Abstract
In dissociated cell and wholemount explant cultures of the embryonic trigeminal pathway NGF promotes exuberant elongation of trigeminal ganglion (TG) axons, whereas NT-3 leads to precocious arborization [J. Comp. Neurol. 425 (2000) 202]. In the present study, we investigated the axonal effects of local applications of NGF and NT-3. We placed small sepharose beads loaded with either NGF or NT-3 along the lateral edge of the central trigeminal tract in TG-brainstem intact wholemount explant cultures prepared from embryonic day 15 rats. Labeling of the TG with carbocyanine dye, DiI, revealed that NGF induces local defasciculation and diversion of trigeminal axons. Numerous axons leave the tract, grow towards the bead and engulf it, while some axons grow away from the neurotrophin source. NT-3, on the other hand, induced localized interstitial branching and formation of neuritic tangles in the vicinity of the neurotrophin source. Double immunocytochemistry showed that axons responding to NGF were predominantly TrkA-positive, whereas both TrkA and TrkC-positive axons responded to NT-3. Our results indicate that localized neurotrophin sources along the routes of embryonic sensory axons in the central nervous system, far away from their parent cell bodies, can alter restricted axonal pathways and induce elongation, arborization responses. D 2004 Elsevier B.V. All rights reserved.
Keywords: NGF, NT-3, Axon elongation, Axon arborization, Trigeminal ganglion
1. Introduction
The NGF family of neurotrophins plays an important role in differentiation of axonal processes of many types of neurons [4,5,45]. In dissociated primary sensory neuron cultures and in explant cultures of the embryonic trigeminal pathway, exogenous application of NGF promotes exuberant axon elongation outside the central trigeminal tract, whereas NT-3 leads to precocious arborization of central trigeminal tract axons [43]. However, the main difficulty in addressing the role of neurotrophins in axonal development has been in differentiating between the morphological and survival effects. Recently, this problem was circumvented by using dissociated dorsal root ganglion (DRG) cell cultures from bax-deficient mice, in which the null mutation of the pro-apoptotic bax gene enables the primary sensory neurons survive in the absence of neurotrophins [23]. Despite differential effects of NGF and NT-3 on primary sensory neurons [23,43], their precise roles on the growth patterns of axonal projections remain unclear. Because, in both studies [23,43], neurotrophins were added to the culture medium at varying concentrations and thus, they were ubiquitously available to all neurons and their axonal processes. Few studies employed local applications of neurotrophins in dissociated cell cultures. Gundersen and Barrett [14] showed that dissociated chick dorsal root ganglion (DRG) cell axons turn towards NGF. Collateral formation from the neurites of dissociated chick DRG cells was noted in the presence of neurotrophin-coated beads [12]. Neurotrophin-coated beads most likely exert their effect upon contact with the axonal processes. Recently, Tucker et al. [39] showed chemotropic effects of ectopic neurotrophin sources on mouse sensory-motor nerves in embryonic slice cultures. They reported that developing limb sensory and motor axons change their trajectories and preferentially grow towards neurotrophin-coated beads that are placed in ectopic loci. Beads coated with neurotrophin function blocking antibodies led to significant reduction of sensory and motor axon growth towards the limb. In the present study, we investigated the effects of localized neurotrophin sources on the behavior of embryonic rat central trigeminal axons in the brainstem. We embedded neurotrophin-soaked beads along the central trigeminal tract in wholemount cultures of the trigeminal pathway and examined axonal effects to test the hypothesis that NGF and NT-3 have differential and localized axonal effects on both TrkA- and Trk-C positive central trigeminal axons.
2. Materials and methods
Timed-pregnant Sprague–Dawley rats were obtained from Taconic Farms (Germantown, NY). Day of sperm positivity was designated as embryonic day (E) 0. In the rat, central trigeminal axons first enter the brainstem on E12 and begin laying down the ascending and descending components of the central trigeminal tract by E13 [9,11]. By E15, central trigeminal tract becomes distinct as a laterally positioned and highly restricted pathway with all of its axons growing in the elongation phase with no branching or arborization [9]. In explant cultures of this pathway, central trigeminal axons retain their E15 characteristics, even after 3 days in vitro [10,43]. For these reasons, we selected to use this embryonic time point for our experiments. E15 rat embryos were removed from the dams under barbiturate anesthesia. All of the protocols used in this study were approved by the LSUHSC IACUC and conformed to the NIH guidelines for use of experimental animals.
2.1. Preparation of trigeminal ganglion (TG)-brainstem intact wholemount explants
E15 embryos were collected in cold Gey's balanced salt solution (Invitrogen, Gaithersburg, MD) supplemented with D-galactose (Sigma, St. Louis, MO, 6.4 gm/l). All of the dissections were performed under a stereomicroscope, using dark field optics, and under sterile conditions. The head of each embryo was removed and rinsed in GBSS. Next the forebrain was removed, and the trigeminal ganglia on both sides and the brainstem up to the cervical spinal cord level were carefully dissected out. The surrounding meninges were removed and the brainstem with TG on both sides was splayed out in an “open book” preparation (Fig. 1A, inset). In these preparations the central trigeminal tract is located superficially at the bottom (ventrally and just below the meningeal surface), and the top of this approximately 150-μm thick explant is the ventricular surface. These wholemounts are laid on to microporous membranes with the ventricular surface up and the trigeminal tract side down, and the beads are implanted on the ventricular surface (Fig. 1A, inset). In several wholemounts (n=20) a single neurotrophin-loaded bead (blue) was placed on one side along the lateral side of the central trigeminal tract, and a control (BSA or PBS-loaded) bead (white) was placed along the lateral side of the central trigeminal tract on the other side. This allowed us to compare axonal effects in the same culture and two sides of each explant. In other explants, NGF or NT-3-loaded beads were placed bilaterally (n=68 for NGF, 54 for NT-3). In a few cases (n=6) a single neurotrophin-soaked bead was implanted along the midline of the brainstem, and TG on both sides was labeled with DiI. The culture preparation is illustrated in Fig. 1A.
Fig. 1.
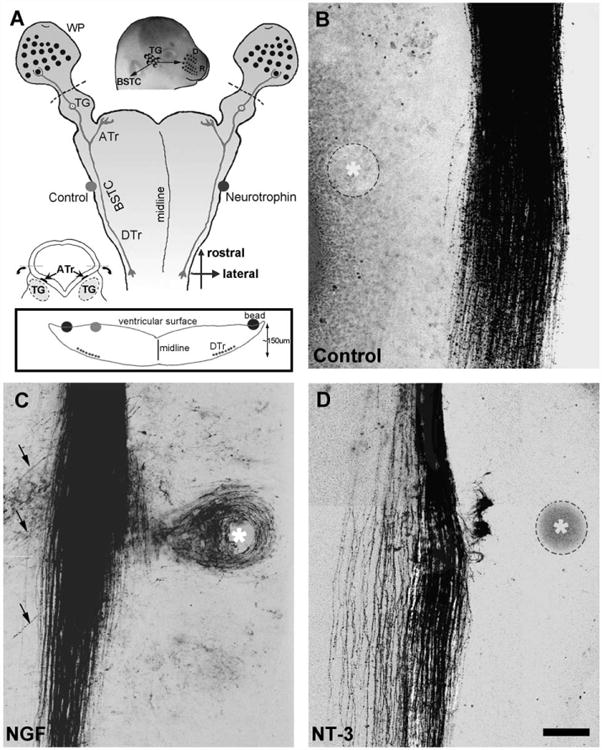
(A) Schematic illustration of the whisker pad-TG-brainstem wholemount cultures and the experimental setup. The whisker pads are sectioned off from dashed lines. Small diagram at the bottom left illustrates the preparation of the “open book” brainstem explants and inset diagram illustrates the relative positions of the tract and the bead. In a few cases the bead was located directly across from the tract (gray bead) on the ventricular surface. ATr: ascending trigeminal tract; DTr: descending trigeminal tract; BSTC: brainstem trigeminal complex, TG: trigeminal ganglion, WP: whisker pad. (B) DiI labeled DTr axons in the presence of a control (BSA-loaded) bead. Note that central trigeminal axons are restricted to the tract and do not show any response to control bead. (C) NGF-loaded sepharose bead induces extensive axon outgrowth both towards and away (arrow) from the source of neurotrophin. Many axons leave the tract and encircle the bead. (D) NT-3-loaded beads, on the other hand, induce attraction, dense arborization and knot-like structures in the vicinity of the bead. Asterisks indicate the beads in (B–D). Scale bar=200 μm for (B–D).
During implantation of the beads, the location of the tract is estimated from previous observations with DiI-labeled specimens, and the beads are positioned as laterally as possible. In a few cases (n=6), the beads lodged just over the trigeminal tract about 100–150 μm away from it on the ventricular surface of the explant. These cases were quite informative about the response of single axons within the tract, as described in the results. All explants were cultured on Millicell (Millipore, Bedford, MA) inserts with microporous membrane. Excess GBSS was suctioned off and the inserts were placed in 6-well culture plates, each well containing 1 ml of serum-free culture medium (SFM) [32,41,43]. The cultures were maintained in a 33°C incubator with humidified air and 5% CO2 for 3 days. At the end of the culture period, the explants were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS, pH=7.4). In order to examine the initial behavior of axons towards the neurotrophin-soaked beads and progression of axonal responses we fixed some cultures (n=8) 24 h, and 48 h (n=8) after incubation.
2.2. Preparation of neurotrophin-loaded beads and assessment of protein release
Color-coded sepharose beads (average diameter 200 μm, gift of K. Muneoka, Tulane University) were washed twice with PBS (pH=7.4) in an eppendorf tube under sterile conditions. Beads were incubated for 30 min at room temperature and excess PBS was removed and they were briefly air-dried under sterile conditions. The white beads were loaded with BSA (100 ng/μl) or were kept in PBS to be used as negative controls. The blue beads were loaded either with 100 ng/μl of NGF or 100 ng/μl NT-3 (Collaborative Biomedical Products, Bedford, MA, and Sigma) at 4 °C overnight on a shaker. For each experiment, the beads were prepared a day in advance and were kept at 4 °C until implantation.
To determine the amount of neurotrophins carried by the beads, we measured the protein concentration of the neurotrophin solution before and after loading the beads using micro BCA protein assay kit (Pierce, Rockford, IL). The assay has a linear working range of 0.5–20 ng/μl of protein. The starting concentration of our neurotrophin solution was 100 ng/μl and after overnight shaking of the beads in it at 4 °C, we collected the neurotrophin solution, prepared different dilutions to find out the final concentration of the neurotrophin. Therefore we used the working range for our measurements and multiplied it with the corresponding dilution factor. In the second part of the experiment where we investigated the neurotrophin release from the beads we used all of the sample since the volume was very small and the concentration was very low, but still within detectable range. We then divided the difference to the number of beads loaded. For each experiment we used 100 beads of the same size. We repeated the experiment three times to get an average. We found that after overnight soaking the neurotrophin level dropped from 100 to 60 ng/μl, and we reasoned that the difference must be distributed within the 100 beads. Since our loading volume was 50 μl, this suggested that each bead (diameter 200 μm, surface area 0.126 mm2, volume 0.04 mm3) is loaded approximately with 20 ng of neurotrophin. To assay the release of protein from the bead, we used a similar approach to culture conditions we used and incubated 100 loaded beads at 33°C in 10 μl of PBS. We collected PBS (10 μl) and replaced it with fresh PBS at daily intervals (day 1, day 2, day 3) and calculated the protein level by using BCA-Micro kit with a slight modification of the manufacturer's instructions since we were using very small volumes. We repeated these experiments twice. We detected protein in the solution after the first day (5–7 ng/μl) and second day (1–3 ng/μl), but protein levels were undetectable after 3 days, suggesting that most of the protein was released by the end of the second day in culture. We used PBS for our release experiments because it was the solution used to wash and prepare the beads. Since the protein detection is very sensitive, SFM or any other solution that contains proteins would introduce confounding variables.
2.3. Analysis of axonal responses to localized neurotrophin sources
After fixation of the cultures, small crystals of carbocyanine dye, DiI (Molecular Probes, Eugene, OR), was inserted into the left and the right TG under a stereomicroscope. The cultures were then incubated at 37 °C for 7 days for the dye to diffuse along the TG axons. When the labeling was complete, the trigeminal tract was photographed with a digital camera attached to a Nikon Micro-phot microscope under epifluorescence using a rhodamine filter set. Select cultures were also examined under a confocal microscope (Nikon TE-300 with Radians 2000 laser scanning system, Bio-Rad) and a z series of photographic images were captured, compressed or saved as 3-D rotational movie files. In several cases the labeled axons were photo converted using the method described by Sandell and Masland [36]. In such cases, the fluorescent dye is photo-oxidized in the presence of diaminobenzidine; the labeled axons turn brown and can be examined under the light microscope without any fluorescence bleaching problems.
For quantitative analyses, axonal labeling was imaged at 20 × magnification and printed on a full page. A transparent sheet with 50 × 50 μm grids was placed on top of the pictures. The labeled central trigeminal tract was designated as zero reference point. For each case a constant length of the central trigeminal tract was used for measurements. Each grid containing an axon was counted as one. Both lateral and medial axon outgrowth was quantified. The number of grids containing axons was scored. The average and standard deviation of grids containing axons were calculated using the Microsoft Excel program. The result was plotted as the number of grids containing axons versus the length in consecutive 50-μm increments for both lateral and medial directions. For statistical evaluation, two pairs of metrics were used: mean length of axons and mean axon density in the medial and lateral directions away from the central trigeminal tract. Statistical comparisons were made by using the one-tailed t-test. Before the application of the proper t-statistic, an F-test was used to detect any difference in the variance of experimental conditions [30]. Error bars in the graphs represent one standard deviation.
2.4. Trk immunocytochemistry in explant cultures
To determine whether different or same classes of TG cells responded to localized sources of neurotrophins we used TrkA and TrkC double immunocytochemistry. In this series of experiments, wholemount cultures were fixed with 4% PFA overnight, and cryoprotected in 30% sucrose in PBS at 4 °C. Some 20 μm thick sections were cut in the horizontal plane using a cryostat and the sections were collected onto subbed slides. All immunocytochemical procedures were performed on the slides.
For immunocytochemistry sections were first washed with PBS and then treated with a blocking solution (10% normal donkey serum in PBS, containing 0.3% TritonX-100) for 45 min at room temperature. Sections were then incubated in a cocktail of rabbit anti TrkA antibody (1:1000) and goat anti TrkC antibody (1:200) in blocking solution at 4 °C overnight. Both antibodies are gifts from L. Reichardt (UCSF). The next day the sections were washed twice for 5 min and then incubated in CY-3 conjugated donkey anti rabbit secondary antibody (Chemicon, Temecula, CA, 1:200) and FITC-conjugated donkey anti goat secondary antibody (Chemicon, 1:200) for 2 h at room temperature in the dark. Control sections were processed the same way, except the primary antibodies were omitted or single antibody was used. The slides were then washed with PBS, and coverslipped using Fluormount (Sigma). The labeling was analyzed with confocal microscopy.
3. Results
The culture paradigm we used mimics many aspects of the embryonic development of the trigeminal pathway in vivo and it also allows us to experimentally manipulate one trigeminal tract while using the contralateral tract as an internal control (Fig. 1A).
In all of our cultures, control beads (BSA-soaked or PBS-washed beads) we saw no alterations in the trigeminal tract (n=20, Figs. 1B and 2A). Axons maintained their unbranched and highly restricted trajectories within the central trigeminal tract. In contrast, the experimental side of the same cultures revealed dramatic changes in the growth patterns of trigeminal axons.
Fig. 2.
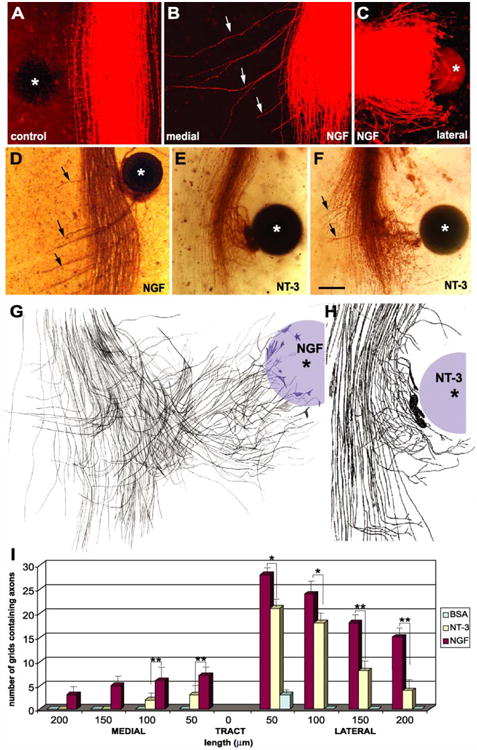
Effects of NGF and NT-3 on descending trigeminal tract axons and quantitative analysis of axon growth. (A) Note that with the exception of a few stray axons laterally, all other axons grow tightly fasciculated and none turn towards the control (BSA) bead. (B) DiI-labeled axons (arrows) growing medially and away from an NGF bead. (C) The lateral side of the same tract showing axons that grow and encapsulate the NGF-loaded bead. (D) Another NGF bead case with DiI labeling photoconverted. Note the axons that grow both towards and away (arrows) from the bead. (E, F), Attraction and axon branching towards NT-3 beads. Note that a few axons are also growing away from the bead in F (arrows). (G, H), camera lucida drawings of axons from single images through z-series obtained with confocal microscopic images illustrating single axons from the parent tract which are turning laterally or medially and growing unbranched, in response to NGF (G) and the nodal branching and some turning of parent trigeminal axons in the tract in response to NT-3 (H). In (B–H) lateral is to the right and rostral is to the top, and in the control case (A) lateral is to the left. Asterisks mark the beads. Scale bar=100 μm for (A–F), 50 μm for (G, H). (I) Bar graph representation of axon outgrowth analysis both towards (lateral) and away (medial) from the neurotrophin source. The thickness of the tract between the medial and lateral edges tract was designated as zero reference point for each case. *p<0.01, **p<0.001.
When an NGF-loaded bead was placed lateral to the central trigeminal tract, many axons left the tract and extended towards the bead, engulfing it by the end of the 3-day culture period (n=68, Figs. 1C, 2C,D and 3E,F). Surprisingly, some axons left the tract and grew medially away from the trigeminal tract, in a direction opposite to the location of the bead (Figs. 1C and 2B,D). Most of the axons responding to localized NGF source were later developing axons that had just reached the level of the bead. This was particularly evident in cases where we followed the axonal responses over 24-h intervals (see below). Thus, localized, high concentrations of NGF along the developing trigeminal tract led to divergence of axons from the well-defined tract, and growth both towards and away from the neurotrophin source. For quantitative assessment of axon growth outside the boundaries of the tract, we compared the densities of axons towards (laterally) or away (medially) from the neurotrophin source (Fig. 2I). We found significantly more axon outgrowth towards the NGF-loaded bead than towards NT-3-loaded beads (p<0.01). With increased distance away from the tract (150 μm and more), this difference became highly significant (p<0.001). Since majority of axons extended longer distances and engulfed the NGF-loaded beads, higher numbers of grids containing axons were present away from the tract. With NT-3 beads, most of the axons left the tract and started to form branches and tangles around the bead. Therefore, the number of grids containing axons was highest at 50 μm away from the tract, and started to diminish after 150 μm distance.
Fig. 3.
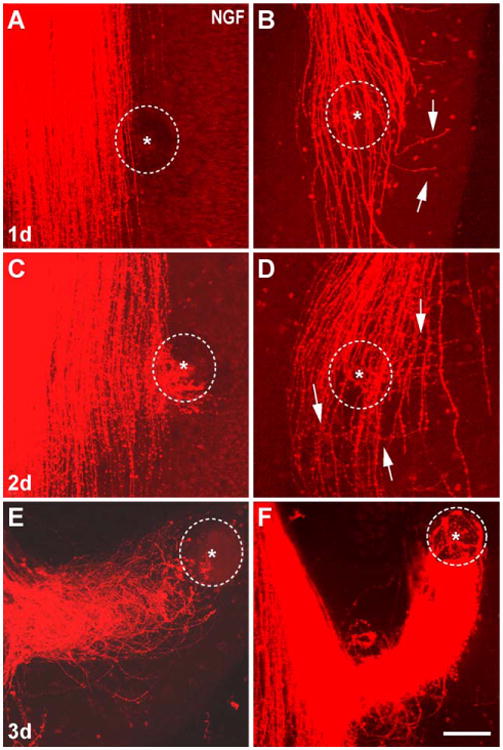
The effects of NGF bead over a three-day culture period. (A) After 24 h most cases did not show any axonal response to the NGF bead. (B) When NGF bead was placed over the trigeminal tract on the ventricular side of the explant, a distinct defasciculation and turning response of axons that have reached this level is seen. Some axons tipped with growth cones (arrows) leave the tract and grow away from the bead. After 48 h in culture, many axons show attraction response to the NGF bead (C). (D) A case with an NGF bead over the tract. A clear turning of the leading edge of immature axons is evident (arrows). After 3 days in culture, central trigeminal axons extended all the way to the most laterally placed beads, forming spiraling funnels (E, F). In these two exemplary cases the central trigeminal tract is located to the left of the micrographs. Dashed circles outline the beads and asterisks mark the center of the beads. Scale bar=150 μm.
Comparison of axon outgrowth away from the NGF or NT-3 source also revealed significantly different responses. In comparison to NT-3-loaded cases, axon outgrowth in medial direction (away from the bead) was highly significant in NGF-loaded cases (p<0.001). While we saw medial axon growth in many NGF bead cases, we saw few such axon outgrowth with NT-3 beads, and these axons did not extend beyond 150 μm (compare heights of the bars for NT-3 cases in Fig. 2I). In control bead cases (PBS or BSA) there was no axon outgrowth away from the central trigeminal tract in either the medial or lateral directions. Occasionally few axons were seen traveling singly and parallel to the tract along the lateral edge (Figs. 1B and 2A,I).
We next examined the time course of trigeminal axonal responses to neurotrophins by fixing cultures after 24, 48 and 72 h in vitro (Figs. 3 and 4). By the end of the first day in culture, there was no noticeable divergence of the axons in the tract towards or away from the NGF bead (Fig. 3A). In a few cases where the NGF bead lodged over the tract on the ventricular surface of the explant, a distinct defasciculation of the tract at this level was noted and single axons tipped with growth cones made right angle turns and left the tract (Fig. 3B, arrows). Such aberrant growth of central trigeminal tract axons was clearly present by the end of the second day (Fig. 3C) in culture. In three cases, where the bead was located over the trigeminal tract on the opposite side of the brainstem explant (see gray bead in Fig. 1A inset for reference), we could also see several parent axons in the trigeminal tract turning medially or laterally and exiting the tract (Fig. 3D). By the end of the third day, numerous axons reached the bead (positioned laterally and away from the tract), and engulfed it (Fig. 3E,F). Others left the tract at right angles, and grew away from the bead (Fig. 2B,D). Confocal microscopic examination of these axons in 3-D allowed us to determine that central trigeminal tract axons that were responding to the localized source of NGF were not branches emitted by the axons that have already extended a long way past the location of the bead. Instead, they were those, which have just reached the level of the bead in the tract, as all of them were tipped with growth cones. Axons that were at the level of the neurotrophin bead defasciculated, took abrupt turns from their normal course, and extended towards the bead. Others left the central tract and grew in a direction opposite to the bead (Fig. 2B,D). Those that grew towards the bead did not extend directly towards the bead, but formed circuitous funnels along a narrow corridor towards the bead (Fig. 3E,F), finally engulfing it. Such spiraling growth towards the bead was clear in 3-D images, and in camera lucida drawings from z-series micrographs (Fig. 2G). In all of the cases, the most dramatic response and axon divergence from the central trigeminal tract was seen within 400–600 μm perimeter of the tract with respect to the bead. Thus, the observed effects were localized to a circumscribed segment of the trigeminal tract in close proximity to the neurotrophin bead, and not present all along the rostrocaudal extent of the tract. For better comparison of NGF and NT-3 effects, statistical evaluations were made using only the cases where loaded beads were placed lateral to the trigeminal tract and cultured for 3 days in vitro. Cases where NGF-loaded bead was placed on the ventricular surface directly facing the tract or cases where cultures were fixed at different time points were not included in our statistical analyses.
Fig. 4.
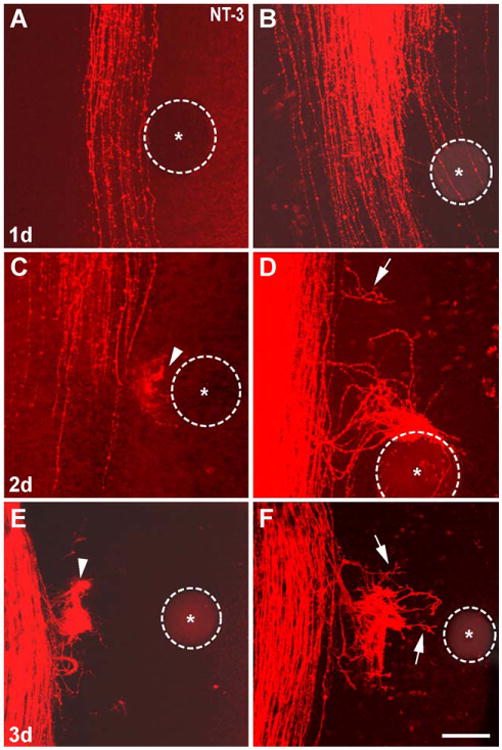
The effects of NT-3 beads over a three-day culture period. (A) After 24 days most cases did not show any axonal response to the NT-3 bead as in NGF cases. (B) In a couple cases axonal responses to the NT-3 bead became apparent by 24 h. Axonal effects were pronounced by the end of the second day (C, D) and not much different from that seen by the end of the third day (E, F). Many axons within the tract formed interstitial branches that extended towards the bead and arborized extensively (arrows D, F) or formed “knots” (arrows, C, E). Dashed circles outline the beads and asterisks mark the center of the beads. Scale bar=150 μm.
Effects of NT-3-loaded beads on central trigeminal axons were quite different than those observed with NGF after 3 days in culture. In these cultures, parent axons in the trigeminal tract emitted interstitial branches, which extended towards the bead and arborized; only few parent axons turned towards the bead and formed arbors (Figs. 1D, 2E,F,H and 4E,F). Occasionally axons growing away from the bead and arborizing along the medial side of the tract were noted (Fig. 2F, arrows). Composite camera lucida drawings of axons responding to NT-3 are illustrated in Fig. 2H.
Most cases did not show any axon growth towards the NT-3 bead after 24 h in culture (Fig. 4A) while some displayed axonal responses at the level of the bead (Fig. 4B). Responses to NT-3 were quite clear by the end of the second day in culture (Fig. 4C,D) and after 3 days (Figs. 2E,F,H and 4E,F). In these cases, few axons with their leading edge at the level of the bead turned towards the bead. Mostly, there was interstitial branching from the central trigeminal tract axons in the vicinity of the bead (n=54, (Figs. 1D, 2E,F,H and 4E,F)). These axons and their branches often formed knot-like tangles facing the NT-3-loaded bead (Figs. 1D, 2E,F,H and 4E,F). As in NGF cases, there was no apparent spatial segregation of axons (within the central trigeminal tract) attracted to or repelled by the bead (Fig. 2G,H). When an NT-3 loaded bead was placed in the midline (n=6), the central trigeminal tract axons did not leave the tract and no axon outgrowth or arborization was observed (data not shown).
We wanted to determine whether both TrkA and TrkC expressing TG neurons were responsive to localized sources of NGF and NT-3. In both TG and DRG, small diameter neurons express TrkA and are dependent on NGF for survival. Large diameter neurons that express TrkC depend on NT-3 for survival. Our double immuno-labeling in 20 μm frozen sections taken from wholemount cultures showed that in both control and experimental sides of the cultures, different populations of TG cells express TrkA and TrkC as has been reported for in vivo development [19]. Differentially TrkA and TrkC expressing neurons were qualitatively similar in cases with control, NGF- or NT-3-loaded beads. An example of double immunocytochemistry in the ganglion, rostral tract and the tract near an NGF-loaded bead is shown in Fig. 5. Upon neurotrophin binding, Trk receptors are internalized and transported towards the cell body (for review, see Ref. [28]). Therefore, TrkA and TrkC immunocytochemistry also labels central trigeminal axons (Fig. 5D–F). Distinct and nonoverlapping populations of TrkA and TrkC-labeled fibers were clearly visible in the central trigeminal tract in both control and experimental (Fig. 5D–I) cultures. As illustrated in Figs. 5G–I and 6A,D, dense axons that leave the tract and engulf the NGF beads are those that are mainly TrkA-positive. In some cases we saw no TrkC-positive axons around the NGF bead (Figs. 5G–I and 6A–C) while in others we saw few TrkC-positive fibers, but none engulfing the bead like the TrkA-positive fibers (Fig. 6D–I). In contrast, both TrkA and TrkC-positive axons showed similar responses to NT-3 beads (Fig. 6J–R). Often, the TrkA- and TrkC-labeled fibers overlapped. Since in all these cases we did not detect double-labeled cells in the ganglion we conclude that these fibers are different populations of axons that have overlapping terminal fields rather than axons that express both Trk receptors. We were not able to detect immunolabeled single axons that leave the tract and extend away from the source of the neurotrophin in any of our cases. This could be attributed to the sensitivity of immunocytochemistry or lack of TrkA or TrkC expression in the axons that preferentially grow away from the neurotrophin source. Further studies with more sensitive antibodies to TrkA and TrkC or antibodies to other neurotrophin receptors might reveal the class of axons responding to localized neurotrophin sources as seen with DiI labeling.
Fig. 5.
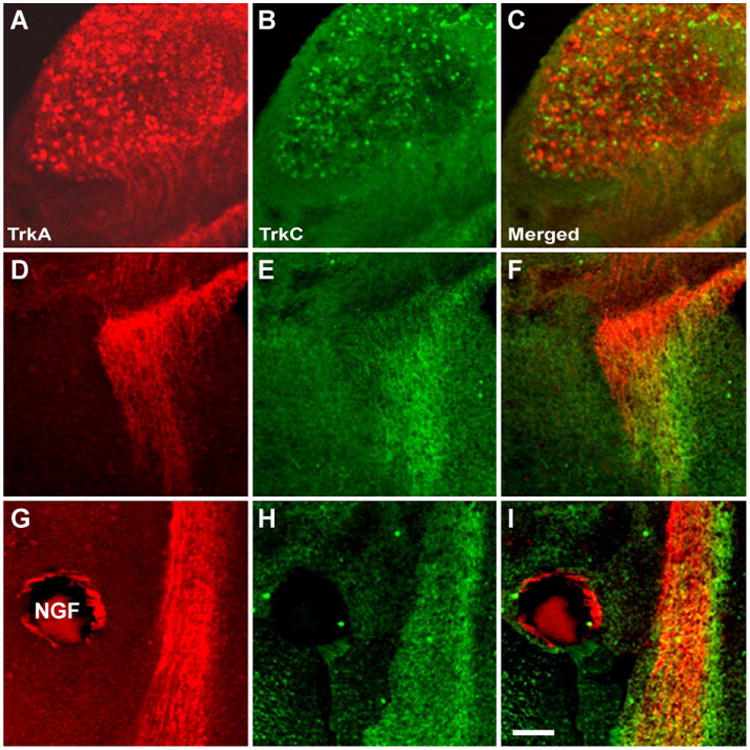
TrkA and TrkC double immunocytochemistry. (A) TrkA and (B) TrkC expression in the same TG from a TG-brainstem wholemount culture with an NGF-bead. Merged images are shown in (C), (F) and (I). Different populations of cells are labeled with either antibody, and there are hardly any cells that express both receptors. (D) and (E) illustrate the initial (rostral) part of the central trigeminal tract, and (G) and (H) at the level of an NGF-loaded bead. Note that at all levels of the tract different populations of trigeminal axons express TrkA and TrkC. Scale bar=100 μm.
Fig. 6.
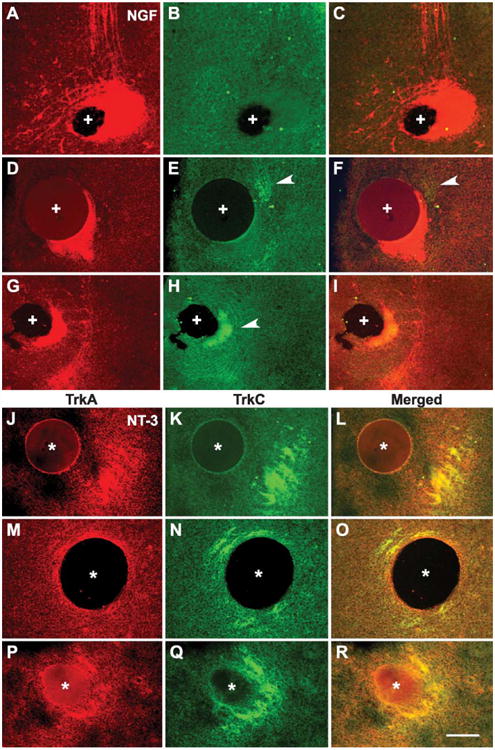
Examples of TrkA and TrkC-labeled axons with respect to neurotrophin bead. (A–I) show TrkA and TrkC-labeled axons with respect to an NGF bead from three different cases. Note that mostly TrkA-labeled axons are around the NGF bead (marked with +). While in some cases there are no TrkC-positive axons (A–C), in others, few axons are seen in the vicinity of the bead (D–I, arrow heads). (J–R), show the presence of both TrkA and TrkC-positive axons near NT-3-loaded beads (marked with *). Since these are cryostat sections through the explants, the size of the bead, and the density of the labeled fibers appear different for each case due to the level of section through the explant. Scale bar=100 μm.
4. Discussion
4.1. Neurotrophin effects on morphological differentiation of axons
Neurotrophin effects on axonal differentiation have been riddled with difficulties due to their trophic effects on cellular viability. Studies in mice with neurotrophin and receptor null mutations have revealed clear defects in neuronal survival but not as much for axonal development. Two other approaches have yielded significant information on neurotrophins and axonal differentiation. One is the use of compartmentalized chambers, wherein neurotrophins can be applied selectively to axons or somata (Refs. [2,3], for reviews see Refs. [16,29]). The second approach is the analyses of neurons that survive independent of neurotrophins. In vitro, sensory neurons that lack the proapoptotic gene Bax, or over express the survival-promoting gene Bcl2, show differential axonal growth when stimulated with neurotrophins [13,23,26]. In Bax and NGF or TrkA double knockout mice central axons of NGF-dependent DRG neurons grow into the spinal cord but their peripheral counterparts fail to develop [34].
In the present study, we adopted a different approach, and examined localized effects of neurotrophins on central trigeminal axons in wholemount cultures of E15 rat embryos. In these cultures, TG cells survive in serum-free culture medium, retain differential expression of Trk receptors, and their axons remain restricted within the boundaries of the trigeminal tract. In recent preliminary experiments we have repeated these cultures using TG-brainstem explants from Bax null mice and the results were indistinguishable (data not shown). Thus, the effects we see with neurotrophin beads most likely reflect axonal responses rather than selective survival of TG cells.
4.2. Target-derived cues that regulate differentiation and patterning of trigeminal sensory axons
Previous work using organotypic trigeminal explant cocultures showed that when embryonic TG axons are presented with a chronologically older brainstem targets, they readily arborize; conversely when TG axons that have already developed terminal arbors are presented with younger brainstem targets, they revert to unbranched growth [10]. Target regulation of trigeminal axon growth patterns has been further demonstrated in studies using xenotypic cocultures of trigeminal tissues from embryonic chick and rat [15]. Clearly these results indicate the presence of target-derived molecular signals (e.g., netrins, semaphorins, ephrins, slits, neurotrophins, extracellular matrix and cell adhesion molecules; for reviews see Refs. [20,31,38]) that regulate axonal differentiation, and further that such signals are preserved across different species.
We recently reported that a member of the Slit family of proteins, Slit-2 can induce arborization of central trigeminal axons at a time when they are in the elongation phase [33]. Slit-2 may not be the only molecule involved in central trigeminal axon growth patterns and arborization. NGF and NT-3 mRNA expression patterns in the developing spinal cord and trigeminal brainstem have been documented [8,22] and the developmental regulation of Trk expression in the TG is well-known [1,19]. Thus, NGF family of neurotrophins, in particular NT-3 may also participate in branching and arborization of central trigeminal axons in vivo.
4.3. Neurotrophins and axon guidance molecules in construction of the trigeminal pathway
Semaphorin 3A (Sema 3A) and neuropilin receptors play a major role in restricting the trigeminal axons along specified routes. In neuropilin or Sema3A knockout mice both peripheral and central TG axons expand out and invade territories they normally would not [21,42]. Studies in the chick also show spatiotemporal regulation of Sema 3A and neuropilin expression in the spinal cord and DRG [35]. In vitro assays indicate that neurotrophins can differentially modify growth cone responses to Sema 3A [40]. NT-3 responsive chick DRG neurons express neuropilin-1 and show growth cone collapse in response to Sema 3A, but they no longer collapse at later ages when they lose neuropilin-1 expression. In contrast, NGF-responsive neurons express progressively high levels of neuropilin-1 and show pronounced collapse response to Sema 3A [35]. Growth cones of E7 chick DRG neurons that have been previously conditioned with exogenous NGF become resistant to collapsing effects of Sema 3A [6]. Furthermore, when an NGF-coated bead is placed adjacent to Sema 3A secreting cells, growth cones do not collapse and they steer towards the NGF-coated bead [7].
Rodent TG cells and their axons express high levels of neuropilin 1 as they form a highly restricted pathway in the brainstem [42]. At later stages, when TG axons leave the tract and invade the brainstem trigeminal nuclei (BSTC), neuropilin 1 expression diminishes. In our experiments we used E15 rat embryos at the time the central trigeminal tract is in its elongation phase with high levels of neuropilin 1 expression. We show that ectopic neurotrophin sources during this stage can disrupt the streamlining of the trigeminal tract and induce axonal growth away from the tract into the BSTC. Present results are in agreement with those seen in chick DRG cell cultures.
4.4. Location and concentration of neurotrophin stimulation
Gradients of various target-secreted molecules and their concentrations at one specific location could play a major role in shaping axonal morphogenesis [16,18,26,37]. A striking example of this is the effects of BDNF on retinal ganglion cells. BDNF application in the Xenopus retina reduces dendritic arborization of ganglion cells but increases axon terminal arbor complexity when applied in the target [24]. Interestingly, increased BDNF levels in the tectum increase dendritic branching of ganglion cells in the retina, without affecting axonal branching in the tectum [25]. BDNF release from cortical neurons also effect morphological differentiation of neighboring cell dendrites in a distance and concentration-dependent manner [17]. Additionally, in embryonic chick DRG cultures described above, neurotrophin-mediated alterations in collapse response to Sema 3A was concentration dependent [6].
We have quantified the amount of protein within the beads and assayed release at 24-h intervals between 1 and 3 days in culture. With our detection system we were not able to determine protein bound to the bead. In culture conditions, any bound neurotrophin molecules would be readily available to axons that contact the bead. Although the release kinetics might be different when the bead is embedded over the ventricular surface of the explants, our release experiments clearly showed that the beads were loaded with neurotrophins and that they release these factors to their vicinity. Indirect evidence also suggests that neurotrophins are concentrated around the bead and do not diffuse far away. Firstly, when neurotrophins are exogenously added to the culture medium, axonal effects can be seen all along the trigeminal pathway [43], with beads, the effects are circumscribed. Second, placement of only NGF beads (but not NT-3 beads) in the brainstem midline elicited some response from the portions of the trigeminal tract facing the bead, and these axons growing away from the tract were never as long as those seen in cases where the bead was next to the tract. Third, in our wholemount explant cultures, the left and the right trigeminal tracts are about 1500–1800 μm apart. NGF or NT-3-loaded beads on one side never elicited any axonal response from the contralateral side. Finally, axons that responded to neurotrophin beads were within a distance of 400–600 μm along the rostrocaudal extent of the tract facing the bead, and no such responses were seen more rostrally, or caudally in the central trigeminal tract. Thus, the effects we report are mostly due to localized actions of neurotrophins on TG axons.
In experiments with NGF beads, placed along the lateral portion of the descending trigeminal tract, axon growth both towards and away from the NGF source was quite intriguing. This type of bidirectional growth might be due to different concentrations of NGF axons become exposed to during the culture period. Our results suggest that in explant cultures if most of the NGF protein is released from the bead during the initial 24 h, central TG axons that first detect these high levels of NGF grow vigorously but away from the bead where NGF concentrations would be lower. On subsequent days, axons that encounter lower levels of NGF emanating from the bead preferentially grow towards it.
Previously we showed that blocking Rac activity virtually eliminates neurotrophin-induced axonal growth outside the trigeminal tract, whereas blocking Rho activity attenuates this response [32]. Differential regulation of Rac and Rho balance within the responding axons or activation of cAMP [13,27] might underlie extensive, unbranched axon growth towards or away from the NGF source. Trk receptor signaling activates not only the Rho family of GTPAses, several small G proteins (e.g., Ras, Rap-1), but also MAP kinase, PI 3-kinase and phospholipase-C- pathways [26,37]. In addition, p75 receptor plays a major role in affecting neurotrophin signaling by Trks, at times cooperatively, at others antagonistically [18] by modifying ligand-binding specificity and affinity. RhoA can be activated by p75, and neurotrophin binding can abolish Rho activity [44]. Thus NGF could induce a variety of effects depending on its concentration and level of binding to Trks and p75 at a given time and locale. Differential gradients of neurotrophin concentration along with other signaling molecules most likely regulate the dynamics of axonal cytoskeletal elements via Rho GTPases and other intracellular signaling molecules.
Acknowledgments
Research supported by NIH/NIDCR. We thank Graham Balcer for his skillful assistance with several of the experiments, Louis Reichardt for Trk antibodies, and Ken Muneoka for sepharose beads. We are also grateful to Luis Marrero for his expert help with confocal imaging at the LSUHSC Morphology and Imaging Core.
References
- 1.Buchman VL, Davies AM. Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development. 1993;118:989–1001. doi: 10.1242/dev.118.3.989. [DOI] [PubMed] [Google Scholar]
- 2.Campenot RB. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campenot RB. NGF and the local control of nerve terminal growth. J Neurobiol. 1994;25:599–611. doi: 10.1002/neu.480250603. [DOI] [PubMed] [Google Scholar]
- 4.Castellani V, Bolz J. Opposing roles for neurotrophin-3 in targeting and collateral formation of distinct sets of developing cortical neurons. Development. 1999;126:3335–3345. doi: 10.1242/dev.126.15.3335. [DOI] [PubMed] [Google Scholar]
- 5.Cohen-Corey S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodeling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- 6.Dontchev VD, Letourneau PC. Nerve growth factor and semaphorin 3A signaling pathways interact in regulating sensory neuronal growth cone motility. J Neurosci. 2002;22:6659–6669. doi: 10.1523/JNEUROSCI.22-15-06659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dontchev VD, Letourneau PC. Growth cones integrate signaling from multiple guidance cues. J Histochem Cytochem. 2003;51:435–444. doi: 10.1177/002215540305100405. [DOI] [PubMed] [Google Scholar]
- 8.Elkabes S, Dreyfus CF, Schaar DG, Black IB. Embryonic sensory development: local expression of neurotrophin-3 and target expression of nerve growth factor. J Comp Neurol. 1994;341:204–213. doi: 10.1002/cne.903410206. [DOI] [PubMed] [Google Scholar]
- 9.Erzurumlu RS, Jhaveri S. Trigeminal ganglion cell processes are spatially ordered prior to the differentiation of the vibrassa pad. J Neurosci. 1992;12:3946–3955. doi: 10.1523/JNEUROSCI.12-10-03946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erzurumlu RS, Jhaveri S. Target influences on the morphology of trigeminal axons. Exp Neurol. 1995;135:1–16. doi: 10.1006/exnr.1995.1061. [DOI] [PubMed] [Google Scholar]
- 11.Erzurumlu RS, Killackey HP. Development of order in the rat trigeminal system. J Comp Neurol. 1983;213:365–380. doi: 10.1002/cne.902130402. [DOI] [PubMed] [Google Scholar]
- 12.Gallo G, Letourneau PC. Localized sources of neurotrophins initiate axon collateral sprouting. J Neurosci. 1998;18:5403–5414. doi: 10.1523/JNEUROSCI.18-14-05403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GT, Barres BA. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- 14.Gundersen LW, Barrett JN. Neuronal chemotaxis: chick dorsal root axons turn towards high concentrations of nerve growth factor. Science. 1979;206:1079–1080. doi: 10.1126/science.493992. [DOI] [PubMed] [Google Scholar]
- 15.Haeberle AS, Erzurumlu RS. Target specific differentiation of peripheral trigeminal axons in rat–chick chimeric explant cocultures. Dev Brain Res. 2001;131:1–8. doi: 10.1016/s0165-3806(01)00235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heerssen HM, Segal RA. Location, location, location: a spatial view of neurotrophin signal transduction. Trends Neurosci. 2002;25:160–165. doi: 10.1016/s0166-2236(02)02144-6. [DOI] [PubMed] [Google Scholar]
- 17.Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002:1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- 18.Huang EJ, Reichardt LF. TRK Receptors: Roles in Neuronal Signal Transduction. Ann Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 19.Huang EJ, Wilkonson GA, Farinas I, Backus C, Zang K, Wong SL, Reichardt LF. Expression of Trk receptors in the developing mouse trigeminal ganglion: in vivo evidence for NT-3 activation of trkA and TrkB in addition to trkC. Development. 1999;126:2191–2203. doi: 10.1242/dev.126.10.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the Growth Cone: Ligand-Receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 21.Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, Yagi T, Fujisawa H. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- 22.Lamballe F, Smeyne RJ, Barbacid M. Developmental expression of trkC, the neurotrophin-3 receptor, in the mammalian nervous system. J Neurosci. 1994;14:14–28. doi: 10.1523/JNEUROSCI.14-01-00014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lentz SI, Knudson CM, Korsmeyer SJ, Snider WD. Neurotrophins support the development of diverse sensory axon morphologies. J Neurosci. 1999;19:1038–1048. doi: 10.1523/JNEUROSCI.19-03-01038.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lom B, Cohen-Cory S. Brain-derived neurotrophic factor differentially regulates retinal ganglion cell dendritic and axonal arborization in vivo. J Neurosci. 1999;19:9928–9938. doi: 10.1523/JNEUROSCI.19-22-09928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lom B, Cogen J, Sanchez AL, Vu T, Cohen-Cory S. Local and target-derived brain-derived neurotrophic factor exert opposing effects on the dendritic arborization of retinal ganglion cells in vivo. J Neurosci. 2002;22:7639–7649. doi: 10.1523/JNEUROSCI.22-17-07639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markus A, Patel TD, Snider WD. Neurotrophic factors and axonal growth. Curr Opin Neurobiol. 2002;12:523–531. doi: 10.1016/s0959-4388(02)00372-0. [DOI] [PubMed] [Google Scholar]
- 27.Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG, Jr, Reichardt LF, Barres BA. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21:681–693. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller FD, Kaplan DR. Neurotrophin signaling pathways regulating neuronal apoptosis. Cell Mol Life Sci. 2001;58:1045–1053. doi: 10.1007/PL00000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller FD, Kaplan DR. On Trk for retrograde signaling. Neuron. 2001;32:767–770. doi: 10.1016/s0896-6273(01)00529-3. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery DC. Design and Analysis of Experiments. 3rd. Wiley; New York: 1991. [Google Scholar]
- 31.Mueller BK. Growth cone guidance: first steps towards a deeper understanding. Annu Rev Neurosci. 1999;22:351–388. doi: 10.1146/annurev.neuro.22.1.351. [DOI] [PubMed] [Google Scholar]
- 32.Ozdinler PH, Erzurumlu RS. Regulation of neurotrophin-induced axonal responses via Rho GTPases. J Comp Neurol. 2001;438:377–387. doi: 10.1002/cne.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozdinler PH, Erzurumlu RS. Slit-2, a branching-arborization factor for sensory neurons in CNS. J Neurosci. 2002;22:4540–4549. doi: 10.1523/JNEUROSCI.22-11-04540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel TD, Jackman A, Rice FL, Kucera J, Snider WD. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron. 2000;25:345–357. doi: 10.1016/s0896-6273(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 35.Pond A, Roche FK, Letourneau PC. Temporal regulation of neuro-pilin-1 expression and sensitivity to semaphorin 3A in NGF- and NT3-responsive chick sensory neurons. J Neurobiol. 2002;51:43–53. doi: 10.1002/neu.10041. [DOI] [PubMed] [Google Scholar]
- 36.Sandell JH, Masland RH. Photoconversion of some fluorescent markers to a diaminobenzidine product. J Histochem Cytochem. 1988;36:555–559. doi: 10.1177/36.5.3356898. [DOI] [PubMed] [Google Scholar]
- 37.Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu Rev Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- 38.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 39.Tucker KL, Meyer M, Barde YA. Neurotrophins are required for nerve growth during development. Nat Neurosci. 2001;4:29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- 40.Tuttle R, O'Leary DD. Neurotrophins rapidly modulate growth cone response to the axon guidance molecule, collapsin-1. Mol Cell Neurosci. 1998;11:1–8. doi: 10.1006/mcne.1998.0671. [DOI] [PubMed] [Google Scholar]
- 41.Ulupinar E, Erzurumlu RS. Peripheral target-specific influences on enbryonic neurite growth vigor and patterns. J Comp Neurol. 1998;399:427–449. doi: 10.1002/(sici)1096-9861(19981005)399:4<427::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 42.Ulupinar E, Datwani A, Behar O, Fujisawa H, Erzurumlu RS. Role of semaphorin III in the developing rodent trigeminal system. Mol Cell Neurosci. 1999;13:281–292. doi: 10.1006/mcne.1999.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulupinar E, Jacquin MF, Erzurumlu RS. Different effects of NGF and NT-3 on embryonic trigeminal axon growth patterns. J Comp Neurol. 2000;425:202–218. doi: 10.1002/1096-9861(20000918)425:2<202::aid-cne4>3.0.co;2-t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamashita T, Tucker KL, Barde YA. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24:585–593. doi: 10.1016/s0896-6273(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Schmidt RE, Yan Q, Snider WD. NGF and NT-3 have different effects on the growth of dorsal root axons in the developing mammalian spinal cord. J Neurosci. 1994;14:5187–5201. doi: 10.1523/JNEUROSCI.14-09-05187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


