Abstract
Efficacy of cisplatin versus cetuximab with radiation in locally advanced head and neck cancer (LAHNC) was evaluated. A total of 96 patients with newly diagnosed LAHNC treated at our institution between 2006 and 2011 with concurrent radiation and cisplatin (group A, n = 45), cetuximab (group B, n = 24), or started with cisplatin but switched to cetuximab because of toxicity (group C, n = 27) were reviewed. Chi-square test, analysis of variance, and log-rank test were used for analysis. The three groups had similar baseline characteristics, except for median age, T stage, albumin levels, hemoglobin levels, performance status, and comorbidities. A complete response (CR) was seen in 77%, 17%, and 67% of patients (P < 0.001), respectively. There was no significant difference in median overall survival (OS) between groups A and C. The median OS for groups A and C was not reached (>65 months), even though it was significantly longer than median OS for group B (11.6 months; P ≤ 0.001). The 2-year OS in groups A and C is significantly higher than that in group B (70% for groups A and C, 22% for group B). There is no significant difference in progression-free survival (PFS) between groups A and C. The median PFS for these groups was not reached (>62 months), and is significantly longer than that for group B (4.3 months; P ≤ 0.001). The 2-year PFS of group A (67%) and group C (76%) was significantly longer than that of group B (20%). Cisplatin with radiation appears to be more efficacious even in suboptimal dosing than cetuximab with radiation in LAHNC but the two groups were not well matched.
Keywords: head and neck cancer, cetuximab, cisplatin, radiation therapy, overall survival
Introduction
Head and neck cancer is a common malignancy with an estimated 500,000 new cases diagnosed worldwide every year.1 At the time of diagnosis, 60% of patients have advanced locoregional disease.2 The current standard of care for patients with locally advanced, unresectable head and neck cancer (LAHNC) is concurrent radiation therapy (RT) and chemotherapy with cisplatin. A meta-analysis of this regimen showed that it improves overall survival (OS) by 6.5% compared with radiation alone but that it has considerable toxicity.3
In the search for more tolerable and efficacious regimens, cetuximab (Erbitux, ImClone Systems, New Jersey, USA), an IgG1 monoclonal antibody against the ligand-binding domain of EGFR that enhances the cytotoxic effects of radiation in squamous cell carcinoma, was developed. In a phase III trial by Bonner et al, patients with LAHNC were randomized to receive cetuximab and RT or RT alone, and results showed that the addition of cetuximab improved locoregional control and OS while preserving quality of life.4
However, currently it is not known whether cisplatin or cetuximab is superior when combined with RT in LAHNC. Koutcher et al compared the addition of either cetuximab or cisplatin to radiation in LAHNC in a single-institution, retrospective study. The locoregional failure rate was at 5.7% in the cisplatin and radiation arm compared to 39.9% in the cetuximab and radiation arm (P < 0.001), and 2-year OS (92.8% vs 66.6%; P < 0.001)5 also favored the cisplatin/radiation arm. Another retrospective study by Ley et al demonstrated superiority of the cisplatin/RT arm over the cetuximab/RT arm with improved disease-free survival (79% vs 27%; P < 0.001) and 30-month OS (72% vs 25%; P < 0.001).6 In another single-institution retrospective review comparing cisplatin/RT with cetuximab/RT, Caudell et al found no significant differences in locoregional control, distant metastasis-free survival, disease-specific survival, or OS.7
The TREMPLIN study was a phase II randomized trial in which patients with LAHNC received three cycles of induction chemotherapy with docetaxel and fluorouracil and were randomized to receive treatment with cetuximab/RT or cisplatin/RT.8 Results failed to show superiority of one regimen over the other in terms of OS or local progression. However, that study was limited by use of prior induction chemotherapy and by its small patient population. In a phase III RTOG 0522 trial, the addition of cetuximab to chemoradiation with cisplatin in LAHNC patients did not result in increased OS (Hazard Ratio (HR), 0.87; 95% CI, 0.66–1.15; P = 0.17) or progression-free survival (PFS) (HR, 1.05; 95% CI, 0.84–1.29; P = 0.66).9 However, the combination led to more grade 3 and 4 toxicities. To date, there have been no randomized trials published that have directly compared cetuximab/RT with cisplatin/RT in LAHNC. This issue is being addressed in the RTOG 10–16 trial, for which accrual has been completed.9
We performed a retrospective review at our institution comparing the outcomes of patients with LAHNC treated with either cisplatin/RT or cetuximab/RT or in whom both treatments were offered sequentially secondary to cisplatin toxicity.
Patients and Methods
We reviewed medical records of 184 patients diagnosed with LAHNC (squamous cell carcinoma of the oral cavity, oropharynx, larynx, and hypopharynx) who were treated at Louisiana State University Health, Shreveport, USA, between January 1, 2006, and June 30, 2011. The research was exempted from Institutional Review Board approval by the Louisiana State University Health Sciences Center IRB, #E11-036. Patients were excluded for receiving induction chemotherapy, any clinical trial participation, any additional concurrent systemic therapy besides cetuximab and cisplatin, prior active malignancy, any weekly cisplatin regimens, nasopharyngeal carcinomas, Eastern Cooperative Oncology Group performance status (ECOG PS) ≥3, and the presence of metastatic disease or recurrent disease. Patients who smoked for any amount of time until the diagnosis were categorized as smokers. Patient who drank ≥7 drinks per week were categorized as drinkers. A total of 96 patients were included in the analysis and were categorized into three different groups. Group A consisted of patients treated with cisplatin and radiation only. Group B consisted of patients treated with cetuximab and radiation only. Group C consisted of patients treated with cisplatin initially but who were switched to cetuximab during the remainder of their RT because of poor tolerance or toxicity.
Treatment
Baseline 18-fluorodeoxyglucose positron emission tomography and computerized tomography (FDG PET/CT) or CT scan was performed in all patients to aid in treatment planning and identification of suspected head and neck neoplastic disease. Dental evaluation was performed on all patients before initiating external beam radiotherapy. Fractionated megavoltage (6 MV photon beam from a Linear Accelerator treatment unit) was administered daily on five consecutive days each week. A 3-field technique (consisting of “shrinking” opposed lateral ports, including a lower neck treatment portal) was used for irradiation. The selected total dose ranged from 66 to 70 Gy given in 33–35 fractions for gross disease and 50 Gy in 25 fractions for subclinical disease. Cisplatin was administered at a dose of 100 mg/m2 intravenously every 3 weeks for a maximum of three doses. Intravenous cetuximab was administered at an initial dose of 400 mg/m2 followed by 250 mg/m2 weekly. The total number of cetuximab doses was capped off at seven during radiotherapy. Patients were assessed after the completion of treatment by a physical examination and imaging studies, including FDG PET/CT.
Statistical Considerations
PFS was defined as the time period from the date of diagnosis to radiological progression, deterioration in performance status rendering patient ineligible for further treatment, or death. OS was defined as the time period between date of diagnosis and the date of death or date of last contact if the exact date of death was unavailable. Responses were determined according to RECIST version 1.0. The Kaplan–Meier method was used to estimate median PFS and median OS. The log-rank test was used to compare survival among various factors. Adjustment for multiple comparisons among the groups for log-rank test was performed if the P value was markedly less than 0.05. Univariate and multivariate Cox regression was used to perform survival analysis in order to estimate the hazard ratio for various factors. All P-values <0.05 were considered statistically significant. SAS system v9.3 (SAS Institute, Inc., Cary, NC, USA) was used to perform all the analyses.
Results
A total of 96 patients were included in the final analysis; 45 in group A, 24 in group B, and 27 in group C. The median follow-up time for surviving patients was 23.96 months, 11.48 months, and 25.14 months in groups A, B, and C, respectively. Of the 24 patients treated with cetuximab, three had renal insufficiency and two had liver dysfunction. Six patients were ≥68 years old, and four of these were ≥80 years old. Eight patients had multiple comorbidities and were clinically deemed not able to tolerate cisplatin with radiation. Baseline characteristics of each group are shown in Table 1. Patients in group B were significantly older than those in groups A and C (59 vs 55 and 56 years; P = 0.028, respectively). There were more patients with T4 disease in group B (75%) than in group A (44%) and group C (40%) (P = 0.024). Mean serum albumin and hemoglobin were also lower in group B (P = 0.018 for albumin; P = 0.015 for hemoglobin) than in groups A and C.
Table 1.
Baseline characteristics of all groups.
| VARIABLE | GROUP A (45) N (%) |
GROUP B (24) N (%) |
GROUP C (27) N (%) |
P VALUE | |
|---|---|---|---|---|---|
| Sex | Female | 12 (26.7) | 7 (29.2) | 6 (22.2) | 0.845* |
| Male | 33 (73.3) | 17 (70.8) | 21 (77.8) | ||
| Age in years (mean) | 55 (6.5) | 61a (12.0) | 56 (7.6) | 0.028# | |
| Race | African American | 15 (33.3) | 11 (45.8) | 11 (40.7) | 0.574* |
| White | 30 (66.7) | 13 (54.2) | 16 (59.3) | ||
| T stage | T3 | 25 (55.6) | 6 (25.0) | 16 (59.3) | 0.024* |
| T4 | 20 (44.4) | 18 (75.0) | 11 (40.7) | ||
| N stage | 0 | 8 (17.8) | 6 (25.0) | 7 (25.9) | 0.2415* |
| 1 | 4 (8.9) | 2 (8.3) | 6 (22.2) | ||
| 2 | 29 (64.4) | 16 (66.7) | 11 (45.8) | ||
| 3 | 4 (8.9) | 0 (0.0) | 3 (11.1) | ||
| Site | Oral cavity | 9 (20.0) | 8 (33.3) | 3 (11.1) | 0.347* |
| Oropharynx | 17 (37.8) | 8 (33.3) | 11 (40.7) | ||
| Larynx | 16 (35.5) | 5 (20.9) | 12 (44.4) | ||
| Hypopharynx | 3 (6.67) | 3 (12.5) | 1 (3.7) | ||
| ECOG PF | 0 | 6 (13.6) | 1 (4.2) | 2 (7.4) | 0.132* |
| 1 | 35 (79.6) | 17 (70.9) | 23 (85.2) | ||
| 2 | 3 (6.8) | 6 (25.0) | 2 (7.4) | ||
| Tobacco use, current and former | 45 (100.0) | 24 (100.0) | 27 (100.0) | >0.99* | |
| Alcohol | Current and former | 36 (80.0) | 15 (62.5) | 14 (51.8) | 0.329* |
| Never | 9 (20.0) | 9 (37.5) | 13 (48.2) | ||
| Albumin, mean (SD) g/dl | 3.5 (0.5) | 3.2a (0.5) | 3.7 (0.4) | 0.018# | |
| Hemoglobin, mean (SD) g/dl | 12.7 (1.8) | 11.5a (1.9) | 13 (1.3) | 0.015# | |
| CCI Score | 0 | 20 (44.5) | 7 (25.9) | 8 (33.3) | 0.4137* |
| 1 | 15 (33.3) | 12 (44.5) | 7 (29.1) | ||
| 2 | 10 (22.2) | 8 (29.6) | 9 (37.5) | ||
Notes:
ANOVA;
Chi-square test;
different/statistically significant from other groups (P < 0.05).
Abbreviation: CCI, Charlson comorbidity Index.
In group A, 17.8% of patients had stage III disease, 55.8% had stage IVA disease, and 24.4% had stage IVB disease. The primary sites of involvement in this group were the oropharynx (56%), larynx (35%), oral cavity (20%), and hypopharynx (7%). For treatment, 64.4% of patients received all three cycles of cisplatin, 28.9% received two cycles, and 4.1% received only one cycle. In group B, 12.5% of patients had stage III disease, 50.0% had stage IVA disease, and 37.5% had stage IVB disease. The primary sites of involvement in this group were the oropharynx (33.3%), oral cavity (33.3%), larynx (20.9%), and hypopharynx (12.5%). In this group, 70.7% of patients completed the full course of cetuximab, 8.3% completed five treatments, and 20% completed <3 treatments. In group C, 29.7% of patients had stage III disease, 51.8% had stage IVA disease, and 18.5% had stage IVB disease. The primary sites of involvement in this group were the larynx (44.4%), oropharynx (40.7%), oral cavity (11.2%), and hypopharynx (3.7%). In this group, 52% of patients received two cycles of cisplatin, and 48% completed one cycle of cisplatin before switching treatment to cetuximab. Patients in this arm received an average of 3.8 cycles of cetuximab after cisplatin. Concurrent RT achieving full target doses was completed in 87.3%, 75%, and 92.6% in groups A, B, and C, respectively. The baseline comorbidities of each group are described in Table 2. Patients in group B were more likely to have baseline renal failure (25%) and chronic liver disease (8%) than those in groups A and C.
Table 2.
Baseline comorbidities.
| COMORBIDITY | CI | FREQUENCY OF COMORBIDITIES, NO. (%) | |||
|---|---|---|---|---|---|
| GROUP A | GROUP B | GROUP C | |||
| 1 | Anemia | 1 | 17 (37) | 18 (75) | 13 (48) |
| 2 | Hypertension | 1 | 15(33) | 9 (37) | 9 (33) |
| 3 | Diabetes mellitus | 1 | 2 (4) | 6 (25) | 4 (15) |
| 4 | Hepatitis B/C | 1 | 2 (4) | 5 (21) | 4 (15) |
| 5 | CAD*/CVD** | 1 | 3 (7) | 7 (29) | 0 (0) |
| 6 | COPD*** | 1 | 2 (4) | 1 (4) | 2 (7) |
| 7 | HIV | 3 | 2 (4) | 3 (12) | 0 (0) |
| 8 | End-stage liver disease | 2 | 0 (0) | 2 (8) | 0 (0) |
| 9 | Others | 1 | 11 (24) | 15 (62) | 15 (55) |
| 10 | No comorbidities | 0 | 20 (44) | 6 (25) | 7 (26) |
Notes:
Coronary artery disease
cerebrovascular disease
chronic obstructive pulmonary disease.
Human Papilloma Virus (HPV) Data
Among the 96 patients in the study, only 13 were tested for HPV (using the Ventana HPV III In-Situ Hybridization system). Six patients were HPV positive and seven were HPV negative: three of eight in group A, one of one in group B, and two of four in group C were HPV positive.
Treatment-related toxicities
Acute renal failure was the most common toxicity in group A (31%) and group C (33.4%) but was not seen in group B (0%). Grade ≥2 nausea was seen in 24.4% of patients in group A, 14.8% in group C, and 0% in group B. Cetuximab-induced acne-form rash was seen in 37.5% of patients in group B and 33.4% of patients in group C but was not seen in group A. Detailed treatment-related toxicity profiles of the groups are listed in Table 3.
Table 3.
Toxicity profile in each group.
| Toxicity | Group A, No. (%) | Group B, No. (%) | Group C, No. (%) |
|---|---|---|---|
| Acute renal failure | 14 (31.0) | 0 (0.0) | 9 (33.4) |
| Nausea and vomiting | 11 (24.4) | 0 (0.0) | 4 (14.8) |
| Ototoxicity | 0 (0.0) | 0 (0.0) | 4 (14.8) |
| Bacteremia/sepsis | 1 (2.2) | 1 (4.16) | 0 (0.0) |
| Rash | 0 (0.0) | 9 (37.5) | 9 (33.4) |
| Neutropenia | 2 (4.4) | 0 (0.0) | 3 (11.2) |
| Hyponatremia | 1 (2.2) | 0 (0.0) | 0 (0.0) |
| No toxicities | 12 (26.7) | 5 (20.9) | 3 (11.2) |
Response assessment and survival data
Overall survival
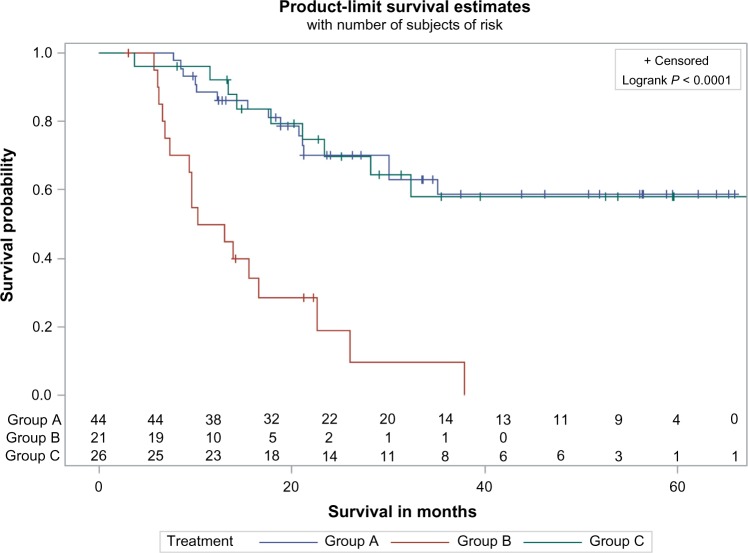
At the first evaluation, a complete response was seen in 77.3% of patients in group A, 17.3% in group B, and 66.7% in group C (P < 0.001 for group B vs the other groups). There was no significant difference in median OS between groups A and C. The median OS for groups A and C was not reached (>65 months), even though it was significantly longer than the median OS for group B (11.6 months; P ≤ 0.001). The 2-year OS in groups A and C was significantly higher than that in group B (70% for groups A and C, 22% for group B) (Fig. 1). When multivariate analysis was used to address other imbalances, there was no difference in median OS between groups A and C (HR, 0.9; 95% CI, 0.4–2.1). Risk of death was statistically higher in group B compared to groups A and C (HR, 4.08, 95% CI: 1.79–9.26, P = 0.0008). Direct adjusted survival by treatment group was estimated by using multivariate Cox regression after adjusting for variables, and adjusted median OS was found to be >37.8 months in groups A and C. Adjusted median OS was at 14.26 months for group B. Multivariate Cox regression analysis using variables such as chemotherapy group, gender, race, ECOG PS, cancer site, albumin, hemoglobin, T stage, and Charlson comorbidity index score demonstrates that the effects of treatment are significant between groups A and C compared to group B (Supplemental Table 1). By using univariate Cox regression analysis, the patients receiving cisplatin had 82% risk reduction of dying compared to cetuximab alone. HR for group A vs B: 0.184 (0.089–0.376) and group C vs B: 0.184 (0.08–0.421). However, there was no difference between group A and group C (HR: 0.996 [0.436–2.277]). Multivariate comparisons among different variables were performed, and the only factor which demonstrated statistically significant changes in OS was the type of chemotherapy used (Supplemental Table 2).
Figure 1.
Univariate OS among patients treated with cisplatin (group A), cetuximab (group B), or cisplatin followed by cetuximab (group C). The Kaplan–Meier method was used to estimate OS.
Progression-free survival
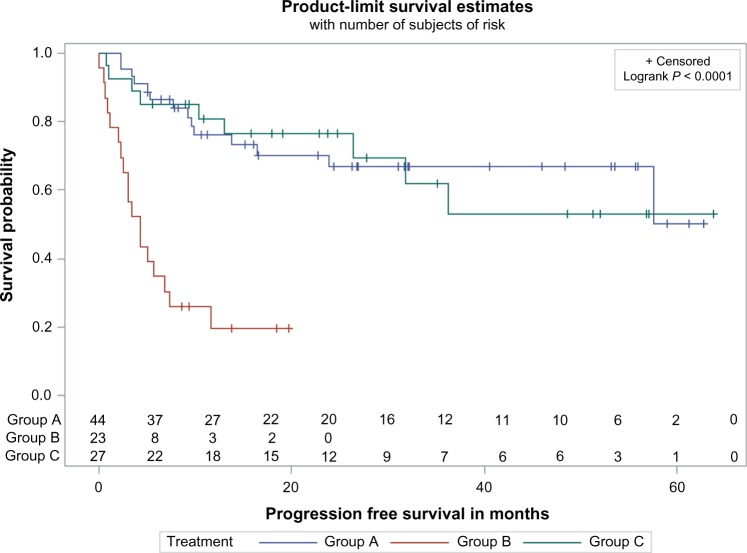
There was no significant difference in PFS between groups A and C. The median PFS for these groups was not reached (>62 months), even though it was significantly longer than that for group B (4.3 months; P ≤ 0.001). The 2-year PFS of group A (67%) and group C (76%) was significantly longer than that of group B (20%) (Fig. 2). In multivariate analysis, there was no difference in PFS between groups A and C (HR, 0.7; 95% CI, 0.3–1.8). There was a greater risk of progression in group B compared to groups A (HR, 5.5; 95% CI, 2.3–13.0) and C (HR, 7.9; 95% CI, 2.9–21.3).
Figure 2.
Univariate PFS among patients treated with cisplatin (group A), cetuximab (group B), or cisplatin followed by cetuximab (group C). The Kaplan–Meier method was used to estimate PFS.
Relapse pattern
In group A, 10 (22.2%) patients had disease relapse of which 4 patients had locoregional relapse and 6 patients had distant metastases (6 pulmonary recurrences). In group B, 13 (54.2%) patients had disease relapse of which 8 patients had locoregional relapse and 5 patients had distant metastases (four pulmonary, one bone and liver). In group C, 7 (25.9%) patients had disease relapse of which 3 patients had locoregional relapse and 4 had distant metastases.
Discussion
For patients with LAHNC undergoing radiotherapy, both cisplatin and cetuximab are valid treatment options.4,10,11 This benefit of adding chemotherapy to radiotherapy has been shown to be consistent in all the sites of head and neck cancer as demonstrated by Blanchard et al.12 The 5-year absolute benefits associated with concomitant chemotherapy range from 4% to 8.9% depending on the site of cancer involved.12 Currently, there is no direct comparison between cisplatin and cetuximab to show superiority of one over the other. Recently, a systemic review and meta-analysis of concomitant platinum-based chemotherapy or cetuximab with radiation in LAHNC, involving 15 trials (12 retrospective and 3 prospective) and 1808 patients, was performed. The results demonstrated significant improved 2-year OS (Relative Risk [RR] = 0.66; 95% CI 0.46–0.94, P = 0.02), 2-year PFS (RR = 0.68; 95% CI 0.53–0.87, P = 0.002), and 2-year locoregional relapse (RR = 0.63, 95% CI 0.45–0.87, P = 0.005) in chemoradiotherapy with cisplatin compared to cetuximab and radiation.13 Our study results are consistent with these results. Like our study, six of the studies included in the meta-analysis had patients who were older in the cetuximab group as compared to the cisplatin group, and one study had patients with poorer PS in the cetuximab group.
Our study is different from previously published similar retrospective reviews5–7 in that we included three groups instead of two. In clinical practice, patients who have poor tolerance to cisplatin are often switched to cetuximab during the remainder of the radiation course as both agents are approved in head and neck cancers. There are no randomized trials carried out to date to substantiate this approach. However, as can be seen from our results, most of the benefit seems to be from using cisplatin. In our study, tolerance to cisplatin was poor and clearly associated with more toxicity compared to cetuximab. In group A, only 64.4% of patients could complete all three doses of cisplatin, and in group C, 48% of patients received only two cycles of cisplatin. A third of patients in groups A and C receiving cisplatin had renal failure. By contrast, patients receiving cetuximab were more likely to develop skin rash. Because of the higher toxicity and poor tolerance to high-dose cisplatin (100 mg/m2 cisplatin), a weekly dose regimen has also been used with comparable results.14 Another option is to combine weekly cisplatin and cetuximab concurrent with radiation, which provides a less toxic alternative to high-dose cisplatin.15
This leads to a question – does the combination of cisplatin and cetuximab with radiation leads to improvement in efficacy as compared to each agent alone? This was evaluated in a recently published trial RTOG 0522.16 Patients with stage III and IV locally advanced stage head and neck cancer were randomly assigned to receive radiation and cisplatin at 100 mg/m2 for two cycles without (Arm A) or with cetuximab (Arm B). No difference was found between arms A and B in 3-year PFS (61.2% vs 58.9%, respectively; P = 0.76), 3-year OS (72.9% vs 75.8%, respectively; P = 0.32), locoregional failure (19.9% vs 25.9%, respectively; P = 0.97), or distant metastasis (13.0% vs 9.7%, respectively; P = 0.08). Moreover, cetuximab plus cisplatin–radiation arm resulted in more frequent interruptions in RT (26.9% vs 15.1%, respectively), and more grade 3 to 4 radiation mucositis (43.2% vs 33.3%, respectively), rash, fatigue, anorexia, and hypokalemia. Thus, addition of cetuximab to cisplatin does not add to efficacy but adds to toxicity when used concurrently with radiation. The same results were demonstrated in a single-institution retrospective study where patients receiving definitive concurrent chemoradiotherapy for LAHNC were stratified into three groups: patients receiving cetuximab only, chemotherapy and cetuximab combination, or platinum-based chemotherapy without cetuximab. This study also concluded that platinum-based concurrent chemoradiotherapy is superior to cetuximab-based monotherapy for definite treatment of LAHNC.17 These results are surprising because cetuximab when combined with cisplatin-based chemotherapy in recurrent or metastatic head and neck cancer leads to improvement in response rate, PFS, and OS.18
In recent years, the demographics of head and neck cancer have also undergone a gradual shift, and HPV is now an important risk factor. Patients with HPV-positive head and neck cancer tend to have a better prognosis and may require less intense treatment for which cetuximab may be a useful candidate.19 In fact, in the recently published meta-analysis of cisplatin and cetuximab with radiation in LAHNC there was no difference in the efficacy of the two treatments.12 Unfortunately, in our dataset very few patients underwent HPV testing, so it is difficult to draw any conclusions about this population.
Overall, our study sheds light on treatment patterns in LACHNC patients receiving cisplatin or cetuximab concurrent with radiation. Cisplatin clearly has a better response rate and leads to better PFS and OS. However, because of its toxicity it also has poor tolerance, and many patients cannot complete all three cycles of chemotherapy. For this reason, many oncologists use cetuximab in older patients and those with poor performance status or comorbidities. Hence, it is difficult to conclude in a retrospective review whether cetuximab is inferior to cisplatin. A direct comparison between the two agents is currently under way in the RTOG 10–16 clinical trial in HPV-positive oropharyngeal cancer. Other ways of improving outcome in LAHNC, including induction chemotherapy followed by chemoradiotherapy, have also been studied, but recent clinical trials have shown no improvement in OS.20–22 Another interesting observation from our study would raise two questions: first, would suboptimal dosing of cisplatin (one or two cycles compared to three cycles) provide an alternate in select patients with poor performance status instead of cetuximab? Second, would one or two cycles of cisplatin be as good as three cycles of cisplatin in patients with good performance status? Only randomized trials would shed more light on these unanswered questions.
The retrospective nature of this study, imbalances in comparison groups, small study population, lack of HPV status on most of the patients, and lack of median dose of radiation received in each group are major setbacks of this study and, therefore, mandate cautious interpretation of the results. In conclusion, our study would suggest that cisplatin and radiation might be more efficacious even in suboptimal dosing when compared to cetuximab and radiation. Only the results of the ongoing prospective randomized clinical trial will shed much-needed light on the comparison between the two regimens. In the meantime, both cisplatin and cetuximab remain optimal candidates for treatment in combination with radiation in patients with LAHNC and choice of treatment should be individualized.
Supplementary Material
Supplemental Table 1. Multivariate Cox regression analysis for survival after adjusting for variables.
Supplemental Table 2. Multivariate Cox regression comparing multiple variables.
Footnotes
Author Contributions
Conceived and designed the experiments: PP, SHJ. Analyzed the data: RS, PP, SHJ. Wrote the first draft of the manuscript: PP, SHJ. Contributed to the writing of the manuscript: PP, BN, FA, GMM, SHJ. Agree with manuscript results and conclusions: PP, SHJ, RS. Jointly developed the structure and arguments for the paper: PP, SHJ. Made critical revisions and approved final version: GMM, PP, SHJ. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: William CS Cho, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Monnerat C, Faivre S, Temam S, Bourhis J, Raymond E. End points for new agents in induction chemotherapy for locally advanced head and neck cancers. Ann Oncol. 2002;13:995–1006. doi: 10.1093/annonc/mdf172. [DOI] [PubMed] [Google Scholar]
- 3.Pignon J-P, le Maître A, Maillard E, Bourhis J, MACH-NC Collaborative Group Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 5.Koutcher L, Sherman E, Fury M, et al. Concurrent cisplatin and radiation versus cetuximab and radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):915–22. doi: 10.1016/j.ijrobp.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Ley J, Mehan P, Wildes TM, et al. Concurrent cisplatin vs. cetuximab with definitive radiation therapy (RT) for head and neck squamous cell carcinoma (HNSCC): a retrospective comparison; Multidisciplinary Head and Neck Cancer Symposium; January 26–8, 2012; Phoenix, AZ. Abstract 163. [Google Scholar]
- 7.Caudell JJ, Sawrie SM, Spencer SA, et al. Locoregionally advanced head and neck cancer treated with primary radiotherapy: a comparison of addition of cetuximab or chemotherapy and the impact of protocol treatment. Int J Radiat Oncol Biol Phys. 2008;71(3):676–81. doi: 10.1016/j.ijrobp.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 8.Lefebvre J, Pointreau Y, Rolland F, et al. Sequential chemoradiotherapy (SCRT) for larynx preservation (LP): results of the randomized phase II TREMPLIN study. J Clin Oncol. 2012;30((360 s) suppl 15) abstract 5501. [Google Scholar]
- 9.Trotti A, Gillison M. Phase III trial of radiotherapy plus cetuximab versus chemoradiotherapy in HPV-associated oropharynx cancer. RTOG 0106. 2014. Available at http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1016.
- 10.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21(1):92–8. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–8. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard P, Baujat B, Holostenco V, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumour site. Radiother Oncol. 2011;100(1):33–40. doi: 10.1016/j.radonc.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 13.Petrelli F, Coinu A, Riboldi V, Borgonovo K, Ghilardi M, Cabiddu M, et al. Concomitant platinum-based chemotherapy or cetuximab with radiotherapy for locally advanced head and neck cancer: a systematic review and meta-analysis of published studies. Oral Oncol. 2014;50(11):1041–8. doi: 10.1016/j.oraloncology.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Merlano M, Russi E, Benasso M, et al. Cisplatin-based chemoradiation plus cetuximab in locally advanced head and neck cancer: a phase II clinical study. Ann Oncol. 2011;22(3):712–7. doi: 10.1093/annonc/mdq412. [DOI] [PubMed] [Google Scholar]
- 15.Espeli V, Zucca E, Ghielmini M, et al. Weekly and 3-weekly cisplatin concurrent with intensity-modulated radiotherapy in locally advanced head and neck squamous cell cancer. Oral Oncol. 2012;48(3):266–71. doi: 10.1016/j.oraloncology.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Ang KK, Zhang Q, Rosenthal DI. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32(27):2940–50. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang C, Chan C, Jiang W, et al. Concurrent cetuximab versus platinum-based chemoradiation for the definitive treatment of locoregionally advanced head and neck cancer. Head Neck. 2014 doi: 10.1002/hed.23609. In Press. [DOI] [PubMed] [Google Scholar]
- 18.Vermorken JB, Mesia R, Rivera F. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 19.Pajares B, Perez-Villa L, Trigo JM, et al. Concurrent radiotherapy plus epidermal growth factor receptor inhibitors in patients with human papillomavirus-related head and neck cancer. Clin Transl Oncol. 2013;16(4):418–24. doi: 10.1007/s12094-013-1099-9. [DOI] [PubMed] [Google Scholar]
- 20.Haddad R, O’Neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomized phase 3 trial. Lancet Oncol. 2013;14(3):257–64. doi: 10.1016/S1470-2045(13)70011-1. [DOI] [PubMed] [Google Scholar]
- 21.Cohen EE, Karrison TG, Kocherginsky M, et al. DeCIDE: a phase III randomized trial of docetaxel (D), cisplatin (P), 5-Fluorouracil (F) (TPF) induction chemotherapy (IC) in patients with N2/N3 locally advanced squamous cell carcinoma of the head and neck (SCCHN) J Clin Oncol. 2012;30(356s, suppl) abstract 5500. [Google Scholar]
- 22.Cohen EEW, Karrison T, Kocherginksy M, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32(25):2735–43. doi: 10.1200/JCO.2013.54.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Multivariate Cox regression analysis for survival after adjusting for variables.
Supplemental Table 2. Multivariate Cox regression comparing multiple variables.