Abstract
Osteopathia striata with cranial sclerosis (OS-CS) is characterized by linear bone dysplasia at the long bone radiographically and sclerotic change at the cranium. The purpose of this case report is to study the symptoms and treatments of osteomyelitis in a patient with OS-CS. A 41-year-old patient had pus discharge from a fistula at the mental region and increase in radiolucencies with sequestra in panoramic radiograph images. Computed tomography (CT) as well as radiograph images for the whole skeleton were taken. The patient was diagnosed with OS-CS. Sequestrectomy and fistulectomy were performed. The patient recovered and no relapse occurred within six months after surgery. For diagnosis of OS-CS, CT and additional radiograph images for the whole skeleton are required. Because of the increased bone density, this patient is prone to relapse after sequestrectomy. Therefore, the surgeon must minimize trauma with the least incision and exfoliation, and preoperative antibiotics.
Keywords: Osteosclerosis, Osteopathia striata caranial sclerosis, Osteomyelitis
Introduction
First described by Voorhoeve[1] in 1924, osteopathia striata (OS) can occur in isolation or as part of a syndrome. The disease in combination with cranial sclerosis (CS) was first described by Hurt in 1953[2]. OS with CS (OS-CS) is a rare sclerosing bone dysplasia characterized by radio-paque longitudinal striation at the metaphysis of the tubular bones and its equivalent of the axial bones is concurrent with craniofacial hyperostosis. Some affected individuals show only radiologic peculiarity and others have minor facial dysmorphism and/or symptoms due to cranial nerve encroachment[3]. Originally, CS is frequently disabled as it may lead to severe hearing loss and nerve palsies. Most patients of OS-CS present with the following clinical features related to the cranial vault: macrocephaly, wide nasal bridge, ocular hypertelorism, and abnormalities of the palate. Mixed and sensorineural hearing losses are reported in up to half of cases with OS-CS[4].
While half of the reported cases of this disease were sporadic, the etiology of the disease was not known for a long time[5]. Recently, the WTX gene, an inhibitor of WNT signaling, was identified as one of the disease-causing genes[6,7]. WNT signaling plays an anabolic role in bone formation by osteoblasts, and interference of this pathway is known to be involved in other sclerosing bone dysplasias as well[7–9].
To the best of our knowledge, fewer than 100 cases have been reported, most of which demonstrated radio-graphic features. Dental anomalies have been reported in 30.7% of OS-CS patients[2]. However, reports on osteomyelitis in OS-CS patients are extremely rare. Therefore, this study describes osteomyelitis in both maxilla and mandible in an OS-CS patient and its treatment.
Case Report
This 41-year-old male patient visited with the chief complaint of discomfort in the mandible and pus discharge from below the mandible. He was diagnosed with osteomyelitis four years ago at his first visit and agreed to a surgery under general anesthesia, but was transferred due to the long distance (Fig. 1). At the transferred hospital, he was under antibiotic and conservative treatments after sequestrectomy to prevent complications, such as secondary infection.
Fig. 1.
Preoperative panoramic view (1st visit). Radiolucent jaw lesion of maxilla and mandible (2010-01-04).
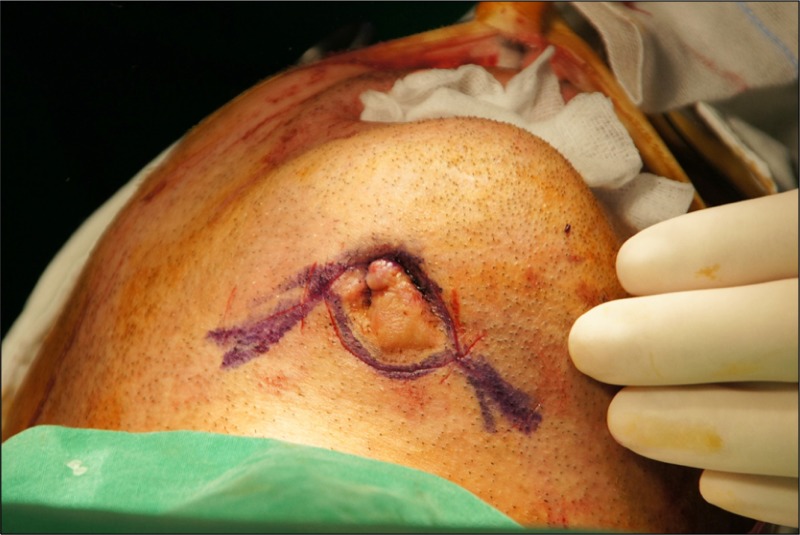
On extraoral examination, a fistula had formed at the mental region and pus discharge was observed from that fistula (Fig. 2). The patient had no other medical conditions and had been prescribed antibiotics from another hospital for osteomyelitis.
Fig. 2.
Fistula formation of mental region.
This patient showed numerous abnormalities, including macrocephaly, frontal bossing, broad nasal bridge, and hypertelorism, but no hearing loss or facial nerve palsy was observed.
On intraoral examination, maxillary teeth were highly mobile due to overall osteomyelitis on maxilla and sequestra were also found at the midsagittal region of the mandible. He had retained a deciduous tooth and also had a few permanent teeth. The patient had very poor oral hygiene and had an anterior crossbite and a narrow maxillary arch.
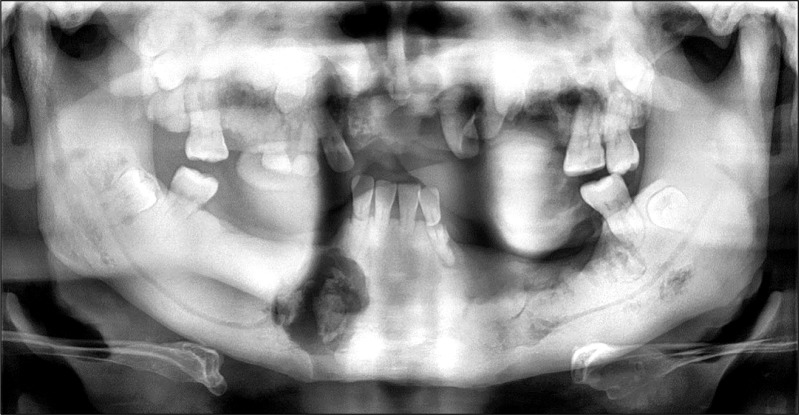
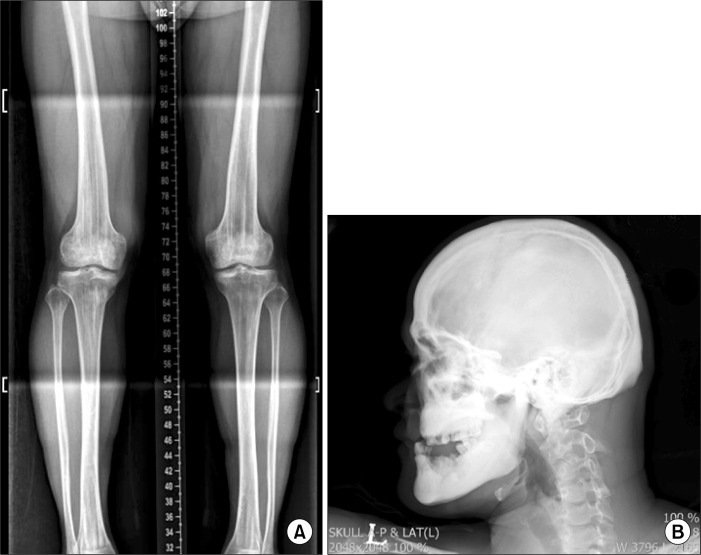
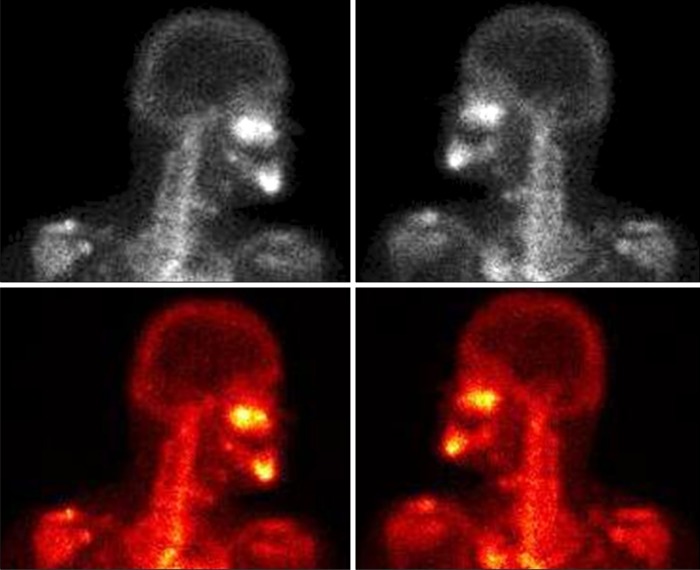
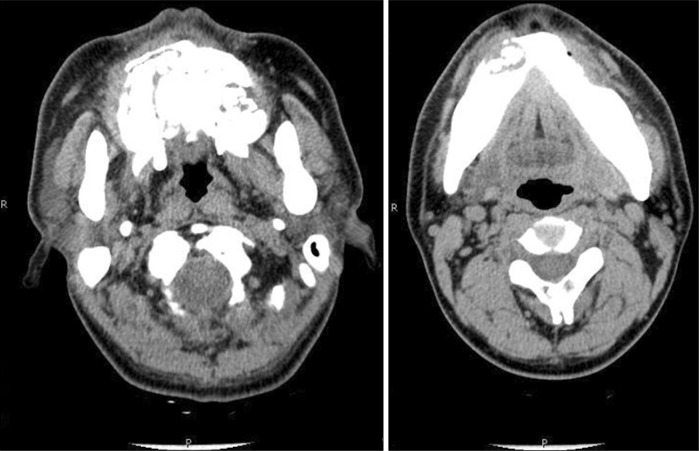
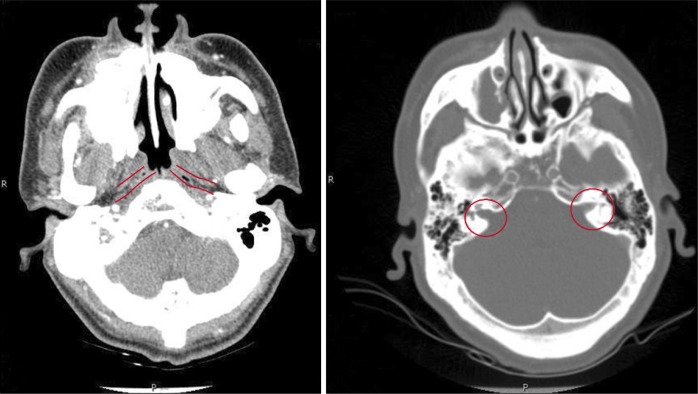
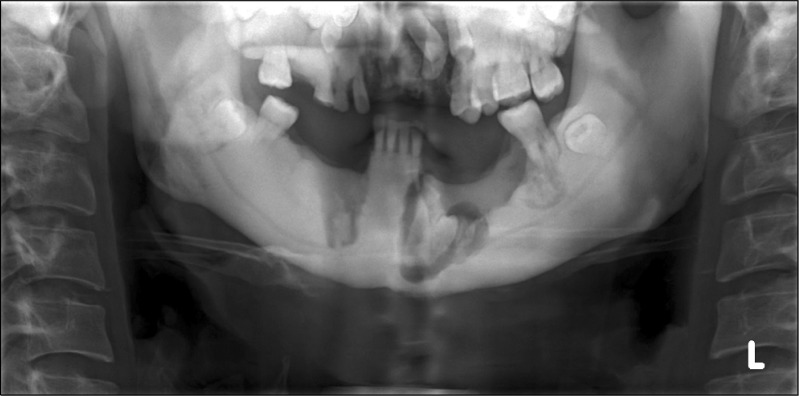
In panoramic radiograph images, radiolucencies at the left mandible had decreased compared to that from four years ago but radiolucencies on the right side had increased (Fig. 3). Also, increased radiopacities were observed for both maxilla and mandible. A number of permanent teeth were impacted at the maxilla. For accurate differential diagnosis, computed tomography (CT) and bone scan were taken and an additional radiographic image for the whole skeleton was taken. Linear patterns were found at the long bone and hot spots were observed at both maxilla and mandible, overall (Fig. 4, 5). On CT, the locations of sequestra at the jaws were shown and clear sclerotic regions at the Eustachian tube and the internal auditory canal, which are concerned with auditory sense were observed (Fig. 6, 7). Finally, the patient was diagnosed with OS-CS and bimaxillary osteomyelitis. In addition, results of pre-operative complete blood cell count test were normal but no testing for WTX gene was performed.
Fig. 3.
Preoperative panoramic view (2nd visit). Generalized radiopaque appearance and osteomyelitis of maxilla and right mandible.
Fig. 4.
(A) Antero-posterior view of lower limbs. Characteristic longitudinal sclerotic striations of osteopathia striata in the metaphyses of the long bones. (B) Lateral view of the skull. Thickened calvarial bones. Particularly dense base of skull. Under-developed maxillary sinuses and frontal bossing.
Fig. 5.
Bone scans showing areas of hyperactivity in the left skull base region.
Fig. 6.
Osteomyelitis of maxilla and right mandible.
Fig. 7.
Undefined calcification of Eustachian tubes and internal acoustic canals.
The treatment plans were sequestrectomy for both jaws under general anesthesia, extraction of mobile teeth and removal of fistula at the mental region with pus discharge. Primary closure was performed at sequestrectomy for maxilla to prevent the opening to the maxillary sinus and nasal sinus. Postoperative antibiotics were prescribed for 10 days (Fig. 8).
Fig. 8.
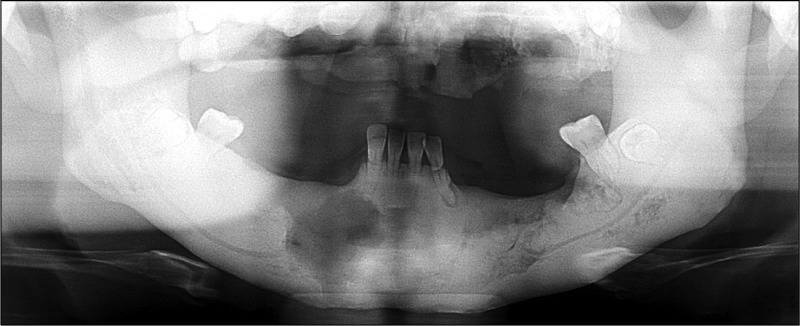
Postoperative panoramic view showing removal of sequestra.
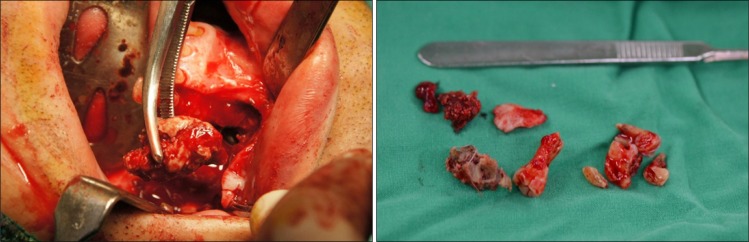
Sequestra were removed and retained teeth were extracted with no challenge (Fig. 9). Pus discharge from the removed fistula area healed completely. Dentures are being fabricated for both maxilla and mandible at the Department of Prosthodontics and the patient is being followed-up.
Fig. 9.
(A) Removal of sequestrum. (B) Sequestra and extracted tooth.
Discussion
OS-CS is a rare skeletal dysplasia characterized by longitudinal striations of the metaphysis of the long bone and sclerosis of the cranial vault and base. More than 100 cases of OS-CS have been reported to date. It is an inherited syndrome thought to be autosomal dominant with complete penetrance and variable expression; sex-linked inheritance has been suggested as well[10]. Cases that may have arisen sporadically have also been reported[11]. About one third of the cases are sporadic, whereas the remaining cases show familial clustering[12]. Since there were no family members with resembling symptoms, this case would be considered sporadic rather than an inherited syndrome. Prevalence is less than 0.1 in 1 million, with predominantly female phenotype[4]. In fact, OS-CS is 2.5 times more common in female with more severe complications than male patients[13]. The linear striations of the long bones typically first appear between five months and six years of age and are usually of little clinical significance[12]. These manifestations are less conspicuous in infancy and generally become overt in childhood. Typical physical presentations of this disorder are a square-like skull, frontal bossing, flat nasal bridge, palate abnormalities, and hearing loss. Facial nerve palsy has been described rarely, with cleft palate reported in 50% of cases[13]. Mental retardation has been present in many patients with OS-CS, but this patient was mentally normal.
The most striking clinical symptom resulting from this disorder in the temporal bone is deafness, which in most cases is conductive or mixed rather than sensorineural. Temporal bone CT was useful for evaluation of the various etiologies of the wide gap in air conduction on the left side of this patient, such as bone sclerosis of mastoid cells, narrowing of the middle ear cavity, mastoid antrum, Eustachian canal, and abnormal ossicular fixation to the bone surface of the middle ear cavity. Conductive hearing loss is thus presumed to be secondary to mural bone over-growth and encroachment with subsequent impaired mobility of the ossicles. Fortunately, this patient did not complain of hearing loss and sclerotic regions, including parts of Eustachian tube and internal auditory canal, were observed on CT images.
Differential diagnosis includes other generalized skeletal diseases characterized by bone sclerosis involving the base of the skull and cause cranial foramen stenosis, such as osteopetrosis, pycnodysostosis, sclerosteosis, craniometaphyseal dysplasia (Pyle disease), fibrous dysplasia, hyperostosis corticalis generalisata/endosteal hyperostosis (van Buchem disease), Camurati-Engelmann disease, frontometaphyseal dysplasia, dysosteosclerosis, and hyperostosis cranialis interna. Apart from fibrous dysplasia, all other pathologies are characterized by an abnormal quantity of compact bony tissue, appearing hyperdense on CT and hypointense on both T1- and T2-weighted magnetic resonance images, but none of these conditions have the pathognomonic bilateral longitudinal sclerotic striations extending from the metaphysis of the tubular bones to the diaphysis seen in OS-CS[4,11,13]. As it is not always possible to distinguish between the various kinds of sclerotic bone dysplasia on the basis of cranial involvement on CT scans, for the majority of them it is often necessary to perform a radiologic examination of the whole skeleton. In our patient, radiographs of the femur and pelvis showed the typical striations of OS-CS and allowed us to make a diagnosis.
The existence of overlapping syndromes suggests that not all sclerosing bone dysplasias are distinct entities, and that there may be some common factor in their pathogenesis and inheritance. More than 20 cases with varying combinations of melorheostosis, osteopoikilosis, and OS have been described in the literature. Walker[14], in 1964, invented the term mixed sclerosing dysplasias to describe two patients with simultaneous radiographic findings of melorheostosis, osteopoikilosis, and OS. This term is nowadays used to describe the presence of more than one osteosclerosis in the same individual[15].
Goltz-Gorlin syndrome, or focal dermal hypoplasia, is a congenital malformation syndrome which can be associated with OS and which seems to be an X-linked dominant trait, perhaps a manifestation of functional mosaicism[13]. Radioclinical features include skin abnormalities (poikiloderma with focal dermal hypoplasia), papillomas of the mucous membranes, abnormalities of the eyes, kidneys, and dental abnormalities, as well as several osseous deformities, such as syndactyly, oligodactyly, polydactyly, hypoplasia of the craniofacial skeleton, rib anomalies, and segmentation abnormalities of the vertebrae[16–18].
In this case, overall osteomyelitis was observed even at the richly vascularized maxilla. Although antibiotics were taken for a considerable amount of time, the condition did not show improvement and surgical intervention was required. The reason for the infection of both maxilla and mandible is the decrease in intraosseous vascularization due to increased bone density. Poor bone vascularization, as well as reduced local defenses, does not allow an adequate therapeutic level of antibiotics to occur at the infection site in osteomyelitis in OS. Therefore, appropriate local and systemic antimicrobial therapy may not result in a satisfactory clinical outcome. Osteomyelitis requires prolonged and adequate antimicrobial treatment; fluoroquinolones and lincomycin are considered useful antibacterial agents for treatment of this infection[19,20]. Amoxicillin, sultamicillin, ornidazole, clindamycin, and cefuroxime axetil are the antibacterial agents used for treatment of the patient in this case.
Surgical intervention as an adjunct to medical treatment may be necessary, and timing is critical for treatment of osteomyelitis. Initially, it should be limited to removal of very loose teeth and bone fragments as well as incision and drainage of fluctuant areas. Once chronicity is established, a decision is required concerning further surgical intervention, including removal of persistently loose teeth or sequestra. If drainage persists despite appropriate antibiotic treatment, as evidenced by repeated cultures and sensitivity testing, closed irrigation, sequestrectomy, saucerization, decortication, or resection, then reconstruction must be considered. Many skilled clinicians urge conservative treatment of sequestra in conjunction with supportive antibiotic therapy. Once the sequestrum has completely formed, it can be removed with minimum surgical trauma. In the case presented, we followed the same surgical procedures. To minimize postoperative infection, third generation cephalosporin was injected intravenously before the surgery and the patient was instructed to take antibiotics for 10 days after surgery. During the operation, surgical intervention was minimized with the least incision and bone removal. After three months of follow up, no symptom of infection was observed and the fistula of the mental region was completely healed.
Osteomyelitis in OS-CS patients has been reviewed in this case report. Upon diagnosis of OS-CS, CT and additional radiograph images for the whole skeleton were a great help, and osteomyelitis was finally diagnosed based on bone scan results. When evaluating an OS-CS patient, increase in bone density and its complications such as hearing loss and cerebral nerve palsy are to be carefully reviewed and evaluated. Maxilla or mandible with above normal bone densities lacks adequate blood supply and local defenses, and as a result, is prone to infection.
Therefore, postoperative osteomyelitis or other infections can be prevented with minimal surgical intervention.
References
- 1.Voorhoeve N. L’image radiologique non encore décrite d’une anomalie du squelette: ses rapports avec la dyschondroplasie et l’osteopathia condensais disseminata. Acta Radiol. 1924;3:407–27. [Google Scholar]
- 2.Viot G, Lacombe D, David A, et al. Osteopathia striata cranial sclerosis: non-random X-inactivation suggestive of X-linked dominant inheritance. Am J Med Genet. 2002;107:1–4. doi: 10.1002/ajmg.10028. [DOI] [PubMed] [Google Scholar]
- 3.Kondoh T, Yoshinaga M, Matsumoto T, et al. Severe cervical kyphosis in osteopathia striata with cranial sclerosis: case report. Pediatr Radiol. 2001;31:659–62. doi: 10.1007/s002470100486. [DOI] [PubMed] [Google Scholar]
- 4.Berenholz L, Lippy W, Harrell M. Conductive hearing loss in osteopathia striata-cranial sclerosis. Otolaryngol Head Neck Surg. 2002;127:124–6. doi: 10.1067/mhn.2002.124852. [DOI] [PubMed] [Google Scholar]
- 5.Kornreich L, Grunebaum M, Ziv N, Shuper A, Mimouni M. Osteopathia striata, cranial sclerosis with cleft palate and facial nerve palsy. Eur J Pediatr. 1988;147:101–3. doi: 10.1007/BF00442625. [DOI] [PubMed] [Google Scholar]
- 6.Perdu B, de Freitas F, Frints SG, et al. Osteopathia striata with cranial sclerosis owing to WTX gene defect. J Bone Miner Res. 2010;25:82–90. doi: 10.1359/jbmr.090707. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins ZA, van Kogelenberg M, Morgan T, et al. Germline mutations in WTX cause a sclerosing skeletal dysplasia but do not predispose to tumorigenesis. Nat Genet. 2009;41:95–100. doi: 10.1038/ng.270. [DOI] [PubMed] [Google Scholar]
- 8.Pellegrino JE, McDonald-McGinn DM, Schneider A, Markowitz RI, Zackai EH. Further clinical delineation and increased morbidity in males with osteopathia striata with cranial sclerosis: an X-linked disorder? Am J Med Genet. 1997;70:159–65. [PubMed] [Google Scholar]
- 9.Kubota T, Michigami T, Ozono K. Wnt signaling in bone metabolism. J Bone Miner Metab. 2009;27:265–71. doi: 10.1007/s00774-009-0064-8. [DOI] [PubMed] [Google Scholar]
- 10.Bueno AL, Ramos FJ, Bueno O, Olivares JL, Bello ML, Bueno M. Severe malformations in males from families with osteopathia striata with cranial sclerosis. Clin Genet. 1998;54:400–5. doi: 10.1111/j.1399-0004.1998.tb03753.x. [DOI] [PubMed] [Google Scholar]
- 11.Odrezin GT, Krasikov N. CT of the temporal bone in a patient with osteopathia striata and cranial sclerosis. AJNR Am J Neuroradiol. 1993;14:72–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Ward LM, Rauch F, Travers R, et al. Osteopathia striata with cranial sclerosis: clinical, radiological, and bone histological findings in an adolescent girl. Am J Med Genet A. 2004;129A:8–12. doi: 10.1002/ajmg.a.30107. [DOI] [PubMed] [Google Scholar]
- 13.Piechowiak H, Goebel FD, Hirche U, Tyrell R. Cranial sclerosis with striated bone disease (osteopathia striata) Klin Padiatr. 1986;198:418–24. doi: 10.1055/s-2008-1033900. [DOI] [PubMed] [Google Scholar]
- 14.Walker GF. Mixed sclerosing bone dystrophies. Two case reports. J Bone Joint Surg Br. 1964;46:546–52. [PubMed] [Google Scholar]
- 15.Ghai S, Sharma R, Ghai S. Mixed sclerosing bone dysplasia: a case report with literature review. Clin Imaging. 2003;27:203–5. doi: 10.1016/s0899-7071(02)00516-8. [DOI] [PubMed] [Google Scholar]
- 16.Vanhoenacker FM, De Beuckeleer LH, Van Hul W, et al. Sclerosing bone dysplasias: genetic and radioclinical features. Eur Radiol. 2000;10:1423–33. doi: 10.1007/s003300000495. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura G, Okada T, Tachibana K, et al. Osteopathia striata, short stature, and characteristic facies: a previously unknown skeletal dysplasia. Eur J Pediatr. 1997;156:631–5. doi: 10.1007/s004310050680. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K, Nakada Y, Nakada D. Unclassified sclerosing bone dysplasia with osteopathia striata, cranial sclerosis, metaphyseal undermodeling, and bone fragility. Am J Med Genet. 1998;76:389–94. [PubMed] [Google Scholar]
- 19.Carolino J, Perez JA, Popa A. Osteopetrosis. Am Fam Physician. 1998;57:1293–6. [PubMed] [Google Scholar]
- 20.Vacek V, Hejzlar M, Salvík M, Pavlanský R. Penetration of Clindamycin into bone in man. Preliminary report. Chemotherapy. 1972;17:22–5. doi: 10.1159/000220835. [DOI] [PubMed] [Google Scholar]