Abstract
There is no adequate animal model of restless legs syndrome (RLS) and periodic leg movements disorder (PLMD), disorders affecting 10% of the population. Similarly, there is no model of rapid eye movement (REM) sleep behavior disorder (RBD) that explains its symptoms and its link to Parkinsonism. We previously reported that the motor inhibitory system in the brainstem extends from the medulla to the ventral mesopontine junction (VMPJ). We now examine the effects of damage to the VMPJ in the cat. Based on the lesion sites and the changes in sleep pattern and behavior, we saw three distinct syndromes resulting from such lesions; the rostrolateral, rostromedial and caudal VMPJ syndromes. The change in sleep pattern was dependent on the lesion site, but was not significantly correlated with the number of dopaminergic neurons lost. An increase in wakefulness and a decrease in slow wave sleep (SWS) and REM sleep were seen in the rostrolateral VMPJ lesioned animals. In contrast, the sleep pattern was not significant changed after lesion in rostromedial and caudal VMPJ lesioned animals. All 3 groups of animals showed a significant increase in periodic and isolated leg movements in SWS and increased tonic muscle activity in REM sleep. Beyond these common symptoms, an increase in phasic motor activity in REM sleep, resembling that seen in human RBD, was found in the caudal VMPJ lesioned animals. In contrast, the increase in motor activity in SWS in rostral VMPJ lesioned animals is similar to that seen in human RLS/PLMD patients. The proximity of the VMPJ region to the substantia nigra suggests that the link between RLS/PLMD and Parkinsonism, as well as the progression from RBD to Parkinsonism may be mediated by the spread of damage from the regions identified here into the substantia nigra.
Keywords: periodic leg movements, REM sleep behavior disorder, Parkinsonism, pons, retrorubral nucleus, substantia nigra
Introduction
Periodic leg movement disorder (PLMD), is a symptom common in the restless legs syndrome (RLS; Montplaisir et al., 1992) and in rapid eye movement (REM) sleep behavior disorder (RBD; Schenck and Mahowald, 1990). PLMD is an increase in phasic motor activity in slow wave sleep (SWS). RLS, PLMD, and RBD are also commonly seen in Parkinsonism (Schenck et al., 1996; Wetter et al., 2000; Gagnon et al., 2002; Ondo et al., 2002; Boeve et al., 2004; Gatto et al., 2007). It is well known that Parkinsonian patients have damage in the substantia nigra (SN; Gibb et al., 1989; McRitchie et al., 1999; Yekhlef et al., 2003). However, the neuropathology underlying RLS, PLMD and RBD is uncertain.
In normal animals and humans, muscle tone in the postural muscles is low in SWS and absent in REM sleep. The brain mechanisms regulating muscle tone in SWS remain unclear. In contrast, the neural mechanisms responsible for the suppression of muscle tone in REM sleep are fairly well understood. It has been shown that the pontine inhibitory area (PIA) and the medial medulla, including the nuclei gigantocellularis, magnocellularis (NMC), and paramedianus, participate in the regulation of muscle tone during REM sleep (Kanamori et al., 1980; Lai and Siegel, 1988, 1990, 1991, 1999; Siegel et al., 1991; Kodama et al., 1992, 1998, 2003; Lai et al., 1993, 1999a, 2001; Yamuy et al., 1993). Activation of the PIA and medial medulla produces generalized skeletal muscle atonia in chronic and in decerebrate animals (Magoun and Rhines, 1946; George et al., 1964; Lai et al., 1987; Lai and Siegel, 1988; Hajnik et al., 2000). Damage to the PIA or NMC generates REM sleep without atonia in the cat (Henley and Morrison, 1974; Schenkel and Siegel, 1989; Shouse and Siegel, 1992; Holmes and Jones, 1994) and rat (Sanford et al., 2001). However, it has not been reported that phasic muscle activity during SWS is altered by PIA or NMC lesions. Because an increase in leg movement in SWS is seen in RBD patients (Schenck and Mahowald, 1990; Montplaisir et al., 1992), and because there has been no convincing evidence of damage to the PIA or medial medulla in such patients, damage to these areas does not appear to be a likely cause of human RBD.
Our previous studies demonstrated that the ventral mesopontine junction (VMPJ), including the caudal portion of the dopaminergic retrorubral nucleus (RRN), SN and ventral tegmental area (VTA), as well as the rostroventral paralemniscal tegmental field of the pons, is involved in the regulation of motor activity (Lai and Siegel, 1990). Short train stimulation in the VMPJ generates global inhibition of muscle tone during stimulation, and rhythmic activity appears during the inter-stimulus interval in the decerebrate cat (Lai and Siegel, 1990). Neurotoxic N-methyl-D-aspartic acid (NMDA) lesions in the VMPJ produce an increase in spontaneous or tactile stimulation induced rhythmic stepping-like activity or myoclonic jerks in the decerebrate animal (Lai and Siegel, 1997a). We hypothesized that the VMPJ may be involved in the control of muscle activity during sleep.
Experimental Procedures
Surgical preparation
All procedures were approved by the Animal Research Committee of the University of California, Los Angeles and of the VA Greater Los Angeles Healthcare System. Eight female and one male cat (University of California, Davis, California) weighing 2.5–3.5 kg were used in the study. Implantation of electrodes for sleep recording was described in a previous paper (Lai et al., 1999b). Briefly, cats were anesthetized with isoflurane (1.5%) for stereotaxic electrode implantation. Jewelers’ screws were implanted over the sensorimotor cortex (A27, L4, L8 and L10) for recording cortical EEG, and into the orbital bone for the recording of eye movement (electrooculogram; EOG). Flexible multi-stranded stainless steel wires (7935, A-M Systems, Inc., Carlsborg, WA) were inserted into nuchal and forelimb musculature bilaterally for electromyogram (EMG) recording. Seven animals had EMG electrodes implanted in both the neck and limb musculature, whereas the remaining two cats (LC11 and LC12) had EMG electrodes implanted only in the neck muscles. Stainless steel tripolar electrodes were implanted into the lateral geniculate nuclei (A6, L10, H2) to record ponto-geniculo-occipital (PGO) spikes. Guide cannulae (19 G) were implanted 5 mm dorsal to the VMPJ for NMDA injection, bilaterally.
Sleep recording and ventral brainstem lesion
Sleep recording was performed 7 days after electrode implantation, by which time the animal had recovered from surgery. After 3 consecutive 24-h baseline sleep recordings, a 25 G cannula connecting with a one microliter Hamilton microsyringe (Model 7001) was inserted into the VMPJ through the guide cannula. One half microliter of 0.5 M NMDA (Sigma, St. Louis) was injected into the VMPJ over a period of 5 min. The cannula was retained at its position for another 20 min. Sleep recordings were resumed on day 2 post-NMDA injection every day for 14 days. Then, a 3-day sleep recording was performed once every two weeks over a period of 4-months. In our previous study, we found that unilateral lesion of the VMPJ generated motor hyperactivity in sleep in the cat (Lai et al., 1999b; cat number: LC3). Thus, we wanted to determine whether unilateral and bilateral lesions produce motor hyperactivity in sleep. Among 9 cats in the present study, 2 (LC12 and FO5) had bilateral NMDA injection into the VMPJ simultaneously, 2 (FO4 and VJ4) had NMDA bilateral injections with the two injections given 4-months apart, and the remaining five cats had unilateral VMPJ lesions.
Data analysis
Physiological variables were amplified with a Grass polygraph (Model 78D) and digitized by a CED 1401 Spike2 system (Cambridge Electronic, UK). Five sleep-waking states were scored, active waking, quiet waking, SWS stage 1, SWS stage 2 and REM sleep, according to Ursin and Sterman (1981).
Phasic muscle activities occurring in the neck and limb during SWS were divided into two categories, periodic neck/leg movement and isolated neck/leg movement. Periodic motor activities were scored based on the following criteria for humans (Zucconi et al., 2006). 1) Duration of muscle activity was between 0.5 to 5 sec. 2) Inter-jerk intervals ranged between 5 to 80 sec. 3) Amplitude of muscle twitches were at least double that of the existing background EMG. 4) At least 4 consecutive jerks fulfilled criteria 1–3. Phasic muscle activities which meet the above criteria 1–3 but not 4 were described as isolated activities. The periodic neck movement index (PNMI) and periodic leg movement index (PLMI) were defined as the number of periodic neck and limb muscle activities in SWS in 24-h recording (total number of periodic muscle activities/total SWS time in 24-h), respectively. The INMI and ILMI were the number of isolated motor activities in the neck and leg per 24-h of SWS time (total number of isolated muscle activities/total SWS time in 24-h), respectively. Muscle tone (REM sleep without atonia) and phasic muscle activity during REM sleep were analyzed to evaluate changes in muscle activity in REM sleep after VMPJ lesion. Changes in percent of REM sleep without atonia in REM sleep, which was the number of total duration of REM sleep without atonia divided by the total REM sleep in 24-h, after VMPJ lesion, were used to quantify the effect of VMPJ lesions on muscle tone in REM sleep. The baseline level of sleep states and phasic motor activities in each animal was taken from an average of 2 days of recording, and an average of 2 days of recording was used for the analysis of sleep states and motor activity in sleep after lesion.
Histology
Cats were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.v.) at the end of the experiment. Then, animals were perfused intracardially with cold (4° C) saline followed by cold 4% paraformaldehyde in 0.1 M phosphate buffer saline (PBS) solution at pH 7.4. The brainstem was removed and kept in 4% paraformaldehyde solution at 4° C for 2 hours, then, stored in 30% sucrose in 0.1 M PBS at 4° C.
40 µ serial coronal sections of the brainstem were obtained. Brain sections were processed with immunohistochemistry for tyrosine hydroxylase (TH) and choline acetyltransferase (ChAT) to determine the magnitude of the loss of dopaminergic neurons in the RRN and cholinergic neurons in the pedunculopontine nucleus (PPN). For TH and ChAT immunohistochemistry, tissue sections were rinsed with 0.1 M PBS three times and then incubated in 10% normal goat serum for 2 hours. Then, tissue sections were sequentially incubated with primary antibody against TH (1:1,000, MAB5280, Chemicon, Temecula, CA) or ChAT (1:1,000, MAB5270, Chemicon), both were raised from mouse, for 72 hours, biotinylated goat anti-mouse IgG (BA-1000, Vector, Burlingame, CA) for one hour and Vectastain Elite ABC kit (PK-6102, Vector) for another hour. Tissue sections were rinsed with 0.1 M PBS three times between incubations. The final products of TH and ChAT were visualized with 3,3’-diaminobenzidine (DAB, Sigma) solution containing 0.05% DAB and 0.02% hydrogen peroxide in 0.1 M PBS. Alternative tissue sections were also processed with neutral red after immunohistochemical processing to identify the lesion area and to estimate the total number of neurons lost (Lai et al., 1999b). Tissue sections processed with TH, ChAT, and neutral red of 2 cats from our previous anatomical studies (Lai et al., 1999a) served as control.
Tissue sections were observed under a Nikon microscope. The lesion sites were mapped with a NeuroLucida microscope system (MBF Bioscience, Williston, Vermont).
Results
The lesion sites in the brainstem
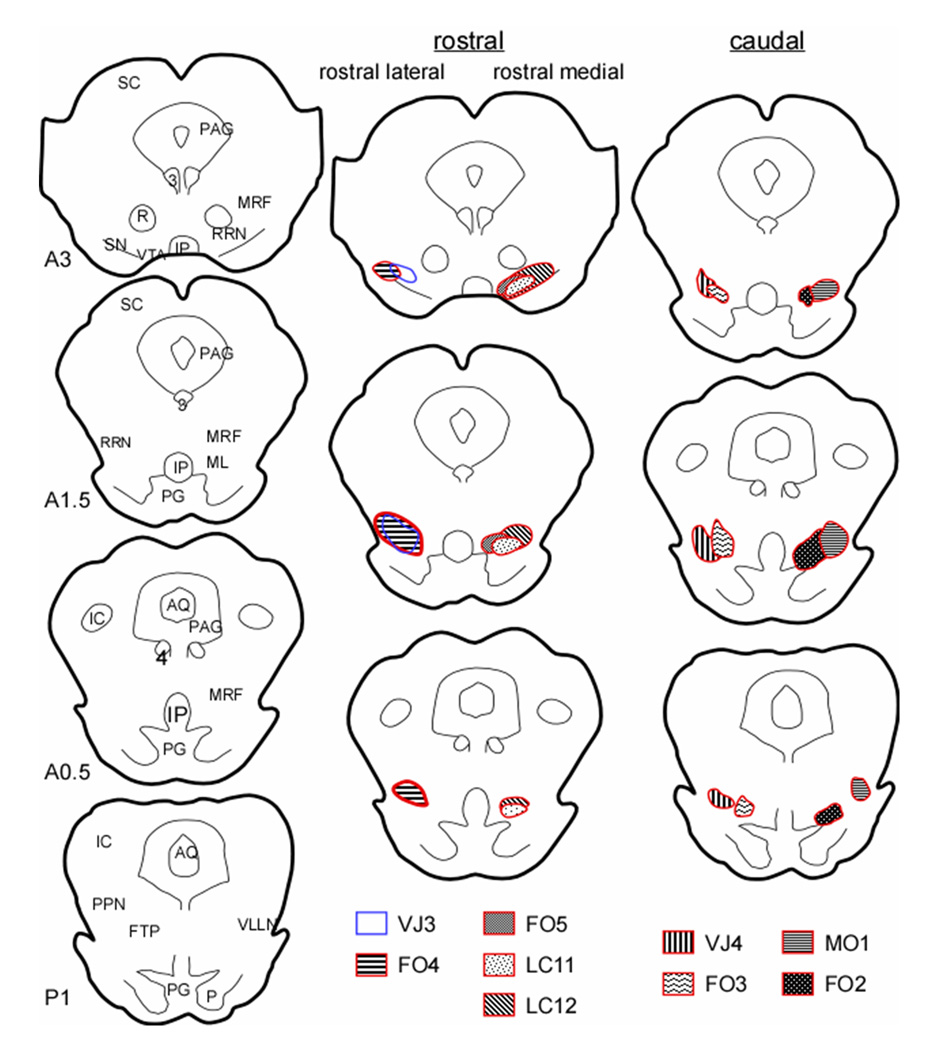
NMDA microinjections killed all neuronal phenotypes (Fig. 1). Although injections were aimed at the VMPJ, the precise lesion locus varied across animals. Animals could be divided into 3 groups, rostral-medial (Group R-M), rostral-lateral (Group R-L), and caudal (C) lesions (Fig. 1). The rostral VMPJ lesioned groups (Groups R-M and R-L) had lesions extending from the caudal red nucleus at their rostral limit to the trochlear nucleus at their caudal limit (Berman, 1968). The lesioned areas in the Group R-M cats (LC11, LC12, and FO5) included the RRN, the ventral portion of the mesencephalic reticular formation (MRF) and the caudal portion of the VTA, whereas, the Group R-L cats (FO4 and VJ3) had lesions in the RRN, MRF, and the caudal portion of the SN. The other 4 cats had lesions in the caudal VMPJ (Group C) including the caudal RRN in the midbrain and the rostral-ventral paralemniscal tegmental field in the pons. Table 1 shows the estimated number of neuron lost in brainstem structures across animals.
Figure 1.
Reconstruction of lesion sites. Two animals had bilateral lesions (LC12 and FO5) and the other 7 had unilateral lesion in the VMPJ. Among the 7 unilateral VMPJ lesioned animals, 2 (FO4 and VJ4) had an additional lesion on the side contralateral to the first lesion 4 months after the first lesion. AQ: aqueduct, FTP: paralemniscal tegmental field, IC: inferior colliculus, IP: interpeduncular nucleus, ML: medial lemniscus, MRF: mesencephalic reticular formation, PAG: periaqueductal gray, PG: pontine gray, PPN: pedunculopontine nucleus, R: red nucleus, RRN: retrorubral nucleus, SC: superior colliculus, SN: substantia nigra, VLLN: ventral nucleus of the lateral lemniscus, VTA: ventral tegmental area. 3: oculomotor nucleus, 4: trochlear nucleus. A and P represent rostral and caudal to the interaural zero.
Table 1.
The total number of neurons lost* after NMDA injection into the VMPJ.
| Cat | VLLN | FTP | PPN | MRF | RRN | VTA | SN | Total |
|---|---|---|---|---|---|---|---|---|
| VJ3 | -- | 60 | -- | 1508 | 1208 | -- | 648 | 3424 |
| FO4(1) | -- | 102 | -- | 935 | 312 | 264 | 858 | 2471 |
| FO4(2) | -- | -- | -- | 480 | 3756 | -- | 2718 | 6954 |
| FO5(1) | -- | -- | -- | 348 | 1032 | -- | 1612 | 2992 |
| FO5(2) | -- | -- | -- | 18 | 1425 | -- | 1932 | 3375 |
| LC11 | -- | -- | -- | 1908 | 852 | 450 | -- | 3210 |
| LC12(1) | -- | -- | -- | 981 | 789 | 870 | 99 | 2739 |
| LC12(2) | -- | -- | -- | 417 | 1936 | 726 | -- | 3079 |
| MO1 | 303 | 745 | 450 | 948 | 1390 | -- | -- | 3836 |
| FO2 | -- | 812 | -- | 684 | 1616 | -- | -- | 3112 |
| FO3 | 108 | 1298 | -- | 856 | 610 | -- | -- | 2872 |
| VJ4(1) | -- | 908 | -- | 1034 | 566 | -- | -- | 2508 |
| VJ4(2) | -- | 1218 | -- | 534 | 552 | -- | -- | 2304 |
including the dopaminergic neurons in the RRN, SN and VTA, as well as the cholinergic neurons in the PPN.
No neurons were lost in the area.
FTP: paralemnical tegmental field (including ventral part of the lateral lemniscus); MRF: mesencephalic reticular formation (including medial lemniscus); PPN: pedunculopontine nucleus, RRN: retrorubral nucleus; SN: substantia nigra; VLLN: nucleus of the lateral lemniscus, ventral portion; VTA: ventral tegmental nucleus.
Immunohistochemical staining showed that one cat (MO1) had a lesion extending into the PPN. However, we estimated that less than 1% of cholinergic neurons in the PPN were removed by the NMDA injection. No PPN lesion was found in the remaining 8 cats. Loss of dopaminergic neurons in the RRN (Fig. 2) was found in all cats to different degrees (Table 2). With the exception of cat FO4, no cat lost more than 30% of the dopaminergic neurons in the VMPJ.
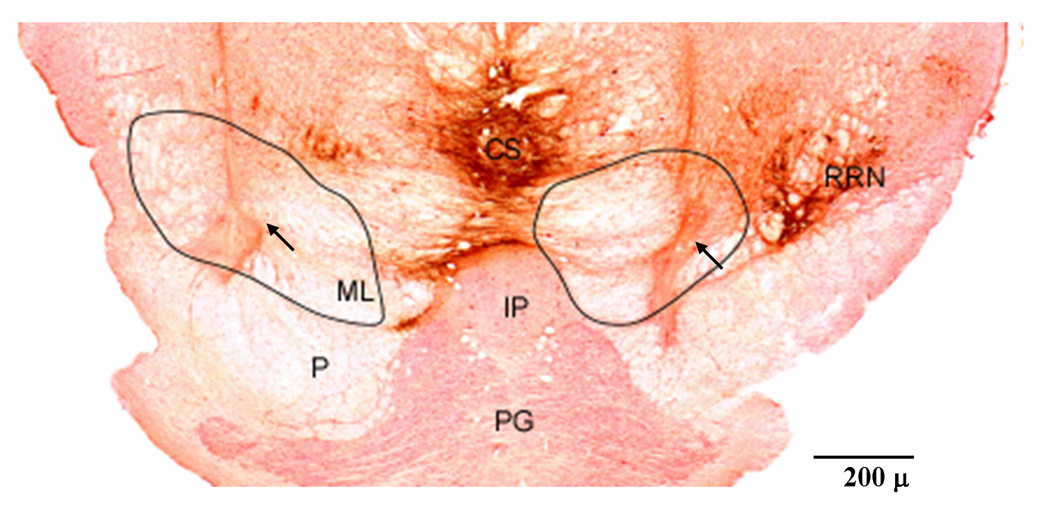
Figure 2.
Example of NMDA lesion at the ventral mesopontine junction (VMPJ). Microphotograph was taken from cat FO4. Tissue section was processed with immunohistochemistry for tyrosine hydroxylase (dark brown) and then with neutral red. The first and second lesion sites were in the rostrolateral and rostromedial VMPJ. Some dopaminergic neurons were found in the lateral (right) and medial (left) retrorubral nucleus (RRN). The arrows indicate the injection sites. The areas within the line represent the lesioned areas. CS: superior central nucleus, IP: interpeduncle nucleus, ML: medial lemniscus, P: pyramidal tract, PG: pontine gray, RRN: retrorubral nucleus.
Table 2.
Percent of dopaminergic neurons lost in the caudal ventral midbrain
| cat | LC11 | LC12 | FO4 | FO5 | VJ3 | FO2 | FO3 | MO1 | VJ4 |
|---|---|---|---|---|---|---|---|---|---|
| % | 14 | 27 | 32 | 22 | 17 | 7 | 5 | 22 | 13 |
Behavior responses to NMDA-VMPJ lesion
Motor activities in waking appeared normal in all animals after NMDA-VMPJ lesions. On day 2 post-NMDA injection, cats were able to stand and walk. Reflex activities, blinking and head turning appeared normal. Eating and drinking behaviors were not affected by NMDA injections.
Change in sleep after neurotoxic lesion in the VMPJ
Visually scored 24-hour sleep recordings showed a change in sleep architecture after VMPJ lesions. Regression analysis between dopaminergic cell loss in the VMPJ and changes in sleep at day 7, 30, 60, 90 and 120 post-lesion indicated that the change in waking, SWS, and REM sleep after VMPJ lesion was not correlated with the degree of dopaminergic neuron loss in the VMPJ (p>0.2, Table 3). In contrast, the duration of sleep-wake stages differed as a function of lesion location (ANOVA, df=4, p<0.05). Although the animals in both of Groups R-M and C showed an increase in SWS and REM sleep after lesion, the change in sleep pattern was not significantly different from the baseline control (Fig. 3, Group R-M: ANOVA, df=21, p>0.05; Group C: ANOVA, df=30, p>0.05). In contrast to Groups R-M and C, a significant increase in wakefulness with reduced SWS and REM sleep was found in cats after rostrolateral VMPJ lesions (Fig. 3, ANOVA, df=14, p<0.05). Thus, a unilateral VMPJ lesion was sufficient to change sleep architecture.
Table 3.
Relationship between dopaminergic neuron degeneration in the VMPJ and changes in sleep pattern. No significant relations were seen.
| D3 | D7 | D14 | D30 | D60 | D90 | D120 | |
|---|---|---|---|---|---|---|---|
| Wake | |||||||
| R value | 0.34 | 0.31 | 0.35 | 0.47 | 0.44 | 0.37 | 0.56 |
| P | 0.37 | 0.42 | 0.36 | 0.2 | 0.23 | 0.33 | 0.15 |
| SWS | |||||||
| R value | −0.1 | −0.1 | −0.28 | −0.43 | −0.43 | −0.37 | −0.56 |
| P | 0.81 | 0.8 | 0.46 | 0.25 | 0.25 | 0.32 | 0.15 |
| REM sleep | |||||||
| R value | −0.14 | −0.21 | −0.37 | −0.48 | −0.41 | −0.33 | −0.3 |
| P | 0.72 | 0.6 | 0.33 | 0.19 | 0.27 | 0.39 | 0.47 |
Figure 3.
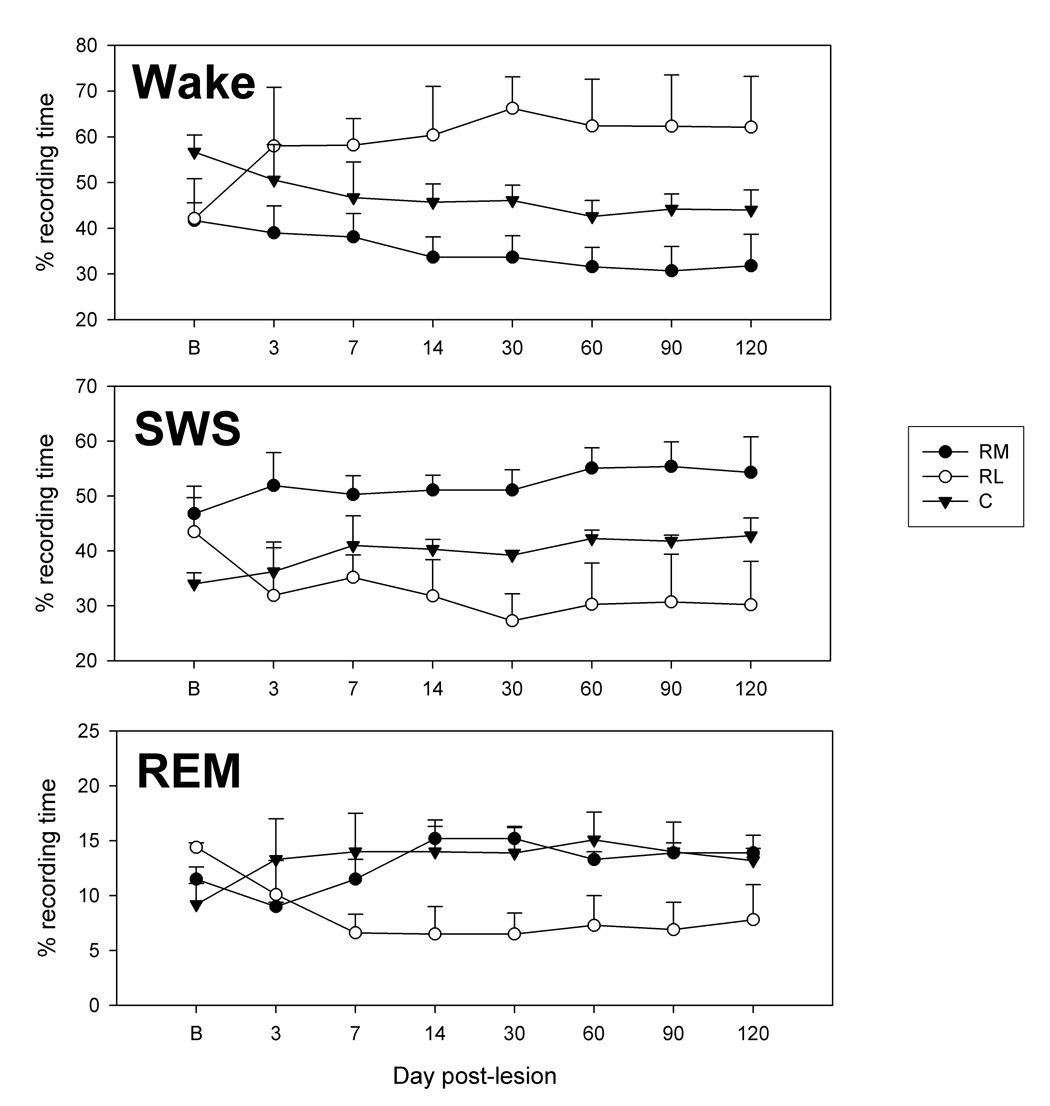
Change in the duration of wake, slow wave sleep (SWS) and REM sleep after VMPJ lesion. A significant increase in wakefulness and decrease in SWS and REM sleep were found in the rostral-lateral VMPJ lesioned cats (Group RL, ANOVA, p<0.05, n=2). On the other hand, the increase in SWS and REM sleep observed in the rostral-medial (Group RM, ANOVA, p>0.05, n=3) and caudal (Group C, ANOVA, p>0.05, n=4) VMPJ lesioned cats, was not significantly different from the baseline sleep pattern. B: baseline.
Motor activity in SWS in the NMDA-VMPJ lesioned animals
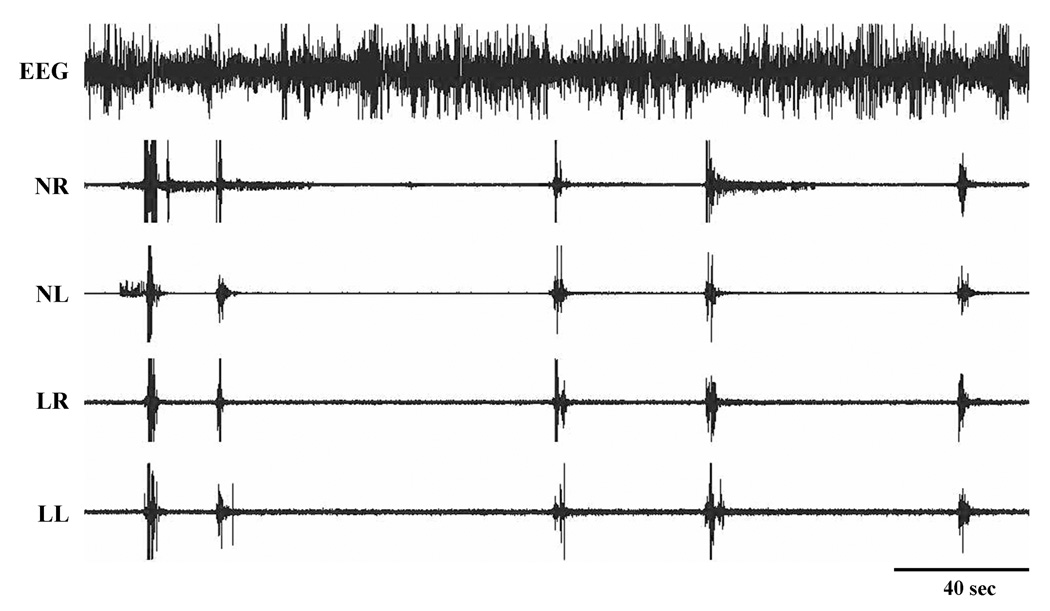
Basal muscle tone was low during SWS in all cats before VMPJ lesions. Basal muscle tone in waking and SWS was unaltered after VMPJ lesions. However, an increase in phasic muscle activity in SWS was seen after lesions in the VMPJ in all animals (Fig. 4; Table 4). The time course of changes in phasic motor activity in SWS could be segregated into 2 phases, early (first week after lesion) and late (after the first week). In the early phase, phasic motor activity appeared as irregular and non-periodic, isolated leg/neck movements. Then, phasic muscle activity in the leg and neck gradually consolidated into regular and periodic leg/neck movements. Thus, the number of isolated leg and neck phasic activities in SWS gradually decreased to a low level by 60 days after VMPJ lesion, whereas, periodic leg and neck phasic activities appeared in the late phase and continued throughout the entire 4-month period of observation (Fig. 5). Periodic leg movements occurred unilaterally and bilaterally. In some cases, these were accompanied by periodic neck muscle activity. Periodic motor events were accompanied by EEG desynchronization in most of cases in the VMPJ lesioned cat (Fig. 4).
Figure 4.
Periodic movements in the neck and leg during SWS after VMPJ lesion. NL and NR: left and right neck muscle EMG; LL and LR: left and right limb muscle EMG.
Table 4.
Isolated and periodic movement index after second VMPJ lesion.
| Cat | Index | B | D3 | D7 | D14 | D30 | D60 | D90 | D120 |
|---|---|---|---|---|---|---|---|---|---|
| FO4 | INMI | 11.4 | 7.6 | 6.3 | 2.3 | 9.6 | 4.4 | 10.0 | 8.2 |
| ILMI | 7.6 | 1.5 | 2.6 | 1.2 | 8.1 | 4.6 | 9.7 | 4.6 | |
| FO4 | PNMI | 29.8 | 19.8 | 31.3 | 27.5 | 30.8 | 25.4 | 28.7 | 24.2 |
| PLMI | 12.9 | 10.8 | 11.4 | 11.2 | 12.6 | 13.1 | 15.7 | 10.1 | |
| VJ4 | INMI | 14.1 | 19.7 | 21.7 | 26.0 | 11.4 | 18.7 | 15.4 | 12.2 |
| ILMI | 11.6 | 17.4 | 9.2 | 16.3 | 12.1 | 9.5 | 14.3 | 13.8 | |
| VJ4 | PNMI | 6.9 | 8.5 | 12.3 | 9.7 | 11.2 | 7.9 | 8.1 | 8.3 |
| PLMI | 22.5 | 19.6 | 17.1 | 25.4 | 26.8 | 27.2 | 21.5 | 25.3 |
Baseline of the second lesion was taken from data taken 4-months after the first lesion.
INMI and ILMI: Isolated neck and leg movements index, respectively.
PNMI and PLMI: Periodic neck and leg movements index, respectively.
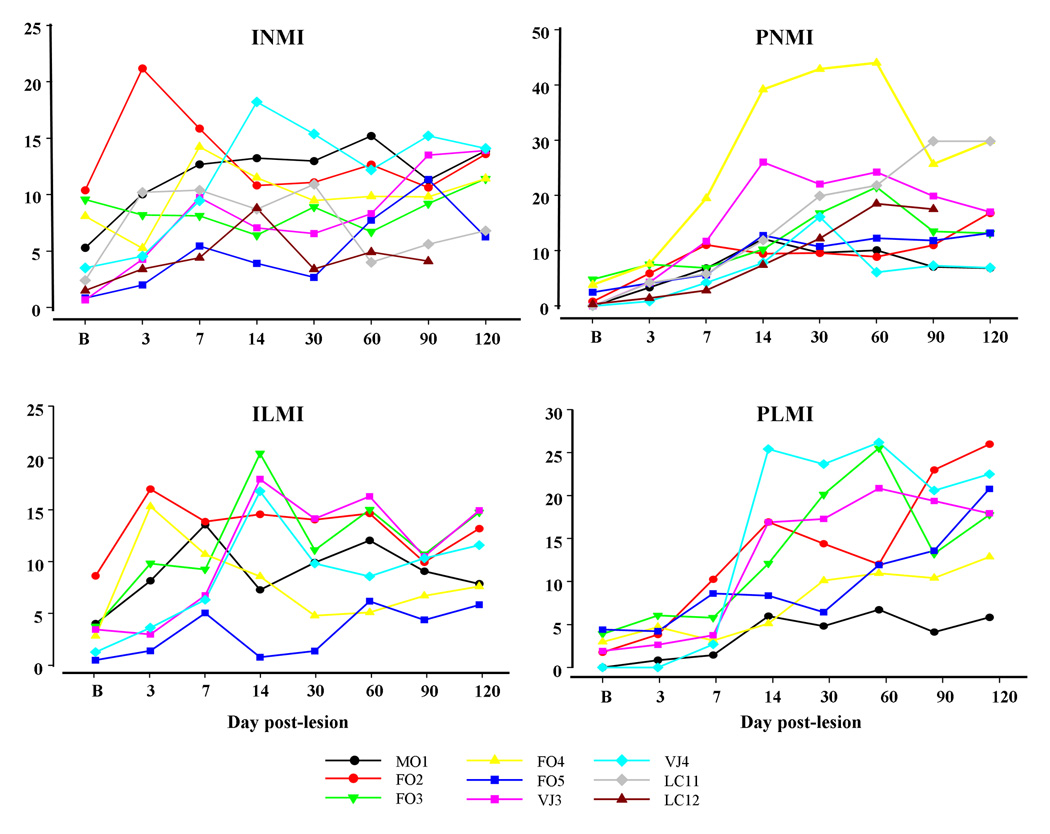
Figure 5.
Changes in periodic and isolated leg and neck muscle activity in SWS after VMPJ lesion. An increase in periodic and isolated leg and neck muscle activity in SWS was seen by day 7 and day 3 after VMPJ lesion and continued for the entire 4-months period. The change in the index of periodic leg (PLMI, bottom right) and neck (PNMI, top right) muscle activity, as well as isolated leg (ILMI, bottom left) and neck (INMI, top left) muscle activity reached a plateau level at 14 days after VMPJ lesion. The Y-axis represents the index of INM, PNM, ILM, and PLM.
Motor activity in REM sleep in the NMDA-VMPJ lesioned animals
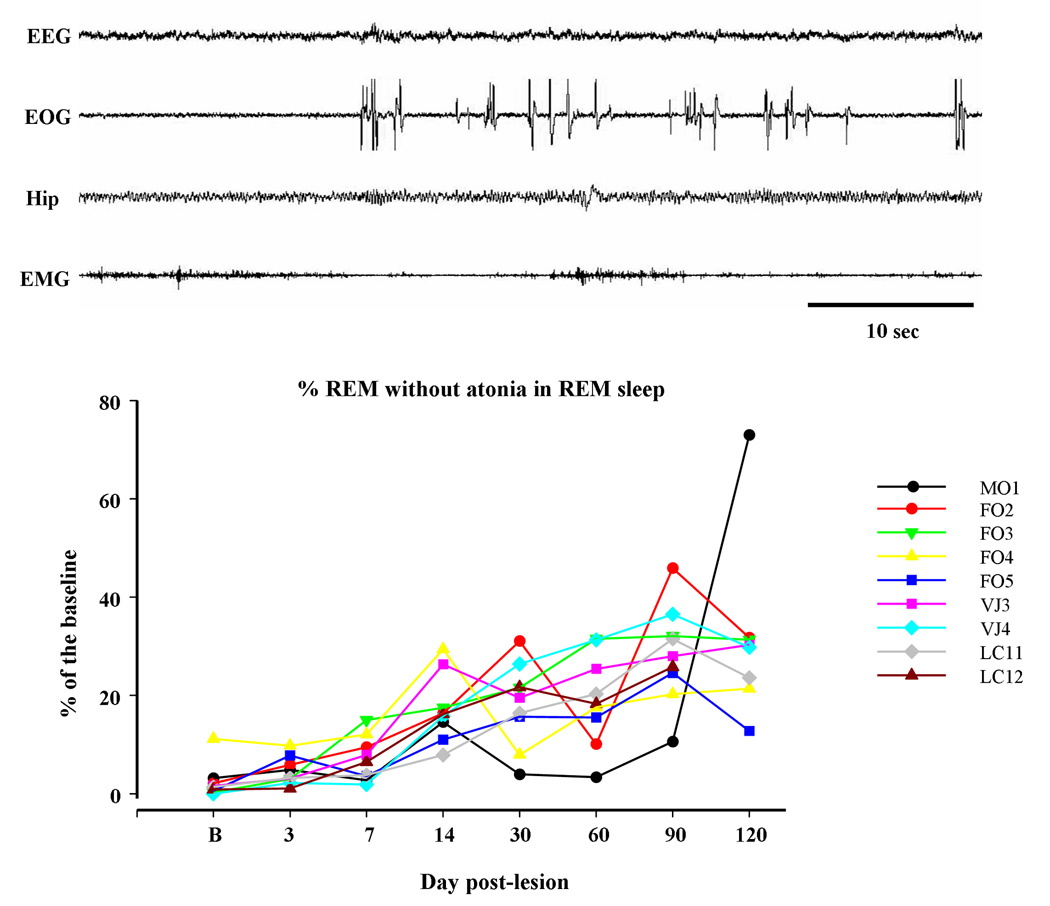
The major change in muscle activity during REM sleep after NMDA-VMPJ lesion was an increase in tonic activity, i.e. REM sleep without atonia (Fig. 6). Although the lesion area differed between animals, an increase in tonic muscle activity in REM sleep was found in all animals in our VMPJ lesion series. REM sleep without atonia appeared in the neck and/or limb musculatures, unilaterally and/or bilaterally. The percent of REM sleep time without atonia gradually increased beginning 7 days after NMDA injection into the VMPJ and reaching a plateau level by 60 days after lesion (Fig. 6).
Figure 6.
REM sleep with and without atonia (upper panel) and change in percent of REM sleep without atonia in REM sleep (bottom panel) after VMPJ lesion. EEG desynchronization, rapid eye movement, and hippocampal theta (Hip) waves with high muscle tone were recorded during REM sleep after VMPJ lesion. Bottom: percent of REM sleep without atonia in REM sleep was gradually increased after VMPJ lesion in all animals. EOG: electrooculograph.
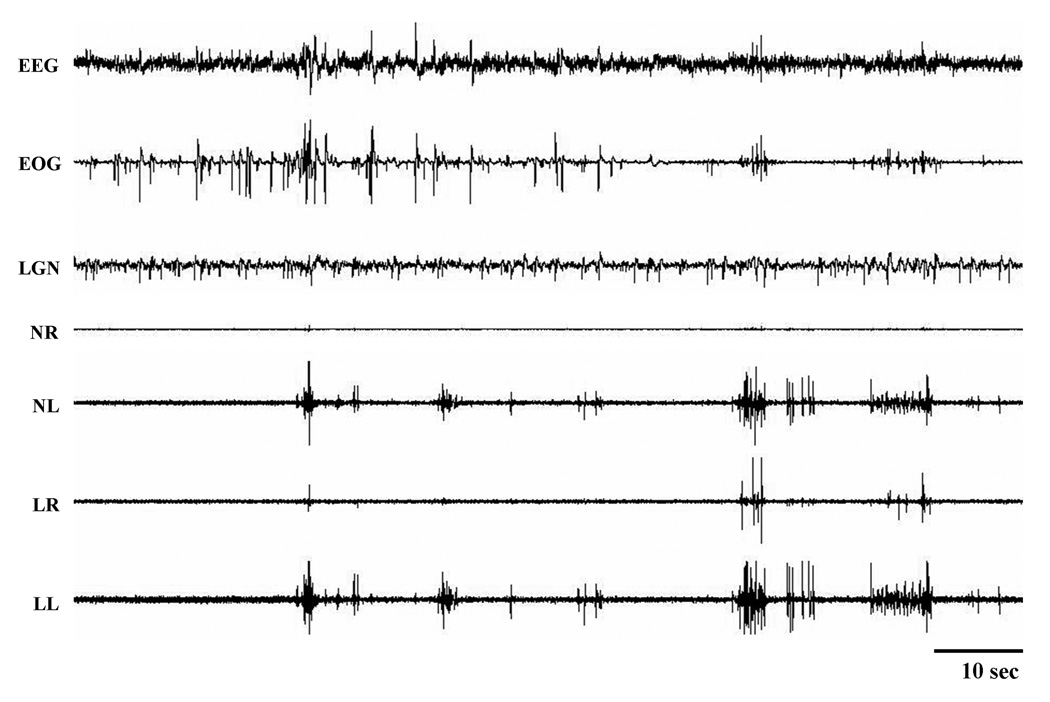
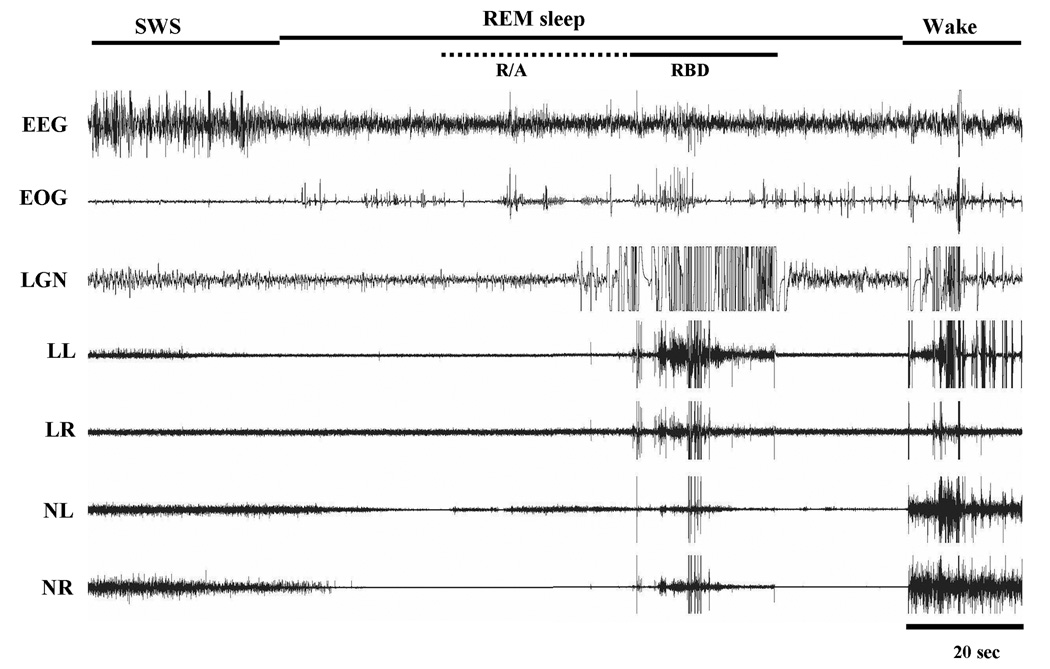
Abnormal phasic motor activity in REM sleep was found in cats that had lesions of the caudal VMPJ (Group C; Table 5). Periodic phasic muscle activities and leg twitching not only appeared in SWS but also occurred in REM sleep (Fig. 7). High amplitude muscle activity abruptly appeared in all recorded muscles in REM sleep, while the EEG remained desynchronized and the EOG recording showed rapid eye movements (Fig. 8). Behavioral activities observed during this episode of REM sleep appeared as jerking, kicking and extending of the leg; raising and moving the head; and lifting of the body. This abnormal REM sleep behavior, which resembled RBD seen in humans, lasted for 20 – 62 seconds with an average of 43 ± 18 seconds. Animals went into REM sleep immediately after the episodes of RBD-like activity (Fig. 8).
Table 5.
Index of phasic motor activity in REM sleep before (B) and after VMPJ lesion.
| Cat | B | D3 | D7 | D14 | D30 | D60 | D90 | D120 |
|---|---|---|---|---|---|---|---|---|
| Group R-M | ||||||||
| LC11 | 5.4 | 4.4 | 5.7 | 12.6 | 18.9 | 11.7 | 5.9 | 7.3 |
| LC12 | 9.6 | 8.2 | 4.7 | 18.2 | 23.4 | 14.1 | 7.9 | N/A |
| FO5 | 2.0 | 8.1 | 16.8 | 3.6 | 5.9 | 6.6 | 2.7 | 3.9 |
| Group R-L | ||||||||
| FO4(1) | 91.8 | 97.1 | 192.9 | 99.4 | 78.8 | 46.4 | 65.6 | 80.8 |
| FO4(2) | 80.8 | 65.4 | 56.1 | 49.2 | 51.9 | 74.8 | 79.2 | 83.1 |
| VJ3 | 3.7 | 5.2 | 8.4 | 5.0 | 4.9 | 4.6 | 3.3 | 4.4 |
| Group C | ||||||||
| FO2 | 19.0 | 22.5 | 29.4 | 48.2 | 79.3 | 37.5 | 39.5 | 46.8 |
| FO3 | 7.3 | 4.5 | 5.3 | 10.1 | 16.4 | 12.9 | N/A | 19.7 |
| MO1 | 7.1 | 12.7 | 17.3 | 29.3 | 10.4 | 20.5 | 41.4 | 46.5 |
| VJ4(1) | 10.4 | 11.7 | 14.7 | 54.4 | 66.0 | 62.6 | 61.7 | 54.9 |
| VJ4(2) | 54.9 | 57.1 | 51.4 | 49.6 | 63.7 | 55.8 | 57.5 | 51.6 |
Baseline of the second lesion was taken from data of 4-month after first lesion.
(1) and (2): the first and second VMPJ lesions.
N/A: data were not available.
Figure 7.
Periodic leg movements in REM sleep after caudal VMPJ lesion. Periodic leg movements during REM sleep was not seen under baseline conditions. LGN: lateral geniculate nucleus activity.
Figure 8.
REM sleep behavior disorder- (RBD) like activity. Muscle tone gradually increased and then, phasic muscle activity was seen in REM sleep. EEG desynchronization and rapid eye movements were seen during motor activity. Leg kicking was observed during the episode of RBD-like activity. Normal REM sleep returned after the episode of RBD-like activity. R/A: REM sleep without atonia.
Effect of second lesion contralateral to the side of first lesion on motor activity in sleep
Two cats (FO4 and VJ4) had a second lesion on the side contralateral to the first lesion. The second lesion failed to alter motor activities in sleep, periodic leg/neck movements, isolated leg/neck movements, REM sleep without atonia and RBD, from the level observed 4 months after the first lesion (Table 4 and Table 5). As was the case with the change in sleep pattern, unilateral lesion of the VMPJ was sufficient to cause motor hyperactivity in sleep.
Discussion
The major finding of the present study is that lesions in the VMPJ induce changes in sleep patterns, as well as in the duration and nature of motor activity during sleep. Changes in sleep patterns were site-dependent with a significant increase in wakefulness in rostral-lateral VMPJ lesioned animals. Dopaminergic mechanisms have been reported to be involved in the regulation of sleep (Gerashchenko et al., 2006; Monti and Monti, 2007), however, our present study found that changes in sleep pattern after VMPJ lesions were not correlated with the number of caudal midbrain dopaminergic neurons lost. Although all VMPJ lesioned animals developed PLMD and REM sleep without atonia, RBD-like behaviors were only seen in cats with caudal VMPJ lesions. We also found that unilateral lesion of the VMPJ was sufficient to elicit motor hyperactivity in sleep and a change in the sleep pattern. A clinical study found that RBD can be induced by a unilateral tumor located at the pontocerebellar angle (Zambelis and Soldatos, 2002).
In RBD patients, motor hyperactivity is not only seen in REM sleep but also found in SWS. An increase in periodic and isolated leg movements during SWS is seen in 60% and 40 % of RBD patients, respectively (Schenck and Mahowald, 1990). Periodic leg movements persist in REM sleep in RBD patients (Lapierre and Montplaisir, 1992). Although loss of muscle atonia in REM sleep is a symptom of RBD, these patients experience periods of atonia intermixed with persistent muscle tone (Schenck et al., 1992; Fantini et al., 2003). Sleep organization is reported to be either changed or unaltered in idiopathic RBD. Schenck et al. (1993) and Massicotte-Marquez et al. (2005) reported that more than 80% of idiopathic RBD patients show an increase in SWS compared with age-matched normal subjects. In contrast, Iranzo et al. (2002) found that SWS is not significantly altered in idiopathic RBD. REM sleep was also reported to be either increased in 43% of RBD patients (Schenck and Mahowald, 1990) or unaltered (Iranzo et al., 2002), as we see in the current study. The decrease in sleep after rostrolateral VMPJ lesions in the present study is consistent with Gerashchenko et al’s findings (2006). They found that rats with lesions in the SN but not in the VTA developed insomnia. The decrease in sleep seen in the rostrolateral VMPJ lesioned cat may mimic that seen in Parkinson’s disease patients.
The physiological role of VMPJ in sleep regulation remains unclear. Fos-expressing neurons have been found in the VMPJ in the phase of REM sleep rebound after REM sleep deprivation (Verret et al., 2006) indicating the VMPJ may be involved in the regulation of REM sleep. Anatomical studies have shown that neurons in the VMPJ project to sleep-related areas including basal forebrain (Sawchenko et al., 1983; Willoughby and Blessing, 1987; Vertes, 1988; Jones and Cuello, 1989), PIA (Lai et al., 1993) and NMC (Luppi et al., 1988; Lai et al., 1999a). Glutamatergic projections from the VMPJ to the PIA and NMC (Lai et al., 1993; 1999a) may be involved in the generation of REM sleep (Onoe and Sakai, 1995; Kodama et al., 1998). Neurons in the rostrolateral VMPJ, perhaps containing γ-aminobutyric acid (GABA), also project to locus coeruleus (LC; Verret et al., 2006). GABA has been shown to inhibit LC neuronal activity (Gervasoni et al., 1998), and its levels in the LC are increased during SWS and REM sleep (Nitz and Siegel, 1997). The dorsal raphe nucleus has been found to project to the medial portion of the VMPJ (Vertes and Kocsis, 1994). However, the role of raphe projections to the VMPJ remains unclear.
The effect of VMPJ lesion on muscle activity in sleep may be mediated through the caudal brainstem and cerebellum. We demonstrated that motor hyperactivity induced by VMPJ lesions can be attenuated or blocked by glutamate injection into the NMC in the decerebrate cat (Lai and Siegel, 1997b). Anatomically, the VMPJ is one of the major sources of projections to the caudal brainstem muscle inhibitory areas, the NMC (Luppi et al., 1988; Lai et al., 1999a) and the PIA (Lai et al., 1993). Axonal fibers from the VMPJ were also found to innervate the LC (Verret et al., 2006), which has been shown to have a role in motor facilitation (Fenik et al., 2005a,b,c; Fung and Barnes, 1981, 1987; Lai et al., 1989; Liu et al., 1995; Wu et al., 1999). Activation of the VMPJ may thus activate neuronal activity in the PIA and NMC via glutamatergic mechanism and suppress LC neuronal activity through GABAergic projections. We hypothesize that the effects of VMPJ on PIA, NMC and LC may contribute to the motor inhibition in sleep.
The PIA has been demonstrated to be involved in the regulation of muscle tone in REM sleep and has been hypothesized to participate in the generation of RBD (Morrison, 1988). Although electrolytic lesion in the PIA generates motor hyperactivity, orienting, walking, and attacking in REM sleep (Morrison et al., 1981), chemical lesions in the PIA in the cat induce REM sleep without atonia but fail to elicit the elaborate motor behavior characteristic of RBD seen with electrolytic lesions (Webster and Jones, 1988; Shouse and Siegel, 1992). Work in the rat reported that REM sleep atonia is preserved during inactivation of PIA neuronal activity by tetrodotoxin injection (Sanford et al., 2005), although electrolytic lesions of the same site induces REM sleep without atonia (Sanford et al., 2001). Therefore, the behavioral activation in REM sleep seen in the PIA-electrolytic lesioned cat may result from damage to passing fibers. Indeed, clinical studies have shown that the PIA appears normal in RBD patients (Schenck and Mahowald, 1996; Mazza et al., 2006), however, dysfunction of the ventral pons has been reported in human RBD (Schenck and Mahowald, 1990; Mazza et al., 2006). Severe neural degeneration in the ventral pons is reported in olivopontocerebellar atrophy patients, who also develop REM sleep without atonia and RBD (Salva and Guilleminault, 1986; Schenck et al., 1993; Tachibana et al., 1995). We found in the present study that the integrity of the rostroventral pons is critical for preventing RBD, whereas, the caudal midbrain is involved in the control of phasic motor activity in SWS and tonic activity in REM sleep. Clinically, dopaminergic and benzodiazepine-related agonists are the most potent drugs in the treatment in RLS/PLMD (Montplaisir et al., 1986; Earley and Allen, 1996) and RBD (Schenck et al., 1986). Our present finding that caudo-ventral midbrain lesions generate PLMD and that the rostroventral pontine lesions generate RBD may be underlie the differences in the effective pharmacological treatment between PLMD and RBD.
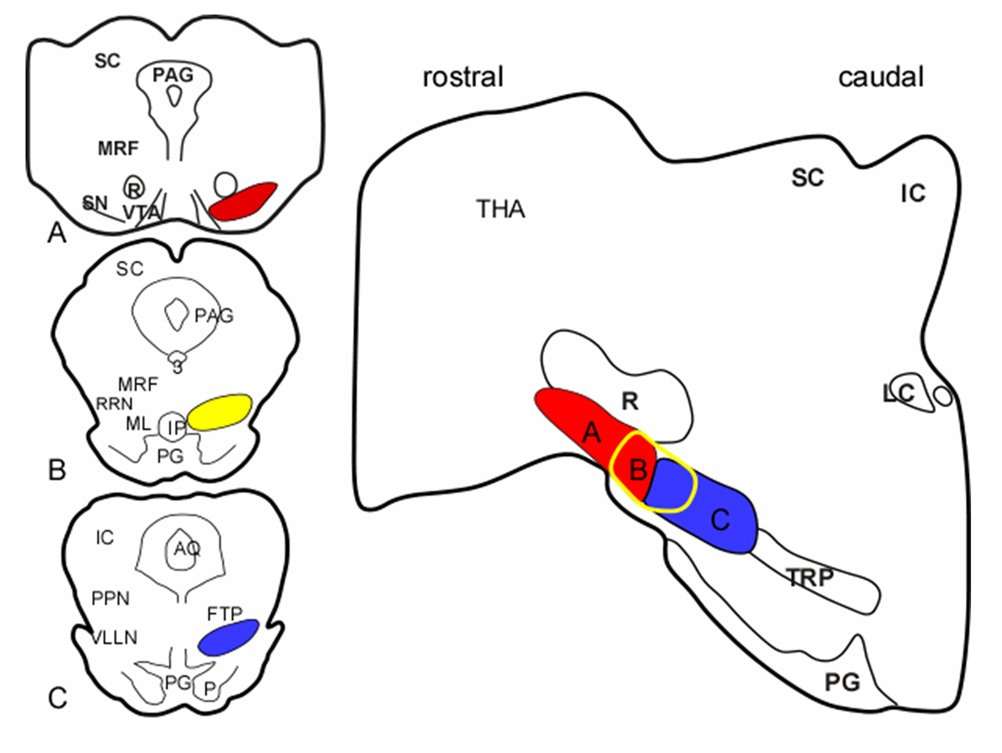
RLS and PLMD are closely associated with Parkinsonism (Wetter et al., 2000; Ondo et al., 2002; Gomez-Esteban et al., 2007). REM sleep without atonia (Wetter et al., 2001; Gagnon et al., 2002) and RBD (Schenck et al., 1996; Montplaisir et al., 1997; Boeve et al., 1998; Wetter et al., 2001; Gagnon et al., 2002; Askenasy, 2003) are commonly seen in Parkinsonism. Recent studies found that patients may be diagnosed simultaneously with Parkinson’s disease and RBD, or diagnosed with Parkinson’s disease and then develop RBD, or vice versa (Schenck et al., 1996; Olson et al., 2000; Eisensehr et al., 2001). However, neuronal degeneration is not found in the SN in idiopathic RBD (Boeve et al., 2007), but it is found in RLS patients (Allen et al., 2001), 85% of which have PLMD (Montplaisir et al., 1997). The association between Parkinson’s disease and RLS/PLMD/RBD has not been explained. The findings of the current and our previous studies (Lai et al., 1999b) suggest that there might be an anatomical link between Parkinson’s disease and RLS/PLMD/RBD (Fig. 9). Our present study showed that REM sleep without atonia and RBD-like behavior can be seen in cats with lesions in the rostral (B in Fig. 9) and caudal (C in Fig. 9) VMPJ, respectively. In conjunction with our previous findings that lesions in the rostroventral midbrain of the SN (A in Fig. 9), which is located rostral and adjacent to the VMPJ, induce sleep fragmentation and insomnia (Lai et al., 1999b), we hypothesize that neuronal degeneration can be generated in either part of the ventral brainstem, the rostral ventral midbrain and the VMPJ, and progressively extend to the caudal or rostral part of the brainstem (Lai and Siegel, 2003). Parkinson’s disease will develop first if the lesion starts in the rostral ventral midbrain, whereas REM sleep without atonia and/or RBD will be seen first if neuronal degeneration begins in the VMPJ (Lai and Siegel, 2003). The loss of dopaminergic neurons in the rostral ventral midbrain would be expected to cause further sleep disruption (Gerashchenko et al., 2006).
Figure 9.
Hypothetical anatomical link between motor disorders in sleep and Parkinsonism. The frontal sections (left) represent the corresponding areas shown on the sagittal section (right), and show the interface between the rostral midbrain and VMPJ. Neuronal degeneration in A (red area), which included substantia nigra, developed motor symptoms of Parkinsonism. Animals with neuronal degeneration in C (blue area), which is located in the caudal VMPJ, developed RBD. Whereas, neuronal degeneration in B (yellow circled), which is located in the rostral VMPJ generated PLMD and REM sleep without atonia. We hypothesize that neuronal degeneration may begin at either part of the ventral brainstem and then, extend to the rostral or caudal brainstem. IC: inferior colliculus, LC: locus coeruleus, PG: pontine gray, PPN: pedunculopontine nucleus, R: red nucleus, SC: superior colliculus, THA: thalamus, TRP: tegmental reticular nucleus, peripheral division.
Our present study found that neurotoxic lesion in the rostral and caudal VMPJ produced PLMD and REM sleep without atonia and RBD-like activity in the cat. Investigation of the neuronal activity and neurochemistry of the cell groups responsible for theses effects will further our understanding of both RBD and Parkinsonism and may suggest more effective treatments.
Acknowledgments
Funding: National Institutes of Health (NS042566 to Y.Y.L., HL041370 to J.M.S.) and Restless Leg Syndrome Foundation (Y.Y.L.) and the Medical Research Service of the Department of Veterans Affairs.
Abbreviations
- AQ
aqueduct
- ChAT
choline acetyltransferase
- CS
superior central nucleus
- DAB
3,3’-diaminobenzidine
- EMG
electromyogram
- EOG
electrooculogram
- FTP
paralemniscal tegmental field
- GABA
γ-aminobutyric acid
- Hip
hippocampal theta
- IC
inferior colliculus
- ILMI
isolated leg movement index
- INMI
isolated neck movement index
- IP
interpeduncular nucleus
- LC
locus coeruleus
- LGN
lateral geniculate nucleus
- LL
left limb muscle EMG
- LR
right limb muscle EMG
- ML
medial lemniscus
- MRF
mesencephalic reticular formation
- NL
left neck muscle EMG
- NMC
nucleus magnocellularis
- NMDA
N-methyl-D-aspartic acid
- NR
right neck muscle EMG
- P
pyramidal tract
- PAG
periaqueductal gray
- PBS
phosphate buffer saline
- PG
pontine gray
- PGO
ponto-geniculo-occipital
- PIA
pontine inhibitory area
- PLMD
periodic leg movements disorder
- PLMI
periodic leg movement index
- PNMI
periodic neck movement index
- PPN
pedunculopontine nucleus
- R
red nucleus
- R/A
REM sleep without atonia
- RBD
REM sleep behavior disorder
- REM
rapid eye movement
- RLS
restless legs syndrome
- RRN
retrorubral nucleus
- SC
superior colliculus
- SN
substantia nigra
- SWS
slow wave sleep
- TH
tyrosine hydroxylase
- THA
thalamus
- TRP
tegmental reticular nucleus, peripheral division
- VLLN
ventral nucleus of the lateral lemniscus
- VMPJ
ventral mesopontine junction
- VTA
ventral tegmental area
- 3
oculomotor nucleus
- 4
trochlear nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen RP, Barker PB, Wehrl F, Song HK, Earley CJ. MRI measurement of brain iron in patients with restless legs syndrome. Neurology. 2001;56:263–265. doi: 10.1212/wnl.56.2.263. [DOI] [PubMed] [Google Scholar]
- Askenasy JJM. Sleep disturbances in Parkinsonism. J Neural Transm. 2003;110:125–150. doi: 10.1007/s007020300001. [DOI] [PubMed] [Google Scholar]
- Berman AL. The brain stem of the cat. Madison: Univ of Wisconsin Press; 1968. [Google Scholar]
- Boeve BF, Silber MH, Ferman TJ, Kokmen E, Smith GE, Ivnik RJ, Parisi JE, Olson EJ, Petersen RC. REM sleep behavior disorder and degenerative dementia. Neurology. 1998;51:363–370. doi: 10.1212/wnl.51.2.363. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Silber MH, Ferman TJ. REM sleep behavior disorder in Parkinson’s disease and dementia with Lewy bodies. J Geriatr Psychiat Neurol. 2004;17:146–157. doi: 10.1177/0891988704267465. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Dickson DW, Olson EJ, Shepard JW, Silber MH, Ferman TJ, Ahlskog JE, Benarroch EE. Insights into REM sleep behavior disorder pathophysiology in brainstem-predominant Lewy body disease. Sleep Med. 2007;8:60–64. doi: 10.1016/j.sleep.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley CJ, Allen RP. Pergolide and carbidopa/levodopa treatment of the restless legs syndrome and periodic leg movements in sleep in a consecutive series of patients. Sleep. 1996;19:801–810. doi: 10.1093/sleep/19.10.801. [DOI] [PubMed] [Google Scholar]
- Eisensehr I, Lindeiner H, Jager M, Noachtar S. REM sleep behavior in sleep-disordered patients with versus without Parkinson’s disease: is there a need for polysomnography? J Neurol Sci. 2001;186:7–11. doi: 10.1016/s0022-510x(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Fantini ML, Gagnon JF, Filipini D, Montplaisir J. The effects of pramipexole in REM sleep behavior disorder. Neurology. 2003;61:1418–1420. doi: 10.1212/wnl.61.10.1418. [DOI] [PubMed] [Google Scholar]
- Fenik VB, Davies RO, Kubin L. Noradrenergic, serotonergic and GABAergic antagonists injected together into the XII nucleus abolish the REM sleep-like depression of hypoglossal motoneuronal activity. J Sleep Res. 2005a;14:419–429. doi: 10.1111/j.1365-2869.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005b;172:1322–1330. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik VB, Ogawa H, Davies RO, Kubin L. Carbachol injections into the ventral pontine reticular formation activate locus coeruleus cells in urethane-anesthetized rats. Sleep. 2005c;28:551–559. doi: 10.1093/sleep/28.5.551. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Barnes CD. Evidence of facilitatory coerulospinal action in lumbar motoneurons of cats. Brain Res. 1981;216:299–311. doi: 10.1016/0006-8993(81)90132-3. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Barnes CD. Membrane excitability changes in hindlimb motoneurons induced by stimulation of the locus coeruleus in cats. Brain Res. 1987;402:230–242. doi: 10.1016/0006-8993(87)90029-1. [DOI] [PubMed] [Google Scholar]
- Gagnon JF, Bedard MA, Fantini ML, Petit D, Panisset M, Rompre S, Carrier J, Montplaisir J. REM sleep behavior disorder and REM sleep without atonia in Parkinson’s disease. Neurology. 2002;59:585–589. doi: 10.1212/wnl.59.4.585. [DOI] [PubMed] [Google Scholar]
- Gatto EM, Uribe Roca MC, Martinez O, Valiensi S. Rapid eye movement (REM) sleep without atonia in two patients with corticobasal degeneration (CBD) Parkinsonism Related Disorders. 2007;13:130–132. doi: 10.1016/j.parkreldis.2006.07.012. [DOI] [PubMed] [Google Scholar]
- George R, Haslett WL, Jenden DJ. A cholinergic mechanism in the brainstem reticular formation: induction of paradoxical sleep. Int J Neuropharmacol. 1964;3:541–552. doi: 10.1016/0028-3908(64)90076-0. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Blanco-Centurion CA, Miller JD, Shiromani PJ. Insomnia following hypocretin2-saporin lesions of the substantia nigra. Neuroscience. 2006;137:29–36. doi: 10.1016/j.neuroscience.2005.08.088. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Darracq L, Fort P, Souliere F, Chouvet G, Luppi PH. Electrophysiological evidence that noradrenergic neurons of the rat locus coeruleus are tonically inhibited by GABA during sleep. Eur J Neurosci. 1998;10:964–970. doi: 10.1046/j.1460-9568.1998.00106.x. [DOI] [PubMed] [Google Scholar]
- Gibb WRG, Luthert PJ, Marsden CD. Corticobasal degeneration. Brain. 1989;112:1171–1192. doi: 10.1093/brain/112.5.1171. [DOI] [PubMed] [Google Scholar]
- Gomez-Esteban JC, Zarranz JJ, Tijero B, Velasco F, Barcena J, Rouco I, Lezcano E, Lachen MC, Jauregui A, Ugarte A. Restless legs syndrome in Parkinson’s disease. Move Disorders. 2007;22:1912–1916. doi: 10.1002/mds.21624. [DOI] [PubMed] [Google Scholar]
- Hajnik T, Lai YY, Siegel JM. Atonia-related regions in the rodent pons and medulla. J Neurophysiol. 2000;84:1942–1948. doi: 10.1152/jn.2000.84.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley K, Morrison AR. A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat. Acta Neurobiol Exp. 1974;34:215–232. [PubMed] [Google Scholar]
- Holmes CJ, Jones BE. Importance of cholinergic, GABAergic, serotonergic and other neurons in the medullary reticular formation for sleep-wake states studied by cytotoxic lesions in the cat. Neuroscience. 1994;62:1179–1200. doi: 10.1016/0306-4522(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Santamaria J, Pujol J, Moreno A, Deus J, Tolosa E. Brainstem proton magnetic resonance spectroscopy in idiopathic REM sleep behavior disorder. Sleep. 2002;25:867–870. [PubMed] [Google Scholar]
- Jones BE, Cuello AC. Afferents to the basal forebrain cholinergic cell area from pontomesencephalic-catecholamine, serotonin, and acetylcholine-neurons. Neurosci. 1989;31:37–61. doi: 10.1016/0306-4522(89)90029-8. [DOI] [PubMed] [Google Scholar]
- Kanamori N, Sakai K, Jouvet M. Neuronal activity specific to paradoxical sleep in the ventromedial medullary reticular formation of unrestrained cats. Brain Res. 1980;189:251–255. doi: 10.1016/0006-8993(80)90024-4. [DOI] [PubMed] [Google Scholar]
- Kodama T, Lai YY, Siegel JM. Enhancement of acetylcholine release during REM sleep in the caudomedial medulla as measured by in vivo microdialysis. Brain Res. 1992;580:348–350. doi: 10.1016/0006-8993(92)90967-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T, Lai YY, Siegel JM. Enhanced glutamate release during REM sleep in the rostromedial medulla as measured by in vivo microdialysis. Brain Res. 1998;780:178–181. [PMC free article] [PubMed] [Google Scholar]
- Kodama T, Lai YY, Siegel JM. Changes in inhibitory amino acid release linked to pontine-induced atonia: an in vivo microdialysis study. J Neurosci. 2003;23:1548–1554. doi: 10.1523/JNEUROSCI.23-04-01548.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Siegel JM. Medullary regions mediating atonia. J Neurosci. 1988;8:4790–4796. doi: 10.1523/JNEUROSCI.08-12-04790.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Siegel JM. Muscle tone suppression and stepping produced by stimulation of midbrain and rostral pontine reticular formation. J Neurosci. 1990;10:2727–2734. doi: 10.1523/JNEUROSCI.10-08-02727.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Siegel JM. Pontomedullary glutamate receptors mediating locomotion and muscle tone suppression. J Neurosci. 1991;11:2931–2937. doi: 10.1523/JNEUROSCI.11-09-02931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Siegel JM. Brainstem-mediated locomotion and myoclonic jerks. I. Neural substrates. Brain Res. 1997a;745:257–264. doi: 10.1016/s0006-8993(96)01177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Siegel JM. Brainstem-mediated locomotion and myoclonic jerks. II. Pharmacological effects. Brain Res. 1997b;745:265–270. doi: 10.1016/s0006-8993(96)01180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Siegel JM. Muscle atonia in REM sleep. In: Mallick BN, Inoue S, editors. Rapid eye movement sleep. New Delhi: Narosa Pub.; 1999. pp. 69–90. [Google Scholar]
- Lai YY, Siegel JM. Physiological and anatomical link between Parkinson-like disease and REM sleep behavior disorder. Mole Neurobiol. 2003;27:137–151. doi: 10.1385/MN:27:2:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Kodama T, Siegel JM. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: an in vivo microdialysis study. J Neurosci. 2001;23:1548–1554. doi: 10.1523/JNEUROSCI.21-18-07384.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Siegel JM, Wilson WJ. Effect of blood pressure on medial medullainduced muscle atonia. Am J Physiol. 1987;252:H1249–H1257. doi: 10.1152/ajpheart.1987.252.6.H1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Strahlendorf HK, Fung SJ, Barnes CD. The actions of two monoamines on spinal motoneurons from stimulation of the locus coeruleus in the cat. Brain Res. 1989;484:268–272. doi: 10.1016/0006-8993(89)90369-7. [DOI] [PubMed] [Google Scholar]
- Lai YY, Clements JR, Siegel JM. Glutamatergic and cholinergic projections to the pontine inhibitory area identified with horseradish peroxidase retrograde transport and immunohistochemistry. J Comp Neurol. 1993;336:321–330. doi: 10.1002/cne.903360302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Clements JR, Wu XY, Shalita T, Wu JP, Kuo JS, Siegel JM. Brainstem projections to the ventromedial medulla in cat: retrograde transport horseradish peroxidase and immunohistochemical studies. J Comp Neurol. 1999a;408:419–436. doi: 10.1002/(sici)1096-9861(19990607)408:3<419::aid-cne8>3.0.co;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Shalita T, Hajnik T, Wu JP, Kuo JS, Chia LG, Siegel JM. Neurotoxic N-methyl-D-aspartate lesion of the ventral midbrain and mesopontine junction alters sleep wake organization. Neuroscience. 1999b;90:469–483. doi: 10.1016/s0306-4522(98)00429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42:1371–1374. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- Liu RH, Fung SJ, Reddy VK, Barnes CD. Localization of glutamatergic neurons in the dorsolateral pontine tegmentum projecting to the spinal cord of the cat with a proposed role of glutamate on lumbar motoneuron activity. Neuroscience. 1995;64:193–208. doi: 10.1016/0306-4522(94)00354-8. [DOI] [PubMed] [Google Scholar]
- Luppi LH, Sakai K, Fort P, Salvert D, Jouvet M. The nuclei of origin of monoaminergic, peptidergic, and cholinergic afferents to the cat nucleus reticularis magnocellularis: a double-labeling study with cholera toxin as a retrograde tracer. J Comp Neruol. 1988;277:1–20. doi: 10.1002/cne.902770102. [DOI] [PubMed] [Google Scholar]
- Magoun HW, Rhines R. An inhibitory mechanism in the bulbar reticular formation. J Neurophysiol. 1946;9:165–171. doi: 10.1152/jn.1946.9.3.165. [DOI] [PubMed] [Google Scholar]
- Massicotte-Marquez J, Carrier J, Decary A, Mathieu A, Vendette M, Petit D, Montplaisir J. Slow-wave sleep and delta power in rapid eye movement sleep behavior disorder. Ann Neurol. 2005;57:277–282. doi: 10.1002/ana.20373. [DOI] [PubMed] [Google Scholar]
- Mazza S, Soucy J, Gravel P, Michaud M, Postuma R, Massicotte-Marquez J, Decary A, Montplaisir J. Assessing whole brain perfusion changes in patients with REM sleep behavior disorder. Neurology. 2006;67:1618–1622. doi: 10.1212/01.wnl.0000242879.39415.49. [DOI] [PubMed] [Google Scholar]
- McRitchie DA, Reid WGJ, Halliday GM, Hely MA, Brooks WS, Morris JGL. Neuropathology of three clinical cases prospectively diagnosed as dementia with Lewy bodies. J Clin Neurosci. 1999;6:149–154. doi: 10.1054/jocn.1998.0009. [DOI] [PubMed] [Google Scholar]
- Monti JM, Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev. 2007;11:113–133. doi: 10.1016/j.smrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Gobuout R, Poirier G, Bedard MA. Restless legs syndrome and periodic movements in sleep: physiopathology and treatment with L-Dopa. Clin. Neuropharmacol. 1986;9:456–463. doi: 10.1097/00002826-198610000-00006. [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Lapierre O, Warnes H, Pelletier G. The treatment of the restless leg syndrome with or without periodic leg movements in sleep. Sleep. 1992;15:391–395. [PubMed] [Google Scholar]
- Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Move Disord. 1997;12:61–65. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- Morrison AR. Paradoxical sleep without atonia. Arch Ital Biol. 1988;126:275–289. [PubMed] [Google Scholar]
- Morrison AR, Mann GL, Hendricks JC. The relationship of excessive exploratory behavior in wakefulness to paradoxical sleep without atonia. Sleep. 1981;4:247–257. [PubMed] [Google Scholar]
- Nitz D, Siegel JM. GABA release in the locus coeruleus as a function of sleep/wake state. Neurosci. 1997;78:795–801. doi: 10.1016/s0306-4522(96)00549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123:331–339. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- Ondo WG, Vuong KD, Jankovic J. Exploring the relationship between Parkinson disease and restless legs syndrome. Arch Neurol. 2002;59:421–424. doi: 10.1001/archneur.59.3.421. [DOI] [PubMed] [Google Scholar]
- Onoe H, Sakai K. Kainate receptors: a novel mechanism in paradoxical (REM) sleep generation. NeuroReport. 1995;6:353–356. [PubMed] [Google Scholar]
- Salva MAQ, Guilleminault C. Olivopontocerebellar degeneration, abnormal sleep, and REM sleep without atonia. Neurology. 1986;36:576–577. doi: 10.1212/wnl.36.4.576. [DOI] [PubMed] [Google Scholar]
- Sanford L, Cheng C, Tang X, Silvestri A, Mann G, Ross R, Morrison AR. Sleep and behavior in rats with pontine lesions producing REM sleep without atonia. Sleep Res Online. 2001;4:1–5. [Google Scholar]
- Sanford L, Yang L, Tang X, Ross R, Morrison AR. Tetrodotoxin inactivation of pontine regions: influence on sleep-wake states. Brain Res. 2005;1044:42–50. doi: 10.1016/j.brainres.2005.02.079. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Steinbusch HWM, Verhostad AAJ. The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res. 1983;277:355–360. doi: 10.1016/0006-8993(83)90945-9. [DOI] [PubMed] [Google Scholar]
- Schenck CH, Mahowald MW. Polysomnographic, neurologic, psychiatric, and clinical outcome report on 70 consecutive cases with REM sleep behavior disorder (RBD): sustained clonazepam efficacy in 89.5% of 57 treated patients. Cleveland Clin J Med. 1990;57(Suppl):S9–S23. [Google Scholar]
- Schenck CH, Mahowald MW. REM sleep parasomnias. Neurologic Clinics. 1996;14:697–720. doi: 10.1016/s0733-8619(05)70281-4. [DOI] [PubMed] [Google Scholar]
- Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology. 1996;46:388–393. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- Schenck CH, Hopwood J, Duncan E, Mahowald MW. Preservation and loss of REM atonia in human idiopathic REM sleep behavior disorder (RBD): quantitative polysomnographic (PSG) analyses in 17 patients. Sleep Res. 1992;21:16. [Google Scholar]
- Schenck CH, Hurwitz TD, Mahowald MW. REM sleep behavior disorder: an update on a series of 96 patients and a review of the world literature. J Sleep Res. 1993;2:224–231. doi: 10.1111/j.1365-2869.1993.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Schenkel E, Siegel JM. REM sleep without atonia after lesions of the medial medulla. Neurosci. Lett. 1989;98:159–165. doi: 10.1016/0304-3940(89)90503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouse MN, Siegel JM. Pontine regulation of REM sleep components in cats: integrity of the pedunculopontine tegmentum (PPT) is important for phasic events but unnecessary for atonia during REM sleep. Brain Res. 1992;571:50–63. doi: 10.1016/0006-8993(92)90508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer HM, Paul R, Shiromani P, Dement WC, Mignot E, Chiu C. Neuronal activity in narcolepsy: identification of cataplexy-related cells in the medial medulla. Science. 1991;252:1315–1318. doi: 10.1126/science.1925546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana N, Kimura K, Kitajima K, Nagamine T, Kimura J, Shibasake H. REM sleep without atonia at early stage of sporadic olivopontocerebellar atrophy. J Neurol Sci. 1995;132:28–34. doi: 10.1016/0022-510x(95)00119-m. [DOI] [PubMed] [Google Scholar]
- Ursin R, Sterman MB. A manual for standardized scoring of sleep and waking states in the adult cat. Los Angeles: UCLA/BRI; 1981. [Google Scholar]
- Verret L, Fort P, Gervasoni D, Leger L, Luppi PH. Localization of the neurons active during paradoxical (REM) sleep and projecting to the locus coeruleus noradrenergic neurons in the rat. J Comp Neurol. 2006;495:573–386. doi: 10.1002/cne.20891. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Brainstem afferents to the basal forebrain in the rat. Neurosci. 1988;24:907–935. doi: 10.1016/0306-4522(88)90077-2. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Projections of the dorsal raphe nucleus to the brainstem: PHA-L analysis in the rat. J Comp Neurol. 1994;340:11–26. doi: 10.1002/cne.903400103. [DOI] [PubMed] [Google Scholar]
- Webster HH, Jones BE. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. II. Effects upon sleep-waking states. Brain Res. 1988;458:285–302. doi: 10.1016/0006-8993(88)90471-4. [DOI] [PubMed] [Google Scholar]
- Wetter TC, Collado-Seidel V, Pollmacher T, Yassouridis A, Trenkwalder C. Sleep and periodic leg movement patterns in drug-free patients with Parkinson’s disease and multiple system atrophy. Sleep. 2000;23:361–367. [PubMed] [Google Scholar]
- Wetter TC, Trenkwalder C, Gershanik O, Hogl B. Polysomnographic measures in Parkinson's disease: a comparison between patients with and without REM sleep disturbances. Wien Klin Wochenschr. 2001;113:249–253. [PubMed] [Google Scholar]
- Willoughby JO, Blessing WW. Origin of serotonin innervation of the arcuate and ventromedial hypothalamic region. Brain Res. 1987;428:170–173. doi: 10.1016/0006-8993(87)90975-9. [DOI] [PubMed] [Google Scholar]
- Wu MF, Gulyani SA, Yau E, Mignot E, Phan B, Siegel JM. Locus coeruleus neurons: cessation of activity during cataplexy. Neurosci. 1999;91:1389–1399. doi: 10.1016/s0306-4522(98)00600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamuy J, Mancillas JR, Morales FR, Chase MH. C-fos expression in the pons and medulla of the cat during carbachol-induced active sleep. J Neurosci. 1993;13:2703–2718. doi: 10.1523/JNEUROSCI.13-06-02703.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekhlef F, Ballan G, Macia F, Delmer O, Sourgen C, Tison F. Routine MRI for the differential diagnosis in Parkinson’s disease, MSA, PSP, and CBD. J Neural Transm. 2003;110:151–169. doi: 10.1007/s00702-002-0785-5. [DOI] [PubMed] [Google Scholar]
- Zambelis T, Soldatos CR. REM sleep behavior disorder associated with a neurinoma of the left pontocerebellar angle. J Neurol Neurosurg Psychiatry. 2002;72:821–822. doi: 10.1136/jnnp.72.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucconi M, Ferri R, Allen R, Baier PC, Bruni O, Chokroverty S, Ferini-Strambi L, Fulda S, Garcia-Borreguero D, Hening WA, Hirshkowitz M, Hogl B, Hornyak M, King M, Montagna P, Parrino L, Plazzi G, Terzano MG. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–183. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]