Introduction
The US HIV epidemic is characterized by low HIV prevalence in the general population and cases densely concentrated in the most socially disenfranchised and marginalized communities (El-Sadr, Mayer, & Hodder, 2010). Conditions of poverty including substance abuse, unstable housing, lack of transportation, and food insecurity are therefore prevalent among people living with HIV and are associated with poor health outcomes (Adler & Ostrove, 1999; Adler & Stewart, 2010). For people living with HIV, poverty can influence HIV treatment adherence with even modest disruptions in treatment yielding significant clinical impacts (Parienti, Das-Douglas, Massari et al., 2008). There is general agreement that patients should be told to take every dose of their medications and that those with adherence less than 85% can risk developing viral resistance (Bangsberg, Kroetz, & Deeks, 2007).
A significant factor contributing to medication non-adherence among the poor is food insecurity, defined as limited access to nutritious food to meet dietary needs for an active and healthy life (Ivers, Cullen, Freedberg et al., 2009). For example, nearly half of HIV positive individuals receiving drug treatment in British Columbia, Canada are food insecure, a rate that is five times greater than the Canadian population (Normen, Chan, Braitstein et al., 2005). Food insecurity can directly contribute to HIV infection by compromising health in general and indirectly impacting HIV disease progression through treatment non-adherence (van der Sande, Schim van der Loeff, Aveika et al., 2004). Food insecurity can interfere with the absorption of medications and the pharmacokinetics of antiretroviral therapy (ART) (Alghamdi, Sheth, Manowski, Djoleto, & Bhatnagar, 2009). Food insecurity among people living with HIV also increases the likelihood of medical hospitalizations more than two-fold and nearly as much for emergency room visits (Weiser, Hatcher, Frongillo et al., 2012).
Another reliable predictor of HIV treatment non-adherence is alcohol consumption. Alcohol use can impair memory, disrupt organizational skills, disturb sleep patterns, and interfere with managing medications (Hendershot, Stoner, Pantalone, & Simoni, 2009). Alcohol consumption also has adverse health effects and is associated with malnutrition in its own right. Alcohol use and food insecurity therefore pose significant threats to the health of people living with HIV, including their impacts on medication adherence. Although alcohol use and food insecurity co-occur in impoverished communities, we are not aware of previous research investigating the impact of food insecurity on treatment adherence among people living with HIV who drink alcohol.
The current study examined food insecurity in relation to ART adherence and health outcomes among HIV positive men and women who drink alcohol and are taking ART. We hypothesized that food insecurity would predict ART non-adherence and HIV non-suppression over and above alcohol use. We also hypothesized that among people living with HIV who drink alcohol, experiencing food insecurity would be related to a greater number of medical and psychiatric hospitalizations.
Methods
Participants
People living with HIV who were currently receiving ART and drinking alcohol (N = 183) were reached through community recruitment strategies to infectious disease clinics and social service agencies serving people living with HIV in Atlanta, GA. Interested persons contacted our research program to schedule an intake assessment appointment. The study entry criteria were (a) 18 years of age or older, (b) HIV positive and prescribed ART, and (c) drank alcohol in the past week.
Measures
Participants provided four sources of data. First, participants completed audio-computer assisted self-interviews (ACASI) and were weighed and measured for height at the start of the study. Second, we assessed medication adherence using monthly phone-based unannounced pill counts. In addition to the pill counts, participants completed monthly interviews to assess food security, depression, stressors, and emotional distress. Third, participants responded to cell-phone-delivered interactive text message assessments of daily alcohol use. Finally, we collected HIV-1 RNA copies/Ml (c/Ml) and CD4 cell counts at the final assessment using a participant assisted chart abstraction procedure. The specific measures are described below.
Computerized Interviews
Demographic characteristics
Participants reported basic demographic information including their age, gender, race, marital status, education, income, and employment status.
Medical history
Participants reported the year they first tested HIV positive and the number of times they had been hospitalized for conditions related to their HIV infection. We also assessed 14 HIV-related symptoms of 2-weeks duration using a measure reported in previous research (Kalichman, Rompa, & Cage, 2000); symptoms included shortness of breath; dry cough; oral or throat sores; Thrush, Candida, or oral white patches; fatigue; unintentional weight loss; recurring fever; and night sweats. In addition, participants were weighed and height measured to calculate their body-mass index (BMI). We used standard categories to define underweight (< 18.5), normal weight (18.5 to 24.9), overweight (25 to 29.9) and obese (> 30).
Substance use and psychiatric treatment history
To assess global alcohol use and problem drinking we administered the Alcohol Use Disorders Identification Test (AUDIT), a 10-item scale designed to measure alcohol consumption and identify risks for alcohol abuse and dependence (Saunders, Aasland, Babor, DeLaFuente, & Grant, 1993). Scores on the AUDIT range from 0 – 40 and the AUIDT has demonstrated acceptable reliability and validity. We also asked participants if they were currently using other drugs, including marijuana, cocaine/crack, inhalants (e.g., poppers), amphetamines, and other drugs. We computed an index of non-alcohol drug use by summing the number of drugs used in the previous four months. In addition, participants reported whether they had ever been hospitalized for a mental health/psychiatric condition, received treatment for depression, and received substance abuse treatment.
Monthly-Unannounced Cell-Phone Interviews
Participants received monthly-unannounced phone assessments over the course of the study. These interviews included pill counts to determine ART adherence and assessed food insecurity, depression, stressors, and emotional distress during the previous month.
Medication adherence
Participants consented to monthly-unannounced telephone-based pill counts for the duration of the study, constituting a prospective measure of adherence. Unannounced pill counts are reliable and valid in assessing medication adherence when conducted in homes and on the telephone (Bangsberg, Hecht, Charlebois, Chesney, & Moss, 2001; Haberer et al., 2011; Kalichman et al., 2008). In this study we conducted unannounced cell-phone based pill counts. Participants were provided with a free cell phone that restricted service for project contacts and emergency use. Following office-based training in the pill counting procedure, participants were called every 21 to 35 days at unscheduled times by a phone assessor. Adherence data represents the percentage of pills taken as prescribed as determined by the following formula:
We computed adherence as a continuous variable representing the percent of prescribed doses taken for each individual antiretroviral medication using the above formula (Grymonpre, Didur, Montgomery, & Sitar, 1998). We then computed the average adherence across antiretroviral medications within each month. We defined monthly non-adherence as having taken less than 85% of doses.
The monthly interviews also assessed the reasons participants stated for missing medications including drinking alcohol, depression, running out of medications, and lack of transportation to the pharmacy. These items were adapted from a previous study of barriers to ART adherence (Catz, Kelly, Bogart, Benotsch, & McAuliffe, 2000). Reasons for missing medications were recorded dichotomously as having occurred or not occurred. We summed the number of months that participants indicated each reason for missing medications to compute a rate of occurrence over 12-months.
Food insecurity
To measure food insecurity we adapted items from a standard measure (Coates, Swindale, & Bilinsky, 2007). Four items were included in the monthly telephone interviews that indicate availability and consistent access to food in the previous month. The specific items were: “Did you not eat for a whole day because there wasn’t enough money for food?”; “Did the food that you bought just not last and you didn’t have money to get more?”, “Did you have to choose between paying for medicine or buying food?”, and “Were you hungry, but didn’t eat, because you couldn’t afford enough food?”. Each experience of food insecurity in the previous month was indicated as having occurred or not occurred. Months when food insecurity had occurred were summed to provide an index of months with food insecurity.
Depression
Monthly interviews included the 10-item cognitive and affective subscale of the Centers for Epidemiological Studies Depression Scale (CESD) (Van Dam & Earleywine, 2011). Participants were asked how often they experienced specific thoughts, feelings and behaviors in the past 7 days, responding 0 = no days, 1 = 1-2 days, 2 = 3-4 days, 3 = 5-7 days. We used the cognitive and affective depression subscale to avoid confounding somatic symptoms of depression with the physical symptoms of HIV and other chronic health conditions. The scale was scored for each month by taking the average responses, which were subsequently averaged across months to estimate depression over the course of the study.
HIV-related stressors and distress
Participants were asked whether they experienced 15 HIV-related stressful life events in the previous month. Stressors were related to social relations (e.g., starting and ending relationships, disclosing HIV status), life circumstances (e.g., financial problems, transportation, having a place to stay), and health concerns (e.g., being hospitalized, experiencing an illness, starting a new medication). These HIV-related stressors have been reported in previous studies (Chesney, Folkman, & Chambers, 1996; Kalichman, DiMarco, Austin, Luke, & DiFonzo, 2003). Stressors were responded to dichotomously for having occurred (yes/no). We computed a monthly stress index by summing the number of stressors reported. For each stressful event endorsed, participants also rated the amount of distress they attributed to the experience on a 3-point scale, 0 = no stress, 1 = a little stress, 2= a lot of stress. Mean distress severity ratings were computed to create an emotional distress index.
Electronic Alcohol Diary
We used a cell-phone delivered interactive text-diary assessment to collect day-level alcohol use. Brief daily assessments were delivered using interactive short message system (SMS) response. Assessments occurred every-other month during the study. Participants received a text-prompt to initiate and answer questions about their alcohol use during the previous day. The questions specifically asked about whether participants drank alcohol yesterday and if so, how much alcohol they drank that day. Daily alcohol drinking was recorded by entering numerical responses using the cell phone keypad. The data were stored on a central secured server. Alcohol use assessments were administered daily for 10 consecutive days, 6 times during the 12-month study, resulting in 60 days of alcohol drinking data.
Chart Abstracted HIV-1 RNA c/Ml and CD4 Cell Count
We used a participant assisted method for collecting chart abstracted HIV-1 RNA c/Ml and CD4 cell counts from participants' medical records. Participants were given a form that requested their doctor’s office to provide results and dates of their most recent HIV-1 RNA c/Ml and CD4 cell counts. The form included a place for the provider's office stamp or signature to assure authenticity. Participants collected their chart data at the end of the study, proximal to the final assessment.
Procedures
Following written informed consent, participants completed the ACASI assessment. Participants were then provided with a text-enabled study cell-phone and instructed in its use for both voice and text message functions. We trained participants in the steps required to compete the monthly-unannounced pill counts as well as the bimonthly interactive text-response alcohol diaries. Alcohol diary assessments were conducted every-other month to reduce participant burden and potential assessment reactivity. Participants were provided with cash reimbursements for the baseline ACASI ($40), monthly-unannounced pill counts ($20/month), electronic diary assessments ($2/day), and providing their HIV-1 RNA c/Ml and CD4 counts from medical records ($25). Data were collected between November 30, 2009 and June 29, 2011 and the University Institutional Review Board approved the study.
Data Analyses
Food insecurity was defined as having experienced any of the four food insecurity indicators in a given month. Participants who experienced food insecurity during the course of the study were considered food insecure. To dichotomize adherence, we used 85% of pills taken as prescribed as the cut-off for non-adherence. In addition, we defined adherence over the entire study period as having achieved at least 85% adherence for 9/12 (75%) of the observation months. For chart abstracted viral suppression, we defined viral suppressed as 400 HIV-1 RNA copies/Ml.
We compared food secure and food insecure participants on demographic, substance use, health, mental health characteristics and months indicating reasons for missing medication using t-tests for continuous variables and contingency table chi-square (X2) tests for categorical variables. Differences between food security groups for monthly ART adherence were examined using multivariate analysis of variance (MANOVA) with food insecurity group treated as a between subjects factor and monthly adherence included as the within subjects dependent variable. Multivariable analyses also tested the association of food insecurity with demographic and substance use variables and all other non-redundant variables found significant (p < .05) in the bivariate analyses. Multiple variables were tested as predictors of ART adherence and HIV-1 RNA c/Ml. We used logistic regressions to test associations with dichotomously coded medication adherent/non-adherent and HIV-1 RNA detectable/undetectable. For both the adherence and viral suppression models, we included in regression models participant gender, age, education, employment status, years since testing HIV positive, AUDIT scores, number of days they drank alcohol and the number of alcohol drinks they consumed on drinking days obtained from electronic diary, number of months participants were unable to get to the pharmacy, drug use, depression, HIV-related stress, and food insecurity. Finally, we examined whether associations between food insecurity and HIV RNA suppression occur independent of ART adherence by repeating the logistic regression model for HIV-1 RNA with ART adherence included among predictors. For all analyses, statistical significance was defined as p < .05.
Results
A total of 449 persons were screened for the study, of which 183 met the entry criteria for currently taking ART and drinking alcohol. Monthly assessments over the 12-months were completed by 93%, chart abstracted HIV-1 RNA data were available for 85%, and daily drinking alcohol diaries were completed by 81% of participants. Forty-three percent of participants (N = 85) experienced food insecurity and 122 (57%) participants did not report any indication of food insecurity during the study. Results found that the most prevalent indication of food insecurity was running out of food and being unable to get more, experienced by 35% of participants. In addition, 28% of participants reported hunger during at least one month of the study and 16% of participants had to choose between food and medications. Overall, 43% of participants experienced at least one indicator of food insecurity during at least one month of the study. (see Table 1)
Table 1.
Monthly indicators of food insecurity among HIV positive adults who drink alcohol.
| Had to choose between food or medication |
Ran out of food and could not get more |
Hungry but didn’t eat because Could not afford food |
Didn’t eat for a whole day because no money for food |
Any indicator of food insecure |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Months Reported |
N | % | N | % | N | % | N | % | N | % |
| 0 | 166 | 84 | 128 | 65 | 142 | 72 | 155 | 78 | 112 | 57 |
| 1 | 15 | 8 | 27 | 14 | 33 | 17 | 26 | 13 | 28 | 14 |
| 2 | 5 | 2 | 13 | 7 | 4 | 2 | 6 | 3 | 20 | 10 |
| 3 | 1 | 1 | 11 | 5 | 6 | 3 | 3 | 2 | 12 | 6 |
| 4 | 3 | 2 | 3 | 2 | 2 | 1 | 1 | 1 | 4 | 2 |
| 5 | 0 | 4 | 2 | 4 | 2 | 2 | 1 | 6 | 3 | |
| 6 or more | 7 | 3 | 11 | 5 | 6 | 3 | 4 | 2 | 15 | 8 |
| Any Month | 31 | 16 | 68 | 35 | 55 | 28 | 43 | 22 | 85 | 43 |
Demographic and Substance Use Characteristics
Table 2 shows the demographic characteristics and substance use of participants defined as food secure and food insecure. Participants who experienced food insecurity did not differ from their food secure counterparts in age, education, years living with HIV, gender, race, income, or employment status. In addition, the groups did not differ in their drug use, global alcohol use, or daily drinking.
Table 2.
Demographic and substance use characteristics of HIV positive food secure and insecure adults who drink alcohol.
| Food Secure N = 112 |
Food Insecure N = 85 |
||||
|---|---|---|---|---|---|
| Characteristic | M | SD | M | SD | t |
| Age | 45.9 | 7.3 | 46.0 | 7.5 | 0.1 |
| Education | 12.4 | 1.4 | 12.6 | 1.3 | 1.2 |
| Years HIV+ | 13.1 | 6.5 | 13.3 | 6.8 | 0.7 |
| Days drinking alcohol |
11.7 | 13.4 | 10.9 | 12.9 | 0.4 |
| Number of alcohol drinks per day |
0.7 | 0.8 | 0.7 | 0.8 | 0.3 |
| AUDIT Score | 5.4 | 5.7 | 6.4 | 6.8 | 1.07 |
|
|
|||||
| N | % | N | % | X2 | |
|
|
|||||
| Men | 88 | 78 | 66 | 77 | |
| Women | 24 | 22 | 19 | 23 | 0.8 |
| Married | 26 | 23 | 21 | 25 | 1.2 |
| African-American | 107 | 95 | 76 | 89 | |
| White | 5 | 4 | 5 | 6 | |
| Other ethnicity | 0 | 4 | 5 | 5.6 | |
| Income < $10,000 | 64 | 57 | 54 | 64 | 0.3 |
| Employed | 81 | 72 | 64 | 75 | 0.6 |
| Drug use | 63 | 56 | 50 | 58 | 0.1 |
Health and Mental Health Characteristics
Food insecure participants were significantly worse off than those who were food secure along multiple dimensions of health and mental health outcomes. Food insecurity was associated with unsuppressed HIV, clinically low CD4 cell counts, greater HIV symptoms, and having multiple hospitalizations for HIV-related conditions. In addition, results showed that food insecure participants reported greater depression, stress, and emotional distress and were significantly more likely to have been treated for depression and hospitalized for psychiatric conditions. (see Table 3)
Table 3.
Health and mental health characteristics of HIV positive food secure and insecure adults who drink alcohol.
| Food Secure N = 112 |
Food Insecure N = 85 |
||||
|---|---|---|---|---|---|
| Characteristic | M | SD | M | SD | t |
| HIV symptoms | 2.3 | 2.8 | 3.8 | 3.2 | 3.2** |
| Log HIV-1 RNA c/ml | 1.9 | 0.8 | 2.2 | 1.1 | 1.9* |
| ART adherence | 84.1 | 14.1 | 77.9 | 20.0 | 2.5** |
| Depression | 0.9 | 0.3 | 1.1 | 0.3 | 3.6** |
| Stressors | 3.2 | 0.8 | 4.2 | 1.3 | 6.9** |
| Distress | 8.8 | 7.2 | 17.0 | 10.9 | 6.3** |
|
|
|||||
| N | % | N | % | X2 | |
|
|
|||||
| CD4 cells < 200 | 8 | 8 | 14 | 18 | 3.8* |
| Detectable HIV-1 | 11 | 11 | 16 | 21 | 2.9+ |
| RNA c/MI | |||||
| 5+ HIV-related hospitalizations |
10 | 9 | 16 | 19 | 4.1* |
| Psychiatric hospitalization | 24 | 21 | 32 | 38 | 6.2** |
| Treated depression | 51 | 45 | 59 | 69 | 11.1** |
| Treated substance abuse |
42 | 37 | 39 | 45 | 1.4 |
| BMI Underweight | 1 | 1 | 1 | 1 | |
| Normal weight | 49 | 43 | 39 | 46 | |
| Over weight | 37 | 33 | 25 | 30 | |
| Obese | 25 | 22 | 19 | 22 | 0.2 |
Note:
p < .05,
p < .01
ART Adherence
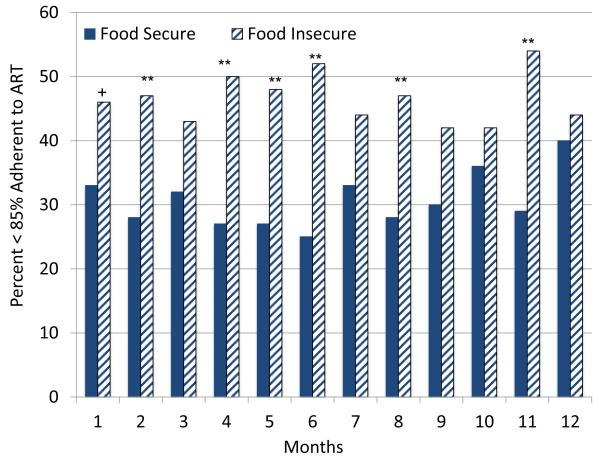
Overall, food insecure participants had poorer adherence during the course of the study. (see Table 3) Figure 1 shows the proportion of food insecurity groups failing to achieve 85% adherence for each month of the study. The association between number of months participants experienced food insecurity and number of months of non-adherence was significant, Spearman’s rho (197) = −.21, p < .01. Bivariate analyses showed that food insecure participants were more likely to have poor ART adherence every month of the study, with the difference reaching statistical significance in six of the 12 months. Multivariate analysis of variance showed that the food insecurity groups differed significantly in ART adherence over the study period, F = 1.86, p < .05.
Figure 1.
Proportion of food secure and food insecure participants that were less than 85% adherent to medications during each month of the study.
With respect to reasons for missing medications, there were no differences between groups for the number of months reporting missing medications when drinking alcohol or when depressed. However, food insecure participants experienced significantly more months when they missed ART because they ran out of medications; 48% of food insecure participants had run out of medications during at least one month compared to 32% of food secure participants. In addition, 51% of food insecure participants did not have transportation to the pharmacy compared to 20% of food secure participants. (see Table 4)
Table 4.
Number of months experiencing reasons for missing ART among HIV positive food secure and insecure adults who drink alcohol.
| Number of months each event recorded |
Food Secure N = 112 |
Food Insecure N = 85 |
|||
|---|---|---|---|---|---|
| N | % | N | % | X2 | |
| Missed medications when drinking |
|||||
| 0 | 81 | 72 | 47 | 55 | |
| 1 | 13 | 11 | 18 | 21 | |
| 2 | 9 | 8 | 8 | 9 | |
| 3+ | 9 | 8 | 12 | 14 | 6.7+ |
| Missed medications when depressed |
|||||
| 0 | 76 | 68 | 44 | 52 | |
| 1 | 17 | 15 | 20 | 23 | |
| 2 | 14 | 12 | 15 | 17 | |
| 3+ | 5 | 4 | 6 | 7 | 5.3 |
| Ran out of medications | |||||
| 0 | 94 | 84 | 66 | 77 | |
| 1 | 14 | 12 | 5 | 6 | |
| 2 | 4 | 3 | 8 | 9 | |
| 3+ | 0 | 6 | 7 | 13.0** | |
| Did not have transportation to the pharmacy |
|||||
| 0 | 90 | 80 | 42 | 49 | |
| 1 | 15 | 13 | 25 | 29 | |
| 2 | 3 | 3 | 5 | 6 | |
| 3+ | 4 | 3 | 13 | 15 | 21.9** |
Note:
p < .10,
p < .01
Multivariable Models
Results of the logistic regression models predicting ART adherence and HIV-1 RNA are shown in Table 5. For the logistic regression predicting treatment adherence over the course of the study, results showed the number of alcohol drinking days, number of alcohol drinks per day, and food insecurity predicted non-adherence; participants who experienced food insecurity were significantly more likely to be non-adherent over the study period over and above the other variables.
Table 5.
Multi-variable regression models predicting ART adherence and detectable HIV-1 RNA c/Ml over 12-months.
| ART Adherence | HIV-1 RNA c/Ml | |||
|---|---|---|---|---|
|
| ||||
| Variable | OR | 95% CI | OR | 95% CI |
| Gender | 1.00 | 0.45 - 2.24 | 2.21 | 0.74 - 6.54 |
| Age | 1.00 | 0.96 - 1.05 | 0.93 | 0.86 - 1.00 |
| Education | 1.29 | 0.99 - 1.67 | 1.03 | 0.73 - 1.46 |
| Employment status | 1.92 | 0.90 - 4.09 | 0.48 | 0.18 - 1.29 |
| Years HIV+ | 1.05 | 0.99 - 1.10 | 1.09* | 1.01 - 1.17 |
| Months unable to get to pharmacy |
0.89 | 0.67 - 1.19 | 1.04 | 0.75 - 1.43 |
| AUDIT Score | 0.99 | 0.93 - 1.05 | 0.99 | 0.90 - 1.08 |
| Days drinking alcohol | 1.06** | 1.01 - 1.11 | 0.96 | 0.91 - 1.02 |
| Number of alcohol drinks per day |
0.49** | 0.25 - 0.96 | 1.61 | 0.69 - 3.74 |
| Drug use | 0.58 | 0.29 - 1.15 | 1.76 | 0.65 - 4.76 |
| Depression | 1.00 | 0.99 - 1.02 | 1.00 | 0.98 - 1.01 |
| Stressors | 0.98 | 0.93 - 1.01 | 0.96 | 0.90 - 1.03 |
| Food insecurity | 0.46* | 0.22 0.94 | 2.96* | 1.0- - 8.00 |
Note:
p < .05,
p < .01
For the logistic regression predicting viral non-suppression (detectable HIV-1 RNA c/Ml), we found that the number of years since HIV diagnosis and food insecurity predicted detectable HIV-1 RNA. Participants who were food insecure were nearly three times as likely to have unsuppressed HIV-1 RNA. Finally, when ART adherence was included in the model, we found adherence was significantly related to HIV-1 RNA (OR = 0.22, 95%CI 0.08-0.62) and food insecurity no longer significantly predicted HIV-1 RNA (OR = 1.83, 95%CI 0.63-5.29).
Discussion
Results of the current study replicated past research by finding high-rates of food insecurity among people living with HIV in US cities. Forty-three percent of participants experienced going an entire day without food in at least one month of the study and 28% reported hunger without access to food. Food insecurity in combination with alcohol use among people living with HIV/AIDS may be particularly problematic given the empty caloric value of alcohol, and the adverse effects of both alcohol use and malnutrition on the immune system. However, we found that the association between food insecurity and HIV-1 RNA was not significant after accounting for ART non-adherence, suggesting that food insecurity primarily may exert its influence on viral suppression through adherence. People who drink alcohol and lack access to food are confronted by two of the most robust obstacles to medication adherence, suggesting a confluence of challenges to maintaining the viral suppressive effects of ART.
In any given month we found that one in three HIV positive alcohol drinkers obtained less than 85% ART adherence. In every month of observation, individuals who had experienced food insecurity were less likely to achieve clinically optimal ART adherence. In addition, suboptimal adherence was reflected in the poorer health status observed among those who were food insecure. Food insecurity was associated with poorer suppression of HIV-1 RNA, a greater likelihood of clinically significant low-CD4 cell counts, and greater numbers of HIV-related symptoms. Multivariate models found that food insecurity predicted ART non-adherence and viral non-suppression over and above demographic characteristics, substance use, and indicators of mental health problems. We therefore conclude that food insecurity represents a marker for severe risk of non-adherence and poor viral suppression in people living with HIV.
Our findings also replicate and extend previous research concerning greater numbers of medical hospitalizations among people living with HIV who experience food insecurity (Weiser, Hatcher, et al., 2012). We found that participants who experience food insecurity over the course of the study were more likely to have been hospitalized for HIV-related conditions. Supporting our hypothesis, we also found that food insecurity was associated with a having been treated for depression and having been hospitalized for psychiatric conditions. Thus, the poor adherence we observed in this study occurs in the context of a complex array of factors that foster non-adherence, including alcohol use and food insecurity, as well as multiple-morbidities, both related to HIV infection and mental health conditions.
Findings from the current study extend previous research showing that food insecurity has detrimental effects on HIV treatment outcomes. For example, in a study of homeless and marginally housed people living with HIV/AIDS in San Francisco found that one in three persons living with HIV in unstable housing or homeless shelters were severely food insecure (Weiser, Bangsberg, et al., 2009). Among persons taking ART, more than half were food insecure and food insecurity was associated with incomplete adherence and viral non-suppression (Weiser, Frongillo, et al., 2009). Food insecurity in US urban centers extends beyond the homeless and marginally housed to individuals with stable housing (Franke et al., 2011; Vogenthaler et al., 2011). Ultimately, food insecurity in combination with being underweight or overweight contributes to the mortality of people living with HIV/AIDS (Kim et al., 2012; Weiser, Fernandes, et al., 2009).
These findings should be interpreted in light of the study limitations. First, we relied on a convenience sample that cannot be considered representative of people living with HIV infection. The sample also came from a wide-range of clinical services that likely varied in adherence assistance and counseling regarding alcohol use. Explaining some of our findings also requires additional information unavailable to us. For example, the association between years since testing HIV positive and HIV-1 RNA may be accounted for by health status at the time of diagnosis (e.g., CD4 nadir), longer-term engagement in care, and general health factors related to aging. The association between number of days alcohol was drank in relation to adherence was in the opposite direction of what is expected; greater number of drinking days was associated with greater adherence. The nature of this unexpected finding cannot be discerned from our current study measures. In addition, participants in this study were taking a variety of ART combinations and for various lengths of time. Although we collected alcohol data using a time-stamped daily electronic diary, these data may still be subject to self-report biases. Socially sensitive behaviors such as alcohol use assessed by self-report may be underreported, suggesting that rates of drinking alcohol in this study should be considered lower-bound estimates. Unannounced pill counts are also limited by their potential to under-estimate adherence (Grymonpre et al., 1998). Another limitation was our definition of non-adherence applied to all medication regimens, which differ in their demand for optimal adherence. We selected 85% adherence as a cut-off because most combination ART regimens risk resistance at this level of adherence or lower (Bangsberg & Deeks, 2002; Kobin & Sheth, 2011). With these limitations in mind, we believe that the current study results have important implications for improving HIV treatment adherence among people living under adverse conditions.
Food insecurity is resolvable in resource rich countries such as the United States. Unlike developing countries, food is not scarce in the city where this study was conducted. However, the most marginalized and disenfranchised individuals lack access to food and experience hunger. Providing sustained nutritional support to people living with HIV/AIDS will come at a considerable expense over the long run. Research has shown, however, that the costs of providing stable housing to people living with HIV are offset by greater adherence and improved health outcomes (Holtgrave et al., 2012). Increasing access to food and improving the nutrition of people living with HIV will be cost saving when balanced against the costs of hospitalizations, emergency room visits, increased infectiousness, and emerging treatment resistant viral strains (Weiser, Hatcher, et al., 2012; Weiser, Tsai, et al., 2012). Increasing the availability of food and nutritional support to people living with HIV has demonstrated significant improvements in adherence and health (Cantrell et al., 2008). Increasing access to food through food policy changes and structural interventions should be considered a central part of efforts to improve ART adherence and health outcomes for people living with HIV in resource rich as well as resource poor contexts.
Acknowledgments
This research was supported by an America Reinvestment and Recovery Act (ARRA) Challenge Grant from the National Institute of Alcohol Abuse and Alcoholism (NIAAA) RC1AA018983.
References
- Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don't. Annals of the New York Academy of Sciences. 1999;896:3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- Adler NE, Stewart J. Health disparities across the lifespan: meaning, methods, and mechanisms. Annals of the New York Academy of Sciences. 2010;1186:5–23. doi: 10.1111/j.1749-6632.2009.05337.x. [DOI] [PubMed] [Google Scholar]
- Alghamdi AA, Sheth T, Manowski Z, Djoleto OF, Bhatnagar G. Utility of Cardiac CT and MRI for the Diagnosis and Preoperative Assessment of Cardiac Paraganglioma. Journal of Cardiac Surgery. 2009;24(6):700–701. doi: 10.1111/j.1540-8191.2009.00857.x. [DOI] [PubMed] [Google Scholar]
- Bangsberg D, Deeks SG. Is average adherence to HIV antiretroviral therapy enough? Journal of General Internal Medicine. 2002;17(10):812–813. doi: 10.1046/j.1525-1497.2002.20812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsberg D, Hecht FM, Charlebois ED, Chesney M, Moss A. Comparing objective measures of adherence to HIV antiretroviral therapy: Electronic medication monitors and unannounced pill counts. AIDS and Behavior. 2001;5:275–281. [Google Scholar]
- Bangsberg D, Kroetz DL, Deeks S. Adherence-resistance relationships to combination HIV antiretroviral therapy. Current HIV/AIDS Reports. 2007;4:65–72. doi: 10.1007/s11904-007-0010-0. [DOI] [PubMed] [Google Scholar]
- Cantrell RA, Sinkala M, Megazinni K, Lawson-Marriott S, Washington S, Chi BH, Stringer JS. A pilot study of food supplementation to improve adherence to antiretroviral therapy among food-insecure adults in Lusaka, Zambia. Journal of Acquired Immune Deficienct Syndromes. 2008;49(2):190–195. doi: 10.1097/QAI.0b013e31818455d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychology. 2000;19(2):124–133. [PubMed] [Google Scholar]
- Chesney M, Folkman S, Chambers D. Coping effectiveness training for men living with HIV: preliminary findings. International Journal of STD and AIDS. 1996;7(Suppl 2):75–82. doi: 10.1258/0956462961917690. [DOI] [PubMed] [Google Scholar]
- Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide USAID. Academy for Educational Development; Washington, DC: 2007. [Google Scholar]
- El-Sadr WM, Mayer KH, Hodder SL. AIDS in America--forgotten but not gone. New England Journal of Medicine. 2010;362(11):967–970. doi: 10.1056/NEJMp1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke MF, Murray MB, Munoz M, Hernandez-Diaz S, Sebastian JL, Atwood S, Shin SS. Food insufficiency is a risk factor for suboptimal antiretroviral therapy adherence among HIV-infected adults in urban Peru. AIDS and Behavior. 2011;15(7):1483–1489. doi: 10.1007/s10461-010-9789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grymonpre RE, Didur CD, Montgomery PR, Sitar DS. Pill count, self-report, and pharmacy claims data to measure medication adherence in the elderly. Annals of Pharmacotherapy. 1998;32(7-8):749–754. doi: 10.1345/aph.17423. [DOI] [PubMed] [Google Scholar]
- Haberer JE, Robbins GK, Ybarra M, Monk A, Ragland K, Weiser SD, Bangsberg DR. Real-Time Electronic Adherence Monitoring is Feasible, Comparable to Unannounced Pill Counts, and Acceptable. AIDS and Behavior. 2011;16:375–382. doi: 10.1007/s10461-011-9933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. Journal of Acquired Immune Deficiency Syndromes. 2009;52(2):180–202. doi: 10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtgrave DR, Wolitski RJ, Pals SL, Aidala A, Kidder DP, Vos D, Bendixen AV. Cost-Utility Analysis of the Housing and Health Intervention for Homeless and Unstably Housed Persons Living with HIV. AIDS and Behavior. 2012;17:1626–1631. doi: 10.1007/s10461-012-0204-3. [DOI] [PubMed] [Google Scholar]
- Ivers LC, Cullen KA, Freedberg KA, Block S, Coates J, Webb P. HIV/AIDS, undernutrition, and food insecurity. Clinical Infectious Diseases. 2009;49(7):1096–1102. doi: 10.1086/605573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman S, Amaral CM, Cherry C, Flanagan JA, Pope H, Eaton L, Schinazi R. Monitoring Antiretroviral adherence by unannounced pill counts conducted by telephone: Reliability and criterion-related validity. HIV Clinical Trials. 2008;9:298–308. doi: 10.1310/hct0905-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman S, DiMarco M, Austin J, Luke W, DiFonzo K. Stress, social support, and HIV-status disclosure to family and friends among HIV-positive men and women. Journal of Behavioral Medicine. 2003;26(4):315–332. doi: 10.1023/a:1024252926930. [DOI] [PubMed] [Google Scholar]
- Kalichman S, Rompa D, Cage M. Reliability and validity of self-reported CD4 lymphocyte count and viral load test results in people living with HIV/AIDS. International Journal of STD and AIDS. 2000;11(9):579–585. doi: 10.1258/0956462001916551. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Westfall AO, Chamot E, Willig AL, Mugavero MJ, Ritchie C, Willig JH. Multimorbidity Patterns in HIV-Infected Patients: The Role of Obesity in Chronic Disease Clustering. Journal of Acquired Immune Deficiency Syndromes. 2012;15:600–605. doi: 10.1097/QAI.0b013e31827303d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobin AB, Sheth NU. Levels of adherence required for virologic suppression among newer antiretroviral medications. Annals of Pharmacothery. 2011;45(3):372–379. doi: 10.1345/aph.1P587. [DOI] [PubMed] [Google Scholar]
- Normen L, Chan ES, Braitstein P, Annema A, Bondy G, Montaner J. Food inseurity and hunger are prevalent among HIV-positive indivudals in Britsh Columbia, Canada. Journal of Nutrition. 2005;135:820–825. doi: 10.1093/jn/135.4.820. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, DeLaFuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption II. Addictions. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Van Dam NT, Earleywine M. Validation of the Center for Epidemiologic Studies Depression Scale--Revised (CESD-R): pragmatic depression assessment in the general population. Psychiatry Research. 2011;186(1):128–132. doi: 10.1016/j.psychres.2010.08.018. [DOI] [PubMed] [Google Scholar]
- van der Sande MA, Schim van der Loeff MF, Aveika AA, Sabally S, Togun T, Sarge-Njie R, Whittle HC. Body mass index at time of HIV diagnosis: a strong and independent predictor of survival. Journal of Acquired Immune Deficiency Syndromrd. 2004;37(2):1288–1294. doi: 10.1097/01.qai.0000122708.59121.03. [DOI] [PubMed] [Google Scholar]
- Vogenthaler NS, Hadley C, Rodriguez AE, Valverde EE, del Rio C, Metsch LR. Depressive symptoms and food insufficiency among HIV-infected crack users in Atlanta and Miami. AIDS and Behavior. 2011;15(7):1520–1526. doi: 10.1007/s10461-010-9668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser SD, Bangsberg DR, Kegeles S, Ragland K, Kushel MB, Frongillo EA. Food Insecurity Among Homeless and Marginally Housed Individuals Living with HIV/AIDS in San Francisco. AIDS and Behavior. 2009;13:841–848. doi: 10.1007/s10461-009-9597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser SD, Fernandes KA, Brandson EK, Lima VD, Anema A, Bangsberg DR, Hogg RS. The Association Between Food Insecurity and Mortality Among HIV-Infected Individuals on HAART. Journal of Acquired Immune Deficiency Syndromes. 2009;52:352–349. doi: 10.1097/QAI.0b013e3181b627c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser SD, Frongillo EA, Ragland K, Hogg RS, Riley ED, Bangsberg DR. Food insecurity is associated with incomplete HIV RNA suppression among homeless and marginally housed HIV-infected individuals in San Francisco. Journal of General Internal Medicine. 2009;24(1):14–20. doi: 10.1007/s11606-008-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser SD, Hatcher A, Frongillo EA, Guzman D, Riley ED, Bangsberg DR, Kushel MB. Food Insecurity Is Associated with Greater Acute Care Utilization among HIV-Infected Homeless and Marginally Housed Individuals in San Francisco. Journal of General Internal Medicine. 2012;28:91–98. doi: 10.1007/s11606-012-2176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser SD, Tsai AC, Gupta R, Frongillo EA, Kawuma A, Senkungu J, Bangsberg D. Food insecurity is associated with morbidity and patterns of healthcare utilization among HIV-infected individuals in a resource-poor setting. AIDS. 2012;26(1):67–75. doi: 10.1097/QAD.0b013e32834cad37. [DOI] [PMC free article] [PubMed] [Google Scholar]