, 1983), and motor speed (Ghozlan and Widlocher, 1989; Lapierre and Butter, 1980). More recently, investigators have employed measures of handwriting movement to more precisely quantify fine motor activity associated with psychomotor retardation in patients with depressive disorders (Sabbe et al., 1996; 1999; Tucha et al., 2002; Pier et al. 2004a; 2004b). Comprehensive reviews of this work have been previously published (Schrijvers et al., 2008; Sobin and Sackeim, 1997).
What has been generally lacking from both traditional and recent approaches to the study of motor function in depression are more precise quantitative assessment methods that yield findings which can be related to pathophysiological processes associated with dysfunction in specific neural circuits and neurotransmitters. Over the past twenty-five years we have developed and refined quantitative instrumental approaches to measuring abnormalities in motor function in neuropsychiatric disorders (Caligiuri and Ellwanger, 2000; Caligiuri et al., 1988; Caligiuri and Lohr, 1989, 1990, 1994; Lohr and Caligiuri, 2006). The two main methods we have developed assess the ability to maintain steady-state force (Caligiuri and Lohr, 1994) and the ability to scale velocity with distance (Caligiuri et al., 1998). Although we have observed that patients with clinically observable movement disorders show abnormalities on these measures (with abnormalities in force steadiness being seen more in movement disorders marked by a hyperdopaminergic state, and velocity scaling abnormalities being associated more with hypodopaminergic conditions), we also have found that patients without observable clinical movement difficulty can demonstrate abnormalities on these measures. In particular, we have reported that patients with schizophrenia may show abnormalities on these measures without clinical evidence of tardive dyskinesia or antipsychotic-induced parkinsonism (Caligiuri and Lohr, 1994; Caligiuri et al., 1993).
Another important issue is that, although we and others have previously demonstrated that patients with depression manifest problems in motor control and performance, most previous studies of motor impairment in major depressive disorder included patients who were currently receiving treatment with psychotropic medications, including antidepressant and mood stabilizing medications. It is unclear the extent to which these medications may impact motor performance directly, apart from effects on depression, and with the exception of Pier et al (2004a; 2004b), relatively few previous studies have investigated motor function in unmedicated depressed patients.
For these reasons we undertook the present study with the following hypothesis: (1) Patients with major depression who have not been treated for at least one month exhibit disturbances in motor physiology as assessed by measures of force steadiness, reaction time, movement time, or velocity scaling. Because the assessment measures in this study may yield information related to specific neurotransmitters or neural circuits, we also decided to assess whether there was a relationship between performance on these measures and affective state. Therefore, a second hypothesis was: (2) Performance on measures of force steadiness, reaction time, movement time and velocity scaling measures in unipolar disorder patients are related to the severity of affective symptoms as measured by the Hamilton Depression Rating Scale (Hamilton, 1960).
Methods
Subjects and Medication Status
We studied 40 individuals (26 men and 14 women) meeting DSM-IV criteria for unipolar major depressive disorder and 40 healthy comparison subjects (22 men and 18 women). The demographic characteristics of the sample are presented in Table 1. We recruited the healthy comparison subjects through advertisements placed in the local media and Internet sites for research recruitment, and also through fliers and other advertisements for the study placed in the UCSD Psychiatric Outpatient Services or from outpatient psychiatric clinics at the VA San Diego Healthcare System. All subjects signed Institutional Review Board-approved consent for voluntary participation in this research. All patients were enrolled at UCSD Psychiatric Outpatient Services or from outpatient psychiatric clinics at the VA San Diego Healthcare System.
Table 1.
Demographic characteristics of the study subjects.
| Major Depression | Comparison Group | Statistic | |
|---|---|---|---|
| N | 40 | 40 | |
| Age (SD) | 40.3 (9.6) | 36.3 (13.1) | t=1.54, p=NS |
| Age Range | 22–59 | 19–67 | |
| Gender F:M | 14:26 | 18:22 | |
| Ham-D total (SD) | 21.15 (5.54) | ||
| Ham-D range | 9–31 |
Patients were excluded if they had received any psychotropic medications within the past month, including antidepressant medications such as selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), or mood stabilizers such as lithium, carbamazepine, valproate, lamotrigine or second generation antipsychotic drugs. Patients were also excluded if they were on any non-psychiatric medications known to produce motor side effects, or, if there was any clinical evidence of neuroleptic-induced side effects such as parkinsonism, tardive dyskinesia, akathisia, or tremor that might be related to antipsychotic medication treatments in the past, even in the absence of history or documentation of such treatment. These were determined by record review, interview with patients, and also interview with any significant others where appropriate, as well as examination of the patient. Other inclusion and exclusion criteria for patients with depression were: (1) Must currently meet DSM-IV criteria for major depressive episode in the context of unipolar depressive disorder as determined by SCID, (2) Must have had a diagnosis of major depressive disorder for at least five years, (3) may be of either gender, (4) may be either left- or right-handed, (5) must have no history of previous treatment with typical (first generation) antipsychotic medications, (6) must have no history for drug or alcohol dependence in the past year and no history of alcohol or drug abuse within the past month as determined by DSM-IV criteria, (7) must have no clinical evidence of current significant medical illness such as diabetes, thyroid disease, or cardiac, hepatic or renal disease, (8) must have no current clinical evidence or past history of cerebral neurological impairment (including strokes, tumors, trauma leading to loss of consciousness for longer than 10 minutes), and (9) must be able to give informed consent. For healthy comparison subjects the same criteria were applied, except of course the criteria relating to having the diagnosis of major depressive disorder (1 and 2 above).
Although we attempted to determine the number of previous episodes of depression in this group, we found the information unreliable, as many of the patients (and family members of patients) described waxing and waning of depressive symptoms over the years, without a clear-cut remission of symptoms. It was also difficult to determine the exact onset of depression in the lifetime of many of the patients, as they often described some symptoms of depression in adolescence or young adulthood, but it was not clear if these symptoms would meet full criteria for major depressive disorder. To our knowledge, none of the patients were receiving psychotherapy during the time of our assessments, but we did not deliberately exclude patients for this.
Clinical Assessment of Diagnosis and Psychopathology
For diagnosis, all patients underwent a Structured Clinical Interview for DSM-IV (SCID) performed by trained personnel (First et al., 1995), and this was used for both inclusion of patients with depression and exclusion of other psychiatric disorders. The clinical assessment for depression was the 28-item Hamilton Depression Rating Scale, the HAM-D (Hamilton, 1960).
Instrumental Assessment of Motor Function
Force Steadiness
Several investigators have used the phenomenon of force variability to assess neural mechanisms and disease state across different pathologies. For example, investigators have employed measures of force variability to quantify disease progression in Huntington’s disease (Reilmann et al., 2001). Studies in cerebral palsy and stroke have demonstrated that individuals with these illnesses cannot maintain the levels of precise force necessary to perform simple grip tasks, suggesting an important role for the motor cortex in the generation and maintenance of fine muscle force (Hermsdorfer and Mai, 1996; Valvano and Newell, 1998). Investigators have reported elevated levels of force variability in individuals with schizotypal personality disorder (Neumann and Walker, 1999) and schizophrenia (Caligiuri and Lohr, 1994), suggesting disturbances of inhibitory mechanisms regulating motor control. While the measurement of abnormal force steadiness can be used to assess several different aspects of motor control, our specific assessment procedure was initially determined to provide a measure of dyskinesia. As dyskinesia is often associated with basal ganglia disturbance, this method may shed light on the involvement of basal ganglia in various conditions, but, as mentioned, other brain regions, such as the motor cortices, may be involved as well.
Measurements of force steadiness involve having a subject attempt to apply constant pressure on a strain gauge, and measuring the amount of variability in the applied force. Subjects sit facing a computer monitor with one of their hands positioned on top of a platform equipped with the load cell (Sensotec, model 31/1426–02). This load cell transduces the force applied when flexing the index finger downward. A target line equivalent to 350 centiNewtons of force and the subject’s force signal are simultaneously displayed on the monitor. The two waveforms move from left to right across the monitor screen at a constant speed, filling a 20-second window. At the end of each 20-second window, the screen is refreshed.
The subjects were instructed to match the target line with the force signal line generated by maintaining steady levels of finger flexion force. Three 15-second trials were obtained, each separated by a 5-second rest period. Force signals were sampled at 100 samples/second by a laboratory computer and stored for subsequent analysis. The analysis involved identifying the segment with the greater range in force (determined by computing the force minima and maxima over the medial 80% of each segment) and calculating the waveform mean and standard deviation. Both hands were assessed and the average scores for the two hands were used for analysis.
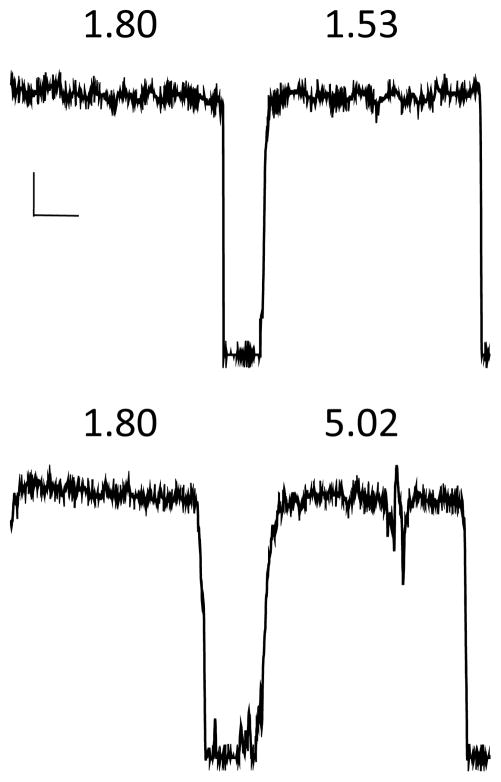
The coefficient of variation (CV) served as the force steadiness score, and was obtained by dividing the standard deviation of the force waveform by the mean force waveform. Higher scores indicate greater error or instability. Force steadiness error is the direct result of irregular muscle contractions that produce changes in measurable force over time. The force steadiness data acquisition procedure, segmentation, and analysis have been shown to be highly reliable and valid (Caligiuri et al., 1997). Figure 1 shows examples of normal (top) and abnormal (bottom) hand force waveforms.
Figure 1.
Examples of normal (top) and abnormal (bottom) performance on the hand force steadiness task. Shown are two right hand trials for each subject with their respective coefficients of variability. Coefficients of variability for the two top trials reflect normal performance; while the second (right) trial of the bottom example reflects abnormal performance. Calibration bars indicate 50 cN (vertical) and 5 seconds (horizontal).
Reaction Time
Reaction time (RT) is a familiar measure of speed of information processing and has been studied in patients with depression (Dantchev and Widlocher, 1998; Liotti et al., 1991; Swann et al., 1999). RT has been reported to be prolonged in patients with depression and is a prominent feature of what has been called psychomotor retardation (Sobin and Sackeim, 1997). While RT has been repeatedly shown to be a reliable measure of psychomotor slowing, findings are mixed in patients with parkinsonian bradykinesia (Camicioli et al., 2008). More recently, Goetz and coworkers (Goetz et al., 2009) reported that patients early in the course of Parkinson’s disease showed steady decline on overall measures of parkinsonian dysfunction, and on measures of tremor, finger-tapping speed and speech, whereas patients actually improved on the RT tests over six months. Thus, while patients with parkinsonism display reduced movement speeds, reaction time is relatively preserved. For the purpose of the present study, we extracted RT measures from the velocity scaling task (described above) to determine whether patients with unipolar depression exhibit psychomotor (prolonged RT) or parkinsonian (normal RT with prolonged movement time) motor impairment.
Reaction time was measured by calculating the time, in milliseconds, from the onset of a visual stimulus (target box) to onset of wrist rotation.
Movement Time
Movement time (MT) is a familiar measure of motor speed and has been studied extensively in Parkinson’s disease (Evarts et al., 1981; Hallett and Khoshbin, 1980), and depression (Hart and Kwentus, 1987; Nebes et al., 1998; Pier et al., 2004a, b; Sabbe et al., 1999). Evarts and colleagues (Evarts et al., 1981) reported that MT impairment was more severe than RT impairment in patients with Parkinson’s disease (PD) and, importantly, that bradykinesia can occur in the absence of impaired RT. This dissociation suggests different processes underlying the initiation and speed components of clinical motor slowing in PD. Movement times can be improved in PD patients treated with levodopa (Lange et al., 2006; Tucha et al., 2006) suggesting further that impaired MT may involve a dopamine-mediated subcortical pathophysiology. With regard to depression, studies have consistently reported prolonged MT. Using quantitative instrumental methods, Sabbe and colleagues (Sabbe et al., 1999) reported that medicated patients with major depressive disorder (MDD) exhibited prolonged RT and MT compared to age comparable healthy individuals (Pier et al., 2004b). These authors concluded that psychomotor slowing in depression consists of both cognitive (initiation) and motor (speed) components, but with the motor impairment being more pronounced. Another study employing the same methods by the same authors included unmedicated patients with severe depression (Pier et al., 2004a). Unlike their prior study, both RT and MT were significantly prolonged in the unmedicated MDD patients. Thus, while parkinsonian slowness in early stages appears to be dominated by abnormalities in MT, slowing in MDD appears to relate to both RT and MT abnormalities. The extent to which medication status, severity of depression, or both contribute to the cognitive component in psychomotor retardation is unclear.
In the present study, MT was measured by calculating the time, in milliseconds, from the onset of wrist rotation to the point in time when the velocity trace cross the zero axis (i.e. when rotation stopped and changed directions).
Velocity Scaling
The velocity scaling measure quantifies the subject’s ability to increase movement velocity with increasing target distance during a wrist flexion task. We initially became interested in this measure because it has been argued for over four decades that a fundamental disturbance in parkinsonian bradykinesia is an inability to modify movement velocity to meet the task demands (Benecke, 1989; Berardelli et al., 1986; Draper and Johns, 1964). One important feature of programmed motor behavior is the principle of isochrony, which refers to the relationship between distance and velocity of hand movements during limb movement. Isochrony stipulates that the average velocity of endpoint movement increases with movement distance (hence preserving the time span, making it isochronous), and is present in many if not most types of movement (Viviani et al., 2009; Viviani and Flash, 1995). For example, when moving the elbow to different degrees of flexion, normal healthy individuals will scale movement velocity in proportion to degrees of flexion, thus maintaining a constant movement time. However, patients with parkinsonian hypokinesia execute all movements, regardless of distance, with a more uniform velocity, thus failing to adhere to the principle of isochrony. We have refined this notion in a previous study of wrist movement (Caligiuri et al., 1998), and have shown that patients with neuroleptic-induced parkinsonism do not exhibit isochrony and instead move to targets of increasing distance with relatively constant velocities, which means that longer distances require more time. The ability to scale velocities with movement distance has been considered a cardinal function of the basal ganglia by some investigators (Smiley-Oyen et al., 2003; Vaillancourt et al., 2004).
For our measure of velocity scaling, the velocity-distance relationship during simple wrist rotation was measured using a platform equipped with a rotation sensor (electrogoniometer) that was attached to a handle. Rotation of the handle through extension or flexion of the wrist produces a continuous signal proportional to the angle of rotation. Using custom software, the digitized signal (sampled at 100 samples/second) is displayed on a computer monitor, with the position of the cursor along the horizontal plane calibrated in degrees of rotation. Along with the cursor, target boxes were displayed on the monitor along a horizontal plane located at 25° or 45° of rotation. These targets were placed to the left of midline for right wrist flexion, and to the right of midline for left wrist flexion. Subjects were instructed to flex the wrist “as quickly and as accurately as possible” when a target box appeared on the screen. The targets were displayed for two seconds, and the inter-stimulus interval was also two seconds. If any subject required clarification, the instructions were repeated with emphasis on “speed of movement.” Thirty-two trials or movements, consisting of 16 trials for each of two randomly presented target locations, were administered for each hand, resulting in a total of 64 movements. At the end of the 32 trials for each hand, the program computed the peak velocity, reaction time, and angular distance for each trial. Trials that did not meet specified criteria (multiple velocity peaks, omissions, changes in movement direction, or anticipated starts) were automatically removed from analyses by the software. Peak velocity and distance difference scores were then obtained by subtracting the respective values for the 25° targets from the 45° targets. The velocity scaling (VS) score was derived from the ratio of the velocity difference over the angular distance difference, scaled in degrees/second/degree. Lower scores represent disturbances in the ability to scale movement velocity with distance. Both hands were assessed, and data from the two hands were averaged for analyses. In previous studies we have determined that test-retest reliability for this measure is very high (Caligiuri and Ellwanger, 2000). Figure 2 shows examples of normal (left) and abnormal (right) rotation (top) and velocity (bottom) waveforms from the VS task. The series of velocity peaks shown in the box demonstrate the lack of velocity scaling observed in individuals with this motor programming disorder.
Figure 2.
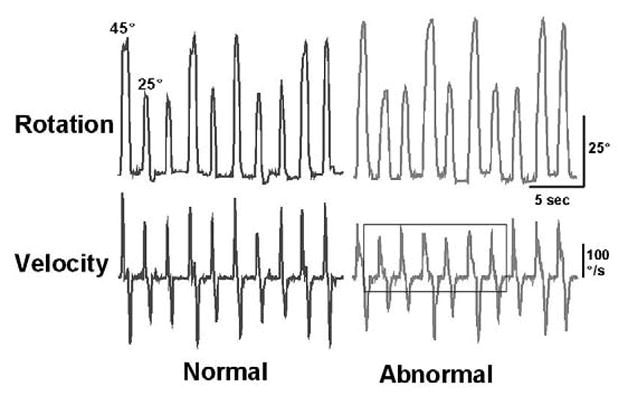
Examples of normal (left) and abnormal (right) rotation (top) and velocity (bottom) waveforms from the VS task. The series of velocity peaks shown in the box demonstrate the lack of velocity scaling observed in individuals with this motor programming disorder.
Statistical Analysis
Group data were examined for homogeneity of variances and normal distributions prior to undertaking parametric statistical analyses. The distributions for the four neuromotor variables were found to be normally distributed. Thus, group comparisons were subjected to Fisher’s exact t-tests to test the hypothesis that unmedicated patients with major depression exhibit disturbances in motor physiology as assessed by measures of force steadiness, reaction time, movement time, or velocity scaling. Separate analyses were performed for the force steadiness, velocity scaling, movement time, and reaction time data.
Bivariate correlational analyses (Pearson’s r) were used to test the hypothesis that performance on the neuromotor measures is related to the severity of affective symptoms as measured by the HAM-D.
Results
Motor Performance
Results from group difference tests are shown in Table 2. Overall, major depressive disorder patients performed significantly more poorly than healthy comparison subjects, with greater variability (CV) on average force steadiness (FS), longer durations of movement time (MT) and, reduced average velocity scaling (VS). There was no significant difference between the groups in terms of reaction time (RT).
Table 2.
Means, standard deviations (in parentheses), and ranges for the instrumental motor measures for the depression and comparison groups.
| Major Depression | Comparison Group | Statistic | |
|---|---|---|---|
| Force Steadiness (FS) | |||
| Average (SD) | 2.41 (0.99) | 1.70 (0.68) | t= 3.73, p<0.0005 |
| FS Range | 0.82–5.72 | 0.87–3.47 | |
| Velocity Scaling (VS) | |||
| Average peak (SD) | 1.38 (0.62) | 2.49 (1.21) | t= −5.16, p<0.000005 |
| VS Range | 0.21–2.79 | 1.19–7.77 | |
| Movement Time (MT) | |||
| Average (SD) | 714 (216) | 586 (194) | t= 2.79, p<0.01 |
| MT Range | 377–1260 | 175–1129 | |
| Reaction Time (RT) | |||
| Average (SD) | 299 (46) | 303 (73) | t= −0.37, p=NS |
| RT Range | 219–382 | 184–490 |
There was no correlation between the FS and VS measures in the entire group (r= −0.16, NS), or in the depression group alone (r= −0.15, NS). There was also no correlation between MT and FS scores for the entire group (r= 0.16, NS) or for the depression group alone (r=0.20, NS). There was, however, a correlation between MT and VS scores for both the entire group (r= −0.62, p<0.001) and the depression group alone (r= −.36, p<0.001).
To address whether hand dominance could affect performance on our motor measures, we compared scores obtained from the dominant hand with nondominant hand. Among the 40 depressed patients, only one subject was left hand dominant; whereas of the 40 healthy subjects 26 were right hand dominant and 14 were left hand dominant. For patients, there were no differences between the two hands for FS (2.49 ± 1.19 vs 2.32 ± 1.10 for dominant vs nondominant hand respectively) and RT (302 ± 56 vs 311 ± 47 ms for dominant vs nondominant hand respectively). However for MT, performance for the nondominant hand was significantly slower (752 ± 220 ms) than for the dominant hand (669 ± 234 ms) (t=3.91; p<0.001). For VS, performance for the nondominant hand was significantly lower (1.10 ± 0.93 deg/sec/deg) than for the dominant hand (1.60 ± 0.89 deg/sec/deg) (t=2.29; p<0.05). For healthy comparison subjects, no differences were found between dominant and nondominant hands for any of the four instrumental motor measures.
Relationship to Severity of Psychopathology
There were no significant correlations of any of the motor measures with the total HAM-D Score, or with any of the subscores, including the mood, motor retardation, agitation, and anergia subscores.
Discussion
We addressed two questions about the nature of motor pathophysiology in unipolar major depressive disorder. We first examined whether patients with major depression who have not been treated for at least one month exhibit disturbances in motor physiology that have been considered to indicate abnormalities in the sub-cortical motor circuits – force steadiness, movement time, reaction time, or velocity scaling. We found significantly poorer performance in the depression group than in the comparison group on the force steadiness, movement time, and velocity scaling measures, but not on the reaction time measure.
The second question was to examine whether disturbances in motor physiology in untreated unipolar disorder patients were related to the affective symptom severity. We found no correlation between any of the motor scores and the severity of depression (as measured by the HAM-D or its subscores).
Although patients in this study demonstrated significant impairments in the ability to maintain steady-state force and the ability to scale velocity with distance, these two findings appeared to be independent (as indicated by a lack of correlation between them in the group as a whole, and in the depression group alone), suggesting that two separate physiological mechanisms relating to basal ganglia circuitry may be involved. Movement time was also not related to FS measures, but it was significantly negatively correlated with VS, which is understandable, considering that VS incorporates movement time in its calculation.
Our findings suggest that there are physiological abnormalities in the motor system of patients with depression, but the exact nature of these is unclear. For example, force steadiness could represent the eruption of unwanted or inappropriate motor control programs into movements when gating mechanisms fail, or when there is insufficient selection of specific programs or insufficient inhibition of alternative or competing motor programs. Alternatively, abnormalities in force steadiness could also be due to problems in such motor control strategies as minimization of jerk (the rate of change of acceleration), which has been proposed as a core strategy underlying the production of smooth reaching movements (Novak et al., 2000; Viviani and Flash, 1995).
Unlike some previous investigations, we did not find a difference between depressed patients and the comparison subjects in terms of reaction time. RT findings in depression are not consistent. While most of the previous research shows delayed initiation or reaction times in depression (Sobin and Sackeim, 1997; Schrijvers et al., 2008), some earlier studies (Cornell et al., 1984; Schwartz et al., 1989), reported normal reaction times. It is possible that this may represent an influence of medications on RT in some previous studies, as our patients were unmedicated. For example, in two prior studies of unmedicated depressed patients, investigators reported normal initiation times among unmedicated dysthymic (Pier et al., 2004a) and nonmelancholic (Pier et al, 2004b) patients. Another possibility is that the RT abnormalities may be associated with very severe depressive illness, and our patients were outpatients who were only moderately depressed. It is also possible that our method of assessing RT could have contributed to the difference in our finding, as we did not specifically assess it using a dedicated task, but rather derived the information from our velocity scaling task. Because the velocity scaling task requires attention, it is possible that if reaction time abnormalities were due to impaired attention in previous studies, these abnormalities would not have shown up in our study. Interestingly, in a neuromotor condition such as Parkinson’s Disease, in contrast to movement time abnormalities, reaction time abnormalities are inconsistently present, and poorly related to bradykinesia (Evarts et al., 1981).
Our findings related to the presence of motor dysfunction in untreated patients with major depression is consistent with other observations on the coexistence of depression and movement problems, such as the frequently observed comorbidity of mood and motor findings in Parkinson’s and Huntington’s Diseases (DeLong, 1990; Folstein et al., 1987; Richards, 2005). The co-occurrence of mood symptoms with parkinsonism and/or choreoathetosis in neurological diseases suggests that there may be common pathophysiological features underlying some motor abnormalities and affective problems, perhaps involving the basal ganglia or dopaminergic mechanisms. Although our results do not definitively implicate the basal ganglia or dopaminergic mechanisms per se, the finding of motor impairment in unmedicated depressed patients that is similar to that observed in neurological disorders is suggestive of an overlap. In particular, force steadiness abnormalities, which often appear with dyskinesia, is suggestive of hyperdopaminergic problems in the basal ganglia, and velocity scaling abnormalities, such as are seen in Parkinson’s disease, are suggestive of hypodopaminergic abnormalities. Additionally, as there was no relationship between force steadiness and any of our hypokinetic measures (velocity scaling, movement time or reaction time), it appears that these problems may be independent in terms of their underlying pathophysiology. This suggests that any underlying motor problems in major depression are potentially complex, and may involve more than a few simple circuits or neurotransmitters.
Interestingly, we did not observe a significant relationship between the severity of depression and severity of motor dysfunction in this cross-sectional study. Although it might be anticipated that there would be a relationship between the severity of depression overall or of specific depressive components with the motor findings, we have generally found either poor or no correlation of depression severity ratings with motor findings in previous studies in bipolar disorder (Lohr and Caligiuri, 2006) and depression (Caligiuri et al., 2003). Several explanations may account for this. First, we suspect that the motor findings may be more related to trait rather than state variables in psychiatric illnesses, which would explain the lack of relationship, since state variables in depression change dramatically over time. Second, traditional depression severity scales are subjective and based largely on patient self-report and history, and this subjectivity could potentially account for the lack of a statistical relationship between motor performance and mood. Another possibility is that major depression is a multigenic disorder, and that motor problems only occur in a subset of patients, which would also obscure any relationship with symptoms.
Although, as mentioned earlier, it is possible that antidepressant and other psychotropic medications may cause motor dysfunction and therefore be potential confounders of attempts to analyze motor disturbance in depression, most previous studies included patients on medication, with a few exceptions (Pier et al., 2004a, b). In the present study, unmedicated unipolar depressed patients exhibited significant impairment on tasks designed to assess neuromotor function (FS, VS and MT), but exhibited relatively normal performance on a cognitive motor task (RT). Prior studies have reported impaired RT among medicated depressed patients (see (Sobin and Sackeim, 1997) for review), suggesting that the cognitive-motor impairment may be partially medication-related. The dissociation showing impaired neuromotor with intact cognitive-motor function sheds light on the potential pathophysiology of major depression, particularly with regard to the basal ganglia. It has been hypothesized that the coexistence of mood disorder in diseases clearly marked by dysfunction of the basal ganglia such as Huntington’s or Parkinson’s disease suggests a shared pathophysiological mechanism. The findings of the present study support this hypothesis.
The present study was able to evaluate possible confounding effects of gender or handedness. Previous studies have not addressed these specific variables that are known to influence some aspects of motor performance (e.g. speed) in healthy subjects. While gender was not significantly different between the groups (although there were slightly more women in the control than in the depression group), hand dominance did affect performance differently for the two subject groups. For FS and RT, there were no handedness effects for either healthy or depressed subjects. However, on the two measures purported to reflect parkinsonian bradykinesia - MT and VS - depressed patients exhibited longer MTs and lower VS scores for the nondominant than dominant hands. For healthy subjects, no differences were found between dominant and nondominant hands for MT or VS. While the potential mechanisms that could lead to differences in lateralized motor performance in our patients are not known, the present findings suggest greater impairment of the right than left hemisphere motor areas in unipolar depression. The notion that there exists an imbalance in the activation of the two hemispheres in depression and other affective disorders has been previously suggested (Hecht, 2010). Based on an extensive review of the literature, Hecht concluded that the two hemispheres are differentially involved in emotional (right) and cognitive (left) processes. The present findings of impaired left hand (right hemisphere) neuromotor performance together with findings linking the right hemisphere with emotional processing, suggest that both motor and emotional processing likely involve a subcortical, possibly dopaminergic mechanism.
There are several caveats and limitations to be noted with this study. We did not have detailed information on the number of depressive episodes in the past, or on psychosocial variables such as educational level, so were unable to determine if these influenced our results. Also, although the motor findings we report are consistent with abnormalities in basal ganglia circuit dysfunction as well as disturbances in dopaminergic transmission in these structures, we did not measure directly any basal ganglia or dopaminergic functions, so all conclusions related to specific pathophysiological processes remain speculative. We also only studied outpatients with moderately severe depression, so our findings must be interpreted accordingly, and we may not have observed abnormalities in motor control that would be seen only with more severely depressed individuals.
Acknowledgments
This research was supported in part from the VA Center of Excellence for Stress and Mental Health (CESAMH), from a grant from NIMH (R01MH-57140), the VA Merit Review program, and an independent investigator award from the National Alliance for Research on Schizophrenia and Depression (NARSAD).
Role of Funding Source
None of these sponsors had a role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors
Dr Lohr was the lead investigator. He contributed in the study design; subject recruitment; clinical assessment, statistical analyses and preparation of the manuscript
Dr Caligiuri was the senior investigator; He contributed in the study design; development of instrumentation; statistical analyses and preparation of the manuscript
Todd May conducted all the motor assessments, assisted in subject recruitment, and conducted the informed consent procedures.
Conflict of Interest
None of the authors of this manuscript have a conflict of interest with the research presented herein. The research was supported by the Dept of Veteran affairs and NARSAD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benecke R. The Pathophysiology of Parkinson’s Disease. In: Quinn NP, Jenner PG, editors. Disorders of Movement. Academic Press; San Diego: 1989. pp. 59–72. [Google Scholar]
- Berardelli A, Dick JP, Rothwell JC, Day BL, Marsden CD. Scaling of the size of the first agonist EMG burst during rapid wrist movements in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1986;49:1273–1279. doi: 10.1136/jnnp.49.11.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MP, Ellwanger J. Motor and cognitive aspects of motor retardation in depression. J Affect Disord. 2000;57:83–93. doi: 10.1016/s0165-0327(99)00068-3. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Harris MJ, Jeste DV. Quantitative analyses of voluntary orofacial motor control in schizophrenia and tardive dyskinesia. Biol Psychiatry. 1988;24:787–800. doi: 10.1016/0006-3223(88)90255-7. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Lohr JB. A potential mechanism underlying the voluntary suppression of tardive dyskinesia. J Psychiatr Res. 1989;23:257–266. doi: 10.1016/0022-3956(89)90031-9. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Lohr JB. Fine force instability: a quantitative measure of neuroleptic-induced dyskinesia in the hand. J Neuropsychiatry Clin Neurosci. 1990;2:395–398. doi: 10.1176/jnp.2.4.395. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Lohr JB. A disturbance in the control of muscle force in neuroleptic-naive schizophrenic patients. Biol Psychiatry. 1994;35:104–111. doi: 10.1016/0006-3223(94)91199-1. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Lohr JB, Jeste DV. Parkinsonism in neuroleptic-naive schizophrenic patients. Am J Psychiatry. 1993;150:1343–1348. doi: 10.1176/ajp.150.9.1343. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Lohr JB, Rotrosen J, Adler L, Lavori P, Edson R, Tracy K. Reliability of an instrumental assessment of tardive dyskinesia: results from VA Cooperative Study #394. Psychopharmacology (Berl) 1997;132:61–66. doi: 10.1007/s002130050320. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Lohr JB, Ruck RK. Scaling of movement velocity: a measure of neuromotor retardation in individuals with psychopathology. Psychophysiology. 1998;35:431–437. [PubMed] [Google Scholar]
- Caligiuri MP, Gentili V, Eberson S, Kelsoe J, Rapaport M, Gillin JC. A quantitative neuromotor predictor of antidepressant non-response in patients with major depression. J Affect Disord. 2003;77:135–41. doi: 10.1016/s0165-0327(02)00107-6. [DOI] [PubMed] [Google Scholar]
- Camicioli RM, Wieler M, de Frias CM, Martin WR. Early, untreated Parkinson’s disease patients show reaction time variability. Neurosci Lett. 2008;441:77–80. doi: 10.1016/j.neulet.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Cornell DG, Suarez R, Berent S. Psychomotor retardation in melancholic and nonmelancholic depression: cognitive and motor components. J Abnorm Psychol. 1984;93:150–157. doi: 10.1037//0021-843x.93.2.150. [DOI] [PubMed] [Google Scholar]
- Dantchev N, Widlocher DJ. The measurement of retardation in depression. J Clin Psychiatry. 1998;59(Suppl 14):19–25. [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Draper IT, Johns RJ. The disordered movement In parkinsonism and the effect of drug treatment. Bull Johns Hopkins Hosp. 1964;115:465–480. [PubMed] [Google Scholar]
- Evarts EV, Teravainen H, Calne DB. Reaction time in Parkinson’s disease. Brain. 1981;104:167–186. doi: 10.1093/brain/104.1.167. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer R, Williams J, Gibbon M. Structured Clinical Interview for DSM-IV (SCID-I) (User’s Guide and Interview) Research Version. Biometric Research Department, New York Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- Folstein SE, Chase GA, Wahl WE, McDonnell AM, Folstein MF. Huntington disease in Maryland: clinical aspects of racial variation. Am J Hum Genet. 1987;41:168–179. [PMC free article] [PubMed] [Google Scholar]
- Foster FG, Kupfer DJ. Psychomotor activity as a correlate of Depression and sleep in acutely disturbed psychiatric inpatients. Am J Psychiatry. 1975;132:928–931. doi: 10.1176/ajp.132.9.928. [DOI] [PubMed] [Google Scholar]
- Ghozlan A, Widlocher D. Decision time and movement time in depression: differential effects of practice before and after clinical improvement. Percept Mot Skills. 1989;68:187–192. doi: 10.2466/pms.1989.68.1.187. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Stebbins GT, Wolff D, DeLeeuw W, Bronte-Stewart H, Elble R, Hallett M, Nutt J, Ramig L, Sanger T, Wu AD, Kraus PH, Blasucci LM, Shamim EA, Sethi KD, Spielman J, Kubota K, Grove AS, Dishman E, Taylor CB. Testing objective measures of motor impairment in early Parkinson’s disease: Feasibility study of an at-home testing device. Mov Disord. 2009;24:551–556. doi: 10.1002/mds.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greden JF, Albala AA, Smokler IA, Gardner R, Carroll BJ. Speech pause time: a marker of psychomotor retardation among endogenous depressives. Biol Psychiatry. 1981;16:851–859. [PubMed] [Google Scholar]
- Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103:301–314. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart RP, Kwentus JA. Psychomotor slowing and subcortical-type dysfunction in depression. J Neurol Neurosurg Psychiatry. 1987;50:1263–1266. doi: 10.1136/jnnp.50.10.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht D. Depression and the hyperactive right-hemisphere. Neurosci Res. 2010;68:77–87. doi: 10.1016/j.neures.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Mai N. Disturbed grip-force control following cerebral lesions. J Hand Ther. 1996;9:33–40. doi: 10.1016/s0894-1130(96)80009-3. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Weiss BL, Foster G, Detre TP, McPartland R. Psychomotor activity in affective states. Arch Gen Psychiatry. 1974;30:765–768. doi: 10.1001/archpsyc.1974.01760120029005. [DOI] [PubMed] [Google Scholar]
- Lange KW, Mecklinger L, Walitza S, Becker G, Gerlach M, Naumann M, Tucha O. Brain dopamine and kinematics of graphomotor functions. Hum Mov Sci. 2006;25:492–509. doi: 10.1016/j.humov.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Lapierre YD, Butter HJ. Agitated and retarded depression. A clinical psychophysiological evaluation. Neuropsychobiology. 1980;6:217–223. doi: 10.1159/000117755. [DOI] [PubMed] [Google Scholar]
- Liotti M, Sava D, Rizzolatti G, Caffarra P. Differential hemispheric asymmetries in depression and anxiety: a reaction-time study. Biol Psychiatry. 1991;29:887–899. doi: 10.1016/0006-3223(91)90055-q. [DOI] [PubMed] [Google Scholar]
- Lohr JB, Caligiuri MP. Abnormalities in motor physiology in bipolar disorder. J Neuropsychiatry Clin Neurosci. 2006;18:342–349. doi: 10.1176/jnp.2006.18.3.342. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Halligan EM, Rosen J, Reynolds CF., 3rd Cognitive and motor slowing in Alzheimer’s disease and geriatric depression. J Int Neuropsychol Soc. 1998;4:426–434. doi: 10.1017/s1355617798455024. [DOI] [PubMed] [Google Scholar]
- Neumann CS, Walker EF. Motor dysfunction in schizotypal personality disorder. Schizophr Res. 1999;38:159–168. doi: 10.1016/s0920-9964(99)00011-0. [DOI] [PubMed] [Google Scholar]
- Novak KE, Miller LE, Houk JC. Kinematic properties of rapid hand movements in a knob turning task. Exp Brain Res. 2000;132:419–433. doi: 10.1007/s002210000366. [DOI] [PubMed] [Google Scholar]
- Pier MP, Hulstijn W, Sabbe BG. No psychomotor slowing in fine motor tasks in dysthymia. J Affect Disord. 2004a;83:109–120. doi: 10.1016/j.jad.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Pier MP, Hulstijn W, Sabbe BG. Psychomotor retardation in elderly depressed patients. J Affect Disord. 2004b;81:73–77. doi: 10.1016/j.jad.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Reilmann R, Kirsten F, Quinn L, Henningsen H, Marder K, Gordon AM. Objective assessment of progression in Huntington’s disease: a 3-year follow-up study. Neurology. 2001;57:920–924. doi: 10.1212/wnl.57.5.920. [DOI] [PubMed] [Google Scholar]
- Richards H. Depression in neurological disorders: Parkinson’s disease, multiple sclerosis, and stroke. J Neurol Neurosurg Psychiatry. 2005;76:48–52. doi: 10.1136/jnnp.2004.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbe B, Hulstijn W, van Hoof J, Zitman F. Fine motor retardation and depression. J Psychiatr Res. 2006;30:295–306. doi: 10.1016/0022-3956(96)00014-3. [DOI] [PubMed] [Google Scholar]
- Sabbe B, Hulstijn W, van Hoof J, Tuynman-Qua HG, Zitman F. Retardation in depression: assessment by means of simple motor tasks. J Affect Disord. 1999;55:39–44. doi: 10.1016/s0165-0327(98)00087-1. [DOI] [PubMed] [Google Scholar]
- Schrijvers D, Hulstijn W, Sabbe BG. Psychomotor symptoms in depression: a diagnostic, pathophysiological and therapeutic tool. J Affect Disord. 2008;109:1–20. doi: 10.1016/j.jad.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Schwartz F, Carr AC, Munich RL, Glauber S, Lesser B, Murray J. Reaction time impairment in schizophrenia and affective illness: the role of attention. Biol Psychiatry. 1989;25:540–548. doi: 10.1016/0006-3223(89)90214-x. [DOI] [PubMed] [Google Scholar]
- Smiley-Oyen AL, Worringham CJ, Cross CL. Motor learning processes in a movement-scaling task in olivopontocerebellar atrophy and Parkinson’s disease. Exp Brain Res. 2003;152:453–465. doi: 10.1007/s00221-003-1570-x. [DOI] [PubMed] [Google Scholar]
- Sobin C, Sackeim HA. Psychomotor symptoms of depression. Am J Psychiatry. 1997;154:4–17. doi: 10.1176/ajp.154.1.4. [DOI] [PubMed] [Google Scholar]
- Swann AC, Katz MM, Bowden CL, Berman NG, Stokes PE. Psychomotor performance and monoamine function in bipolar and unipolar affective disorders. Biol Psychiatry. 1999;45:979–988. doi: 10.1016/s0006-3223(98)00172-3. [DOI] [PubMed] [Google Scholar]
- Tucha O, Aschenbrenner S, Eichhammer P, Putzhammer A, Sartor H, Klein HE, Lange KW. The impact of tricyclic antidepressants and selective serotonin re-uptake inhibitors on handwriting movements of patients with depression. Psychopharmacology (Berl) 2002;159:211–5. doi: 10.1007/s002130100921. [DOI] [PubMed] [Google Scholar]
- Tucha O, Mecklinger L, Thome J, Reiter A, Alders GL, Sartor H, Naumann M, Lange KW. Kinematic analysis of dopaminergic effects on skilled handwriting movements in Parkinson’s disease. J Neural Transm. 2006;113:609–623. doi: 10.1007/s00702-005-0346-9. [DOI] [PubMed] [Google Scholar]
- Ulrich G, Harms K. A video analysis of the non-verbal behaviour of depressed patients before and after treatment. J Affect Disord. 1985;9:63–67. doi: 10.1016/0165-0327(85)90011-4. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Mayka MA, Thulborn KR, Corcos DM. Subthalamic nucleus and internal globus pallidus scale with the rate of change of force production in humans. Neuroimage. 2004;23:175–186. doi: 10.1016/j.neuroimage.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Valvano J, Newell KM. Practice of a precision isometric grip-force task by children with spastic cerebral palsy. Dev Med Child Neurol. 1998;40:464–473. doi: 10.1111/j.1469-8749.1998.tb15397.x. [DOI] [PubMed] [Google Scholar]
- Viviani P, Burkhard PR, Chiuve SC, Corradi-Dell’Acqua C, Vindras P. Velocity control in Parkinson’s disease: a quantitative analysis of isochrony in scribbling movements. Exp Brain Res. 2009;194:259–283. doi: 10.1007/s00221-008-1695-z. [DOI] [PubMed] [Google Scholar]
- Viviani P, Flash T. Minimum-jerk, two-thirds power law, and isochrony: converging approaches to movement planning. J Exp Psychol Hum Percept Perform. 1995;21:32–53. doi: 10.1037//0096-1523.21.1.32. [DOI] [PubMed] [Google Scholar]
- Widlocher DJ. Psychomotor retardation: clinical, theoretical, and psychometric aspects. Psychiatr Clin North Am. 1983;6:27–40. [PubMed] [Google Scholar]
- Wolff EA, 3rd, Putnam FW, Post RM. Motor activity and affective illness. The relationship of amplitude and temporal distribution to changes in affective state. Arch Gen Psychiatry. 1985;42:288–294. doi: 10.1001/archpsyc.1985.01790260086010. [DOI] [PubMed] [Google Scholar]