Abstract
Radiation treatment planning for women with locally advanced cervical cancer (stages IB2-IVA) is often based on positron emission tomography (PET). PET, however, has poor sensitivity in detecting metastases in aortocaval nodes. We have initiated a study that aims to determine if pretherapeutic laparoscopic surgical staging followed by tailored chemoradiation improves survival compared to PET/CT radiologic staging alone followed by chemoradiation. This international, multicenter phase III trial will enroll 600 women with stages IB2-IVA cervical cancer and PET/CT imaging showing fluorodeoxyglucose (FDG)-avid pelvic nodes and FDG-negative paraaortic nodes. Eligible patients will be randomized to either pelvic radiotherapy with chemotherapy (standard-of-care arm) or surgical staging via a minimally invasive extraperitoneal approach followed by tailored radiotherapy with chemotherapy (experimental arm). The primary endpoint is overall survival. Secondary endpoints are disease-free survival, short- and long-term morbidity of pretherapeutic surgical staging, and determination of anatomic locations of metastatic paraaortic nodes in relationship to the inferior mesenteric artery. We believe this study will show that tailored chemoradiation after pretherapeutic surgical staging improves survival compared to chemoradiation based on PET/CT in women with stages IB2-IVA cervical cancer.
Keywords: ervix, surgical staging, PET/CT, laparoscopy
Introduction
Although cancer of the uterine cervix remains a clinically staged disease, it is not uncommon for patients with cervical cancer to undergo computed tomography (CT), magnetic resonance imaging (MRI), and/or positron emission tomography (PET) as part of their work-up. Clinicians may glean important information from these imaging studies. First, the presence of positive paraaortic lymph nodes remains the most important prognostic factor for survival in patients with cervical cancer [1]. In addition, the presence or absence of metastases in the lymph nodes guides treatment planning. This may include the choice of primary surgery or radiotherapy as well as definition of the radiation fields.
Unfortunately, none of the imaging modalities currently available has proven to be particularly sensitive in detecting metastases in paraaortic lymph nodes in patients with locally advanced cervical cancer (stages IB2-IVA). CT has a sensitivity of only 67% [2], and while MRI may perform well in the detection of parametrial, bladder, and rectal invasion from primary cervical tumors, it performs poorly in the detection of lymph node metastasis. In a meta-analysis of MRI for the detection of lymph node metastasis, Scheidler et al. [3] reported an overall sensitivity of MRI of 38%. Other authors found that MRI had a sensitivity of 0% in the detection of positive paraaortic lymph nodes when compared to surgery and pathologic evaluation [4].
Currently, fluorodeoxyglucose (FDG)-PET is being utilized in attempts to better detect paraaortic lymph node spread in patients with cervical cancer. In a combined analysis of results of four prospective studies that compared FDG-PET to the gold standard of surgical staging, Havrilesky et al. [5] found an overall sensitivity of 84% (95% CI, 68–94%). Thus, although PET is an improvement over CT, even with PET, 16% (and as many as 32%) of patients with metastases in paraaortic lymph nodes will be incorrectly classified as node negative. The implications of undertreating these patients may be disastrous, given that the survival rate for patients with histologically positive paraaortic lymph nodes treated with extended-field radiation therapy is as high as 50% [6, 7].
Alternately, in an effort to not undertreat the 16–32% of patients who have falsely negative PET scans for paraaortic disease, radiation oncologists may prophylactically extend the field to include these nodal basins. However, doing so is not without significant additional morbidity. The risk of grade 3 or 4 toxicity with extended field radiation approaches 15%. [7, 8] This includes 9% of patients who will experience at least grade 3 gastrointestinal toxicity which is often chronic and debilitating. In addition, there is significant treatment-related mortality with extending the radiation field. In one large phase III study, 1.5% of patients died from complications of extended-field radiation therapy compared to 0% who received only pelvic radiation with concurrent chemotherapy. [8]
Two recent phase II studies have further evaluated the sensitivity of FDG-PET/CT in the detection of pathologically positive paraaortic nodes [9, 10]. In these studies, all patients had stages IB2-IVA cervical cancer and underwent PET/CT followed by surgical staging via removal of the paraaortic nodes utilizing a laparoscopic extraperitoneal approach. In our study, 60 evaluable patients successfully underwent the procedure. The overall sensitivity of PET scan in detecting metastatic disease to the paraaortic nodes when CT scan or MRI was negative was only 36%. For women with a completely negative PET scan, 12% had disease spread to nodes along the aorta [9]. A similar study was performed in France where 98 patients with locally advanced cervical cancer underwent PET scan followed by laparoscopic extraperitoneal paraaortic lymphadenectomy. For all patients, the sensitivity of the PET/CT scan was 91.6% in detecting disease in the paraaortic region [10].
In both studies, however, for women with PET/CT scans showing probable metastases in pelvic nodes and disease-free paraaortic nodes, 21–24% were found to have positive paraaortic nodes on standard H and E pathologic review of the surgical specimen [9, 10]. Furthermore, when ultrastaging and immunohistochemistry are performed on nodes thought negative after standard H and E pathologic processing, an additional 2–8% of patients will have additional micrometastatic disease in these lymph nodes [11, 12]. Had treatment planning been based solely on best available imaging (PET/CT), a significant number of women with paraaortic metastases would have been treated with pelvic irradiation only and would have been undertreated.
Recent data suggest that in addition to providing important prognostic and therapy-directing information, pre-radiotherapy lymph node dissection provides a therapeutic benefit. Marnitz et al. [13] reported their experience with laparoscopic transperitoneal surgical staging in 84 patients with clinically advanced cervical cancer. They found that patients with microscopically positive paraaortic lymph nodes that were resected laparoscopically had the same survival as patients with pathologically negative paraaortic lymph nodes on surgical staging. LeBlanc et al. [14] reported their data on surgical staging via a laparoscopic extraperitoneal approach in 156 patients with locally advanced cervical cancer. They too found that patients who underwent resection of microscopically positive paraaortic lymph nodes followed by tailored extended-field radiotherapy had the same survival as patients with negative lymph nodes who underwent pelvic irradiation.
Although it is commonly accepted that FDG-PET/CT performs poorly in the detection of microscopic disease spread to the paraaortic nodes in women with locally advanced cervical cancer, it is not known whether surgical staging improves outcomes for these patients. We therefore have initiated LiLACS (Lymphadenectomy in Locally Advanced Cervical Cancer Study), a prospective randomized controlled trial comparing radiologic to surgical staging. The primary objective of this trial is to determine if pretherapeutic paraaortic surgical staging followed by tailored chemoradiation is associated with longer survival than standard radiologic staging with PET/CT followed by whole-pelvis chemoradiation in patients with locally advanced cervical cancer. Secondary objectives are to determine disease-free survival, the short- and long-term morbidity of pretherapeutic surgical staging and radiotherapy (up to 5 years), and the anatomic locations of metastatic paraaortic nodes in relationship to the inferior mesenteric artery. Information about the locations of at-risk paraaortic nodes should help resolve the debate as to whether the upper boundary of surgical staging for cervical cancer should be the inferior mesenteric artery or the renal vessels.
Trial Design
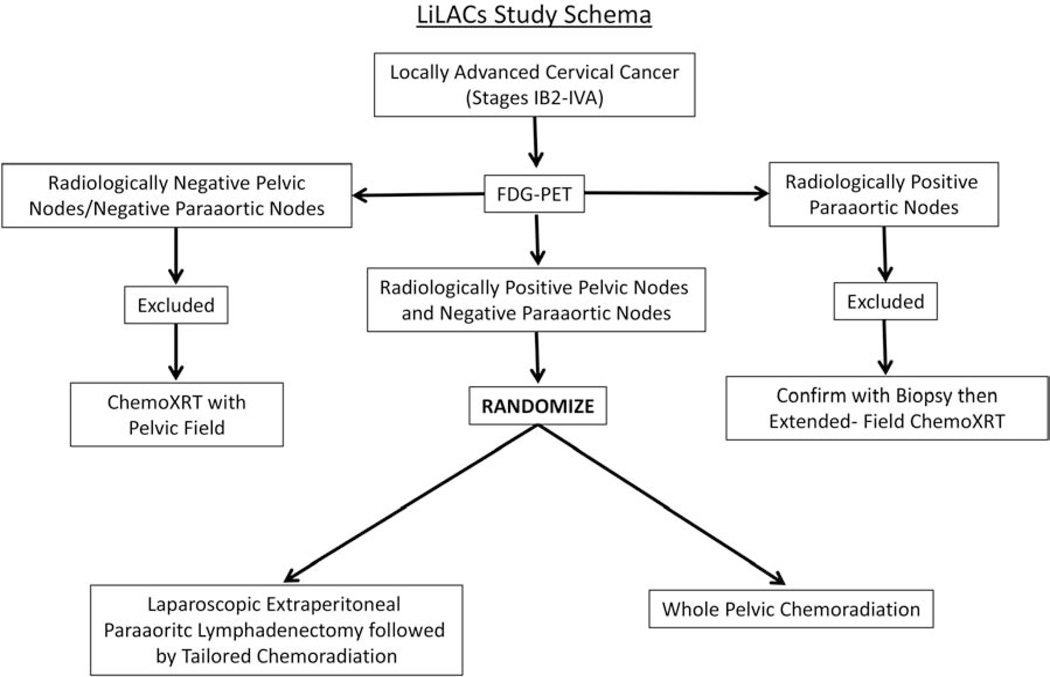
This international, multicenter phase III study will recruit patients with locally advanced cervical cancer (stages IB2-IVA) and PET/CT showing FDG-positive pelvic nodes and FDG-negative paraarotic nodes. Eligible patients will be randomized 1:1 to either standard pelvic chemoradiation (standard-of-care arm) or pretherapeutic paraaortic lymphadenectomy followed by tailored chemoradiation (experimental arm) (Figure 1). All patients will receive platinum-based chemotherapy with definitive radiotherapy. Pretherapeutic lymphadenectomy will be performed via a minimally invasive extraperitoneal approach using either traditional laparoscopy or robotically assisted laparoscopy.
Figure 1.
The study will be partially funded by MD Anderson Cancer Center through donor and institutional funds. For the participating French centers, funding has been obtained through the Programme Hospitalier de Recherche Clinique (Institut National du Cancer).
Eligibility Criteria for Participating Surgeons/Centers
Interested gynecologic oncologists will contact the principal investigators directly. To participate in this trial, surgeons must have performed at least 10 laparoscopic or robotically assisted extraperitoneal lymph node dissections and provide an unedited video of one surgery. Each video will be evaluated and judged by the Trial Management Committee on the basis of surgeon technique and tissue handling, competency in identification and dissection of proper anatomic structures, surgical technique with respect to blood loss and prevention of intraoperative injury, appropriate decision making based on intraoperative findings, and appropriate use of instrumentation for all parts of the procedure. If the investigator and/or his or her institution has published results and outcomes in the peer-reviewed literature that demonstrate skill and acceptable technique in performing laparoscopic or robotically assisted extraperitoneal lymph node dissection, then they may be exempt from providing the above evidence of skill. Once the principal investigator at a site is accredited, he or she can then judge the competency of local collaborating surgeons at his or her institution who wish to enroll patients, ensuring that the above criteria are met.
Eligibility Criteria for Patients
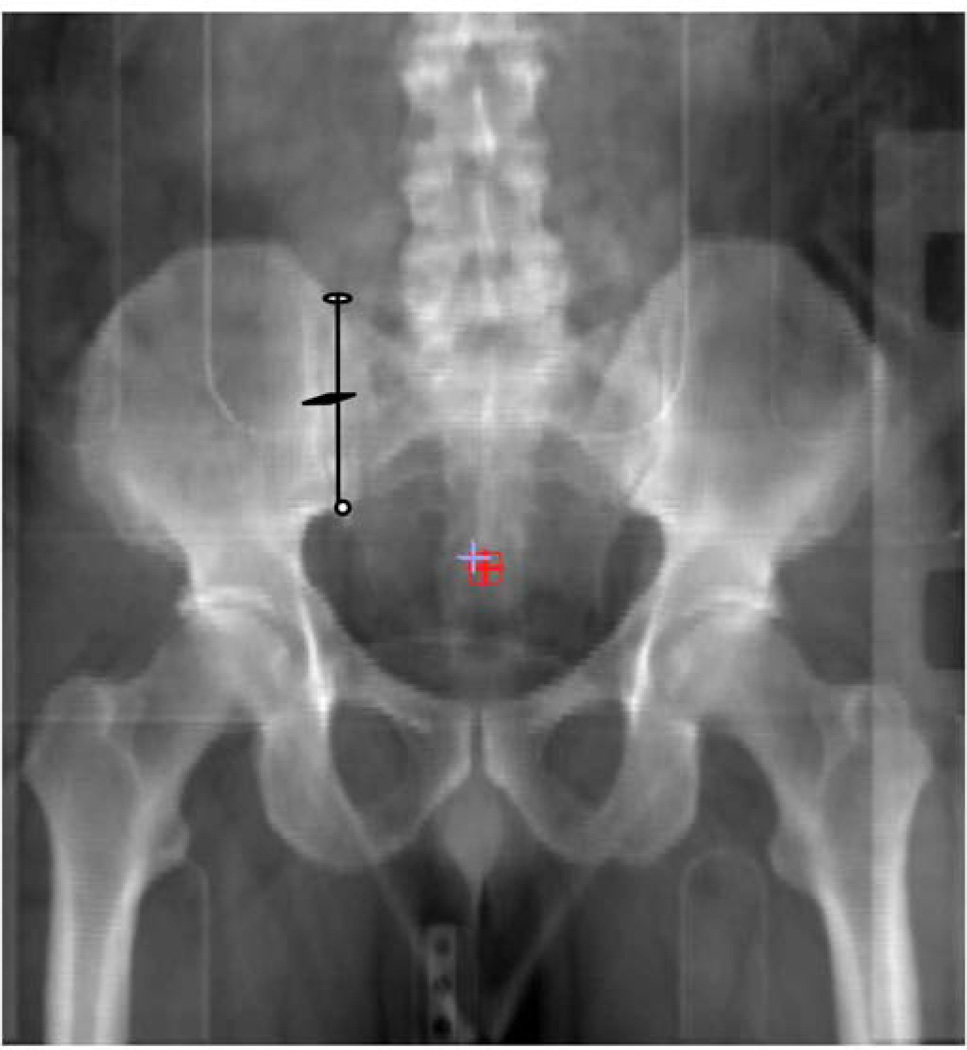
Eligible patients will include women who have clinical stages IB2-IVA cervical squamous cell carcinoma, adenosquamous carcinoma, or adenocarcinoma; have FDG-positive or indeterminate pelvic lymph nodes or indeterminate low common iliac nodes; have FDG-negative paraaortic nodes; and are dispositioned to definitive chemoradiation with the intent to cure. (Definitions of nodal status appear in the next section.) For a patient to be eligible for this study, the highest positive common iliac node must lie inferior to the mid-sacroiliac point (Figure 2). This criterion was specified as ethically we could not justify a pelvic-only radiation field in a patient with a high common iliac node regardless of the absence of FDG avidity along the aorta or vena cava.
Figure 2.
Women will be excluded if they have previously undergone pelvic radiotherapy or retroperitoneal surgery. In addition, women will be excluded if they have undergone simple or radical hysterectomy prior to radiotherapy or if their planned treatment is radiotherapy only (without chemotherapy) or palliative radiotherapy. Finally, women with known disease outside the pelvis or recurrent disease will not be eligible.
Definitions of Positive and Indeterminate Nodes
All scans will be performed on a dedicated PET/CT scanner that will allow fusion of PET and CT images. Any lymph node in the abdomen or pelvis larger than 10 mm on CT will be considered abnormal. Standard imaging protocols for PET/CT will be followed in accordance with Society of Nuclear Medicine guidelines [15]. Noncontrast CT will be used for attenuation correction.
The European Association of Nuclear Medicine procedure guidelines for tumor PET imaging, version 1.0 [16], state that “it is impossible to give universal rules for [quantitative] detection limits” for what should be considered a positive node on PET. The guidelines go on to say that “detection limits obviously depend on the degree of contrast between the tumor and its immediate surroundings. There is no single detection limit for FDG- PET since it depends on many factors. The most significant of these are: histology (FDG avidity of the type of tumor), the volume of vital tumor cells, movement during acquisition (e.g. blurred signals in the case of pulmonary foci), and physiological uptake in the adjacent background” [16]. Therefore, pelvic or paraaortic nodes that are focally more avid than background will be considered positive. Nodes with no detectable FDG uptake above background activity will be considered negative. There is no standard standard uptake value above or below which nodes will be considered positive or negative.
Pretherapeutic Paraaortic Lymphadenectomy
Pretherapeutic paraaortic lymphadenectomy will be performed no later than 3 weeks after PET/CT. The paraaortic lymphadenectomy will be performed through a left-sided laparoscopic extraperitoneal approach [17]. Lymph-node-bearing tissue from the aorta, aortocaval space, vena cava, and superior bilateral common iliac vessels will be completely removed. Nodal specimens removed from the area between the inferior mesenteric artery and the renal vessels will be labeled “supramesenteric lymph nodes”, while nodal specimens removed from the area between the inferior mesenteric artery and the bifurcation of the great vessels will be labeled “inframesenteric lymph nodes” [18, 19].
The lymphadenectomy may be completed via traditional laparoscopy or robotically assisted laparoscopy. If the surgeon believes that the laparoscopic retroperitoneal approach is not feasible, a laparoscopic transperitoneal approach may be attempted to complete the procedure. If a laparoscopic transperitoneal approach is not successful, the patient may undergo an open retroperitoneal dissection, an open transperitoneal dissection, or discontinuation of the staging procedure at the surgeon’s discretion.
If fixed or enlarged (>2 cm) lymph nodes are encountered, pathologic confirmation of metastases will be obtained intraoperatively and attempts will then be made to debulk them laparoscopically. Failing this, either clips will be applied to demarcate the area so that a boost dose of radiotherapy can be delivered later, or the procedure will be converted to an extraperitoneal laparotomy with open debulking.
Radiation Treatment Plan
For women in the experimental arm, further management of the primary cervical cancer will be tailored according to the results of the pretherapeutic staging procedure. Patients with negative paraaortic lymph nodes will be treated with external beam radiotherapy to the pelvis (as defined by the surgical clips applied at the lower limit of the paraaortic node dissection) to a usual dose of 45 Gy. Limited boosts will be indicated individually on clinically involved parametria or pelvic nodes. The external beam radiotherapy will be followed by intracavitary brachytherapy (high dose rate, low dose rate, or pulsed dose rate) with intent to cure. Patients with metastases in paraaortic lymph nodes will receive extended-field external beam radiotherapy followed by intracavitary brachytherapy with intent to cure. Patients who complete both external beam radiotherapy and intracavitary brachytherapy will receive a total dose of 80 to 90 Gy low-dose equivalent to Point A. Concurrent platinum-based chemotherapy will be given with definitive radiotherapy. Chemotherapy will be administered according to each participating institution’s standard practice. Patients with carcinomatosis discovered intraoperatively will be given platinum-based chemotherapy along with palliative radiotherapy if indicated.
For women in the standard-of-care arm, external beam radiotherapy will be delivered to the pelvis to a usual dose of 45 Gy. Limited boosts will be indicated individually on clinically involved parametria or pelvic nodes. The external beam radiotherapy will be followed by intracavitary brachytherapy with intent to cure (high dose rate, low dose rate, or pulsed dose rate). Patients who complete both external beam radiotherapy and intracavitary brachytherapy will receive a total dose of 80 to 90 Gy low-dose equivalent to Point A. Concurrent platinum-based chemotherapy will be given with definitive radiotherapy.
Follow-Up/Surveillance
PET/CT will be performed 3 months after completion of chemoradiation. Thereafter, CT scan of the abdomen and pelvis and plain film chest x-ray will be performed every 6 months for the next 3 years. After completion of chemoradiation, patients will have clinical examinations by an oncologist every 3 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter. Patients will leave the study at 5 years after completion of initial chemoradiation if they are disease free or at the time of death.
Statistical Considerations
Sample Size
Data collected in the phase II study of laparoscopic extraperitoneal paraaortic lymphadenectomy in locally advanced cervical cancer at MD Anderson [9] indicate that approximately 32% of women with locally advanced cervical cancer will meet the inclusion criteria for LiLACS. Of these women, approximately 79% will truly have negative paraaortic lymph nodes. For them, the 3-year overall survival rate with whole-pelvis radiotherapy and concurrent chemotherapy is predicted to be 75%. [20] For the approximately 21% of women with false-negative imaging findings (i.e. negative findings on imaging but pathologically positive paraaortic lymph nodes), the 3-year overall survival rate with whole-pelvis radiotherapy (without extension of the field to cover the paraaortic nodes) and concurrent chemotherapy is predicted to be only 10%. [21] If these metastases were known, however, and the radiation field were extended to cover the paraaortic basins, the 3-year overall survival rate would be expected to improve to 50% [7]. Thus, the expected overall survival rate would be approximately 61% without surgical staging and 70% with preoperative paraaortic surgical evaluation. A sample size of 600 patients will yield 80% power with a 2-sided 0.05 significance level to detect an absolute 9% difference in 3-year overall survival rate (between 61% in the patients without preoperative paraaortic surgical staging and 70% in the patients with preoperative paraaortic surgical staging). We expect that accrual of the 600 patients will be completed in 2018. This sample size calculation includes an interim analysis for efficacy and futility once 174 deaths have been observed following the methods of Lan and DeMets [22].
Randomization
Demographic data, tumor characteristics, and PET/CT imaging results for prospective patients will be entered into a web-based data collection program housed at MD Anderson Cancer Center. Once patients have been deemed eligible by the study coordinators, they will provide written informed consent and then be randomized to one of the two treatment groups with an equal allocation between groups (1:1).
Statistical Analysis
As the primary endpoint is overall survival, we will estimate overall survival with the product limit estimator of Kaplan and Meier [23], and we will estimate the 3-year overall survival for each treatment group with a 95% confidence interval. We will also estimate the median overall survival for each treatment group with a 95% confidence interval. We will use the log-rank test to compare the two treatment groups with respect to overall survival with a nominal significance level of 0.049. We will also use the log-rank test for the interim analysis of overall survival, which will take place after 174 deaths have been observed. If the p value from this test is less than 0.0031, the study will be stopped for efficacy, and if the p value from this test is greater than 0.3217, the study will be stopped for futility.
We will use a Cox [24] proportional hazards regression to model overall survival as a function of treatment group and other well-known and potential prognostic factors, such as clinical stage, histology, and grade. We will estimate the hazard ratio for treatment (preoperative paraaortic surgical staging vs. not) and other potential prognostic factors with 95% confidence intervals. We will perform analyses for disease-free survival similar to the analyses for overall survival.
Discussion
The primary objective of LiLACS is to determine if surgical staging for women with stages IB2-IVA cervical cancer improves overall survival. This international, multicenter phase III trial is powered to show an absolute 9% improvement in the 3-year overall survival rate for women who undergo surgical staging (experimental arm) compared to women who have treatment planning based on PET/CT (standard-of-care arm). In addition, the study will determine progression-free survival as well as the intraoperative and postoperative morbidity of minimally invasive extraperitoneal lymphadenectomy. Finally, this study will determine the anatomic locations of at-risk paraaortic nodes as there remains debate as to whether the upper boundary of surgical staging for cervical cancer should be the inferior mesenteric artery or the renal vessels [18, 19].
Currently, GOG233/ACRIN6671 is evaluating the utility of preoperative PET/CT in detecting lymph node metastases in women with cervical and uterine cancers. For the cervical cancer arm, women with locally advanced cervical cancer (stages IB2, IIA > 4cm, and IIB-IVA) will undergo PET/CT followed by open extra-peritoneal or laparoscopic transperitoneal pelvic and paraaortic lymphadenectomies in an effort to determine the diagnostic sensitivity and specificity of PET/CT in identifying lymph node metastases to pelvic and abdominal lymph nodes. Presumably, this study will confirm all prior studies that PET/CT has a poor sensitivity in detecting paraaortic nodal metastases in women with cervical cancer [5, 9, 10]. GOG233/ACRIN6671 does not include any endpoints for recurrence or survival in these patients. LiLAC is designed to answer the question if reliance only on the poorly sensitive PET/CT for treatment planning translates into a worse survival for women with locally advanced cervical cancer.
Surgical treatment of gynecologic cancers is based largely on anecdotal experience, case series, and retrospective comparisons. There are very few published large, phase III clinical trials on which to base our operative approach to gynecologic malignancies. In fact, fewer than 1% of the published articles in the gynecologic oncology literature describe randomized controlled trials of surgical procedures. [25] The importance of changing care on the basis of appropriately designed prospective studies cannot be overemphasized. Although LiLACS will need to enroll 600 patients and will require an estimated 8 years to complete (5 years to accrue patients and 3 years of follow-up), we believe this trial is necessary to provide the best outcomes for our patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest to disclose
References
- 1.Creasman WT, Kohler MF. Is lymph vascular space involvement an independent prognostic factor in early cervical cancer? Gynecol Oncol. 2004;92:525–529. doi: 10.1016/j.ygyno.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Camilien L, Gordon D, Fruchter RG, Maiman M, Boyce JG. Predictive value of computerized tomography in the presurgical evaluation of primary carcinoma of the cervix. Gynecol Oncol. 1988;30:209–215. doi: 10.1016/0090-8258(88)90026-1. [DOI] [PubMed] [Google Scholar]
- 3.Scheidler J, Hricak H, Yu KK, Subak L, Segal MR. Radiological evaluation of lymph node metastases in patients with cervical cancer. A meta-analysis. Jama. 1997;278:1096–1101. [PubMed] [Google Scholar]
- 4.Chung HH, Lee S, Sim JS, Kim JY, Seo SS, Park SY, Roh JW. Pretreatment laparoscopic surgical staging in locally advanced cervical cancer: preliminary results in Korea. Gynecol Oncol. 2005;97:468–475. doi: 10.1016/j.ygyno.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Havrilesky LJ, Kulasingam SL, Matchar DB, Myers ER. FDG-PET for management of cervical and ovarian cancer. Gynecol Oncol. 2005;97:183–191. doi: 10.1016/j.ygyno.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Goff BA, Muntz HG, Paley PJ, Tamimi HK, Koh WJ, Greer BE. Impact of surgical staging in women with locally advanced cervical cancer. Gynecol Oncol. 1999;74:436–442. doi: 10.1006/gyno.1999.5472. [DOI] [PubMed] [Google Scholar]
- 7.Varia MA, Bundy BN, Deppe G, Mannel R, Averette HE, Rose PG, Connelly P. Cervical carcinoma metastatic to para-aortic nodes: extended field radiation therapy with concomitant 5-fluorouracil and cisplatin chemotherapy: a Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys. 1998;42:1015–1023. doi: 10.1016/s0360-3016(98)00267-3. [DOI] [PubMed] [Google Scholar]
- 8.Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, Rotman M, Gershenson D, Mutch DG. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 9.Ramirez PT, Jhingran A, Macapinlac HA, Euscher ED, Munsell MF, Coleman RL, Soliman PT, Schmeler KM, Frumovitz M, Ramondetta LM. Laparoscopic extraperitoneal para-aortic lymphadenectomy in locally advanced cervical cancer: a prospective correlation of surgical findings with positron emission tomography/computed tomography findings. Cancer. 2011;117:1928–1934. doi: 10.1002/cncr.25739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uzan C, Souadka A, Gouy S, Debaere T, Duclos J, Lumbroso J, Haie-Meder C, Morice P. Analysis of morbidity and clinical implications of laparoscopic para-aortic lymphadenectomy in a continuous series of 98 patients with advanced-stage cervical cancer and negative PET-CT imaging in the para-aortic area. Oncologist. 2011;16:1021–1027. doi: 10.1634/theoncologist.2011-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez A, Mery E, Ferron G, Querleu D. Incidence of micrometastases in histologically negative para-aortic lymph nodes in advanced cervical cancer patients. Gynecol Oncol. 2010;119:76–80. doi: 10.1016/j.ygyno.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Zand B, Euscher ED, Soliman PT, Schmeler KM, Coleman RL, Frumovitz M, Jhingran A, Ramondetta LM, Ramirez PT. Rate of para-aortic lymph node micrometastasis in patients with locally advanced cervical cancer. Gynecol Oncol. 2010;119:422–425. doi: 10.1016/j.ygyno.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marnitz S, Kohler C, Roth C, Fuller J, Hinkelbein W, Schneider A. Is there a benefit of pretreatment laparoscopic transperitoneal surgical staging in patients with advanced cervical cancer? Gynecol Oncol. 2005;99:536–544. doi: 10.1016/j.ygyno.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Leblanc E, Narducci F, Frumovitz M, Lesoin A, Castelain B, Baranzelli MC, Taieb S, Fournier C, Querleu D. Therapeutic value of pretherapeutic extraperitoneal laparoscopic staging of locally advanced cervical carcinoma. Gynecol Oncol. 2007;105:304–311. doi: 10.1016/j.ygyno.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, Townsend DW, Berland LL, Parker JA, Hubner K, Stabin MG, Zubal G, Kachelriess M, Cronin V, Holbrook S. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47:885–895. [PubMed] [Google Scholar]
- 16.Boellaard R, O'Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, Oyen WJ, Kotzerke J, Hoekstra OS, Pruim J, Marsden PK, Tatsch K, Hoekstra CJ, Visser EP, Arends B, Verzijlbergen FJ, Zijlstra JM, Comans EF, Lammertsma AA, Paans AM, Willemsen AT, Beyer T, Bockisch A, Schaefer-Prokop C, Delbeke D, Baum RP, Chiti A, Krause BJ. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37:181–200. doi: 10.1007/s00259-009-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Querleu D, Dargent D, Ansquer Y, Leblanc E, Narducci F. Extraperitoneal endosurgical aortic and common iliac dissection in the staging of bulky or advanced cervical carcinomas. Cancer. 2000;88:1883–1891. [PubMed] [Google Scholar]
- 18.Frumovitz M, Ramirez PT, Macapinlac HA, Klopp AH, Nick AM, Ramondetta LM, Jhingran A. Anatomic location of PET-positive aortocaval nodes in patients with locally advanced cervical cancer: implications for surgical staging. Int J Gynecol Cancer. 2012;22:1203–1207. doi: 10.1097/IGC.0b013e31825e523a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gil-Moreno A, Magrina JF, Perez-Benavente A, Diaz-Feijoo B, Sanchez-Iglesias JL, Garcia A, Cabrera-Diaz S, Puig O, Martinez-Gomez X, Xercavins J. Location of aortic node metastases in locally advanced cervical cancer. Gynecol Oncol. 2012;125:312–314. doi: 10.1016/j.ygyno.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, Rotman M, Gershenson DM, Mutch DG. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 21.Rotman M, Pajak TF, Choi K, Clery M, Marcial V, Grigsby PW, Cooper J, John M. Prophylactic extended-field irradiation of para-aortic lymph nodes in stages IIB and bulky IB and IIA cervical carcinomas. Ten-year treatment results of RTOG 79-20. JAMA. 1995;274:387–393. [PubMed] [Google Scholar]
- 22.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 24.Cox DR. Regression models and life tables (with discussion) Journal of the Royal Statistical Society. 1972;34:187–220. [Google Scholar]
- 25.Obermair A. Is surgical literature relevant? Int J Gynecol Cancer. 2010;20:1300. doi: 10.1111/IGC.0b013e3181f5110b. [DOI] [PubMed] [Google Scholar]