Abstract
Aldosterone exerts its best known sodium homeostasis actions by controlling sodium excretion at the level of the distal tubules via activation of the apical epithelial sodium channel (ENaC) and the basolateral Na+/K+ ATPase pump. Recently, this mineralocorticoid hormone has been demonstrated to act on the heart and blood vessels. Excess release of aldosterone in relation to the salt status induces both genomic and non-genomic effects that by promoting endothelial dysfunction, and vascular and cardio-renal adverse remodeling, contribute to the target organ damage found in hypertension, heart failure, myocardial infarction and chronic renal failure. Mineralocorticoid receptor blockers have been shown to be highly effective in resistant hypertension and to slow down heart failure progression, and in experimental animals, the development of atherosclerosis. Blockade of the action of aldosterone and potentially other mineralocorticoid steroids has been increasingly demonstrated to be an extremely beneficial therapy in different forms of cardiovascular disease. This review provides a summary of the knowledge that exists regarding aldosterone actions in the cardiovascular system and, in providing the translational impact of this knowledge to the clinical arena, illustrates how much more needs to be achieved in exploring the use of mineralocorticoid receptor blockers in less advanced stages of heart, renal, and vascular disease.
Keywords: Hypertension, heart failure, chronic renal failure
INTRODUCTION
The renin-angiotensin-aldosterone (RAAS) system is a powerful hormone regulatory mechanism serving multiple functions as a key determinant of tissue perfusion pressure and cellular homeostasis. Since the original demonstration of renin in renal extracts characterization of the biochemical physiology and mechanisms of action of this system has become one of the major accomplishments of the biomedical research enterprise. It is also a fruitful example of translational medicine, with major impact on the therapy of cardiovascular disease as a consequence of the development of drugs that inhibit the pathological actions of angiotensin II (Ang II) and its interplay with the adrenocortical steroid aldosterone.
The knowledge of how the RAAS functions has taken diverse routes that entail exploring the pathways by which Ang II is produced in blood and tissues, the functions and signaling mechanisms for the action of the hormone, and its link to the processes by which aldosterone is formed and acts through its interplay with Ang II and the neuro-hormonal mechanisms regulating salt and water intake. The translational lessons derived from the original reports of Ang II and aldosterone biosynthesis embodies an extensive medical literature, the highlights of which resulted in the development of angiotensin converting enzyme (ACE) inhibitors, Ang II receptor antagonists (ARBs), direct renin inhibitors (DRI), and mineralocorticoid receptor (MR) antagonists. It would be a futile effort to detail accurately the complete history of these accomplishments, as they constitute a body of literature of several hundred papers and more than one hundred years of exemplary discoveries. This review provides a succinct summary of the translational therapeutic outcomes for aldosterone and Ang II given their key interplay in cardiovascular regulation through a narrative synthesis of accomplishments and the inclusion of the timeline of discoveries documented in Table 1.
Table 1.
| Year | Discovery or Major Basic Research Accomplishment |
|---|---|
| 1563 | Bartolomeo Eustacchio description of the adrenal gland (glandulae Renibus incumentes). 108 |
| 1855 | Addison’s first identification of the lethal association of pathological changes in the suprarenal capsules (adrenal glands) with anemia. 109 |
| 1935 | Development of biologically active extracts free of contaminating compounds from the adrenal medulla. |
| 1939 | Kuizinga and Cartland 110 show potent mineralocorticoid activity in the amorphus fraction of adrenocortical preparations. |
| 1952–1955 | Simpson and Tait 111–115 critical studies of adrenal mineralocorticoid activity culminate with the isolation of electrocortin, later rename as aldosterone. 116 |
| 1954 | Aldosterone structure identified as 11_-21-dihydroxy-18-oxo-pregn-4-ene-3,20-dione 113, 116 |
| 1956 | Luetscher et al. 5 obtain aldosterone crystals from human urine. |
| 1955–1956 | Aldosterone is synthetized by the Ciba group in Basel117, 118. |
| 1956 | Giroud et al. 119, 120 in Montreal, demonstrated that the zona glomerulosa of the rat exclusively produced aldosterone. |
| Clinical Translational Research | |
| 1955 | First description of the primary aldosteronism syndrome 63. |
| 1999 | The Randomized ALdactone Evaluation Study (RALES) trial demonstrates the role for aldosterone antagonists in chronic severe (NYHA class III/IV) systolic HF 90. |
| 2003 | The Eplerenone Post-myocardial infarction Heart failure Efficacy and Survival Study (EPHESUS) documents the benefit of aldosterone receptor antagonists in patients with an EF <40% after MI 91. |
| 2014 | TOPCAT shows moderate effects of spironolactone in heart failure with preserved ejection fraction (HFpEF) since only hospitalization for heart failure benefited in this population from MR antagonism 101. |
Aldosterone Biosynthesis and Functions
The isolation of aldosterone can be traced back to the early 1930s when researchers succeeded in obtaining biologically active extracts free of contaminating compounds from the adrenal medulla. As narrated by Tai et al. 1, this work was accomplished by several teams of investigators working at Columbia University 2, the Mayo Clinic 3 and the Ciba group in Basel 4. Aldosterone crystals were obtained first from human urine collected from two patients with congestive heart failure (HF) 5. Table 1 documents the evolution of the research leading to the final synthesis of aldosterone by the Ciba group of investigators in 1955. Whereas the clinical role of aldosterone in disease processes continues to expand, the classification provided by Genest, Kuchel, and Nowacynski (1973) (Table 2) summarizes the involvement of this adrenal hormone in human hypertension 6.
Table 2.
Adrenal Steroids in Hypertension
| 1. Hypertension caused by overproduction of mineralocorticoid hormones |
| Primary Aldosteronism (Conn’s syndrome) |
| 17-alpha-hydroxylase deficiency (Biglieri’s syndrome) |
| Virilizing adrenal hyperplasia due to 11-beta hydroxylase deficiency |
| Cushing’s syndrome |
| Non-endocrine ACTH producing tumors |
| Adrenocortical carcinoma |
| Iatrogenic (i.e., anovulatory pills, licorice, glucocorticoids, excess salt intake) |
| 2. Hypertension associated with overproduction of mineralocorticoids |
| Malignant hypertension |
| Hypertension in terminal renal failure |
| True renovascular hypertension |
| Essential hypertension |
| Liddle’s syndrome |
| 3. Congenital enzymatic defects associated with hypertension |
| 17-alpha hydroxylase deficiency |
| 11-beta hydroxylase deficiency |
Adapted from reference 6.
Normal adrenal steroidogenesis leads to the production of glucocorticoids, mineralocorticoids, and sex hormones. While glucocorticoid secretion is regulated primarily by pituitary ACTH, Ang II acts as the primary stimulus for aldosterone secretion 7. The mechanisms by which aldosterone interplays with the regulation of blood pressure and the control of renal salt excretion (Figure 1) are mediated by the MR, a ligand-activated transcription factor that is a member of the nuclear receptor superfamily 8. The relatively slow genomic aldosterone actions influence renal gene expression of proteins that regulate Na+/K+ ATPase activity and the renal epithelial sodium and outer medullary potassium channels 9. Of importance, aldosterone has a significant influence in the activation of genes participating in the genesis of adverse cardiac and vascular remodeling. Aldosterone can exert direct vasoconstrictor actions 10, 11 and in normal healthy volunteers the effects of aldosterone in forearm blood flow were dose-dependent 12. In vascular smooth muscle cells (VSMCs), aldosterone induces a proliferative response through increased expression of p53 binding protein 13. Wehling and colleagues 14–16 and Rautureau et al. 8 provide a comprehensive discussion of aldosterone non-genomic effects, which are in the order of minutes rather than hours 17, 18. In addition, aldosterone can upregulate vascular angiotensin receptors 19, a finding that is critically important in understanding vasoconstrictor and inflammatory mechanisms of the interplay among Ang II, aldosterone, and salt intake. Collagen deposition in the vasculature leading to vascular stiffness was prevented in VSMCs from mice with conditional inactivation of the MR 20. The authors concluded that the MR influences vascular elasticity by modulation of cell-matrix attachment proteins independent of major vascular structural changes 20.
Figure 1.
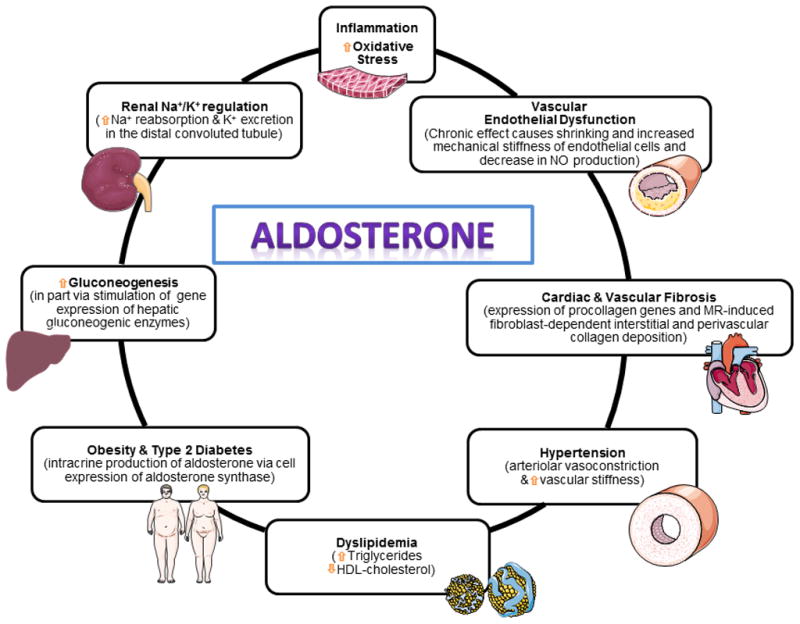
Pleotropic aldosterone actions contributing to the pathogenesis of cardiovascular disease.
The interactions between MRs and angiotensin receptors appear to explain many of the effects found when blocking either of these receptors 21. These signaling pathways are important in explaining the mechanisms by which MRs induce cardiac and vascular collagen deposition, activation of pro-inflammatory cytokines, and increases in oxidative stress 8, 22–27. Recently, evidence has been produced of a role of placental growth factor in the mediation of aldosterone action 28. Furthermore, aldosterone proatherogenic effects may in part be mediated by stimulation of placental growth factor 29. The effects on atherosclerosis are mediated via MRs as demonstrated in mice with deletion of smooth muscle MRs 20 or administration of MR antagonists 30. Furthermore, the aldosterone contribution to atherosclerosis progression may include facilitation of aortic aneurysm formation 31.
Atherogenic effects of aldosterone may relate to its role in inflammation, stimulation of oxidative stress, endothelial dysfunction, and prothrombotic activity 26, 32, 33. Aldosterone, in association with inflammatory mediators such as interferon-γ (IFN-γ) and tumour necrosis factor α (TNF-α), stimulate growth and proliferation of VSMCs, leading to adverse vascular remodeling 34. Moreover, new fascinating data shows prevention of vascular and renal inflammatory responses following administration of T-regulatory lymphocytes both to Ang II-infused 35 and to aldosterone-infused mice 23, 34. The finding that Ang II functions as an obligatory mediator pathway for the formation of aortic fatty streaks in the presence of hypercholesterolemia and no changes in arterial pressure was first demonstrated in monkeys 36. These findings led to the hypothesis that the RAAS is a critical contributor to the pathogenesis of atherosclerosis through stimulation of oxidative stress, inflammatory cytokines, and recruitment of monocytes into the subendothelial vascular spaces 36–39. Proatherogenic aldosterone mechanism occurs via induction of oxidative stress, endothelial dysfunction, activation of adhesion molecules, and up-regulation of ACE and AT1 receptors (see 27 for review). Atherogenic actions of aldosterone can be prevented by eplerenone administration 40–42. In primary aldosteronism, plasma osteopontin (OPN) levels are higher than those in essential hypertensive subjects, and in OPN-knockout mice reduced renal interstitial fibrosis is induced by aldosterone infusions 43, 44. An intriguing study showed that aldosterone can stimulate the release of Willebrand factor and IL-8 via exocytosis of Weibel–Palade bodies 45. This non-genomic effect is a potentially powerful mechanism for the activation of proinflammatory factors. Prothrombotic aldosterone actions include stimulation of plasminogen activator inhibitor-1 (PAI-1), a finding that may contribute to extracellular collagen deposition as PAI-1 inhibits the production of plasmin from plasminogen 46. The opportunity to advance the use of MR antagonists in prevention or management of coronary heart disease is a tantalizing proposition as mortality due to myocardial infarction worsens in the presence of hyperaldosteronism 47–50.
Adrenal glands contain the genes and proteins for local tissue production of bioactive angiotensins 51. Cells in the zona glomerulosa contain the highest levels of renin activity and Ang II. The independence of this tissue system from that of the circulation or the kidney is based on the demonstration that renin activity is not suppressed in anephric rats, while increases in adrenal renin and aldosterone are independent of plasma or kidney renin concentrations 52–54. The role of this adrenal RAAS remains to be elucidated. Nevertheless, the interdependent nature of the interplay between aldosterone and Ang II in the regulation of cardiovascular function entails both a stimulatory action of the peptide in the systemic release of aldosterone and the ability of aldosterone to increase the expression of angiotensin receptors in vascular smooth muscle cells 55. In VSMCs intracellular mobilization of calcium (Ca2+) by Ang II is augmented in the presence of aldosterone via increased expression of extracellular-signal-regulated kinase (ERK1/2) and c-Jun N-terminal kinase (JNK) pathways 15, 56. Moreover, eplerenone blocked mitogen-activated protein kinase (MAPK) activation, radical oxygen species (ROS) generation, and epidermal growth factor receptor (EGFR) transactivation 57. The aldosterone-induced activation of ERK1/2, JNK and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) has been reported to be dependent on the transactivation of AT1a but not AT1b receptors 8, 21. This interpretation agrees with two published studies showing that: a)- eplerenone inhibits aldosterone vasoconstrictor actions and non-genomic effects on the sodium-proton exchanger (NHE1) activity or intracellular Ca2+ responses of rat mesenteric vessels 58; and b)- aldosterone-mediated mesenteric artery vasoconstriction are blunted in AT1a knockout mouse 8, 21, 59.
Mice in which a VSMC-specific deletion of MR (VSMC-MR) has been induced have lower blood pressure as they age 60. This occurs despite the absence of changes in sodium excretion, indicating that the kidney effects of aldosterone do not participate in these effects. There is also absence of vascular remodeling. Interestingly, the aged VSMC-MR mice exhibited a reduction in vascular myogenic tone and Ang II-dependent contraction, and this was associated with reduced expression and activity of L-type Ca2+ channels. Changes in myogenic tone appeared to be related to reduce density of the L-type Ca2+ channel Cav1.2. Thus, VSMC-MR seems to contribute to Ang II–induced vascular oxidative stress, contraction, and elevated blood pressure, and vascular MR-mediated vascular tone may participate in control of blood pressure as well as vascular aging 60.
Salt excess appears to be important for aldosterone to exert its hypertensive action in rodents, especially in rats 61, 62, whereas in mice even in the presence of salt, and despite powerful pro-inflammatory actions, aldosterone exerts little effect on blood pressure 23. The reason for this difference is unclear. However, the effect of salt on aldosterone effects may explain that in essential hypertensive patients with stage 1 hypertension, blockade of MR has a mild antihypertensive action, whereas in uncontrolled or in resistant hypertension, often associated with excess consumption of salt, the effects of MR blockade are much more powerful. Salt consumption by hypertensive and HF patients probably is a confounder of the efficacy of MR blockade in clinical trials.
Translational Correlates
Dysregulation of MR participates in the pathogenesis of cardiovascular disease either via amplification of the proliferative, pro-inflammatory and prothrombotic effects of excess Ang II levels or excess secretion of aldosterone. Knowledge of the direct effects of hyperaldosteronism on cardiovascular function resulted from Conn’s report of the association of high blood pressure and hypokalemia in a woman with a diagnosis of primary aldosteronism 63, 64. However, as early as 1937, Steigler and Reichstein 65 had reported a syndrome of hypokalemia and hypertension associated with the administration of synthetized deoxycorticosterone. A syndrome resembling primary aldosteronism without elevated aldosterone levels is associated with inhibition of 11-β-hydroxysteroid dehydrogenase enzyme (11-β-OHSD) 66–68. This apparent mineralocorticoid excess process results from increased binding of cortisol to MR since the 11-β-OHSD accounts for the conversion of cortisol to cortisone. Nevertheless, the contribution of aldosterone to cardiovascular pathology remained elusive until the publication of a sensitive radioimmunoassay 69 and a more comprehensive understanding of its role in the control of electrolyte homeostasis 70. Availability of a measure of aldosterone activity provided a tool to seek the contribution of this hormone to the pathophysiology of essential hypertension, an effort that led to identification of subsets of hypertensive subjects where aldosterone was associated with sodium retention and hypertension. Williams and Hollenberg 71, 72 characterized a subset of essential hypertensive patients that they have named as “non-modulators”. These individuals are now recognized to associate hypertension with salt-sensitivity, insulin resistance, and RAAS gene polymorphisms 73–75. A state of hyperaldosteronism is also found in diseases associated with important alterations in body fluid volumes, particularly HF, cirrhosis accompanied by ascites, and the nephrotic syndrome 51.
Although most studies in hypertensive subjects have not demonstrated elevations of plasma aldosterone except in few patients 76, quartiles of plasma levels of aldosterone in the Framingham study were directly associated with blood pressure levels 77. Moreover, treatment with MR blockers lowers blood pressure in patients with hypertension 78, and particularly in resistant hypertension 79. The MR blocker spironolactone prevented vascular fibrosis independently of blood pressure reduction in spontaneously hypertensive rats 80–82. The more selective but less potent MR blocker eplerenone also reduces small artery fibrosis and stiffness in stage 1 essential hypertension, possibly contributing to the antihypertensive actions of MR antagonism as well as improvement of tissue perfusion 78. Spironolactone has also been shown to block some of the effects of Ang II 82, indicating that aldosterone mediates some actions often attributed to direct effects of the peptide. The effects with MR blockers are mimicked by aldosterone synthase inhibitors, indicating that spironolactone and eplerenone effects are probably mediated by blockade of aldosterone actions 83.
The important role of aldosterone in the regulation of sodium and potassium balance led to the exploration of the potential clinical benefits that could be derived from blocking the MR. The synthesis of the first MR antagonist (spironolactone) by the chemists at Searle laboratories lagged for almost 30 years behind the identification of aldosterone 84–87. As commented by Delyani, 88, 89 the drug was initially labelled as a potassium-sparing diuretic given that at that time the known aldosterone actions were limited to epithelial ion transport. Spironolactone (7-acetate of the γ-lactone of 17-hydroxy-7-mercapto-3-oxo-17-α-pregn-4-ene-21-carboxylic acid) acts as a competitive antagonist of the MR (but also other steroid receptors such as the androgen receptor, leading to both its adverse side effects and also some additional therapeutic indications as a consequence of its antiandrogenic effects). The drug is rapidly cleared from the circulation (T1/2 10 minutes) to two principal metabolites (canrenoate and canrenone) 86, 87, 89.
The most compelling results for the participation of aldosterone in the pathogenesis of cardiovascular disease derived from the use of MR antagonists in HF. The rationale for the exploration of blocking MRs in HF was based on the observation that ACE inhibitors had only a transient Ang II suppression action and that the same could be demonstrated for plasma aldosterone 89. It was therefore reasoned that addition of spironolactone on top of background ACE therapy could have a greater impact on the progression of HF. The Randomized ALdactone Evaluation Study (RALES) trial established the benefits of aldosterone antagonists in the evolution of severe HF (NYHA class III/IV) 90. In this trial, 1,663 subjects with a diagnosis of severe heart failure (ejection fraction < 35%) were randomized to conventional therapy (ACE inhibitor, loop diuretic and digoxin in most subjects) plus placebo or the combined use of conventional therapy plus spironolactone (25 mg/day). The trial was interrupted after a mean follow-up period of 24 months because an interim analysis showed a significant 35% reduction in all causes of death in the spironolactone-treated group compared to the placebo medicated subjects 90. The beneficial effects of spironolactone were associated in this trial with significant reductions in the frequency of hospitalization for worsening HF and HF symptomatology. Since the greatest benefits were observed in subjects in whom spironolactone was administered on top of background ACE inhibitor therapy, it is clear that combined suppression of Ang II formation and aldosterone production was enhanced through blockade of the MR.
The pleotropic actions of aldosterone on the cardiovascular system, particularly the aldosterone-mediated collagen synthesis that contributes to adverse left ventricular remodeling, prompted an examination of the action of MR antagonists in subjects’ post-myocardial infarction. The trial was in part based on finding that adrenalectomy or eplerenone attenuated the adverse cardiac remodeling in an Ang II model of experimental hypertension 62. The Eplerenone Post-myocardial infarction Heart failure Efficacy and Survival Study (EPHESUS) determined the effect of the MR antagonist eplerenone on morbidity and mortality among patients with acute myocardial infarction 91. Eplerenone (pregn-4-ene-7,21-dicarboxylic acid, 9,11-epoxy-17-hydroxy-3-oxo, γ-lactone, methyl ester) is a selective MR antagonist devoid of the progestational and anti-androgenic side effects of spironolactone and shows minimal activity on P450 enzymes. During a mean 16-month follow-up, patients with acute myocardial infarction complicated by left ventricular dysfunction and HF assigned to eplerenone and associated optimal medical therapy demonstrated significantly reduced morbidity and mortality 91. The beneficial effect of aldosterone blockade in the evolution of HF can be shown even in those subjects with milder forms of the syndrome. This was found in another trial that recruited NYHA class II systolic HF subjects. Patients enrolled in the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) study showed a reduced rate of hospitalization and death when treated with eplerenone 92, 93.
The landmark studies using MR antagonists in HF have in part overshadowed the potential use of these agents in a broader patient population, such as individuals with cardiac arrhythmias, myocardial ischemia secondary to cardiac fibrosis, and diastolic dysfunction 94. Spironolactone can prevent cardiac collagen deposition in two experimental rat models of hypertension independent of the drug effect on blood pressure and cardiac hypertrophy 94, 95. Translational outcomes of these experimental studies are contained in a study where spironolactone improved echocardiographic measurements of myocardial relaxation in patients with exertional dyspnea and abnormal left ventricular filling patterns 96. These important findings need further confirmation as diastolic dysfunction associated with heart failure and preserved ejection fraction (HFpEF) has no known effective treatment 97, 98. The Trial of Aldosterone Antagonist Therapy in Adults with Preserved Ejection Fraction Congestive Heart (TOPCAT) has addressed the potential benefit of spironolactone on 3,445 patients with symptomatic heart failure and HFpEF 99, 100. However, results have been disappointing, since only hospitalization for heart failure was reduced by MR antagonism, whereas other endpoints such as the primary composite outcome of death from cardiovascular causes, aborted cardiac arrest, or hospitalization for the management of HF did not differ statistically with placebo 101.
Other areas where additional research is needed relate to the observation that spironolactone suppresses QT-dispersion 102 and sudden death in subjects that were enrolled in the RALES and EPHESUS trials. It is not known whether these antiarrhythmic actions of aldosterone antagonism are related to conservation of total body potassium and magnesium or reversal of cardiac fibrosis. An attractive alternative hypothesis for the antiarrhythmic effects of MR antagonists has been provided by studies using mice with conditional cardiac-specific overexpression of the human MR 103. In this experimental model, prolongation of ventricular repolarization and presence of severe ventricular arrhythmias due to overexpression of the human MR were prevented by spironolactone administration 103. Additionally, aldosterone arrhythmogenic actions may relate to myocyte gap junction remodeling through its dual effects on the expression of connexin 43 (Cx43) 42, 104. This is clearly an area of potential fruitful research.
Summary
Aldosterone’s main physiological function maintains sodium homeostasis through its direct action on controlling sodium excretion at the level of the distal tubules via activation of the apical epithelial sodium channel (ENaC) and the basolateral Na+/K+ ATPase pump 105. An excess production/release of aldosterone in relation to the salt status induces a genomic and non-genomic effect that in experimental animals leads to vascular remodeling, endothelial dysfunction and cardio-renal adverse remodeling including the structural and functional consequences of increased collagen deposition. In humans, the non-genomic actions of aldosterone through MR are somewhat more varied as vasoconstriction, vasodilatation or no effects have been observed 106. Amphibaric actions of aldosterone in humans may be related to doses employed, the baseline plasma aldosterone levels 107, as well as salt status. The more chronic effects of aldosterone play a critical role in contributing to target organ damage associated with hypertension, HF, myocardial infarction and chronic renal failure 105. MR antagonists are powerful treatment agents having a marked beneficial effect in HF progression and resistant hypertension, and in experimental animals, in atherosclerosis. The pleotropic actions of this mineralocorticoid hormone in human primary essential hypertension and the cardiometabolic syndrome await further investigation as emerging evidence demonstrates that the use of MR antagonists may be associated with potentially impressive outcomes 22,25.
Acknowledgments
The authors recognize the kind and valuable contribution of Jessica VonCannon, B.S. in proofreading the manuscript.
Sources of Funding
The work of ELS was supported by Canadian Institutes of Health Research (CIHR) grants 37917, 82790, and 102606, a Canada Research Chair on Hypertension and Vascular Research from CIHR/Government of Canada, and the Canada Fund for Innovation. The work of CMF is supported by grant HL-051952 from the National Heart, Lung and Blood Institute of the NIH.
Abbreviations
- 11-β-OHSD
11-β-hydroxysteroid dehydrogenase enzyme
- ACE
angiotensin converting enzyme
- Ang II
angiotensin II
- ARBs
angiotensin II receptor blockers
- Ca2+
calcium ion
- Cx43
connexin 43
- DRI
direct renin inhibitors
- EGFR
epidermal growth factor receptor
- ENaC
epithelial sodium channel
- ERK
extracellular signal-regulated kinase
- FMD
flow-mediated vasodilation
- HF
heart failure
- HFpEF
heart failure and preserved ejection fraction
- IFN-γ
interferon γ
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MR
mineralocorticoid receptor
- NHE-1
sodium/hydrogen exchanger 1
- NT
nitrotyrosine
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- Na+/K+ ATPase
sodium-potassium adenosine triphosphatase pump
- OPN
osteopontin
- RAAS
renin angiotensin aldosterone system
- TNF-α
tumor necrosis factor α
- VSMC
vascular smooth muscle cell
Footnotes
Disclosures
CM Ferrario reports that there are no disclosures to be documented. EL Schiffrin reports that there are no disclosures to be documented.
References
- 1.Tait SA, Tait JF, Coghlan JP. The discovery, isolation and identification of aldosterone: reflections on emerging regulation and function. Mol Cell Endocrinol. 2004;217:1–21. doi: 10.1016/j.mce.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Wintersteiner O, Vars HM, Pfiffner JJ. Chemical investigations of the cortical hormone of the adrenal gland. J Biol Chem. 1934;105:100–101. [Google Scholar]
- 3.Mason H, Myers CS, Kendall EC. The chemistry of crystalline substances isolated from the suprarenal gland. J Biol Chem. 1936;114:613–631. [Google Scholar]
- 4.Reichstein T. Uber cortin, da hormon der nebbennieren rinde (X. I. Mitteilung. Helv Chim Acta. 1936;19:29–63. [Google Scholar]
- 5.Luetscher JA, Jr, Neher R, Wettstein A. Isolation of crystalline aldosterone from the urine of patients with congestive heart failure. Experientia. 1956;12:22–23. doi: 10.1007/BF02156988. [DOI] [PubMed] [Google Scholar]
- 6.Genest J, Kuchel O, Nowaczynsli W. Classification of Hypermineralocorticoid Hypertension. In: Onesti G, Kim KI, Moyer JH, editors. Hypertension, Mechanisms and Management. New York and London: Grune & Stratton, Inc; 1973. pp. 463–470. [Google Scholar]
- 7.Funder JW. Aldosterone action. Annu Rev Physiol. 1993;55:115–130. doi: 10.1146/annurev.ph.55.030193.000555. [DOI] [PubMed] [Google Scholar]
- 8.Rautureau Y, Paradis P, Schiffrin EL. Cross-talk between aldosterone and angiotensin signaling in vascular smooth muscle cells. Steroids. 2011;76:834–839. doi: 10.1016/j.steroids.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Viengchareun S, Le MD, Martinerie L, Munier M, Pascual-Le TL, Lombes M. The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl Recept Signal. 2007;5:e012. doi: 10.1621/nrs.05012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman RD, Gros R. Vascular effects of aldosterone: sorting out the receptors and the ligands. Clin Exp Pharmacol Physiol. 2013;40:916–921. doi: 10.1111/1440-1681.12157. [DOI] [PubMed] [Google Scholar]
- 11.Romagni P, Rossi F, Guerrini L, Quirini C, Santiemma V. Aldosterone induces contraction of the resistance arteries in man. Atherosclerosis. 2003;166:345–349. doi: 10.1016/s0021-9150(02)00363-5. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt BM, Montealegre A, Janson CP, Martin N, Stein-Kemmesies C, Scherhag A, Feuring M, Christ M, Wehling M. Short term cardiovascular effects of aldosterone in healthy male volunteers. J Clin Endocrinol Metab. 1999;84:3528–3533. doi: 10.1210/jcem.84.10.6020. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96:643–650. doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- 14.Losel R, Feuring M, Wehling M. Non-genomic aldosterone action: from the cell membrane to human physiology. J Steroid Biochem Mol Biol. 2002;83:167–171. doi: 10.1016/s0960-0760(02)00250-9. [DOI] [PubMed] [Google Scholar]
- 15.Losel R, Schultz A, Boldyreff B, Wehling M. Rapid effects of aldosterone on vascular cells: clinical implications. Steroids. 2004;69:575–578. doi: 10.1016/j.steroids.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Wehling M. Specific, nongenomic actions of steroid hormones. Annu Rev Physiol. 1997;59:365–393. doi: 10.1146/annurev.physiol.59.1.365. [DOI] [PubMed] [Google Scholar]
- 17.Coutinho P, Vega C, Pojoga LH, Rivera A, Prado GN, Yao TM, Adler G, Torres-Grajales M, Maldonado ER, Ramos-Rivera A, Williams JS, Williams G, Romero JR. Aldosterone’s rapid, nongenomic effects are mediated by striatin: a modulator of aldosterone’s effect on estrogen action. Endocrinology. 2014;155:2233–2243. doi: 10.1210/en.2013-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funder JW. Mineralocorticoids, glucocorticoids, receptors and response elements. Science. 1993;259:1132–1133. doi: 10.1126/science.8382375. [DOI] [PubMed] [Google Scholar]
- 19.Schiffrin EL, Franks DJ, Gutkowska J. Effect of aldosterone on vascular angiotensin II receptors in the rat. Can J Physiol Pharmacol. 1985;63:1522–1527. doi: 10.1139/y85-250. [DOI] [PubMed] [Google Scholar]
- 20.Galmiche G, Pizard A, Gueret A, El MS, Ouvrard-Pascaud A, Berger S, Challande P, Jaffe IZ, Labat C, Lacolley P, Jaisser F. Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension. 2014;63:520–526. doi: 10.1161/HYPERTENSIONAHA.113.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemarie CA, Simeone SM, Nikonova A, Ebrahimian T, Deschenes ME, Coffman TM, Paradis P, Schiffrin EL. Aldosterone-induced activation of signaling pathways requires activity of angiotensin type 1a receptors. Circ Res. 2009;105:852–859. doi: 10.1161/CIRCRESAHA.109.196576. [DOI] [PubMed] [Google Scholar]
- 22.Briet M, Schiffrin EL. Vascular actions of aldosterone. J Vasc Res. 2013;50:89–99. doi: 10.1159/000345243. [DOI] [PubMed] [Google Scholar]
- 23.Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension. 2012;59:324–330. doi: 10.1161/HYPERTENSIONAHA.111.181123. [DOI] [PubMed] [Google Scholar]
- 24.Leibovitz E, Ebrahimian T, Paradis P, Schiffrin EL. Aldosterone induces arterial stiffness in absence of oxidative stress and endothelial dysfunction. J Hypertens. 2009;27:2192–2200. doi: 10.1097/HJH.0b013e328330a963. [DOI] [PubMed] [Google Scholar]
- 25.McGraw AP, McCurley A, Preston IR, Jaffe IZ. Mineralocorticoid receptors in vascular disease: connecting molecular pathways to clinical implications. Curr Atheroscler Rep. 2013;15:340–350. doi: 10.1007/s11883-013-0340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiffrin EL. Beyond blood pressure: the endothelium and atherosclerosis progression. Am J Hypertens. 2002;15(10 Pt 2):115S–122S. doi: 10.1016/s0895-7061(02)03006-6. [DOI] [PubMed] [Google Scholar]
- 27.Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47:312–318. doi: 10.1161/01.HYP.0000201443.63240.a7. [DOI] [PubMed] [Google Scholar]
- 28.Jaffe IZ, Newfell BG, Aronovitz M, Mohammad NN, McGraw AP, Perreault RE, Carmeliet P, Ehsan A, Mendelsohn ME. Placental growth factor mediates aldosterone-dependent vascular injury in mice. J Clin Invest. 2010;120:3891–3900. doi: 10.1172/JCI40205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGraw AP, Bagley J, Chen WS, Galayda C, Nickerson H, Armani A, Caprio M, Carmeliet P, Jaffe IZ. Aldosterone increases early atherosclerosis and promotes plaque inflammation through a placental growth factor-dependent mechanism. J Am Heart Assoc. 2013;2:e000018. doi: 10.1161/JAHA.112.000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takai S, Jin D, Muramatsu M, Kirimura K, Sakonjo H, Miyazaki M. Eplerenone inhibits atherosclerosis in nonhuman primates. Hypertension. 2005;46:1135–1139. doi: 10.1161/01.HYP.0000184640.81730.22. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Xie Z, Daugherty A, Cassis LA, Pearson KJ, Gong MC, Guo Z. Mineralocorticoid receptor agonists induce mouse aortic aneurysm formation and rupture in the presence of high salt. Arterioscler Thromb Vasc Biol. 2013;33:1568–1579. doi: 10.1161/ATVBAHA.112.300820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiffrin EL. The many targets of aldosterone. Hypertension. 2004;43:938–940. doi: 10.1161/01.HYP.0000123573.60340.9b. [DOI] [PubMed] [Google Scholar]
- 33.Schiffrin EL. Immune modulation of resistance artery remodelling. Basic Clin Pharmacol Toxicol. 2012;110:70–72. doi: 10.1111/j.1742-7843.2011.00760.x. [DOI] [PubMed] [Google Scholar]
- 34.Idris-Khodja N, Mian MOR, Paradis P, Schiffrin EL. Dual roles of adaptive immunity in hypertension. Eur Heart J. 2014;35:1238–1244. doi: 10.1093/eurheartj/ehu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 36.Strawn WB, Chappell MC, Dean RH, Kivlighn S, Ferrario CM. Inhibition of early atherogenesis by losartan in monkeys with diet-induced hypercholesterolemia. Circulation. 2000;101:1586–1593. doi: 10.1161/01.cir.101.13.1586. [DOI] [PubMed] [Google Scholar]
- 37.Ferrario CM, Smith R, Levy P, Strawn W. The hypertension-lipid connection: insights into the relation between angiotensin II and cholesterol in atherogenesis. Am J Med Sci. 2002;323:17–24. doi: 10.1097/00000441-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98:121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 39.Strawn WB, Ferrario CM. Angiotensin II AT1 receptor blockade normalizes CD11b+ monocyte production in bone marrow of hypercholesterolemic monkeys. Atherosclerosis. 2008;196:624–632. doi: 10.1016/j.atherosclerosis.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keidar S, Hayek T, Kaplan M, Pavlotzky E, Hamoud S, Coleman R, Aviram M. Effect of eplerenone, a selective aldosterone blocker, on blood pressure, serum and macrophage oxidative stress, and atherosclerosis in apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 2003;41:955–963. doi: 10.1097/00005344-200306000-00019. [DOI] [PubMed] [Google Scholar]
- 41.Keidar S, Kaplan M, Pavlotzky E, Coleman R, Hayek T, Hamoud S, Aviram M. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation. 2004;109:2213–2220. doi: 10.1161/01.CIR.0000127949.05756.9D. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki J, Iwai M, Mogi M, Oshita A, Yoshii T, Higaki J, Horiuchi M. Eplerenone with valsartan effectively reduces atherosclerotic lesion by attenuation of oxidative stress and inflammation. Arterioscler Thromb Vasc Biol. 2006;26:917–921. doi: 10.1161/01.ATV.0000204635.75748.0f. [DOI] [PubMed] [Google Scholar]
- 43.Irita J, Okura T, Manabe S, Kurata M, Miyoshi K, Watanabe S, Fukuoka T, Higaki J. Plasma osteopontin levels are higher in patients with primary aldosteronism than in patients with essential hypertension. Am J Hypertens. 2006;19:293–297. doi: 10.1016/j.amjhyper.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 44.Irita J, Okura T, Jotoku M, Nagao T, Enomoto D, Kurata M, Desilva VR, Miyoshi K, Matsui Y, Uede T, Denhardt DT, Rittiling SR, Higaki J. Osteopontin deficiency protects against aldosterone-induced inflammation, oxidative stress, and interstitial fibrosis in the kidney. Am J Physiol Renal Physiol. 2011;301:F833–F844. doi: 10.1152/ajprenal.00557.2010. [DOI] [PubMed] [Google Scholar]
- 45.Jeong Y, Chaupin DF, Matsushita K, Yamakuchi M, Cameron SJ, Morrell CN, Lowenstein CJ. Aldosterone activates endothelial exocytosis. Proc Natl Acad Sci U S A. 2009;106:3782–3787. doi: 10.1073/pnas.0804037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown NJ, Vaughan DE, Fogo AB. Aldosterone and PAI-1: implications for renal injury. J Nephrol. 2002;15:230–235. [PubMed] [Google Scholar]
- 47.Alexandre J, Puddu PE, Simard C, Hof T, Salle L, Guinamard R, Manrique A, Rouet R, Beygui F, Milliez P. Proarrhythmic effects of Aldosterone during myocardial ischemia-reperfusion: implication of the sarcolemmal-KATP channels. J Cardiovasc Pharmacol. 2014;64:134–141. doi: 10.1097/FJC.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 48.Beygui F, Collet JP, Benoliel JJ, Vignolles N, Dumaine R, Barthelemy O, Montalescot G. High plasma aldosterone levels on admission are associated with death in patients presenting with acute ST-elevation myocardial infarction. Circulation. 2006;114:2604–2610. doi: 10.1161/CIRCULATIONAHA.106.634626. [DOI] [PubMed] [Google Scholar]
- 49.Beygui F, Labbe JP, Cayla G, Ennezat PV, Motreff P, Roubille F, Silvain J, Barthelemy O, Delarche N, van BE, Collet JP, Montalescot G. Early mineralocorticoid receptor blockade in primary percutaneous coronary intervention for ST-elevation myocardial infarction is associated with a reduction of life-threatening ventricular arrhythmia. Int J Cardiol. 2013;167:73–79. doi: 10.1016/j.ijcard.2011.11.076. [DOI] [PubMed] [Google Scholar]
- 50.Ivanes F, Susen S, Mouquet F, et al. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur Heart J. 2012;33:191–202. doi: 10.1093/eurheartj/ehr176. [DOI] [PubMed] [Google Scholar]
- 51.Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13(8 Suppl B):9–20. doi: 10.18553/jmcp.2007.13.s8-b.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24:261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 53.van Kats JP, van Meegen JR, Verdouw PD, Duncker DJ, Schalekamp MA, Danser AH. Subcellular localization of angiotensin II in kidney and adrenal. J Hypertens. 2001;19(3 Pt 2):583–589. doi: 10.1097/00004872-200103001-00010. [DOI] [PubMed] [Google Scholar]
- 54.van Kats JP, Chai W, Duncker DJ, Schalekamp MA, Danser AH. Adrenal angiotensin: origin and site of generation. Am J Hypertens. 2005;18:1104–1110. doi: 10.1016/j.amjhyper.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Schiffrin EL, Gutkowska J, Genest J. Effect of angiotensin II and deoxycorticosterone infusion on vascular angiotensin II receptors in rats. Am J Physiol. 1984;246(4 Pt 2):H608–H614. doi: 10.1152/ajpheart.1984.246.4.H608. [DOI] [PubMed] [Google Scholar]
- 56.Wehling M, Neylon CB, Fullerton M, Bobik A, Funder JW. Nongenomic effects of aldosterone on intracellular Ca2+ in vascular smooth muscle cells. Circ Res. 1995;76:973–979. doi: 10.1161/01.res.76.6.973. [DOI] [PubMed] [Google Scholar]
- 57.Mazak I, Fiebeler A, Muller DN, Park JK, Shagdarsuren E, Lindschau C, Dechend R, Viedt C, Pilz B, Haller H, Luft FC. Aldosterone potentiates angiotensin II-induced signaling in vascular smooth muscle cells. Circulation. 2004;109:2792–2800. doi: 10.1161/01.CIR.0000131860.80444.AB. [DOI] [PubMed] [Google Scholar]
- 58.Michea L, Delpiano AM, Hitschfeld C, Lobos L, Lavandero S, Marusic ET. Eplerenone blocks nongenomic effects of aldosterone on the Na+/H+ exchanger, intracellular Ca2+ levels, and vasoconstriction in mesenteric resistance vessels. Endocrinology. 2005;146:973–980. doi: 10.1210/en.2004-1130. [DOI] [PubMed] [Google Scholar]
- 59.Yamada M, Kushibiki M, Osanai T, Tomita H, Okumura K. Vasoconstrictor effect of aldosterone via angiotensin II type 1 (AT1) receptor: possible role of AT1 receptor dimerization. Cardiovasc Res. 2008;79:169–178. doi: 10.1093/cvr/cvn064. [DOI] [PubMed] [Google Scholar]
- 60.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18:1429–1433. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Endemann DH, Touyz RM, Iglarz M, Savoia C, Schiffrin EL. Eplerenone prevents salt-induced vascular remodeling and cardiac fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension. 2004;43:1252–1257. doi: 10.1161/01.HYP.0000128031.31572.a3. [DOI] [PubMed] [Google Scholar]
- 62.Rocha R, Martin-Berger CL, Yang P, Scherrer R, Delyani J, McMahon E. Selective aldosterone blockade prevents angiotensin II/salt-induced vascular inflammation in the rat heart. Endocrinology. 2002;143:4828–4836. doi: 10.1210/en.2002-220120. [DOI] [PubMed] [Google Scholar]
- 63.Conn JW, Louis LH. Primary aldosteronism: a new clinical entity. Trans Assoc Am Physicians. 1955;68:215–231. [PubMed] [Google Scholar]
- 64.Conn JW. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med. 1955;45:3–17. [PubMed] [Google Scholar]
- 65.Steigler M, Reichstein T. Desoxycorticosterone (21-oxyprogesterone aus 5-3-oxy-atio cholensaure) Helv Chim Acta. 1937;20:1164. [Google Scholar]
- 66.White PC, Mune T, Agarwal AK. 11 beta-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev. 1997;18:135–156. doi: 10.1210/edrv.18.1.0288. [DOI] [PubMed] [Google Scholar]
- 67.White PC, Mune T, Rogerson FM, Kayes KM, Agarwal AK. Molecular analysis of 11 beta-hydroxysteroid dehydrogenase and its role in the syndrome of apparent mineralocorticoid excess. Steroids. 1997;62:83–88. doi: 10.1016/s0039-128x(96)00164-x. [DOI] [PubMed] [Google Scholar]
- 68.White PC, Mune T, Rogerson FM, Kayes KM, Agarwal AK. 11 beta-Hydroxysteroid dehydrogenase and its role in the syndrome of apparent mineralocorticoid excess. Pediatr Res. 1997;41:25–29. doi: 10.1203/00006450-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Mayes D, Furuyama S, Kem DC, Nugent CA. A radioimmunoassay for plasma aldosterone. J Clin Endocrinol Metab. 1970;30:682–685. doi: 10.1210/jcem-30-5-682. [DOI] [PubMed] [Google Scholar]
- 70.Williams JS, Williams GH. 50th anniversary of aldosterone. J Clin Endocrinol Metab. 2003;88:2364–2372. doi: 10.1210/jc.2003-030490. [DOI] [PubMed] [Google Scholar]
- 71.Hollenberg NK, Moore T, Shoback D, Redgrave J, Rabinowe S, Williams GH. Abnormal renal sodium handling in essential hypertension. Relation to failure of renal and adrenal modulation of responses to angiotensin II. Am J Med. 1986;81:412–418. doi: 10.1016/0002-9343(86)90291-3. [DOI] [PubMed] [Google Scholar]
- 72.Shoback DM, Williams GH, Moore TJ, Dluhy RG, Podolsky S, Hollenberg NK. Defect in the sodium-modulated tissue responsiveness to angiotensin II in essential hypertension. J Clin Invest. 1983;72:2115–2124. doi: 10.1172/JCI111176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams GH, Dluhy RG, Lifton RP, Moore TJ, Gleason R, Williams R, Hunt SC, Hopkins PN, Hollenberg NK. Non-modulation as an intermediate phenotype in essential hypertension. Hypertension. 1992;20:788–796. doi: 10.1161/01.hyp.20.6.788. [DOI] [PubMed] [Google Scholar]
- 74.Williams GH, Hollenberg NK, Hopkins PM, Jeunemaitre X. The role of intermediate phenotypes in essential hypertension: non-modulation as a model. Endocr Res. 1996;22:675–680. doi: 10.1080/07435809609043762. [DOI] [PubMed] [Google Scholar]
- 75.Williams GH, Fisher ND, Hunt SC, Jeunemaitre X, Hopkins PN, Hollenberg NK. Effects of gender and genotype on the phenotypic expression of nonmodulating essential hypertension. Kidney Int. 2000;57:1404–1407. doi: 10.1046/j.1523-1755.2000.00982.x. [DOI] [PubMed] [Google Scholar]
- 76.Genest J, Lemieux G, Davignon A, Koiw E, Nowaczynski W, Steyermark P. Human arterial hypertension: a state of mild chronic hyperaldosteronism? Science. 1956;123:503–505. doi: 10.1126/science.123.3195.503. [DOI] [PubMed] [Google Scholar]
- 77.Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, Benjamin EJ, Levy D. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33–41. doi: 10.1056/NEJMoa033263. [DOI] [PubMed] [Google Scholar]
- 78.Savoia C, Touyz RM, Amiri F, Schiffrin EL. Selective mineralocorticoid receptor blocker eplerenone reduces resistance artery stiffness in hypertensive patients. Hypertension. 2008;51:432–439. doi: 10.1161/HYPERTENSIONAHA.107.103267. [DOI] [PubMed] [Google Scholar]
- 79.Vaclavik J, Sedlak R, Plachy M, Navratil K, Plasek J, Jarkovsky J, Vaclavik T, Husar R, Kocianova E, Taborsky M. Addition of spironolactone in patients with resistant arterial hypertension (ASPIRANT): a randomized, double-blind, placebo-controlled trial. Hypertension. 2011;57:1069–1075. doi: 10.1161/HYPERTENSIONAHA.111.169961. [DOI] [PubMed] [Google Scholar]
- 80.Benetos A, Lacolley P, Safar ME. Prevention of aortic fibrosis by spironolactone in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 1997;17:1152–1156. doi: 10.1161/01.atv.17.6.1152. [DOI] [PubMed] [Google Scholar]
- 81.Park JB, Schiffrin EL. Cardiac and vascular fibrosis and hypertrophy in aldosterone-infused rats: role of endothelin-1. Am J Hypertens. 2002;15(2 Pt 1):164–169. doi: 10.1016/s0895-7061(01)02291-9. [DOI] [PubMed] [Google Scholar]
- 82.Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002;40:504–510. doi: 10.1161/01.hyp.0000034738.79310.06. [DOI] [PubMed] [Google Scholar]
- 83.Calhoun DA, White WB, Krum H, Guo W, Bermann G, Trapani A, Lefkowitz MP, Menard J. Effects of a novel aldosterone synthase inhibitor for treatment of primary hypertension: results of a randomized, double-blind, placebo- and active-controlled phase 2 trial. Circulation. 2011;124:1945–1955. doi: 10.1161/CIRCULATIONAHA.111.029892. [DOI] [PubMed] [Google Scholar]
- 84.Sadee W, Riegelman S, Jones SC. Plasma levels of spirolactones in the dog. J Pharm Sci. 1972;61:1129–32. doi: 10.1002/jps.2600610720. [DOI] [PubMed] [Google Scholar]
- 85.Sadee W, Riegelman S, Jones SC. Disposition of tritium-labeled spirolactones in the dog. J Pharm Sci. 1972;61:1132–1135. doi: 10.1002/jps.2600610721. [DOI] [PubMed] [Google Scholar]
- 86.Sadee W, Dagcioglu M, Schroder R. Pharmacokinetics of spironolactone, canrenone and canrenoate-K in humans. J Pharmacol Exp Ther. 1973;185:686–695. [PubMed] [Google Scholar]
- 87.Schroder R, Schuren KP, Biamino G, Dennert J, Meyer V, Sadee W. Effect of aldactone on the dynamics and contractility of the heart. Verh Dtsch Ges Kreislaufforsch. 1971;37:438–445. [PubMed] [Google Scholar]
- 88.Delyani JA. Anti-aldosterone therapy in the treatment of heart failure: new thoughts on an old hormone. Expert Opin Investig Drugs. 1998;7:753–759. doi: 10.1517/13543784.7.5.753. [DOI] [PubMed] [Google Scholar]
- 89.Delyani JA. Mineralocorticoid receptor antagonists: the evolution of utility and pharmacology. Kidney Int. 2000;57:1408–1411. doi: 10.1046/j.1523-1755.2000.00983.x. [DOI] [PubMed] [Google Scholar]
- 90.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 91.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 92.Zannad F, McMurray JJ, Drexler H, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pitt B. Rationale and design of the Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure (EMPHASIS-HF) Eur J Heart Fail. 2010;12:617–622. doi: 10.1093/eurjhf/hfq049. [DOI] [PubMed] [Google Scholar]
- 93.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. [Google Scholar]
- 94.Markowitz M, Messineo F, Coplan NL. Aldosterone receptor antagonists in cardiovascular disease: a review of the recent literature and insight into potential future indications. Clin Cardiol. 2012;35:605–609. doi: 10.1002/clc.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brilla CG. Aldosterone and myocardial fibrosis in heart failure. Herz. 2000;25:299–306. doi: 10.1007/s000590050024. [DOI] [PubMed] [Google Scholar]
- 96.Mottram PM, Haluska B, Leano R, Cowley D, Stowasser M, Marwick TH. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;110:558–565. doi: 10.1161/01.CIR.0000138680.89536.A9. [DOI] [PubMed] [Google Scholar]
- 97.Grandi AM. Antihypertensive therapy: role of aldosterone antagonists. Curr Pharm Des. 2005;11:2235–2242. doi: 10.2174/1381612054367418. [DOI] [PubMed] [Google Scholar]
- 98.Venco A, Grandi AM. Aldosterone blockade in essential hypertension. Ital Heart J. 2005;6(Suppl 1):34S–42S. [PubMed] [Google Scholar]
- 99.Desai AS, Lewis EF, Li R, et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–972. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 100.Shah SJ, Heitner JF, Sweitzer NK, et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6:184–192. doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 102.Shah NC, Pringle SD, Donnan PT, Struthers AD. Spironolactone has antiarrhythmic activity in ischaemic cardiac patients without cardiac failure. J Hypertens. 2007;25:2345–2351. doi: 10.1097/HJH.0b013e3282e9a72d. [DOI] [PubMed] [Google Scholar]
- 103.Ouvrard-Pascaud A, Sainte-Marie Y, Benitah JP, et al. Conditional mineralocorticoid receptor expression in the heart leads to life-threatening arrhythmias. Circulation. 2005;111:3025–3033. doi: 10.1161/CIRCULATIONAHA.104.503706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Suzuki S, Ohkusa T, Sato T, Yoshida M, Yasui K, Miwa K, Lee JK, Yano M, Kodama I, Matsuzaki M. Effects of aldosterone on Cx43 gap junction expression in neonatal rat cultured cardiomyocytes. Circ J. 2009;73:1504–1512. doi: 10.1253/circj.cj-08-1065. [DOI] [PubMed] [Google Scholar]
- 105.Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol. 2010;6:261–273. doi: 10.1038/nrneph.2010.30. [DOI] [PubMed] [Google Scholar]
- 106.Toda N, Nakanishi S, Tanabe S. Aldosterone affects blood flow and vascular tone regulated by endothelium-derived NO: therapeutic implications. Br J Pharmacol. 2013;168:519–533. doi: 10.1111/j.1476-5381.2012.02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schmitt M, Gunaruwan P, Frenneaux MP. Rapid nongenomic aldosterone effects in the human forearm? Hypertension. 2004;43:e1. doi: 10.1161/01.hyp.0000105111.06482.7e. [DOI] [PubMed] [Google Scholar]
- 108.Castiglioni A. A history of medicine. New York, N.Y: Alfred A. Knopf; 1941. [Google Scholar]
- 109.Addison T. On the constitutional local effects of disease of the suprarenal capsules. London, UK: P. Highley; 1855. [Google Scholar]
- 110.Kuizinga MH, Cartland GF. Fractionation studies on adrenal cortical extract with notes on the distribution of biological activity among the crystalline and amorphous fractions. Endocrinology. 1939;24:526–535. [Google Scholar]
- 111.Simpson SA, Tait JF. A quantitative method for the bioassay of the effect of adrenal cortical steroids on mineral metabolism. Endocrinology. 1952;50:150–161. doi: 10.1210/endo-50-2-150. [DOI] [PubMed] [Google Scholar]
- 112.Simpson SA, Tait JF, Bush IE. Secretion of a salt-retaining hormone by the mammalian adrenal cortex. Lancet. 1952;2:226–228. doi: 10.1016/s0140-6736(52)91551-1. [DOI] [PubMed] [Google Scholar]
- 113.Simpson SA, Tait JF, Wettstein A, Neher R, Von EJ, Reichstein T. Isolation from the adrenals of a new crystalline hormone with especially high effectiveness on mineral metabolism. Experientia. 1953;9:333–335. doi: 10.1007/BF02155834. [DOI] [PubMed] [Google Scholar]
- 114.Simpson SA, Tait JF. The nature of the circulating hormones of the adrenal cortex in man. Arch Middx Hosp. 1953;3:209–218. [PubMed] [Google Scholar]
- 115.Tait JF, Simpson SA, Grundy HM. The effect of adrenal extract on mineral metabolism. Lancet. 1952;1:122–124. doi: 10.1016/s0140-6736(52)92427-6. [DOI] [PubMed] [Google Scholar]
- 116.Simpson SA, Tait JF, Wettstein A, Neher R, Von EJ, Schindler O, Reichstein T. Constitution of aldosterone, a new mineralocorticoid. Experientia. 1954;10:132–133. doi: 10.1007/BF02158515. [DOI] [PubMed] [Google Scholar]
- 117.Schmidlin J, Anner G, Billeter JR, Wettstein A. Synthesis in aldosterone-series. I. Total synthesis of racemic aldosterone. Experientia. 1955;11:365–368. doi: 10.1007/BF02159929. [DOI] [PubMed] [Google Scholar]
- 118.Vischer E, Schmidlin J, Wettstein A. Microbiological separation of racemic steroids; synthesis of d-aldosterone. Experientia. 1956;12:50–52. doi: 10.1007/BF02164671. [DOI] [PubMed] [Google Scholar]
- 119.Giroud CJ, Saffran M, Schally AV, Stachenko J, Venning EH. Production of aldosterone by rat adrenal glands in vitro. Proc Soc Exp Biol Med. 1956;92:855–859. doi: 10.3181/00379727-92-22635. [DOI] [PubMed] [Google Scholar]
- 120.Giroud CJ, Stachenko J, Venning EH. Secretion of aldosterone by the zona glomerulosa of rat adrenal glands incubated in vitro. Proc Soc Exp Biol Med. 1956;92:154–158. doi: 10.3181/00379727-92-22416. [DOI] [PubMed] [Google Scholar]


