Abstract
The aim of this study was to assess the effects of inoculation of rhizosphere or endophytic bacteria (Psychrobacter sp. SRS8 and Pseudomonas sp. A3R3, respectively) isolated from a serpentine environment on the plant growth and the translocation and accumulation of Ni, Zn, and Fe by Brassica juncea and Ricinus communis on a multi-metal polluted serpentine soil (SS). Field collected SS was diluted to 0, 25, 50, and 75% with pristine soil in order to obtain a range of heavy metal concentrations and used in microcosm experiments. Regardless of inoculation with bacteria, the biomass of both plant species decreased with increase of the proportion of SS. Inoculation of plants with bacteria significantly increased the plant biomass and the heavy metal accumulation compared with non-inoculated control in the presence of different proportion of SS, which was attributed to the production of plant growth promoting and/or metal mobilizing metabolites by bacteria. However, SRS8 showed a maximum increase in the biomass of the test plants grown even in the treatment of 75% SS. In turn, A3R3 showed maximum effects on the accumulation of heavy metals in both plants. Regardless of inoculation of bacteria and proportion of SS, both plant species exhibited low values of bioconcentration factor (<1) for Ni and Fe. The inoculation of both bacterial strains significantly increased the translocation factor (TF) of Ni while decreasing the TF of Zn in both plant species. Besides this contrasting effect, the TFs of all metals were <1, indicating that all studied bacteria–plant combinations are suitable for phytostabilization. This study demonstrates that the bacterial isolates A3R3 and SRS8 improved the growth of B. juncea and R. communis in SS soils and have a great potential to be used as inoculants in phytostabilization scenarios of multi-metal contaminated soils.
Keywords: phytostabilization, plant growth promoting bacteria, serpentine soils, heavy metals, Brassica juncea, Ricinus communis
INTRODUCTION
Remediation of metal contaminated soils using plant based approaches (phytoremediation) is considered a simple, lower cost, environmentally friendly technology that can provide full-scale remediation when compared with existing physicochemical technologies (Ali et al., 2013). The most common phytoremediation method for soil remediation is phytostabilization, which utilizes metal tolerant plants to reduce/prevent the mobility of heavy metals in soils (Bolan et al., 2011). Currently, various plant species such as Erica australis, E. andevalensis, Globularia alypum L., Rosmarinus officinalis L., and Solanum nigrum L. have been used to reduce the mobility of heavy metals in artificially polluted soils (Ferraz et al., 2012; Testiati et al., 2013; Pérez-López et al., 2014). Although these plant species are able to grow on metal polluted soils, their application in phytostabilization practices in metal polluted fields is limited due to their low ability to adapt to adverse environmental conditions (Ma et al., 2011a). In general, multiple stresses including low soil pH, high salinity, low essential nutrients, and high concentrations of metals present in polluted field soils may limit the growth and survival of plants and thus compromise the overall phytoremediation process. Therefore, revegetation of metal polluted soils calls not only for the selection of appropriate plants species that are able to tolerate multiple stresses and accumulate/detoxify heavy metals but also the knowledge on the interactions among plant, soil, metals, and microbes.
In recent years, microbially enhanced methods of phytostabilization have been proposed as a viable strategy for removing/inactivating heavy metals in polluted soils (Grandlic et al., 2008). To reach such a goal of enhanced phytostabilization, the microbial inoculants must alleviate plant toxicity produced by heavy metals and increase plant tolerance. Metal resistant bacteria, particularly the rhizobacteria and endophytes, have been the most common plant associated beneficial microbes used as inoculants in phytostabilization processes of metal polluted soils (Rajkumar et al., 2013a; Jebara et al., 2014). These bacteria are capable of stimulating the plant growth and reducing metal uptake by producing various metabolites including 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase, siderophores, and plant growth hormones, and mobilizing/immobilizing/transforming heavy metals (Zaidi et al., 2006; Dell’Amico et al., 2008; Rajkumar et al., 2012, 2013b; Becerra-Castro et al., 2013; Ma et al., 2013; Zhu et al., 2014). For example, He et al. (2013) have recently investigated the potential of inoculating Brassica napus with ACC utilizing bacteria Rahnella sp. JN6 to alleviate metal stress in plants and showed that plant height, root length, above-ground, and root weight were greatly increased. Similarly, Srivastava and Singh (2014) used the metal immobilizing plant growth promoting bacteria (PGPB) Acinetobacter sp. nbri05 isolated from soil collected from an arsenic contaminated site to improve the plant growth and reduce the heavy metal translocation into plant shoots, thereby improving the phytostabilization potential of Cicer aritenum grown in arsenic contaminated soils. It has also been demonstrated that the use of such stress adapted microbial strains in phytostabilization studies is more effective than applying non-adapted strains (Rajkumar et al., 2013a; Jebara et al., 2014). Recently, Rajkumar et al. (2013a) investigated the phytostabilization potential of Brassica juncea, Luffa cylindrica, and Sorghum halepense after inoculation of Ni resistant serpentine isolate Bacillus megaterium SR28C and found that it was able to alleviate the detrimental effects of Ni by reducing its uptake and translocation to the plants.
Some progress has been made towards understanding the serpentine microbial diversity (Mengoni et al., 2004; Doherty et al., 2008) and their beneficial role on plant growth and phytoremediation in artificially Ni polluted soils (Ma et al., 2009a,b, 2010, 2011b; Rajkumar et al., 2013a), however, little is known about the role of serpentine bacterial isolates on the growth and heavy metal accumulation potential of plants in multi-metal polluted field soils. Heavy metals in general and Ni in particular present in SS were reported to have negative effect on plant mineral nutrition (Duman and Ozturk, 2010) and physiological metabolisms (Velikova et al., 2011) and henceforth the overall growth of plants. With the help of PGPB, the host plants can obtain more nutrients and plant resistance to heavy metal stress can be enhanced (Sessitsch et al., 2013).
In this study using SS as a model for multi-metal polluted field soils, we assessed the effects of inoculation of the metal resistant serpentine bacterial isolates Pseudomonas sp. A3R3 or Psychrobacter sp. SRS8 on the biomass production and Ni, Zn and Fe accumulation potential of B. juncea and R. communis plants. The aim was to evaluate the feasibility of using metal resistant-serpentine bacteria for microbe-assisted phytostabilization of contaminated soil.
MATERIALS AND METHODS
BACTERIAL STRAINS
The endophytic bacteria Pseudomonas spp. A3R3 (GenBank accession no. GU550663), and the rhizobacteria Psychrobacter sp. SRS8 (accession no. FM205059) originally isolated, respectively, from root interior and rhizosphere of Alyssum serpyllifolium grown in SS in Bragança, north–east of Portugal were obtained from the Culture Collection of the Center for Functional Ecology, University of Coimbra (Ma et al., 2009b, 2011b). The selection of these PGPB in this study was based on their ability to tolerate high concentrations of Ni and to produce plant growth promoting (PGP) substances (Table 1).
Table 1.
Characteristic features of the bacterial strains used in this study.
| Plant growth promoting traits |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Bacterial strain | ACC deaminase (μm α-KB mg-1h-1) | P solubilization (mg L-1) | IAA production (mg L-1) | Siderophore production (mg L-1) |
Ni resistance level (mg L-1) | Genbank accession no. | Source | Reference | |
| Catechol | Hydroxamate | ||||||||
| Pseudomonas sp. A3R3 | 67.9 ± 6.2 | 138 ± 21.4 | 69.4 ± 3.2 | 83.3 ± 7.5 | 60.5 ± 6.3 | 750 | GU550663 | Root of Alyssum serpyllifolium | Ma et al. (2011b) |
| Psychrobacter sp. SRS8 | nd | 126 ± 2.2 | 111 ± 1.4 | 899 ± 30.3 | 28.0 ± 2.2 | 1000 | FM205059 | Rhizosphere of A. serpyllifolium | Ma et al. (2009b) |
Average ± SD from three samples. nd, not detected; ACC, 1-aminocyclopropane-1-carboxylic acid; α-KB, α-ketobutyrate; IAA, indole-3-acetic acid.
EXPERIMENTAL PLANTS
Two crop plants, B. juncea (L.) Czern. and Ricinus communis L. were selected for this study based on their demonstrated ability to grow in heavily polluted soil (Bauddh and Singh, 2012) and to accumulate high amounts of heavy metals (Cecchi and Zanchi, 2005; Ma et al., 2009b) together with their potential to produce substantial biomass in a very short time (Safari Sinegani and Khalilikhah, 2008).
MICROCOSM EXPERIMENTS
The SS used in the present study was collected from the Bragança, north-east of Portugal (Freitas et al., 2004), while the pristine soil (PS) was collected from the Botanical Garden of the University of Coimbra, Coimbra, Portugal. The physicochemical properties and heavy metals concentrations of the SS and PS are shown in Table 2. Seeds of B. juncea and R. communis obtained from the Botanical Garden of the University of Coimbra, Portugal, were surface sterilized with 2% Ca(OCl)2 during 2 h and rinsed three times with autoclaved deionized water. The seeds were allowed to germinate in sterilized non-contaminated PS at 25°C under a 16/8 day/night regime. Seedlings were exposed to full sunlight during the day. The soil collected from the serpentine area was mixed with the PS at four different proportions: 0% SS (0% SS + 100% PS), 25% SS (25% SS + 75% PS), 50% SS (50% SS + 50% PS) and 75% SS (75% SS + 25% PS). The mixture was based on the dry weights of the soils. 100% SS (100% SS + 0% PS) was omitted in this study, since in our preliminary microcosm experiments both B. juncea and R. communis grown in 100% SS showed signs of wilting as early as 15 days after the transplantation, and they died during the experiments (data not shown). For inoculation of the seedlings, the bacterial strains were grown in Luria Bertani medium for 18 h at 27°C and 200 rpm. The cultures were then centrifuged at 7000 rpm for 10 min and the pellets were washed with biological saline (0.85% KCl). The pellet was re-suspended in biological saline and the colony forming unit (CFU) was adjusted to 109 mL-1. Fifteen-day-old seedlings were inoculated by soaking the root system in the bacterial culture for 2 h. The roots of control plants were soaked in sterile deionized water. Seedlings were transplanted into plastic pots with a volume of 0.86 cm3, containing 300 g of 0, 25, 50, or 75% SS. The plants were allowed to grow in a greenhouse with an average temperature of 25°C and a 16:8 h day/night regime and watered as required. All treatments were performed in three replicates.
Table 2.
Physicochemical properties of the soils used in this study.
| Parameters | Serpentine soil | Pristine soil | ||
|---|---|---|---|---|
| pH | 7.5 - 8.5 | 7.3 – 7.5 | ||
| Organic matter (%) | 1.3 ± 0.1 | 1.6 ± 0.2 | ||
| Cation exchange capacity [meq(100g)-1] | 1.0 ± 0.2 | 1.5 ± 0.1 | ||
| Electrical conductivity (dS m-1) | 0.8 ± 0.1 | 1.2 ± 0.2 | ||
| Metals (mg kg-1) | Total | Extractable | Total | Extractable |
| Ni | 812 ± 35 | 4.3 ± 0.8 | 18 ± 1 | nd |
| Zn | 160 ± 12.3 | 48.7 ± 2.8 | 86 ± 10.5 | 9.3 ± 1.1 |
| Fe | 6849 ± 215 | 437 ± 12.1 | 653 ± 33 | 75.6 ± 12.1 |
Average ± SD from three samples. nd, not detected.
SOIL ANALYSIS
Both total and diethylenetriaminepentaacetic acid (DTPA)-extractable fractions of heavy metals (Ni, Zn, and Fe) were measured in the soil. To analyze the total metal content, three replicates of 1 g of soil were weighed and 10.5 mL of aqua regia were added to each sample according to Kilburn (2000). The digestion tubes were allowed to stand overnight to equilibrate the aqua regia with the soil and then were put onto a hotplate for 3 h at 110°C. The digests were filtered through Whatman No. 42 filter paper and mixed with deionized water in volumetric flask reaching 25 mL. The concentration of heavy metals in soil extracts was measured by atomic absorption spectrophotometry (AAS; PerkinElmer model 100, MA, USA).
Diethylenetriaminepentaacetic acid-extractable metals were measured by extracting 10 g samples of soil with 20 mL of extracting solution (0.005 M DTPA, 0.01 M CaCl2, 0.1 M Triethanolamine, pH 7.3) according to the procedure of Lindsay and Norvell (1978). The mixtures were agitated on a horizontal shaker for 2 h at room temperature and were then through Whatman No. 42 filter paper prior to analysis by AAS.
PLANT ANALYSIS
After 60 days, plants were harvested and the roots were carefully washed with deionized water and then with 10 mM ethylenediaminetetraacetic acid to remove all attached soil particles. The growth parameters such as root and shoot weight (fresh and dry) were determined. To evaluate the heavy metal concentrations, the washed plant materials were oven dried at 70°C for 3 days and finely ground to a powder. The metal contents (Ni, Zn, and Fe) in the plant tissues were measured by AAS after digestion in a mixture of concentrated HNO3 and HClO4 (4:1, v/v; Ma et al., 2009a).
The determinations of the metal concentration in plant tissues and soils were used to estimate the translocation factor (TF) and the bioconcentration factor (BCF). The TF was calculated by dividing shoot metal concentration by the root metal concentration ([Metal]shoot/[Metal]root; Lotfy and Mostafa, 2014) and the BCF was calculated by dividing the metal concentration in the entire plant by the initial soil metal concentration ([Metal]plant/[Metal]soil; Zayed et al., 1998). Metal concentrations in all compartments were calculated on a dry weight basis.
STATISTICAL ANALYSIS
Data were analyzed by analysis of variance (ANOVA) and treatment means were compared using Fisher’s protected LSD test (p < 0.05). All the analyzes were performed using SPSS 10.0.
RESULTS AND DISCUSSION
PLANT GROWTH
Although B. juncea and R. communis used in this study have been reported to grow in heavy metal contaminated soils (Niu et al., 2007; Bauddh and Singh, 2012), the adverse environmental conditions particularly the low essential nutrients and the elevated levels of various metals in SS (Freitas et al., 2004) may reduce the plant growth and biomass production through impairing their metabolism and interfering with the absorption of essential nutrients (Duman and Ozturk, 2010). Soil properties greatly influence plant growth and root development. The low organic matter (1.3–1.6%) and cation exchange capacity [1.0–1.5 meq(100g)-1] (Table 2) of the soils used in our study may have contributed to the limited growth of the tested plants. Thus in this study, the effect of rhizosphere or endophytic bacteria previously isolated from serpentine environment (Ma et al., 2009b, 2011b) on the growth and metal accumulation potential of B. juncea and R. communis in SS was investigated with the objective of using these species for phytoremediation of multi-metal polluted field soils. In control soils (0% SS), inoculation of B. juncea and R. communis with Pseudomonas sp. A3R3 or Psychrobacter sp. SRS8 showed an increase in plant fresh and dry weight (Figures 1A,B). However, maximum PGP effect was observed in A3R3. In the case of B. juncea, inoculation of A3R3 increased plant fresh and dry weight by 30 and 38%, respectively. Similarly, in R. communis, the strain A3R3 enhanced the fresh and dry weight by 31 and 34%, respectively. Recent studies on the mode of action of PGPB have shown that the increase in plant growth is due to microbial production of various PGP metabolites such as ACC deaminase (which can reduce stress ethylene production in plants through hydrolyzing ethylene precursor ACC to ammonia and α-ketobutyrate, α-KB; Arshad et al., 2007); indole-3-acetic acid (IAA) (which can enhance the plant growth by stimulating plant cell elongation or affecting cell division; Rashotte et al., 2000); nutrient solubilizing metabolites (which can mobilize nutrients – e.g., P and Fe in the rhizosphere; Sessitsch et al., 2013). The maximum plant growth promotion by A3R3 observed in the present study might have been due to the cumulative effects of the production of siderophores, ACC deaminase, IAA and solubilization of P (Table 1).
FIGURE 1.
Influence of Psychrobacter sp. SRS8 and Pseudomonas sp. A3R3 on the fresh weight and dry weight of Brassica juncea (A) and Ricinus communis (B) grown in different proportion of serpentine soil (SS). Each value is the mean of triplicates. Error bars represent SD. Data of columns indexed by the same letter are not significantly different according to Fisher’s protected LSD test (p < 0.05).
The non-inoculated plants exposed to different levels of SS (25–75%), showed a marked inhibition in the growth. The fresh and dry weight of the B. juncea and R. communis generally decreased with increasing the concentrations of SS. In the treatment with 75% SS, the growth of B. juncea was considerably decreased (Figure 1A), with an 84% reduction in fresh weight and 77% reduction in dry weight. Similar responses were also observed in R. communis (Figure 1B), where fresh and dry weight decreased by 78 and 75%, respectively. The low growth observed for plants in SS had already been reported by other authors (Abou-Shanab et al., 2006; Kayama et al., 2006). Plants inoculated with Pseudomonas sp. A3R3 or Psychrobacter sp. SRS8 exhibited an increase in plant growth in the presence of different levels of SS. However, inoculation with Pseudomonas sp. A3R3, a strain that showed ACC deaminase activity in addition to other PGP traits, resulted in higher fresh and dry weight of plants. Pseudomonas sp. A3R3 increased the fresh and dry weight of B. juncea even in the treatment of 75% SS by 82 and 55%, respectively, compared to non-inoculated plants grown with the same treatment of SS. Similarly, in R. communis, Pseudomonas sp. A3R3 enhanced the fresh and dry weight by 45 and 42%, respectively at 75% SS. Similar results have been previously reported by Ma et al. (2011b), Zhang et al. (2011), and Srivastava et al. (2013), suggesting that the production of ACC deaminase by strain A3R3, which can hydrolyze ethylene precursor ACC to α-KB and ammonia, may alleviate the Ni stress-mediated impact on plants. Further, Pseudomonas sp. A3R3 can also produce catechol and hydroxamate type siderophores, IAA and solubilize P (Ma et al., 2011b), indicating that both B. juncea and R. communis might have benefited from this strain. Since Pseudomonas sp. A3R3 was originally isolated from the root tissues of A. serpyllifolium (Ma et al., 2011b), the increased plant growth caused by Pseudomonas sp. A3R3 also implies that the endophytic bacteria with various PGP traits might be preferable for plant growth promotion in metal polluted soils. However, this has to be tested with a broader spectrum of endophytes before a general validity can be assumed since the present study comprised only one endophytic bacterial strain. Based on the above observations, it was inferred that the inoculation of serpentine PGPB improved the establishment of B. juncea and R. communis in Ni rich SS, as reflected by increased growth performance.
HEAVY METAL CONCENTRATIONS IN SOILS AFTER HARVESTING
Influence of plants and PGPB inoculation on the variation of metal mobility in soils was determined after harvesting. Regardless of inoculation with PGPB, a decrease in the total content of all the three metals was observed in root-adhering soils of both B. juncea and R. communis, compared to unplanted (Table 3), as a likely consequence of metal uptake by plants. However, plants inoculated with PGPB were effective at decreasing the total Ni and Zn concentrations in soils compared with respective non-inoculated control. In the treatment with 75% of SS, inoculation of Psychrobacter sp. SRS8 decreased the total Ni and Zn concentrations in the soils of B. juncea by 28 and 25%, respectively, whereas in the soils of R. communis the percentage of decrease was 23 and 22, respectively. This result suggests that with the help of PGPB both B. juncea and R. communis can efficiently take up Ni and Zn from soils and confirm the previous finding that rhizosphere microbes may stimulate heavy metal uptake by plants (Chen et al., 2013; Prapagdee et al., 2013). On the contrary to total Ni and Zn contents, plants inoculated with PGPB significantly increased the concentration of total Fe in soils when compared with the non-inoculated control plants. This may be attributed to a decrease in plant Fe uptake, possibly due to the presence of high concentrations of bioavailable Ni or competition between bioavailable Ni and Fe at the plant uptake site as a consequence of microbial induced Ni mobilization. Previously, plant nutritional imbalances in response to the presence of heavy metals have been reported by Ouzounidou et al. (2006), suggesting that Ni may compete effectively for specific binding sites or by other means block the uptake units, leading to decrease in Fe uptake by plants.
Table 3.
Heavy metals concentrations in soils before planting and after harvesting of Brassica juncea and Ricinus communis inoculated with Psychrobacter sp. SRS8 and Pseudomonas sp. A3R3.
| Treatment | DTPA extractable (mg kg-1 dry soil) |
Total (mg kg-1 dry soil) |
||||
|---|---|---|---|---|---|---|
| Ni | Zn | Fe | Ni | Zn | Fe | |
| Before planting | ||||||
| SS 0% (Pristine soil) | nd | 1.0 ± 0.1 | 35.1 ± 6.4 | 21.6 ± 2.7 | 38.5 ± 6.8 | 556 ± 24.6 |
| SS 25% (without plants ) | nd | 18.7 ± 0.9 | 159 ± 10.7 | 290 ± 46.2 | 180 ± 25.2 | 2290 ± 217 |
| SS 50% (without plants) | 8.9 ± 3.3 | 28.7 ± 6.8 | 220 ± 54.3 | 367 ± 58.9 | 214 ± 56.4 | 3664 ± 190 |
| SS 75% (without plants) | 14.8 ± 3.3 | 33.9 ± 9.7 | 190 ± 23.6 | 418 ± 89.6 | 235 ± 17.5 | 5311 ± 218 |
| After harvesting of B. juncea | ||||||
| SS 0% | nd | 2.2 ± 0.7 g | 54.0 ± 10.5 h | 19.2 ± 1.7 f | 34.4 ± 5.7 e | 395 ± 28.4 i |
| SS 0% + A3R3 | nd | 18.6 ± 4.4 f | 73.8 ± 8.4 g | 14.5 ± 0.9 f | 29.4 ± 2.9 e | 468 ± 68.0 hi |
| SS 0% + SRS8 | nd | 62.3 ± 9.4 c | 83.3 ± 12.6 g | 12.6 ± 1.2 f | 22.7 ± 7.1 e | 502 ± 39.0 h |
| SS 25% | 1.6 ± 0.4 h | 21.3 ± 1.5 f | 202 ± 20.6 f | 252 ± 37.8 d | 167 ± 34.7 c | 1992 ± 367 f |
| SS 25% + A3R3 | 15.5 ± 2.6 f | 51.2 ± 6.4 d | 289 ± 17.3 c | 189 ± 15.3 e | 126 ± 13.0 d | 1923 ± 194 fg |
| SS 25% + SRS8 | 28.0 ± 5.3 e | 76.3 ± 7.9 b | 338 ± 38.5 b | 171 ± 13.5 e | 107 ± 14.1 d | 2070 ± 275 f |
| SS 50% | 10.4 ± 2.6 g | 35.4 ± 5.2 e | 281 ± 31.6 cd | 323 ± 19.3 b | 196 ± 47.2 b | 2775 ± 298 e |
| SS 50% + A3R3 | 43.8 ± 10.0 d | 58.4 ± 9.1 c | 378 ± 23.5 a | 271 ± 12.5 c | 153 ± 18.4 c | 2998 ± 306 d |
| SS 50% + SRS8 | 74.2 ± 10.5 b | 85.5 ± 7.3 a | 384 ± 65.3 a | 243 ± 8.2 d | 127 ± 8.3 d | 3284 ± 271 c |
| SS 75% | 16.8 ± 2.9 f | 38.0 ± 8.0 e | 228 ± 26.1 e | 364 ± 42.2 a | 223 ± 29.1 a | 4335 ± 407 b |
| SS 75% + A3R3 | 51.2 ± 16.2 c | 51.8 ± 8.8 d | 267 ± 37.2 d | 309 ± 13.0 b | 190 ± 10.3 b | 4539 ± 400 a |
| SS 75% + SRS8 | 86.5 ± 7.3 a | 73.0 ± 7.2 b | 272 ± 35.0 cd | 261 ± 15.8 cd | 168 ± 13.3 bc | 4416 ± 278 b |
| After harvesting of R. communis | ||||||
| SS 0% | nd | 16.5 ± 1.7 f | 26.6 ± 5.2 g | 26.8 ± 4.3 h | 18.6 ± 3.5 e | 290 ± 42.8 j |
| SS 0% + A3R3 | nd | 35.3 ± 3.3 e | 43.0 ± 10.0 fg | 20.6 ± 1.6 h | 15.4 ± 3.2 e | 335 ± 31.5 ij |
| SS 0% + SRS8 | nd | 50.9 ± 2.6 cd | 58.6 ± 5.6 f | 19.1 ± 1.9 h | 11.8 ± 1.8 e | 421 ± 31.6 i |
| SS 25% | 0.9 ± 0.2 g | 33.5 ± 3.2 e | 112 ± 12.0 e | 375 ± 20.9 d | 102 ± 18.1 c | 1242 ± 90.3 h |
| SS 25% + A3R3 | 7.5 ± 0.7 f | 81.8 ± 5.7 b | 218 ± 26.2 d | 305 ± 37.8 f | 79.6 ± 12.4 d | 1387 ± 155 g |
| SS 25% + SRS8 | 13.9 ± 1.5 e | 97.6 ± 7.3 a | 218 ± 24.4 d | 247 ± 31.0 g | 78.4 ± 6.0 d | 1542 ± 71.9 f |
| SS 50% | 8.9 ± 1.4 f | 56.5 ± 4.4 c | 217 ± 18.0 d | 449 ± 29.3 b | 125 ± 32.6 b | 2185 ± 244 e |
| SS 50% + A3R3 | 34.3 ± 4.5 d | 79.2 ± 3.5 b | 298 ± 47.0 b | 350 ± 38.4 e | 103 ± 20.9 c | 2404 ± 265 d |
| SS 50% + SRS8 | 56.5 ± 3.9 b | 96.1 ± 7.4 a | 337 ± 24.8 a | 326 ± 20.4 f | 99.9 ± 12.5 c | 2589 ± 225 c |
| SS 75% | 15.8 ± 1.5 e | 62.7 ± 3.8 c | 218 ± 27.0 d | 477 ± 31.4 a | 151 ± 18.0 a | 3644 ± 276 b |
| SS 75% + A3R3 | 37.7 ± 4.7 c | 85.5 ± 6.4 b | 219 ± 53.4 d | 402 ± 22.9 c | 119 ± 17.7 b | 3836 ± 199 a |
| SS 75% + SRS8 | 67.8 ± 3.1 a | 100 ± 11.4 a | 246 ± 14.4 c | 365 ± 45.5 de | 119 ± 8.9 b | 3707 ± 146 b |
Average ± SD from three samples. Data of columns within each plant species indexed by the same letter are not significantly different according to Fisher’s protected LSD test (p < 0.05). nd, not detected; DTPA, diethylenetriaminepentaacetic acid; SS, serpentine soil.
According to Jackson and Alloway (1991), Singh et al. (1996), Rauret et al. (1999), Wang et al. (2004), and Meers et al. (2007), the levels of metals in plant tissues are closely related to the concentrations of DTPA-extractable metals in soils. Therefore, the bioavailable fraction of metals in the rhizosphere soils of B. juncea and R communis was determined using DTPA as extractant. In general, plant roots and rhizosphere microbes strongly influence the physicochemical characteristics of the soil through various metabolic actions/activities [e.g., excretion of organic acids, (phyto)siderophores] resulting in an increase/decrease in metal bioavailability in soils. In the present study, irrespective of inoculation of PGPB, the bioavailable fractions in the soils of all SS treatments increased significantly after the growth of B. juncea or R communis and this was likely due to the ability of plant and/or its associated microbes to increase metal availability (Table 3). However, this effect was higher when the plants were inoculated with PGPB, particularly Psychrobacter sp. SRS8. These results clearly revealed that inoculation of PGPB prompted the release of unavailable metals into soil. In the treatment of 50% SS, inoculation of B. juncea with Psychrobacter sp. SRS8 increased maximum concentrations of DTPA extractable Ni, Zn, and Fe in the soils, which were 7.1-, 2.4-, and 1.4-fold higher than those in the soils of non-inoculated plants, respectively. Similarly, R communis inoculated with Psychrobacter sp. SRS8 increased the concentrations of DTPA extractable Ni, Zn, and Fe in the soil by 6.3-, 1.7-, and 1.6-fold, respectively. The increase in DTPA extractable Ni, Zn, and Fe after bacterial inoculation might be attributed to solubilization of unavailable forms of heavy metal bearing minerals due to complexation reaction as a consequence of metabolites (e.g., organic acids, siderophores) release by PGPB.
METAL UPTAKE BY Brassica juncea AND Ricinus communis
Metal concentrations in roots and shoots of both plants tended to increase in the treatments with higher proportions of SS (Table 4). The maximum accumulation of metals was observed in the root and shoot systems of plants grown in 75% SS. However, the metal concentrations in the shoots of both plants were much lower than in the respective roots. A similar finding was recently reported for B. juncea and R. communis grown on metal contaminated soils (Bauddh and Singh, 2012). Regardless of inoculation of PGPB, B. juncea took up more heavy metals than R. communis under the same conditions, except Zn which concentration was higher in root and shoot tissues of R. communis. Besides, the concentrations of Fe and Zn in the tissues of both plants were found to be higher than Ni and this could be explained by the fact that Zn and Fe are an essential element for plant growth (Desideri et al., 2010).
Table 4.
Heavy metals concentrations in shoots and roots of Brassica juncea and Ricinus communis grown in serpentine soils (SS) and inoculated with Psychrobacter spp. SRS8 and Pseudomonas spp. A3R3.
| Treatment |
Shoot (mg kg-1 dw) |
Root (mg kg-1 dw) |
|||||
|---|---|---|---|---|---|---|---|
| Proportion of SS (%) | PGPB | Ni | Zn | Fe | Ni | Zn | Fe |
| strain | |||||||
| B. juncea | |||||||
| 0 | Control | 2.2 ± 0.2 i | 9.5 ± 0.6 h | 67.0 ± 5.3 f | 13.2 ± 1.3 e | 17.8 ± 2.7 e | 170 ± 23.3 f |
| A3R3 | 4.4 ± 0.5 hi | 10.1 ± 0.8 h | 75.3 ± 4.5 f | 14.9 ± 1.5 e | 24.8 ± 4.5 e | 196 ± 22.2 f | |
| SRS8 | 5.3 ± 0.3 h | 11.1 ± 1.0 h | 75.7 ± 7.3 f | 17.2 ± 2.0 e | 26.0 ± 3.9 e | 215 ± 34.8 f | |
| 25 | Control | 29.7 ± 1.3 g | 37.6 ± 1.9 g | 86.0 ± 9.3 e | 127 ± 6.5 d | 138 ± 17.9 d | 352 ± 32.4 e |
| A3R3 | 34.0 ± 1.9 f | 41.9 ± 4.6 f | 109 ± 15.5 d | 140 ± 2.1 c | 176 ± 16.8 c | 461 ± 51.8 d | |
| SRS8 | 39.7 ± 2.1 e | 44.3 ± 5.1 f | 117 ± 12.5 d | 147 ± 8.3 c | 189 ± 11.3 c | 525 ± 37.3 d | |
| 50 | Control | 36.6 ± 2.0 ef | 58.3 ± 3.4 e | 115 ± 5.3 d | 146 ± 6.5 c | 177 ± 23.4 c | 510 ± 42.9 d |
| A3R3 | 39.7 ± 2.8 e | 61.7 ± 5.2 de | 135 ± 15.2 c | 163 ± 14.3 b | 219 ± 21.8 bc | 640 ± 46.1 bc | |
| SRS8 | 42.8 ± 4.2 d | 64.0 ± 5.5 d | 198 ± 11.0 b | 167 ± 6.5 b | 232 ± 26.8 bc | 667 ± 43.5 bc | |
| 75 | Control | 46.7 ± 1.7 c | 72.3 ± 6.9 c | 188 ± 17.8 b | 173 ± 8.3 b | 261 ± 12.7 b | 615 ± 44.0 c |
| A3R3 | 50.0 ± 1.9 b | 83.3 ± 8.0 b | 234 ± 15.2 a | 187 ± 15.0 ab | 287 ± 35.2 ab | 706 ± 6.4 ab | |
| SRS8 | 54.4 ± 4.1 a | 90.6 ± 5.1 a | 244 ± 25.0 a | 193 ± 13.1 a | 304 ± 35.2 a | 733 ± 86.8 a | |
| R. communis | |||||||
| 0 | Control | 1.7 ± 0.4 e | 11.5 ± 0.8 f | 26.4 ± 4.2 e | 9.5 ± 0.7 f | 28.5 ± 1.6 f | 95.3 ± 3.1 g |
| A3R3 | 3.0 ± 0.9 e | 13.1 ± 1.0 f | 34.1 ± 3.8 e | 12.7 ± 0.8 f | 40.0 ± 3.2 f | 116 ± 8.6 g | |
| SRS8 | 3.3 ± 0.7 e | 14.5 ± 0.9 f | 33.5 ± 4.2 e | 14.2 ± 0.8 f | 44.3 ± 5.4 f | 105 ± 9.2 g | |
| 25 | Control | 13.2 ± 1.2 d | 23.9 ± 3.3 e | 46.9 ± 3.9 d | 90.4 ± 6.9 e | 184.5 ± 8.2 e | 157 ± 11.5 f |
| A3R3 | 19.8 ± 3.4 c | 34.3 ± 4.1 d | 56.3 ± 5.9 c | 109 ± 8.1 d | 220 ± 24.8 d | 187 ± 7.2 e | |
| SRS8 | 21.5 ± 3.4 c | 35.9 ± 3.0 d | 57.9 ± 5.0 c | 112 ± 14.2 d | 234 ± 5.4 d | 205 ± 10.9 de | |
| 50 | Control | 22.1 ± 4.0 c | 41.3 ± 4.9 cd | 58.9 ± 7.9 c | 113 ± 12.3 cd | 219 ± 12.5 d | 224 ± 10.1 d |
| A3R3 | 29.2 ± 1.7 b | 51.1 ± 4.0 b | 70.0 ± 8.6 b | 127 ± 4.9 bc | 283 ± 16.6 c | 265 ± 22.4 c | |
| SRS8 | 30.1 ± 2.9 b | 53.2 ± 3.8 b | 72.5 ± 6.6 b | 129 ± 11.4 b | 329 ± 18.5 b | 273 ± 13.7 c | |
| 75 | Control | 31.4 ± 4.3 b | 52.4 ± 4.7 b | 75.0 ± 5.8 b | 133 ± 18.2 b | 287 ± 22.1 c | 316 ± 21.9 b |
| A3R3 | 38.9 ± 3.7 a | 60.4 ± 8.4 a | 92.0 ± 6.0 a | 147 ± 9.4 ab | 344 ± 16.0 ab | 379 ± 20.3 a | |
| SRS8 | 40.5 ± 15.2 a | 61.7 ± 8.2 a | 93.9 ± 10.8 a | 159 ± 9.6 a | 358 ± 13.7 a | 393 ± 12.0 a | |
Average ± SD from three samples. Data of columns within each plant species indexed by the same letter are not significantly different according to Fisher’s protected LSD test (p < 0.05). dw, dry weight; PGPB, plant growth promoting bacteria.
Inoculation of plants with PGPB greatly enhanced the quantity of accumulation of Ni in root and shoot tissues compared with respective non-inoculated controls (Table 4). However, the maximum effect was observed in Psychrobacter sp. SRS8. In the treatment with 75% of SS, Psychrobacter sp. SRS8 increased the Ni concentrations in the shoot and root tissues of B. juncea by 16 and 11%, respectively, whereas in R. communis the percentage of increase was 29 and 20, respectively. The increased Ni accumulation in both plants by Psychrobacter sp. SRS8 might be due to its efficiency of mobilizing the Ni at the soil-root interface. Our result is in agreement with the observations of Chen et al. (2013) and Prapagdee et al. (2013) who reported enhanced uptake of metals in B. napus and H. annuus due to inoculation of metal mobilizing PGPB suggesting that the inoculation of Psychrobacter sp. SRS8 enhances the heavy metal availability in the rhizosphere (Ma et al., 2009b) and thereby increases the uptake of Ni by plants (Table 4).
Zn and Fe concentrations in the roots of both plants inoculated with or without PGPB also showed similar trends to those described for Ni concentrations (Table 4). This finding is in accordance with other observation reported in Ma et al. (2010) and Babu et al. (2013), where Zn and Fe concentrations in the plant roots were greater than those in the shoots. The non-inoculated plants grown with various SS treatments showed low Zn and Fe concentrations, but PGPB inoculation increased Zn and Fe concentrations in the shoots and roots of both plant species grown under the same condition (Table 4). This may be attributed to the microbial metabolites/actions such as production of siderophores and P solubilization. Although both Psychrobacter sp. SRS8 and Pseudomonas sp. A3R3 showed similar trends on the production of siderophores and solubilization of P (Table 2), the rhizosphere isolate Psychrobacter sp. SRS8 was more effective than the endophytic strain A3R3 in stimulating heavy metal accumulation in the tissues of both plants. Most likely, Psychrobacter sp. SRS8 may have produced metal mobilizing metabolites in the rhizosphere and thus may have increased heavy metal availability to plant roots as they are usually in close contact with the root surface and heavy metals in the soils, and improved its uptake. Further work is, however, required to characterize the microbial metabolites and to elucidate the factors that induce heavy metal accumulation in plants.
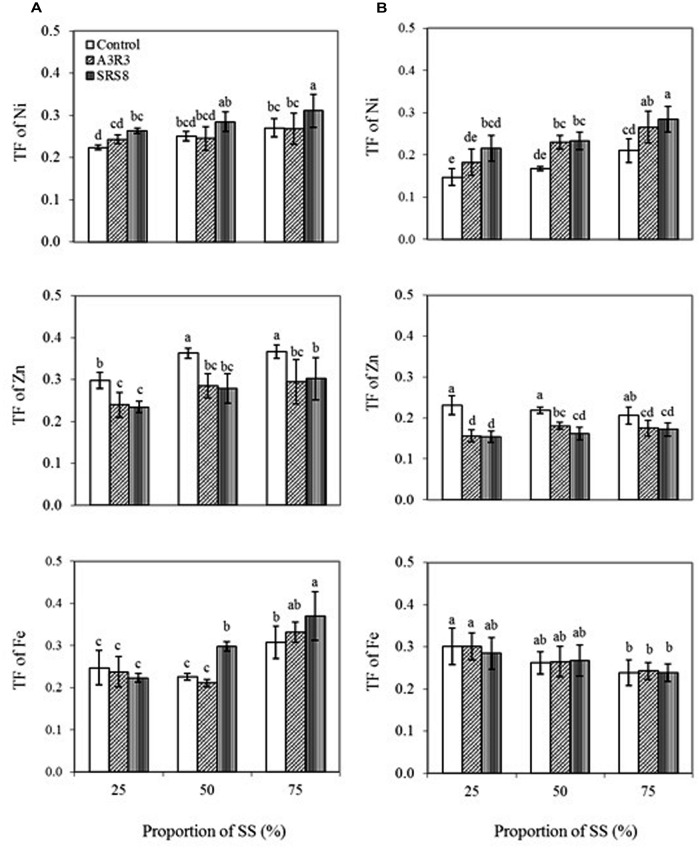
TRANSLOCATION OF METALS FROM ROOTS TO SHOOTS
TF was calculated to assess the efficiency of PGPB inoculation on the translocation of heavy metals from roots to shoots. Plant species with a TF > 1 are considered appropriate for phytoextraction. The results showed that B. juncea transported relatively more Ni to the shoots than R. communis (Figure 2). This indicates that the transfer ability of Ni in B. juncea is better than that in R. communis, so the B juncea has higher potential for phytoextraction. Besides, a slight increase in TF of Ni in both plants was observed with the increasing proportions of SS. Moreover, the inoculation of PGPB strains also increased the TF of Ni in both plants grown in soils with various proportions of SS indicating that the inoculated PGPB played an important role on Ni accumulation in plant shoots. Although both Pseudomonas sp. A3R3 and Psychrobacter sp. SRS8 led to higher values of the TF, the later was more effective than the former in stimulating the translocation of Ni from roots to shoots. A recent study by Prapagdee et al. (2013) reported that the inoculation of metal mobilizing rhizobacteria Micrococcus sp. MU1 and Klebsiella sp. BAM1 increased Cd uptake into the shoot by 25 and 21%, respectively, compared with non-inoculated plants during the fourth week of plantation and indicated that heavy metal uptake/accumulation in plants strongly depended on the heavy metal mobilization potential of rhizobacteria.
FIGURE 2.
Influence of Psychrobacter sp. SRS8 and Pseudomonas sp. A3R3 on the translocation factor (TF) of Ni, Zn, and Fe in B. juncea (A) and R. communis (B). Each value is the mean of triplicates. Error bars represent SD. Data of columns indexed by the same letter are not significantly different according to Fisher’s protected LSD test (p < 0.05).
In contrast to Ni, it is apparent that inoculation of PGPB reduced Zn TF in both plants (Figures 2A,B). For instance, the strain Pseudomonas sp. A3R3 reduced the TF of Zn in B. juncea and R. communis by 22 and 14%, respectively, at 75% SS, compared to the corresponding non-inoculated controls (Figures 2A,B). This result is in agreement with a previous report describing decreased translocation of Zn in Nicotiana tabacum inoculated with Sanguibacter sp. S_d2 grown in Cd- and Zn-enriched soil (Mastretta et al., 2009). Similarly, Rajkumar et al. (2013a) found that inoculation of B. juncea, Luffa aegyptiaca, and Sorghum halepense with the PGPR Bacillus megaterium SR28C had no effect on or reduced Ni accumulation in the shoot tissues when compared with those non-inoculated control. They attributed this effect to a direct dilution of metal concentrations by an increased shoot biomass. In our study, the significant decrease in TF of Zn caused by PGPB inoculation is probably due to the dilution effect since both plants grown with PGPB inoculation had much larger shoot biomass than those grown without PGPB inoculation. However, in case of Fe TF, the bacterial inoculation posed different effects on plants (Figures 2A,B). For instance, compared with non-inoculated control, inoculation of Pseudomonas sp. A3R3 and Psychrobacter sp. SRS8 increased the TF of Fe in B. juncea by 6 and 19%, respectively, when plants were grown in 75% SS (Figure 2A). On the contrary, in R. communis, both strains had no significant effects on the TF of Fe (Figure 2B). Similar results were reported by Li and Ramakrishna (2011), where the metal mobilizing PGPB Pseudomonas sp. TLC 6-6.5-4 was more effective on enhancing the heavy metal accumulation in maize than in sunflower. It is likely that in the present study the decrease or variation in the accumulation of Zn and Fe by the inoculated plants was not only due to the effect of heavy metals dilution but may have also been associated with other factors, including microbial populations and their response to environmental conditions in the rhizosphere, plant, and soil type, since the plants growing in metal polluted soils may modulate their growth and metal accumulation response to various physico-chemico-biological properties of the environment (Ma et al., 2011a; Becerra-Castro et al., 2012; Rajkumar et al., 2013b; Sessitsch et al., 2013; Cabello-Conejo et al., 2014). For instance, Jiang et al. (2008) recently reported that different plants, i.e., Indian mustard, maize and tomato inoculated with metal mobilizing PGPB vary in their ability to accumulate/translocate heavy metals, and found that tomato accumulated more heavy metals than other plant species from the soil. Similarly, Israr et al. (2011) reported that single metal uptake by Sesbania drummondii was affected by the presence of other metals. The authors attributed this result to competition between the metals for the plant uptake sites. Further studies, however, as to why these plants with PGPB have different abilities on the translocation of Fe are needed.
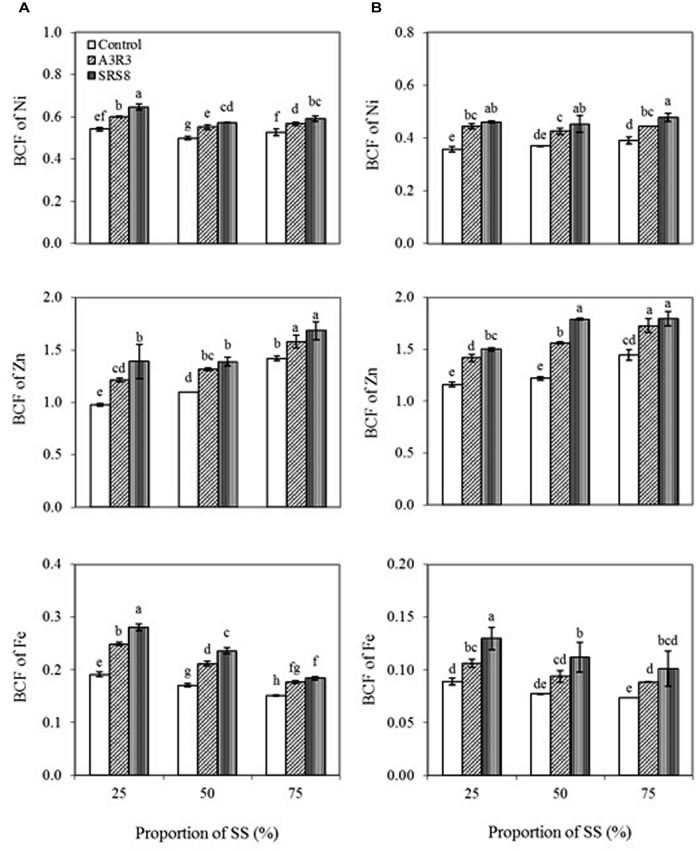
BIOCONCENTRATION OF METALS
The BCF is commonly used to measure the ability of a plant to remove metals from soils (Zayed et al., 1998). In this study, BCF of Ni, Zn, and Fe in both plants increased with increasing proportion of SS (Figure 3), indicating that concentrations of heavy metals in soils have significant effects on the concentrations in plant tissues. Besides, inoculation of plants with Pseudomonas sp. A3R3 or Psychrobacter sp. SRS8 significantly increased the BCF of the metals in both B. juncea and R. communis compared with respective non-inoculated control. However, Psychrobacter sp. SRS8 was more effective on increasing the BCF of Ni, Zn, and Fe in both plants than Pseudomonas sp. A3R3. Based on the BCF values, overall Zn was the metal most accumulated in both plants, followed by Ni, with accumulation of Fe being very low. The increased BCF value caused by PGPB inoculation could indicate that both Pseudomonas sp. A3R3 and Psychrobacter sp. SRS8 played a significant role on heavy metal uptake and accumulation in plants. Actually, the bioavailable fractions of Ni, Zn, and Fe in SS soils where the inoculated plants grew was significantly higher than that found in the respective soils from non-inoculated plants (Table 3), suggesting that the PGPB increased metal solubilization also leading to a subsequent increase in plant metal uptake and translocation.
FIGURE 3.
Influence of Psychrobacter sp. SRS8 and Pseudomonas sp. A3R3 on the bioconcentration factor (BCF) of Ni, Zn, and Fe in B. juncea (A) and R. communis (B). Each value is the mean of triplicates. Error bars represent SD. Data of columns indexed by the same letter are not significantly different according to Fisher’s protected LSD test (p < 0.05).
In general, plants exhibiting TF and BCF values less than one are suitable for heavy metal phytostabilization programs (Shi et al., 2011; Wu et al., 2011) because low values indicate that a given species is unable to extract large amounts of metal from the soil and translocate it to the shoots. In our study, though PGPB inoculation were capable of stimulating heavy metal accumulation in plants tissues to some extent, both B. juncea and R. communis had lower values of TF and BCF (<1) for all the three metals, indicating that these plant species with serpentine PGPB inoculation could be used for the revegetation or phytostabilization purposes.
CONCLUSION
Although the phytoremediation of metal polluted soils is becoming a feasible alternative to physicochemical clean up technologies, it is still a challenging task because of limited plant growth and phytotoxic metal levels in soils (Kavamura and Esposito, 2010). Our study demonstrates that B. juncea and R. communis inoculated with serpentine PGPB can be potentially used for revegetation and phytostabilization of metal polluted field soils. Microcosm experiments demonstrated that inoculation of Pseudomonas sp. A3R3 or Psychrobacter sp. SRS8 improved plant biomass production and heavy metal accumulation. The former had a better performance on plant biomass production, whereas the latter on heavy metal accumulation in plants. However, both B. juncea and R. communis exhibited lower values of TF and BCF (<1) for Ni and Fe, regardless of inoculation of PGPB and proportion of SS. Overall, the increased growth response of plants in SS caused by PGPB indicates that the inoculation of Pseudomonas sp. A3R3 or Psychrobacter sp. SRS8 alleviated the toxicity of heavy metals and provided a better environment for plant growth probably through producing various plant growth beneficial metabolites. Such serpentine isolates with various PGP traits are therefore good inoculants for enhancing the phytostabilization process in metal polluted field soils. Further research will be carried out to determine the co-inoculation effects of Pseudomonas sp. A3R3 and Psychrobacter sp. SRS8 on the plant growth and heavy metal phytoremediation in degraded ecosystems.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Ying Ma and Rui S. Oliveira wish to acknowledge the support of Fundação para a Ciência e a Tecnologia (FCT) through the research grants SFRH/BPD/76028/2011 and SFRH/BPD/85008/2012 and Fundo Social Europeu. Mani Rajkumar acknowledges the financial support received in the form of Ramalingaswami re-entry fellowship from Department of Biotechnology (DBT), Government of India. Inês Rocha was supported by the FCT grant BI-EXPL/AGR-TEC/1204/2013. This work was supported by National Funds through FCT under the project EXPL/AGR-TEC/1204/2013, financed by Fundo Europeu de Desenvolvimento Regional (FEDER), Eixo I do Programa Operacional Fatores de Competitividade (POFC) of QREN (COMPETE: FCOMP-01-0124-FEDER-041572), and the project PEst-OE/BIA/UI4004/2014.
REFERENCES
- Abou-Shanab R. A. I., Angle J. S., Chaney R. L. (2006). Bacterial inoculants affecting nickel uptake by Alyssum murale from low, moderate and high Ni soils. Soil Biol. Biochem. 38 2882–2889 10.1016/j.soilbio.2006.04.045 [DOI] [Google Scholar]
- Ali H., Khan E., Sajad M. A. (2013). Phytoremediation of heavy metals–concepts and applications. Chemosphere 91 869–881 10.1016/j.chemosphere.2013.01.075 [DOI] [PubMed] [Google Scholar]
- Arshad M., Saleem M., Hussain S. (2007). Perspectives of bacterial ACC deaminase in phytoremediation. Trends Biotechnol. 25 356–362 10.1016/j.tibtech.2007.05.005 [DOI] [PubMed] [Google Scholar]
- Babu A. G., Kim J. D., Oh B. T. (2013). Enhancement of heavy metal phytoremediation by Alnus firma with endophytic Bacillus thuringiensis GDB-1. J. Hazard. Mater. 250–251, 477–483 10.1016/j.jhazmat.2013.02.014 [DOI] [PubMed] [Google Scholar]
- Bauddh K., Singh R. P. (2012). Cadmium tolerance and its phytoremediation by two oil yielding plants Ricinus communis (L.) and Brassica juncea (L.) from the contaminated soil. Int. J. Phytoremediation 14 772–785 10.1080/15226514.2011.619238 [DOI] [PubMed] [Google Scholar]
- Becerra-Castro C., Kidd P., Kuffner M., Prieto-Fernández A., Hann S., Monterroso C., et al. (2013). Bacterially induced weathering of ultramafic rock and its implications for phytoextraction. Appl. Environ. Microbiol. 79 5094–5103 10.1128/AEM.02268-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra-Castro C., Monterrosob C., Prieto-Fernández A., Rodríguez-Lamas L., Loureiro-Viñas M., Acea M. J., et al. (2012). Pseudometallophytes colonising Pb/Zn mine tailings: a description of the plant–microorganism–rhizosphere soil system and isolation of metal-tolerant bacteria. J. Hazard. Mater. 217–218, 350–359 10.1016/j.jhazmat.2012.03.039 [DOI] [PubMed] [Google Scholar]
- Bolan N. S., Park J. H., Robinson B., Naidu R., Huh K. Y. (2011). Phytostabilization: a green approach to contaminant containment. Adv. Agron. 112 145–204 10.1016/B978-0-12-385538-1.00004-4 [DOI] [Google Scholar]
- Cabello-Conejo M. I., Becerra-Castro C., Prieto-Fernández A., Monterroso C., Saavedra-Ferro A., Mench M., et al. (2014). Rhizobacterial inoculants can improve nickel phytoextraction by the hyperaccumulator Alyssum pintodasilvae. Plant Soil 379 35–50 10.1007/s11104-014-2043-7 [DOI] [Google Scholar]
- Cecchi C. G. S., Zanchi C. (2005). Phytoremediation of soil polluted by nickel using agricultural crops. Environ. Manag. 36 675–681 10.1007/s00267-004-0171-1 [DOI] [PubMed] [Google Scholar]
- Chen Z. J., Sheng X. F., He L. Y., Huang Z., Zhang W. H. (2013). Effects of root inoculation with bacteria on the growth, Cd uptake and bacterial communities associated with rape grown in Cd-contaminated soil. J. Hazard. Mater. 244–245, 709–717 10.1016/j.jhazmat.2012.10.063 [DOI] [PubMed] [Google Scholar]
- Dell’Amico E., Cavalca L., Andreoni V. (2008). Improvement of Brassica napus growth under cadmium stress by cadmium-resistant rhizobacteria. Soil Biol. Biochem. 40 74–84 10.1016/j.soilbio.2007.06.024 [DOI] [Google Scholar]
- Desideri D., Meli M. A., Roselli C. (2010). Determination of essential and non-essential elements in some medicinal plants by polarised X ray fluorescence spectrometer (EDPXRF). Microchem. J. 95 174–180 10.1016/j.microc.2009.11.010 [DOI] [Google Scholar]
- Doherty J. H., Ji B. M., Casper B. B. (2008). Testing nickel tolerance of Sorghastrum nutans and its associated soil microbial community from serpentine and prairie soils. Environ. Pollut. 151 593–598 10.1016/j.envpol.2007.04.002 [DOI] [PubMed] [Google Scholar]
- Duman F., Ozturk F. (2010). Nickel accumulation and its effect on biomass, protein content and antioxidative enzymes in roots and leaves of watercress (Nasturtium officinale R.). Br. J. Environ. Sci. 22 526–532 10.1016/S1001-0742(09)60137-6 [DOI] [PubMed] [Google Scholar]
- Ferraz P., Fidalgo F., Almeida A., Teixeira J. (2012). Phytostabilization of nickel by the zinc and cadmium hyperaccumulator Solanum nigrum L. Are metallothioneins involved? Plant Physiol. Biochem. 57 254–260 10.1016/j.plaphy.2012.05.025 [DOI] [PubMed] [Google Scholar]
- Freitas H., Prasad M. N. V., Pratas J. (2004). Analysis of serpentinophytes from northeast of Portugal for trace metal accumulation relevance to the management of mine environment. Chemosphere 54 1625–1642 10.1016/j.chemosphere.2003.09.045 [DOI] [PubMed] [Google Scholar]
- Grandlic C. J., Mendez M. O., Chorover J., Machado B., Maier R. M. (2008). Plant growth-promoting bacteria for phytostabilization of mine tailings. Environ. Sci. Technol. 42 2079–2084 10.1021/es072013j [DOI] [PubMed] [Google Scholar]
- He H. D., Ye Z. H., Yang D. J., Yan J. L., Xiao L., Zhong T., et al. (2013). Characterization of endophytic Rahnella sp. JN6 from Polygonum pubescens and its potential in promoting growth and Cd, Pb, Zn uptake by Brassica napus. Chemosphere 90 1960–1965 10.1016/j.chemosphere.2012.10.057 [DOI] [PubMed] [Google Scholar]
- Israr M., Jewell A., Kumar D., Sahi S. V. (2011). Interactive effects of lead, copper, nickel and zinc on growth, metal uptake and antioxidative metabolism of Sesbania drummondii. J. Hazard. Mater. 186 1520–1526 10.1016/j.jhazmat.2010.12.021 [DOI] [PubMed] [Google Scholar]
- Jackson A. P., Alloway B. J. (1991). The bioavailability of cadmium to lettuce and cabbage in soils previously treated with sewage sludges. Plant Soil 132 179–186 10.1007/BF00010398 [DOI] [Google Scholar]
- Jebara S. H., Saadani O., Fatnassi I. C., Chiboub M., Abdelkrim S., Jebara M. (2014). Inoculation of Lens culinaris with Pb-resistant bacteria shows potential for phytostabilization. Environ. Sci. Pollut. Res. Int. 10.1007/s11356-014-3510-7 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Jiang C. Y., Sheng X. F., Qian M., Wang Q. Y. (2008). Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere 72 157–164 10.1016/j.chemosphere.2008.02.006 [DOI] [PubMed] [Google Scholar]
- Kavamura V. N., Esposito E. (2010). Biotechnological strategies applied to the decontamination of soils polluted with heavy metals. Biotechnol. Adv. 28 61–69 10.1016/j.biotechadv.2009.09.002 [DOI] [PubMed] [Google Scholar]
- Kayama M., Choi D., Tobita H., Utsugi H., Kitao M., Maruyama Y., et al. (2006). Comparison of growth characteristics and tolerance to serpentine soil of three ectomycorrhizal spruce seedlings in northern Japan. Trees 20 430–440 10.1007/s00468-006-0057-3 [DOI] [Google Scholar]
- Kilburn M. (2000). Aqua regia digestion for soils/sludges. Anal. Methods Manual H 115. [Google Scholar]
- Li K. F., Ramakrishna W. (2011). Effect of multiple metal resistant bacteria from contaminated lake sediments on metal accumulation and plant growth. J. Hazard. Mater. 189 531–539 10.1016/j.jhazmat.2011.02.075 [DOI] [PubMed] [Google Scholar]
- Lindsay W. L., Norvell W. A. (1978). Development of a DTPA test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 42 421–428 10.2136/sssaj1978.03615995004200030009x [DOI] [Google Scholar]
- Lotfy S. M., Mostafa A. Z. (2014). Phytoremediation of contaminated soil with cobalt and chromium. J. Geochem. Explor. 144 367–373 10.1016/j.gexplo.2013.07.003 [DOI] [Google Scholar]
- Ma Y., Prasad M. N. V., Rajkumar M., Freitas H. (2011a). Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol. Adv. 29 248–258 10.1016/j.biotechadv.2010.12.001 [DOI] [PubMed] [Google Scholar]
- Ma Y., Rajkumar M., Luo Y. M., Freitas H. (2011b). Inoculation of endophytic bacteria on host and non-host plants – effects on plant growth and Ni uptake. J. Hazard. Mater. 196 230–237 10.1016/j.jhazmat.2011.08.034 [DOI] [PubMed] [Google Scholar]
- Ma Y., Rajkumar M., Freitas H. (2009a). Improvement of plant growth and nickel uptake by nickel resistant-plant growth promoting bacteria. J. Hazard. Mater. 166 1154–1161 10.1016/j.jhazmat.2008.12.018 [DOI] [PubMed] [Google Scholar]
- Ma Y., Rajkumar M., Freitas H. (2009b). Isolation and characterization of Ni mobilizing PGPB from serpentine soils and their potential in promoting plant growth and Ni accumulation by Brassica spp. Chemosphere 75 719–725 10.1016/j.chemosphere.2009.01.056 [DOI] [PubMed] [Google Scholar]
- Ma Y., Rajkumar M., Luo Y., Freitas H. (2013). Phytoextraction of heavy metal polluted soils using Sedum plumbizincicola inoculated with metal mobilizing Phyllobacterium myrsinacearum RC6b. Chemosphere 93 1386–1392 10.1016/j.chemosphere.2013.06.077 [DOI] [PubMed] [Google Scholar]
- Ma Y., Rajkumar M., Vicente J., Freitas H. (2010). Inoculation of Ni-resistant plant growth promoting bacterium Psychrobacter sp. strain SRS8 for the improvement of nickel phytoextraction by energy crops. Int. J. Phytoremediation 13 126–139 10.1080/15226511003671403 [DOI] [PubMed] [Google Scholar]
- Mastretta C., Taghavi S., Van der Lelie D., Mengoni A., Galardi F., Gonnelli C., et al. (2009). Endophytic bacteria from seeds of Nicotiana tabacum can reduce cadmium phytotoxicity. Int. J. Phytoremediation 11 251–267 10.1080/15226510802432678 [DOI] [Google Scholar]
- Meers E., Du Laing G., Unamuno V., Ruttens A., Vangronsveld J., Tack F. M. G., et al. (2007). Comparison of cadmium extractability from soils by commonly used single extraction protocols. Geoderma 141 247–259 10.1016/j.geoderma.2007.06.002 [DOI] [Google Scholar]
- Mengoni A., Grassi E., Barzanti R., Biondi E. G., Gonnelli C., Kim C. K., et al. (2004). Genetic diversity of bacterial communities of serpentine soil and of rhizosphere of the nickel-hyperaccumulator plant Alyssum bertolonii. Microb. Ecol. 48 209–217 10.1007/s00248-003-0149-1d [DOI] [PubMed] [Google Scholar]
- Niu Z. X., Sun L. N., Sun T. H., Li Y. S., Wang H. (2007). Evaluation of phytoextracting cadmium and lead by sunflower, ricinus, alfalfa and mustard in hydroponic culture. J. Environ. Sci. 19 961–967 10.1016/S1001-0742(07)60158-2 [DOI] [PubMed] [Google Scholar]
- Ouzounidou G., Moustakas M., Symeonidis L., Karataglis S. (2006). Response of wheat seedlings to Ni stress: effects of supplemental calcium. Arch. Environ. Contam. Toxicol. 50 346–352 10.1007/s00244-005-5076-3 [DOI] [PubMed] [Google Scholar]
- Pérez-López R., Márquez-García B., Abreu M. M., Nieto J. M., Córdoba F. (2014). Erica andevalensis and Erica australis growing in the same extreme environments: phytostabilization potential of mining areas. Geoderma 230–231, 194–203 10.1016/j.geoderma.2014.04.004 [DOI] [Google Scholar]
- Prapagdee B., Chanprasert M., Mongkolsuk S. (2013). Bioaugmentation with cadmium-resistant plant growth-promoting rhizobacteria to assist cadmium phytoextraction by Helianthus annuus. Chemosphere 92 659–666 10.1016/j.chemosphere.2013.01.082 [DOI] [PubMed] [Google Scholar]
- Rajkumar M., Ma Y., Freitas H. (2013a). Improvement of Ni phytostabilization by inoculation of Ni resistant Bacillus megaterium SR28C. J. Environ. Manag. 128 973–980 10.1016/j.jenvman.2013.07.001 [DOI] [PubMed] [Google Scholar]
- Rajkumar M., Prasad M. N. V., Swaminathan S., Freitas H. (2013b). Climate change driven plant–metal–microbe interactions. Environ. Int. 53 74–86 10.1016/j.envint.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Rajkumar M., Sandhya S., Prasad M. N. V., Freitas H. (2012). Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol. Adv. 30 1562–1574 10.1016/j.biotechadv.2012.04.011 [DOI] [PubMed] [Google Scholar]
- Rashotte A. M., Brady S. R., Reed R. C., Ante S. J., Muday G. K. (2000). Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol. 122 481–490 10.1104/pp.122.2.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauret G., López-Sánduz J. F., Sahuquillo A. (1999). Improvement of the BCR three-step sequential extraction procedure prior the certification of new sediment and soil reference materials. J. Environ. Monit. 1 57–61 10.1039/a807854h [DOI] [PubMed] [Google Scholar]
- Safari Sinegani A. A., Khalilikhah F. (2008). Phytoextraction of lead by Helianthus annuus: effect of mobilizing agent application time. Plant Soil Environ. 54 434–440. [Google Scholar]
- Sessitsch A., Kuffner M., Kidd P., Vangronsveld J., Wenzel W. W., Fallmann K., et al. (2013). The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol. Biochem. 60 182–194 10.1016/j.soilbio.2013.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Zhang X., Chen G., Chen Y., Wang L., Shan X. (2011). Seedling growth and metal accumulation of selected woody species in copper and lead/zinc mine tailings. J. Environ. Sci. 23 266–274 10.1016/S1001-0742(10)60402-0 [DOI] [PubMed] [Google Scholar]
- Singh D. B., Prasad G., Rupainwar D. C. (1996). Adsorption technique for the treatment of As (V) rich effluents. Colloid Surf. A Physicochem. Eng. Asp. 111 49–56 10.1016/0927-7757(95)03468-4 [DOI] [Google Scholar]
- Srivastava S., Singh N. (2014). Mitigation approach of arsenic toxicity in chickpea grown in arsenic amended soil with arsenic tolerant plant growth promoting Acinetobacter sp. Ecol. Eng. 70 146–153 10.1016/j.ecoleng.2014.05.008 [DOI] [Google Scholar]
- Srivastava S., Verma P. C., Chaudhry V., Singh N., Abhilash P. C., Kumar K. V., et al. (2013). Influence of inoculation of arsenic-resistant Staphylococcus arlettae on growth and arsenic uptake in Brassica juncea (L.) Czern. Var. R-46. J. Hazard. Mater. 262 1039–1047 10.1016/j.jhazmat.2012.08.019 [DOI] [PubMed] [Google Scholar]
- Testiati E., Parinet J., Massiani C., Laffont-Schwob I., Rabier J., Pfeifer H. R., et al. (2013). Trace metal and metalloid contamination levels in soils and in two native plant species of a former industrial site: evaluation of the phytostabilization potential. J. Hazard. Mater. 248–249, 131–141 10.1016/j.jhazmat.2012.12.039 [DOI] [PubMed] [Google Scholar]
- Velikova V., Tsonev T., Loreto F., Centritto M. (2011). Changes in photosynthesis, mesophyll conductance to CO2, and isoprenoid emissions in Populus nigra plants exposed to excess nickel. Environ. Pollut. 159 1058–1066 10.1016/j.envpol.2010.10.032 [DOI] [PubMed] [Google Scholar]
- Wang H. L., Magesan G., Bolan N. S. (2004). An overview of the environmental effects of land application of farm effluents. N. Z. J. Agri. Res. 47 389–403 10.1080/00288233.2004.9513608 [DOI] [Google Scholar]
- Wu Q. H., Wang S. Z., Thangavel P., Li Q. F., Zheng H., Bai J., et al. (2011). Phytostabilization potential of Jatropha Curcas L. in polymetallic acid mine tailings. Int. J. Phytoremediation 13 788–804 10.1080/15226514.2010.525562 [DOI] [PubMed] [Google Scholar]
- Zaidi S., Usmani S., Singh B. R., Musarrat J. (2006). Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 64 991–997 10.1016/j.chemosphere.2005.12.057 [DOI] [PubMed] [Google Scholar]
- Zayed A., Gowthaman S., Terry N. (1998). Phytoaccumulation of trace elements by wetland plants: I. Duckweed. J. Environ. Q. 27 715–721 10.2134/jeq1998.00472425002700030032x [DOI] [Google Scholar]
- Zhang Y. F., He L. Y., Chen Z. J., Wang Q. Y., Qian M., Sheng X. F. (2011). Characterization of ACC deaminase-producing endophytic bacteria isolated from copper-tolerant plants and their potential in promoting the growth and copper accumulation of Brassica napus. Chemosphere 83 57–62 10.1016/j.chemosphere.2011.01.041 [DOI] [PubMed] [Google Scholar]
- Zhu L. J., Guan D. X., Luo J., Rathinasabapathi B., Ma L. Q. (2014). Characterization of arsenic-resistant endophytic bacteria from hyperaccumulators Pteris vittata and Pteris multifida. Chemosphere 113 9–16 10.1016/j.chemosphere.2014.03.081 [DOI] [PubMed] [Google Scholar]