Abstract
Ventricular assist devices have become standard therapy for patients with advanced heart failure either as a bridge to transplantation or destination therapy. Despite the functional and biologic evidence of reverse cardiac remodeling, few patients actually proceed to myocardial recovery, and even fewer to the point of having their device explanted. An enhanced understanding of the biology and care of the mechanically supported patient has redirected focus on the possibility of using ventricular assist devices as a bridge to myocardial recovery and removal. Herein, we review the current issues and approaches to transforming myocardial recovery to a practical reality.
Keywords: Circulatory support, myocardial remodeling, heart failure
Introduction
Traditionally, left ventricular assist devices (LVADs) are used to assist the advanced heart failure (HF) patient as bridge to transplant (BTT) or as destination therapy (DT) for lifetime use. Current continuous flow LVADs (CF-LVAD) have become routine therapy for patients with end-stage HF, reproducibly improving quality of life and enhancing survival in both the BTT and DT population [1–3]. In addition, a small, but not insignificant, group of patients improve their heart function to the point where the LVAD can be removed, often referred to as bridge to recovery. Such reports often describe younger patients with shorter duration of disease, such as witnessed with viral myocarditis. Importantly, some centers have observed recovery of patients with chronic HF as well [4–8].
Cumulatively, there is a growing enthusiasm from basic, translational, and clinical perspectives to pursue strategies aimed at not just implanting LVADs, but rather, removing them. What if the LVAD became an intervention for the failing heart, instead of a final therapeutic option? What if the LVAD was used as a tool to promote its removal? The advanced HF community is poised to challenge the notion that end-stage heart failure is end-stage, and furthermore unleash the possibility that it may actually be reversible. This review will examine some of the issues – scientifically, medically, and surgically – involved with this paradigm shift towards using mechanical support as a bridge to LVAD removal.
Reverse Remodeling versus Recovery
Medical and device therapies reduce HF morbidity and mortality as well as reduce left ventricular (LV) volume and mass. Changes in myocyte size, structure, and organization result in a return of normal LV shape and a shift in the LV end-diastolic pressure-volume curve toward normal. These molecular, cellular, and anatomic changes are globally referred to as “reverse remodeling” [9, 10]. This definition suggests a multifactorial process of returning the heart towards its normal phenotype.
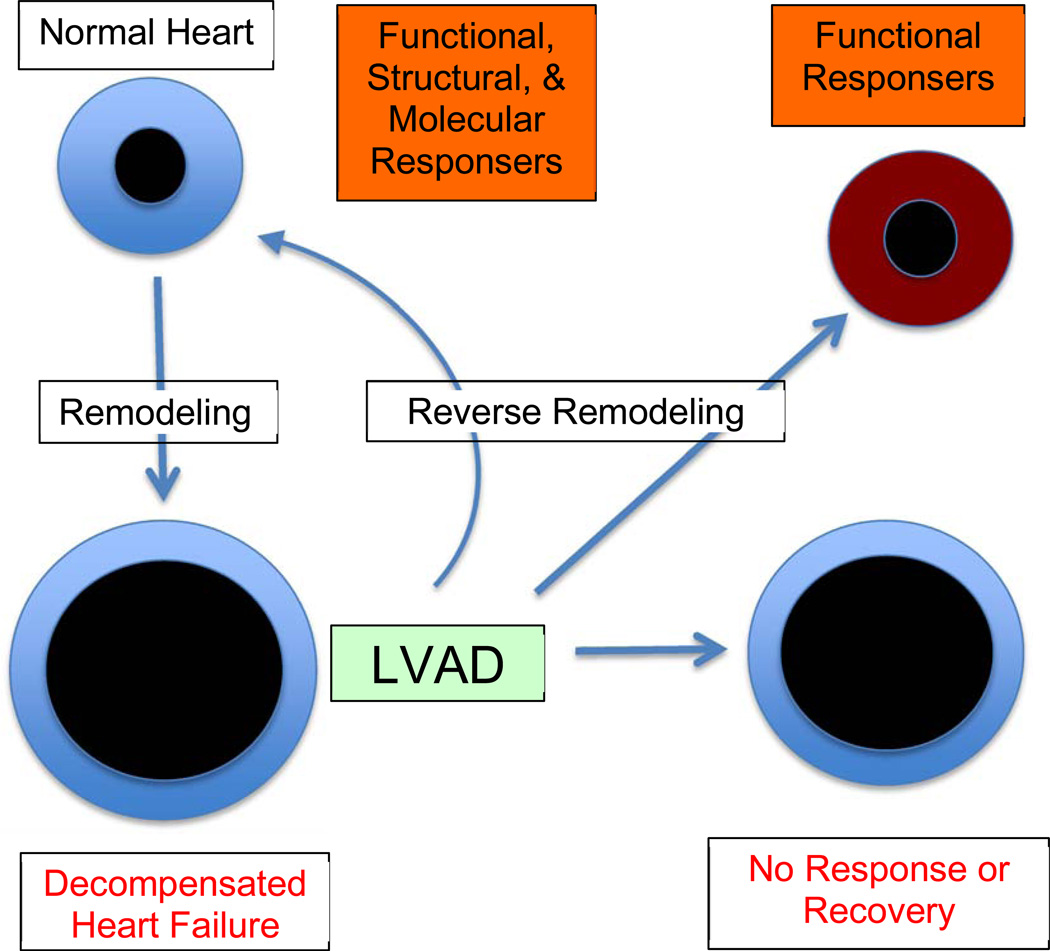
The paradox is as follows: while structural changes associated with HF, may reverse in some patients, normal myocardial function might not be restored; conversely, on occasion myocardial function can return to normal, with structural components still in disarray. A definition of myocardial recovery was recently proposed as, “the normalization of the molecular, cellular, myocardial and LV geometric changes that provoked cardiac remodeling, that allow the heart to maintain preserved LV structure and function in the face of normal and/or perturbed hemodynamic loading conditions” [11]. The advantage of this statement is that it clearly incorporates systolic and diastolic function into the definition of myocardial recovery (Figure 1).
Figure 1.
Bridge to removal. LVADs are placed in patients with pathologic remodeling and development of advanced heart failure. After implantation, the heart undergoes variable levels of reverse remodeling. If inadequate (non-responder), then continued support and/or transplantation remain the mainstay of therapy. If cellular, metabolic, architectural, and functional reversal occurs to normal, then these patients exhibit myocardial recovery. Another, perhaps more common group, are those patients that demonstrate functional improvement without structural and/or molecular normalization. These latter two groups (in orange) are both considered “responders” to therapy and are candidates for consideration of LVAD removal.
The distinction between remodeling and recovery provides substrate for academic pursuit, but may not be clinically relevant. Indeed, whether or not a patient needs to have full myocardial recovery prior to weaning and explanting their LVAD is subject to debate. Many patients with moderately reduced LV function can live good, long lives with medical therapy. If they are able to achieve this functional state, why do they need to have an LVAD? Indeed, if the goal of LVAD therapy is to induce “remission” of heart failure, rather than full recovery, then we are likely underestimating the population of LVAD patients that could potentially live without their pumps [12].
Bridge to Recovery: The Candidates
Over the last 15 years, multiple investigators, either as single centers or multi-institutional working groups, have reported successful cases of LVAD removal (Table 1). Within the latest INTERMACS dataset of over 6500 FDA-approved device implants, bridge to recovery accounted for only 1.2% of these patients [13]. Similarly, in the cohort of 1,108 patients enrolled in the Heartmate II BTT and DT trials, the rate of myocardial recovery sufficient to allow for device explanation was 1.8% (20 patients, 10 BTT, 10 DT) [14]. This study found device explantation to be more likely in younger patients (< age 40) with non-ischemic cardiomyopathy of short duration (< 1 year). In those that did undergo device explant, survival was 95% and 85% at 1 and 3 years, respectively. Importantly, freedom from recurrent heart failure requiring reimplantation or transplantation was 74% at 3.5 years. This study confirmed some of the previous characteristics seen in explanted patients that received pulsatile pumps [15].
Table 1.
LVAD bridge to recovery studies (adapted from [12]).
| Study | Design | N | Standardized Med Therapy |
Duration (mos) |
Recovery* n (%) |
Durability** |
|---|---|---|---|---|---|---|
| LVAD Working Group, 2007 [45] | P | 67 | N | 4.5 | 6 (9) | 100/6m |
| Berlin, 2008 and 2011 [46, 47] | P | 188 | N | 4 | 35 (19) | 74/3y; 66/5y |
| Harefield, 2006 [6] | P | 15 | Y | 11 | 11 (73) | 100/1y; 89/4y |
| Harefield, 2011 [5] | P | 20 | Y | 9 | 12 (60) | 88/3y |
| Athens-Harefield, 2007 [48] | P | 8 | Y | 7 | 4 (50) | 100/2y |
| Vancouver, 2011 [49] | P | 17 | N | 7 | 4 (23) | 100/2y |
| Gothenburg, 2007 [50] | P | 18 | N | 7 | 3 (17) | 33/8y |
| Pittsburgh, 2003 [51] | R | 18 | N | 8 | 6 (33) | 67/1y |
| Osaka, 2005 [52] | R | 11 | N | 15 | 5 (45) | 100/8–29m |
| Pittsburgh, 2010 [53] | R | 102 | N/A | 5 | 14 (14) | 71/5y |
| Multicenter, 2002 [54] | R | 271 | N/A | 2 | 22 (8) | 77/3y |
| Columbia, 1998 [55] | R | 111 | N/A | 6 | 5 (4.5) | 20/15m |
| Montefiore, 2013 [20] | P | 21 | Y | ? | 5 (23%) | 100/>3y |
P, prospective; R, retrospective; NA, not applicable; HF, heart failure.
Recovery defined as LVAD explantation as a result of functional myocardial recovery.
Durability defined as freedom from LVAD reimplantation or transplant - percentage over mean years of follow-up.
Importantly, these low rates of recovery are seen in patient populations who (1) were generally not expected to recover, (2) may not have been actively surveyed for evidence of recovery, and (3) may not have been treated with adjuvant therapy specifically aimed at improving myocardial recovery.
When patients with favorable characteristics are systematically treated and prospectively evaluated with a goal of recovery in mind, much higher rates of BTR have been achieved. The seminal report came from Yacoub’s group in England where patients with non-ischemic cardiomyopathy and no evidence of active myocarditis underwent an intense period of medical management followed by adjuvant pharmacologic therapy with clenbuterol (see below). This approach allowed for explantation in 11 of 15 (73%) patients with pulsatile LVADs and 12 of 20 (60%) of patients with CF-LVADs [5, 6]. Similarly, the Berlin Heart Institute group has explanted nearly 100 patients with idiopathic dilated cardiomyopathy by using serial “turn down” echocardiograms to closely monitor changes in function and geometry so as to actively identify potential recovery candidates [7, 16].
Although this review is more clinically focused, there is significant potential to drive decisions regarding candidacy for explantation based on biology. Indeed, many secrets are hiding within the apical core as well as in serum. We and others have utilized gene arrays, proteomics, and other inductive and deductive approaches to identify characteristics of patients that respond to LVAD unloading compared to the nonresponders. While many of these features have yet to be validated, they offer a promising and appealing avenue for future investigation[12].
Despite the inherent limitations of small sample sizes, varied periods of LVAD support, and diverse medical management, the available clinical studies cumulatively identify many reproducible themes. Characteristics consistently associated with higher likelihood of recovery include: younger age, shorter duration of HF, and non-ischemic HF etiology. As demonstrated by both the Harefield and Berlin groups, concerted efforts aimed at identifying patients, a priori, as recovery candidates, coupled with active surveillance, use of adjuvant medical therapy, and standardized weaning protocols may increase the rate of successful pump removal.
Medical Therapy use with LVADs
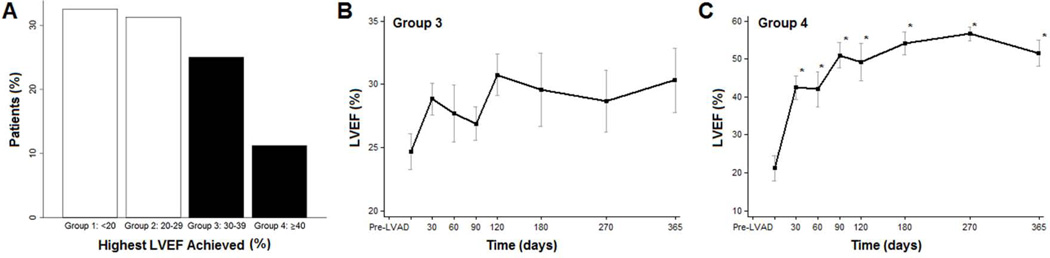
Within the mindset of BTT or DT, it is tempting to think of VAD insertion as the final therapeutic intervention for advanced HF. As such, adjuvant medical therapies are utilized, for the most part, to treat blood pressure or arrhythmias, rather than to influence reverse remodeling and myocardial recovery. Indeed, just the mechanical unloading of the weakened ventricle may be a powerful tool to promote myocardial recovery. In order to understand the influence of unloading alone, we serially followed 81 CF-LVAD patients with turn-down echocardiograms [17]. Within this group, nearly 20% of patients had significant recovery of LV function (EF>40%). Importantly, most of the return of function occurred in the first 6 months after LVAD implant (Figure 2). This study is one of the largest to prospectively link mechanical unloading with functional recovery and provides the framework with which to build adjuvant medical strategies to enhance the frequency with which recovery takes place.
Figure 2.
Highest left ventricular ejection fraction achieved after LVAD. (A) Highest EF achieved after LVAD unloading. (B) Changes in LVEF over time with highest EF achieved 30%–39% (group 3). (C) Changes in LVEF over time for patients with highest LVEF achieved >40% (group 4). Data is presented as percentages, means, and confidence intervals. *p<0.01 vs Pre-LVAD. Reproduced from [17] with permission.
The Harefield group catalyzed interest in pharmacologic manipulation of the LVAD patient. A prospective study aimed at improving recovery following pulsatile LVAD placement aggressively treated 15 patients with high-dose angiotensin converting enzyme inhibitors, beta-blockers, angiotensin receptor blockers, digoxin, and spironolactone [6]. Once left ventricular end-diastolic size had decreased below 6.0cm, carvedilol was replaced by bisoprolol (selective beta 1-blocker) and clenbuterol (beta 2-agonist) was initiated. Using this protocol, 11 of the 15 patients (73%) recovered sufficient myocardial function to undergo LVAD explantation. At follow-up, patients who underwent VAD removal for myocardial recovery demonstrated a durable recovery with good quality of life [4, 18]. A subsequent study confirmed the effectiveness of this protocol (in non-ischemic cardiomyopathy patients) utilizing the Heartmate II LVAD. In this study, 12 of 19 (63.2%) patients underwent device explantation after a mean 286 (±97) days [5].
Attempts to repeat this experience in the United States were less successful. Using the Harefield protocol, the HARPS trial enrolled 17 patients in 6 US centers for which only 1 patient successfully had her LVAD removed [19]. While the reasons for this disparity in results are unclear, differences related to demographics, chronicity of HF, and medication up-titration point to the difficulty of expanding these demanding studies. That said, when a single institution actively engages in intense medical therapy – with biweekly neurohormonal titration – return of LV function may be present in a larger proportion of patients [17, 20].
The Remission from Stage D Heart Failure study (RESTAGE-HF, ClinicalTrials.gov NCT01774656) has since been designed to investigate the influence of aggressive medical intervention on selected patients receiving HeartMate II LVADs. The primary outcome is the proportion of patients in whom their LVADs can be removed and remain free from additional mechanical support or transplant for at least 3 years. Importantly, these patients will be rigorously followed with serial imaging to not only better define predictors of explantation, but to also chart the course of durable recovery after LVAD removal.
Finally, a discussion of medical therapy would be incomplete without mentioning the vast potential of adjuvant biologic therapy to both enhance reverse remodeling and augment contractile function. Several previous attempts at investigating the role of stem cells in LVAD patients have been abandoned by the NHLBI for administrative reasons. That said, individual centers are injecting both autologous (University of Minnesota, ClinicalTrials.gov NCT00869024) and allogeneic mesenchymal stem cells (MSC) in LVAD patients (AHEPA University Hospital, Greece, ClinicalTrials.gov NCT01759212). The NIH-sponsored Cardiothoracic Surgery Network (CTSN) recently reported a 30 patient pilot trial of intramyocardial MSC injection at the time of LVAD placement. There were no safety issues and potential efficacy signals were observed. A follow-up trial with more centers and a recruitment goal of 120 patients is currently being initiated (CTSN LVAD MPC-II). Adjuvant therapy studies are not limited to stem cells, but also include growth factor (e.g. SDF) and gene (e.g. SERCA2a) based therapies. Cumulatively, the LVAD patient provides a platform that allows for a multitude of creative, biologic interventions.
VAD and Patient Selection
When approaching an advanced heart failure patient with the preoperative notion of recovery rather than BTT or DT, does the type of pump make a difference? In general, both continuous and pulsatile device types have the capacity to provide similar degrees of unloading [21, 22]. Because of their ease of insertion, smaller size, and excellent outcomes, CF-LVADs have essentially replaced their pulsatile counterparts on the VAD shelf. However, when compared to continuous flow devices, pulsatile devices are associated with a greater reduction in brain natriuretic peptide and greater improvement in left ventricular ejection fraction [23].
Whether differences in the quality and quantity of unloading by a particular device translate into clinically meaningful variances in recovery remains controversial. In a retrospective review of 387 patients with end-stage idiopathic dilated cardiomyopathy (IDCM) who underwent LVAD placement, 25% of patients treated with a pulsatile device recovered and underwent device explantation versus only 3.29% of those treated with a non-pulsatile device. Multivariate analysis identified pulsatile device and age (younger) as independent factors associated with recovery to LVAD explantation [24]. Despite some of the perceived advantages of early LVAD generation unloading, the modern reality is that most patients will receive implantable, continuous flow devices. Some of the more exciting, ongoing experimental work is focused on making continuous flow pumps pulsatile by linking software with the cardiac cycle.
Smaller and less invasive VAD technology may find a niche in patients with less advanced symptoms as a strategy for recovery. For example, he Circulite Synergy pump is a small continuous flow device that sits like a pacemaker in the infraclavicular fossa, can provide 2–3 liters/minute of flow, and can provide partial support [25]. A particularly appealing aspect of this pump is the ease of implantation and explantation [26]. Ultimately, a key component of any recovery pump is the ability to provide sustained and significant unloading to allow for reverse cardiac remodeling. Whether or not that reversed phenotype will translate into functional recovery likely remains less of an issue of the pump, but rather the biology of the patient.
Weaning Protocols
Though several groups have published their experience with VAD weaning, no universal criteria for weaning readiness or universal weaning protocol exist. Compared to pulsatile LVADS, CF-LVADS present distinct challenges when weaning. Pulsatile VADs have one-way valves, thus preventing reversal of flow when LVAD support is discontinued. Weaning or stopping a CF-LVAD risks reversal of LVAD flow and acute LV volume overload. LVAD flows are decreased to the point where reversal of flow begins to be seen during diastole. Others have performed full off-pump testing by inflating an endovascular balloon placed retrograde into the outflow graft. This procedure is done concomitantly with a right heart catheterization, often with on-table exercise, to best assess the loading conditions of the unsupported heart.
The Texas Heart Institute group has championed a strategy based on normalization of of the cardiac cycle to guide eligibility for pump removal. Once hearts are adequately unloaded (dimensions within normal range, minimal mitral regurgitation), patients are serially evaluated at minimal pump speeds for normalization of aortic valve opening time. With this reconditioning approach, they have removed pumps from over 30 patients (personal communication, O.H. Frazier, MD – manuscript in revision stage).
The Montefiore group recently published their prospective use of 3-step testing for select patients deemed as candidates for recovery. Restoration of myocardial function was assessed 4 weeks after patients reached maximally tolerated doses of HF medicines [27]. If echocardiography at rest and with diminished support (“turn-down”) demonstrated an EF>40% (step 1), then they proceed to cardiopulmonary stress test (step 2) and right heart catheterization (step 3). Of 34 patients, 21 subjects made it to step 1 testing, with 16 showing no evidence of improved function. Of the remaining 5 who “normalized” function: 1 elected to keep their LVAD; 1 had an exercise-induced increase in PCWP and not deemed and explant candidate; and 3 went on to explantation and are free from major HF events 3–5 years after explant [20].
In summary, there are no generally accepted criteria for VAD weaning or explantation and no proposed criteria have been tested in a prospective multi-institutional fashion. As such, any criteria simply represent an integration of published experience from single center studies and research protocols. Table 2 may serve as guidance for consideration of LVAD explantation in patients clinically deemed suitable for this strategy. Regardless of which criteria are used, some patients may develop recurrent heart failure following LVAD removal. However applying explant criteria that are too strict carries a risk that some patients who would benefit from device explantation could be excluded.
Table 2.
Potential criteria for LVAD explantation. All studies assessed (1) at rest and with exercise/stress and (2) at full and partial or no LVAD support (“turn-down” or “pump-off” for 30 minutes) after achieving maximal heart failure medicine doses. (LVED, left ventricular end diastolic dimension in (d) diastole or (s) systole; VE/VCO2, minute ventilation relative to production of carbon dioxide production; PCWP, pulmonary capillary wedge pressure; CI, cardiac index)
|
Surgical Approaches
Insertion
While the variability of clinical presentation will often determine the specifics for surgical implantation, prospectively setting the goal of recovery implies the need to fix structural heart defects at the time of the initial implant. Fundamentally, the surgical team has to ask the question, how will the heart work when the pump is removed – after LV function has been restored. The most common scenario is related to valve disorders, and much has been written about management of concomitant valve disease with patients requiring VAD [28–30].
Aortic valve competence is required for proper VAD function. Depending on the speed setting and level of unloading, continuous flow LVADs result in aortic valves that are either closed or infrequently opening. This situation can provoke aortic valve commissural fusion, with subsequent development of aortic stenosis and/or aortic insufficiency (AI). Pre-existing AI is known to worsen in the setting of VAD support and it is recommended that AI be corrected at the time of VAD placement [31, 32]. Some centers have taken an aggressive approach either by coapting the aortic leaflets or fully oversewing the valve [33]. Within the recovery paradigm, the concept of replacing an oversewn aortic valve at the time of LVAD explant is not appealing. Acknowledging a lack of data to support a particular approach, potential explant patients with indications for aortic valve intervention are likely best served with a bioprosthetic aortic valve replacement. This strategy would extend to patients that have an existing mechanical aortic valve or native valve aortic stenosis.
The management of tricuspid valve regurgitation (TR) is more controversial. Patients referred for VAD placement will often have bi-ventricular dilation and subsequent development of mitral regurgitation (MR) and TR [34]. While TR is a marker for potential RV dysfunction, it is unclear if fixing TR at the time of VAD implantation results in improved outcomes. While TR has been associated with longer post-implant inotropic support and length of hospital stay, tricuspid valve repair plus VAD implantation has shown similar outcomes compared to LVAD implantation alone [34]. This has led some to question whether repair is necessary. Although no firm recommendation regarding TR can be made for the potential removal patient, the general concept remains on repairing structural defects that could influence remodeling, including that of the right ventricle.
Mitral regurgitation is common in patients with heart failure secondary to the deleterious effects of remodeling. In addition to annular dilation, ventricular enlargement pulls the papillary muscles, distorts their anatomy, and tethers chordal support of the valve [35]. One could argue that MR will improve with LVAD unloading on its own. Indeed, the decrease in LV dimensions will enhance leaflet coaptation. Unfortunately, some ventricles might not shrink down, and others might have some re-dilation after LVAD explantation [7, 36].
As such, a more aggressive approach to repair may be indicated. Mitral intervention, either with repair or replacement, at the time of LVAD will incrementally decrease pulmonary vascular resistance and potentially protect a fragile right ventricle [37]. We and others have routinely used the transapical edge-to-edge approach to treat severe MR in LVAD patients [38]. Although traditional mitral valve repair approaches can be safely used, there is an obligate increase in operative time and dissection. The edge-to-edge technique is fairly straightforward and adds only minutes to the procedure. As a technical point, the repair is more easily performed prior to putting a rigid sewing ring on the apical defect. For example, with the HeartWare device, we will make the core, perform the repair, and then attach the sewing ring. The ability to expose the mitral leaflets is greatly enhanced in this manner. While the ease of the transapical repair is appealing, the approach to mitral repair is patient-dependent and there are no data to help guide its use. As another example, in a patient that has multiple factors favoring recovery, yet has a 4.8 cm mitral annulus, we would take a more traditional approach to repair with an annuloplasty ring inserted through a left atrial approach.
Explantation
Traditionally, LVAD removal is performed through a midline sternotomy with complete extirpation of the device and outflow graft. Cardiopulmonary bypass without cardiac arrest is used to repair the apical defect either primarily or with patch closure. The outflow graft is transected at the level of the aorta either with suture or vascular stapling device. However, redo-sternotomy and explantation can place a patient at risk for other morbidity that might jeopardize a fragile, recovering myocardium, including blood transfusions and direct cardiac injury. As such, most groups have adopted less invasive techniques for VAD removal. All approaches need to consider anatomic issues including need for structural valve repair and presence of LV thrombus.
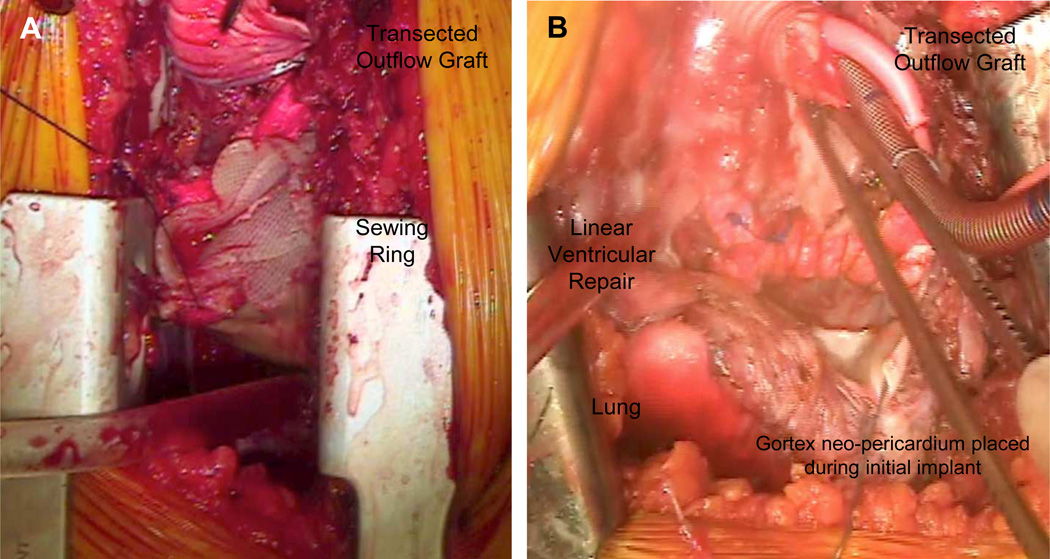
Multiple techniques have been described to facilitate VAD removal without requiring extensive surgical repair of the ventricle, thereby allowing minimally invasive approaches. These often require femoral-femoral bypass on either the beating or fibrillating heart. Even through a mini-thorocotomy, the apical defect can be fixed directly as with a linear aneurysmorraphy repair. With the more flexible sewing rings (e.g. Jarvik 2000), the closure can actually incorporate the sewing ring in this suture (Figure 3).
Figure 3.
Apical closure without plug. Intraoperative view demonstrating (A) primary closure associated with removal of the Jarvik 2000 Flowmaker LVAD. The prolene suture is seen as it goes through felt strips that include the flexible sewing ring. (B) Primary closure with felt strips following removal of the rigid HeartWare sewing ring. Notice the arterial cannula in the transected outflow graft.
With the HeartWare LVAD, the sewing ring can be removed and repaired primarily, or a specially manufactured titanium plug (Fittkau GmbH, Berlin, Germany) can be directly inserted [39, 40]. With the HeartMate II LVAD, a felt plug can be fashioned intra-operatively using a spare sewing ring. This plug is inserted into the attached sewing ring to completely fill the apical defect, thus eliminating the need for extensive ventricular repair and minimizing operative time [41]. We have also wrapped this felt plug with CorMatrix, a biologic material, with the thought of creating a more biocompatible interface. The pump can be removed and the plug inserted on the beating heart or on peripheral cardiopulmonary bypass.
While some might argue that leaving a stiff sewing ring on the apex of the heart will decrease apical involvement in contractile function, most explanted hearts will have a portion of the apex that is either akinetic or hypokinetic, even with a geometric repair. One potential advantage to leaving the sewing ring intact is if the patient requires LVAD reimplantation. In this case, rather than exposing large portions of the apex to replace a sewing ring, the existing plug can be removed and the inflow cannula can be reinserted relatively easily.
Other minimally invasive techniques for VAD removal include leaving a portion or all of the inflow or outflow cannulas/grafts in situ. Here separate small incisions may be utilized to avoid a midline sternotomy [42]. A subxiphoid and separate left and right anterior mini thoracotomies are used to access the VAD, the inflow cannula, and outflow graft. Cardiopulmonary bypass is established using femoral venous cannulation and cannulation of the VAD outflow graft. The inflow VAD cannula and VAD can then be removed. The outflow graft is oversewn near the bypass cannula leaving a small piece of outflow graft in-situ.
Recently, even less invasive approaches for VAD removal have been described. With the HeartMate II LVAD, the inflow graft present in the articulating elbow can be exposed by mini-thorocotomy. This graft can then be clamped, divided, and over sewn. Alternatively, we have used an endovascular stapling device to transect this graft. As such, the entire inflow and outflow components of the device are left in situ [43]. Finally, some have even advocated simply dividing the driveline and leaving the entire pump in situ, ultimately allowing the pump to fully develop a contained thrombus.
Potential complications of leaving portions of the VAD grafts in situ include thromboembolic events or infections. Little data is available regarding the frequency of these complications. Residual graft infections have been described, but overall the limited available reports would suggest that infection or thromboembolic events are uncommon [44]. Outside of a few case reports, the fate of a retained left ventricular inflow cannula, for example, is purely speculative.
Currently, we recommend approaching VAD removal in BTR patients using one of the described minimally invasive techniques to avoid the need for redo sternotomy and potentially avoid the need for cardiopulmonary bypass. This strategy is particularly useful for HeartMate II explants, but potentially more challenging with other durable LVADs. The need for cardiopulmonary bypass should not dissuade attempts at LVAD explantation, as long as the patient is appropriately selected. There is much room for creativity in the surgical approaches for these patients. As our understanding and management of myocardial recovery improves, many of the surgical techniques will continue to evolve.
Post Removal Follow-up & Outcomes
Patients selected for VAD removal should continue to undergo close clinical follow-up, serial cardiac functional imaging, and a structured cardiac rehabilitation program. These individuals remain advanced heart failure patients. Thus, aggressive heart failure medication regimens remain the cornerstone of therapy.
As depicted in Table 1, outcomes after LVAD removal vary greatly. Unfortunately, the cumulative numbers are small, with inconsistent protocols of weaning and imaging. Furthermore, the denominator does not fully represent all of the potential patients screened. Therefore, durability of myocardial recovery facilitated by LVAD unloading remains largely unknown and provides a ripe area for investigation. The RESTAGE-HF trial will likely provide the most uniformly followed post-explant protocol. In this study, medications will be swiftly uptitrated after LVAD removal. Serial echocardiograms will be performed out to 3 years, in addition to other studies including cardiopulmonary stress tests and right heart catheterization as driven by the clinical condition. Ultimately, these protocol-driven studies will give providers a sense of the natural history of these post-explant patients.
Conclusions
While the field of mechanical circulatory support was once singularly focused on how to make a good and lasting heart pump, mechanical issues related to LVADs are now less of a concern. For this field to move forward, a paradigm shift must occur. Myocardial recovery exists, but you have to look for it and strive to obtain it. All of the studies that have demonstrated rates of recovery <2% are in cohorts of patients that are not systemically approached with recovery in mind. In particular, there were no consistent methods to monitor heart function, they lacked protocols for adjuvant HF therapies, and there were no criteria for defining recovery.
Whether it is identifying molecular signatures of recovery, incorporating adjuvant biologic therapies, or designing creative surgical approaches, detailed studies related to reverse remodeling and myocardial recovery are needed. With a better understanding of this biology, all patients will be considered “bridge to removal” candidates, and transplantation and destination therapy will be reserved for those who do not demonstrate evidence of functional recovery despite optimal adjuvant therapy.
Acknowledgements
This work is funded, in part, by the NIH/NHLBI 4R01 HL089592 (CHS), Bridge to Removal (ATS/2014/452136 – R1) - 13 Doris Duke Foundation Clinical Scientist Grant 2013108 (SGD), VA Merit Review Award, 1I01CX000710-01A1 (JS, SGD), Deseret Foundation #00571 (SGD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 2.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 3.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 4.Birks EJ, George RS, Firouzi A, et al. Long-term outcomes of patients bridged to recovery versus patients bridged to transplantation. J Thorac Cardiovasc Surg. 2012;144(1):190–196. doi: 10.1016/j.jtcvs.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Birks EJ, George RS, Hedger M, et al. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: A prospective study. Circulation. 2011;123(4):381–390. doi: 10.1161/CIRCULATIONAHA.109.933960. [DOI] [PubMed] [Google Scholar]
- 6.Birks EJ, Tansley PD, Hardy J, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355(18):1873–1884. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- 7.Dandel M, Weng Y, Siniawski H, et al. Pre-explant stability of unloading-promoted cardiac improvement predicts outcome after weaning from ventricular assist devices. Circulation. 2012;126(11 Suppl 1):S9–S19. doi: 10.1161/CIRCULATIONAHA.111.084640. [DOI] [PubMed] [Google Scholar]
- 8.Dandel M, Weng Y, Siniawski H, Potapov E, Lehmkuhl HB, Hetzer R. Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist devices. Circulation. 2005;112(9 Suppl):I37–I45. doi: 10.1161/CIRCULATIONAHA.104.525352. [DOI] [PubMed] [Google Scholar]
- 9.Kass DA, Baughman KL, Pak PH, et al. Reverse remodeling from cardiomyoplasty in human heart failure. External constraint versus active assist. Circulation. 1995;91(9):2314–2318. doi: 10.1161/01.cir.91.9.2314. [DOI] [PubMed] [Google Scholar]
- 10.Levin HR, Oz MC, Chen JM, Packer M, Rose EA, Burkhoff D. Reversal of chronic ventricular dilation in patients with end-stage cardiomyopathy by prolonged mechanical unloading. Circulation. 1995;91(11):2717–2720. doi: 10.1161/01.cir.91.11.2717. [DOI] [PubMed] [Google Scholar]
- 11.Mann DL, Barger PM, Burkhoff D. Myocardial recovery and the failing heart: Myth, magic, or molecular target? J Am Coll Cardiol. 2012;60(24):2465–2472. doi: 10.1016/j.jacc.2012.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drakos SG, Kfoury AG, Stehlik J, et al. Bridge to recovery: Understanding the disconnect between clinical and biological outcomes. Circulation. 2012;126(2):230–241. doi: 10.1161/CIRCULATIONAHA.111.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth intermacs annual report: Risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32(2):141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein DJ, Maybaum S, MacGillivray TE, et al. Young patients with nonischemic cardiomyopathy have higher likelihood of left ventricular recovery during left ventricular assist device support. Journal of cardiac failure. 2012;18(5):392–395. doi: 10.1016/j.cardfail.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Simon MA, Kormos RL, Murali S, et al. Myocardial recovery using ventricular assist devices: Prevalence, clinical characteristics, and outcomes. Circulation. 2005;112(9 Suppl):I32–I36. doi: 10.1161/CIRCULATIONAHA.104.524124. [DOI] [PubMed] [Google Scholar]
- 16.Dandel M, Knosalla C, Hetzer R. Contribution of ventricular assist devices to the recovery of failing hearts: A review and the berlin heart center experience. European journal of heart failure. 2014;16(3):248–263. doi: 10.1002/ejhf.18. [DOI] [PubMed] [Google Scholar]
- 17.Drakos SG, Wever-Pinzon O, Selzman CH, et al. Magnitude and time course of changes induced by continuous-flow left ventricular assist device unloading in chronic heart failure: Insights into cardiac recovery. J Am Coll Cardiol. 2013;61(19):1985–1994. doi: 10.1016/j.jacc.2013.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George RS, Yacoub MH, Bowles CT, et al. Quality of life after removal of left ventricular assist device for myocardial recovery. J Heart Lung Transplant. 2008;27(2):165–172. doi: 10.1016/j.healun.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 19.Aaronson KD, Pagani FD, Maybaum SW, et al. Combination therapy with pulsatile left ventricular assst device, heart failure medication and clenbuterol in chronic heart failure: Results from harps. J Heart Lung Transplant. 2011;30(4S):S8–S9. [Google Scholar]
- 20.Patel SR, Saeed O, Murthy S, et al. Combining neurohormonal blockade with continuous-flow left ventricular assist device support for myocardial recovery: A single-arm prospective study. J Heart Lung Transplant. 2013;32(3):305–312. doi: 10.1016/j.healun.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Garcia S, Kandar F, Boyle A, et al. Effects of pulsatile- and continuous-flow left ventricular assist devices on left ventricular unloading. J Heart Lung Transplant. 2008;27(3):261–267. doi: 10.1016/j.healun.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Klotz S, Deng MC, Stypmann J, et al. Left ventricular pressure and volume unloading during pulsatile versus nonpulsatile left ventricular assist device support. Ann Thorac Surg. 2004;77(1):143–149. doi: 10.1016/s0003-4975(03)01336-5. [DOI] [PubMed] [Google Scholar]
- 23.Kato TS, Chokshi A, Singh P, et al. Effects of continuous-flow versus pulsatile-flow left ventricular assist devices on myocardial unloading and remodeling. Circulation Heart failure. 2011;4(5):546–553. doi: 10.1161/CIRCHEARTFAILURE.111.962142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krabatsch T, Schweiger M, Dandel M, et al. Is bridge to recovery more likely with pulsatile left ventricular assist devices than with nonpulsatile-flow systems? Ann Thorac Surg. 2011;91(5):1335–1340. doi: 10.1016/j.athoracsur.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 25.Meyns BP, Simon A, Klotz S, et al. Clinical benefits of partial circulatory support in new york heart association class iiib and early class iv patients. Eur J Cardiothorac Surg. 2011;39(5):693–698. doi: 10.1016/j.ejcts.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 26.Mohite PN, Sabashnikov A, Garcia D, Zych B, Simon AR. Staged and effortless explantation of circulite synergy micropump. J Artif Organs. 2014 doi: 10.1007/s10047-014-0763-3. [DOI] [PubMed] [Google Scholar]
- 27.Formica P, Murthy S, Edwards P, Goldstein D, Maybaum S. A structured 3-step approach to evaluate cardiac recovery with continuous flow circulatory support. J Heart Lung Transplant. 2010;29(12):1440–1442. doi: 10.1016/j.healun.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 28.John R, Naka Y, Park SJ, et al. Impact of concurrent surgical valve procedures in patients receiving continuous-flow devices. J Thorac Cardiovasc Surg. 2014;147(2):581–589. doi: 10.1016/j.jtcvs.2013.10.024. discussion 589. [DOI] [PubMed] [Google Scholar]
- 29.Rao V, Slater JP, Edwards NM, Naka Y, Oz MC. Surgical management of valvular disease in patients requiring left ventricular assist device support. Ann Thorac Surg. 2001;71(5):1448–1453. doi: 10.1016/s0003-4975(01)02479-1. [DOI] [PubMed] [Google Scholar]
- 30.Ozturk P, Engin C, Ayik F, et al. Valvular procedures during ventricular assist device implantation. Transplantation proceedings. 2012;44(6):1732–1734. doi: 10.1016/j.transproceed.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 31.Atkins BZ, Hashmi ZA, Ganapathi AM, et al. Surgical correction of aortic valve insufficiency after left ventricular assist device implantation. J Thorac Cardiovasc Surg. 2013;146(5):1247–1252. doi: 10.1016/j.jtcvs.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Rajagopal K, Daneshmand MA, Patel CB, et al. Natural history and clinical effect of aortic valve regurgitation after left ventricular assist device implantation. J Thorac Cardiovasc Surg. 2013;145(5):1373–1379. doi: 10.1016/j.jtcvs.2012.11.066. [DOI] [PubMed] [Google Scholar]
- 33.McKellar SH, Deo S, Daly RC, et al. Durability of central aortic valve closure in patients with continuous flow left ventricular assist devices. J Thorac Cardiovasc Surg. 2014;147(1):344–348. doi: 10.1016/j.jtcvs.2012.09.098. [DOI] [PubMed] [Google Scholar]
- 34.Piacentino V, 3rd, Williams ML, Depp T, et al. Impact of tricuspid valve regurgitation in patients treated with implantable left ventricular assist devices. Ann Thorac Surg. 2011;91(5):1342–1346. doi: 10.1016/j.athoracsur.2011.01.053. discussion 1346–1347. [DOI] [PubMed] [Google Scholar]
- 35.Selzman CH, Bhati RS, Sheridan BC, Stansfield WE, Mill MR. Surgical therapy for heart failure. Journal of the American College of Surgeons. 2006;203(2):226–239. doi: 10.1016/j.jamcollsurg.2006.04.022. quiz A259–260. [DOI] [PubMed] [Google Scholar]
- 36.Kitada S, Kato TS, Thomas SS, et al. Pre-operative echocardiographic features associated with persistent mitral regurgitation after left ventricular assist device implantation. J Heart Lung Transplant. 2013;32(9):897–904. doi: 10.1016/j.healun.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taghavi S, Hamad E, Wilson L, et al. Mitral valve repair at the time of continuous-flow left ventricular assist device implantation confers meaningful decrement in pulmonary vascular resistance. Asaio J. 2013;59(5):469–473. doi: 10.1097/MAT.0b013e31829be026. [DOI] [PubMed] [Google Scholar]
- 38.Russo MJ, Merlo A, Johnson EM, et al. Transapical approach for mitral valve repair during insertion of a left ventricular assist device. The Scientific World Journal. 2013;2013:925310. doi: 10.1155/2013/925310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sajjad M, Butt T, Oezalp F, et al. An alternative approach to explantation and exchange of the heartware left ventricular assist device. Eur J Cardiothorac Surg. 2013;43(6):1247–1250. doi: 10.1093/ejcts/ezs585. [DOI] [PubMed] [Google Scholar]
- 40.Schweiger M, Potapov E, Vierecke J, Stepanenko A, Hetzer R, Krabatsch T. Expeditious and less traumatic explantation of a heartware lvad after myocardial recovery. Asaio J. 2012;58(5):542–544. doi: 10.1097/MAT.0b013e3182640dce. [DOI] [PubMed] [Google Scholar]
- 41.Cohn WE, Gregoric ID, Radovancevic B, Frazier OH. A felt plug simplifies left ventricular assist device removal after successful bridge to recovery. J Heart Lung Transplant. 2007;26(11):1209–1211. doi: 10.1016/j.healun.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 42.Haj-Yahia S, Birks EJ, Dreyfus G, Khaghani A. Limited surgical approach for explanting the heartmate ii left ventricular assist device after myocardial recovery. J Thorac Cardiovasc Surg. 2008;135(2):453–454. doi: 10.1016/j.jtcvs.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Stawinski GV, Mountis MM, Cohn WE, Frazier OH. Inflow graft interruption as a simple method for left ventricular assist device removal after successful bridge to recovery. J Card Surg. 2012;27(3):397–399. doi: 10.1111/j.1540-8191.2012.01447.x. [DOI] [PubMed] [Google Scholar]
- 44.Cohn WE, Fikfak V, Gregoric ID, Frazier OH. Retention of left ventricular assist device outflow grafts after transplantation. J Heart Lung Transplant. 2008;27(8):865–868. doi: 10.1016/j.healun.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 45.Maybaum S, Mancini D, Xydas S, et al. Cardiac improvement during mechanical circulatory support: A prospective multicenter study of the lvad working group. Circulation. 2007;115(19):2497–2505. doi: 10.1161/CIRCULATIONAHA.106.633180. [DOI] [PubMed] [Google Scholar]
- 46.Dandel M, Weng Y, Siniawski H, et al. Prediction of cardiac stability after weaning from left ventricular assist devices in patients with idiopathic dilated cardiomyopathy. Circulation. 2008;118(14 Suppl):S94–S105. doi: 10.1161/CIRCULATIONAHA.107.755983. [DOI] [PubMed] [Google Scholar]
- 47.Dandel M, Weng Y, Siniawski H, et al. Heart failure reversal by ventricular unloading in patients with chronic cardiomyopathy: Criteria for weaning from ventricular assist devices. European heart journal. 2011;32(9):1148–1160. doi: 10.1093/eurheartj/ehq353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drakos SG, Terrovitis JV, Anastasiou-Nana MI, Nanas JN. Reverse remodeling during long-term mechanical unloading of the left ventricle. Journal of molecular and cellular cardiology. 2007;43(3):231–242. doi: 10.1016/j.yjmcc.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 49.Lamarche Y, Kearns M, Josan K, et al. Successful weaning and explantation of the heartmate ii left ventricular assist device. The Canadian journal of cardiology. 2011;27(3):358–362. doi: 10.1016/j.cjca.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Liden H, Karason K, Bergh CH, Nilsson F, Koul B, Wiklund L. The feasibility of left ventricular mechanical support as a bridge to cardiac recovery. European journal of heart failure. 2007;9(5):525–530. doi: 10.1016/j.ejheart.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Gorcsan J, 3rd, Severyn D, Murali S, Kormos RL. Non-invasive assessment of myocardial recovery on chronic left ventricular assist device: Results associated with successful device removal. J Heart Lung Transplant. 2003;22(12):1304–1313. doi: 10.1016/s1053-2498(03)00056-1. [DOI] [PubMed] [Google Scholar]
- 52.Matsumiya G, Monta O, Fukushima N, et al. Who would be a candidate for bridge to recovery during prolonged mechanical left ventricular support in idiopathic dilated cardiomyopathy? J Thorac Cardiovasc Surg. 2005;130(3):699–704. doi: 10.1016/j.jtcvs.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 53.Simon MA, Primack BA, Teuteberg J, et al. Left ventricular remodeling and myocardial recovery on mechanical circulatory support. Journal of cardiac failure. 2010;16(2):99–105. doi: 10.1016/j.cardfail.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farrar DJ, Holman WR, McBride LR, et al. Long-term follow-up of thoratec ventricular assist device bridge-to-recovery patients successfully removed from support after recovery of ventricular function. J Heart Lung Transplant. 2002;21(5):516–521. doi: 10.1016/s1053-2498(01)00408-9. [DOI] [PubMed] [Google Scholar]
- 55.Mancini DM, Beniaminovitz A, Levin H, et al. Low incidence of myocardial recovery after left ventricular assist device implantation in patients with chronic heart failure. Circulation. 1998;98(22):2383–2389. doi: 10.1161/01.cir.98.22.2383. [DOI] [PubMed] [Google Scholar]