Abstract
This review article discusses the mechanisms of cardiomyogenesis in the adult heart. They include the reentry of cardiomyocytes into the cell cycle; dedifferentiation of preexisting cardiomyocytes which assume an immature replicating cell phenotype; transdifferentiation of hematopoietic stem cells into cardiomyocytes; and cardiomyocytes derived from activation and lineage specification of resident cardiac stem cells. The recognition of the origin of cardiomyocytes is of critical importance for the development of strategies capable of enhancing the growth response of the myocardium; in fact, cell therapy for the decompensated heart has to be based on the acquisition of this fundamental biological knowledge.
Keywords: cardiomyogenesis, stem cells, dedifferentiation, transdifferentiation
Introduction
Several organs are characterized by dividing and non-dividing cells. Non-dividing cells can rest in G0 and reenter the cell cycle upon stimulation, or become terminally-differentiated and die at the end of their lifespan without further division. This latter cell population is not dormant in G0; it has lost permanently the ability to replicate and generate cells with identical properties. For several decades, cardiomyocytes have been considered to belong to this cell category. The notion that myocytes cannot divide originated from the difficulty of identifying mitotic nuclei1 and from the negligible level of DNA synthesis measured by 3H-thymidine incorporation.2 The lack of DNA replication and the failure to recognize mitotic cells has led to the conclusion that myocyte renewal is absent in the adult heart. The dogma was introduced that adult cardiomyocytes are terminally-differentiated cells, which are irreversibly withdrawn from the cell cycle. These cells are unable to proliferate, but can perform their physiological functions, undergo cellular hypertrophy, and ultimately die by apoptosis or necrosis.
The widely accepted paradigm is that the heart is an organ characterized primarily by a fixed number of myocytes, which is maintained throughout life until death of the organism.3 The turnover of structural proteins has been considered the mechanism involved in the preservation of myocyte performance and the youth of the cell. Differences with this philosophy have been rejected as scientifically incorrect, or the product of technical errors.3-5 The most common argument brought forward against the regenerative potential of the myocardium is that the heart does not repair itself after infarction. The statement is universally made without considering the fact that whether parenchymal cells proliferate or not, the outcome of infarction is identical in all organs including the bone marrow, the testis, the skin, the kidney, the brain and the intestine.6-10 In these self-renewing organs, stem cells do not normally migrate and home to the damaged area replacing the infarcted tissue.
According to the traditional view, the age of myocytes corresponds to the age of the organ and organism. All cardiomyocytes must age at the same pace and, at any given time, the heart should be composed largely of a homogeneous population of cells of identical age. Because of this assumption, the principle of cellular senescence has never been applied to the heart. This process reflects the close relationship between number of cell divisions, telomeric shortening, oxidative stress and replicative senescence in vivo. A mitotic clock regulates the lifespan of cells, which is independent from organ and organism age and lifespan.11 The heterogeneity in the properties of myocytes, together with evidence in favor of the regeneration of the young, adult and aged myocardium,12-19 has questioned the conventional concept of myocardial biology and offered a novel perspective of the growth dynamics of the heart and its myocyte compartment.
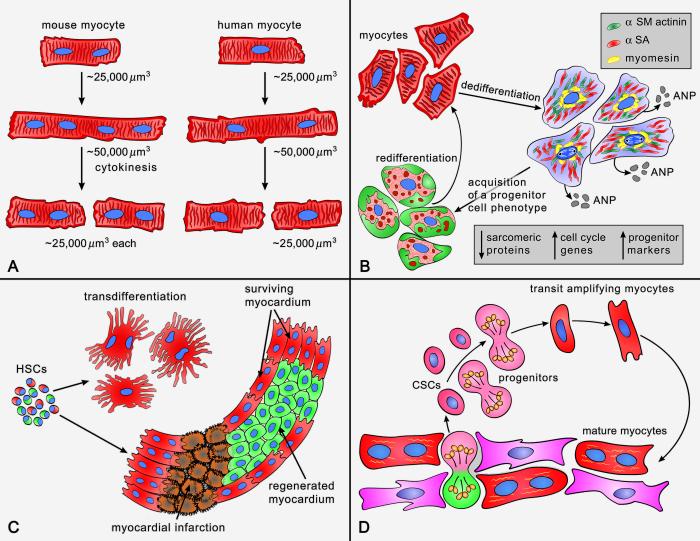
The recognition that myocyte apoptosis and myocyte necrosis are natural components of the wear and tear of the organ, and increase dramatically with age and cardiac pathologies,20-22 has raised the challenging question concerning the origin of the newly-formed cardiomyocytes needed for the preservation of the structure and function of the myocardium. There are five possibilities that have to be considered as potential sources of cardiomyocytes in the adult heart (Figure 1 and Table 1): a) Cardiomyocytes are not post-mitotic terminally-differentiated cells and can re-enter the cell cycle and divide; b) Cardiomyocytes dedifferentiate in vivo reacquiring a primitive cell phenotype and then multiply; c) Cardiomyocytes derive from the engraftment and commitment of circulating hematopoietic stem cells (HSCs); d) Cardiomyocytes constitutes the progeny of resident cardiac stem cells (CSCs), which control cell turnover physiologically and cardiac repair following injury; and e) Cardiomyocytes are generated by a combination of these four cellular mechanisms. The recognition of the origin of cardiomyocytes is of critical importance for the development of strategies capable of enhancing the growth response of the myocardium; in fact, cell therapy for the decompensated heart has to be based on the acquisition of this fundamental biological knowledge.
Figure 1. Potential mechanisms of cardiomyogenesis.
See text.
Table 1.
Levels of Myocyte Regeneration and Cell of Origin.
| Myocyte Source |
Extent of myocardial regeneration in vivo | Assessed by | Reference # |
|---|---|---|---|
| Mitotic Myocytes |
0.12 % of total myocytes | Presence of dividing myocytes in wild type control groups | 48, Fig 5 |
| Negligible | Qualitative observation of Azan staining | 49, Fig 1 | |
| Negligible | Presence of dividing myocytes in untreated control groups | 50, Fig 3 | |
| 0.01% of total myocytes | Presence of dividing myocytes in untreated control groups | 51, Fig 3 | |
| Dedifferentiate d Myocytes |
Un-documented. 43% of myocytes dedifferentiated | Presence of cells exhibiting characteristic of hibernating myocardium |
68, Fig 2 |
| Un-documented. | Presence of markers of fetal-like gene expression program | 69, Fig 4 | |
| Bone Marrow Cells |
68% of infarcted area at day 9 | Presence of sex-mismatched myocytes | 70, Fig 1 |
| consistent with ref 66 | Presence of sex-mismatched myocytes | 88, Fig 4 | |
| Negligible | Absence of myocytes carrying ubiquitous fluorescent reporter | 97, Table 1 | |
| Negligible | Absence of myocytes carrying ubiquitous or myocyte-restricted fluorescent reporter |
98, Table 1 | |
| 25% of total myocytes after 8 weeks | Relative abundance of lineage tagged and un-tagged myocytes | 101, Fig 1 | |
| Cardiac Stem Cells |
10% and 40% of total myocytes in young and old respectively |
Detection of dividing myocytes after 12 weeks chase period | 34, Fig 1 |
| 15% of total myocytes | Detection of dividing myocytes after 10 weeks chase period | 112, Fig 5 | |
| 6% of total myocytes in old hearts untreated | Detection of dividing myocytes after 8 weeks chase period | 40, Fig 8 | |
| 13-22% of infarcted area after 10-20 days | Detection of differentiated cardiac progenitor cells | 107, Fig 1 | |
| 25% of infarcted area when using engineered cells | Detection of virally tagged myocytes | 123, Fig 4 | |
| 33% of infarcted area when using engineered cells | Detection of virally tagged myocytes | 132, Fig 4 | |
| 4% and 8.5% of total myocytes after isoproterenol damage from endogenous and injected progenitors respectively |
Detection of lineage-tagged myocytes | 47, Fig 2 | |
| 0.0055%-0.016% of total myocytes | Detection of lineage-tagged myocytes | 161, Fig 2-3 |
This article discusses information obtained in humans and animals in favor or against each of these potential origins of myocytes.
Myocyte Regeneration in Humans: Complexity of the Problem
The possibility of taking advantage of the growth reserve of the myocardium to improve the management of human heart failure (HF) clinically requires understanding of the level of cardiomyocyte regeneration occurring in the intact and damaged organ.
Difficulties exist in the evaluation of myocyte proliferation by various methodologies. Ideally, in the absence of cell death, the measurement of the number of ventricular myocytes would appear to be the only approach that can demonstrate unequivocally the degree of myocyte cellular hyperplasia. However, the concomitant presence of widespread myocyte loss, together with multiple foci of myocardial injury and tissue scarring, complicates this type of analysis resulting in an underestimation of the extent of cell replacement in the decompensated heart.
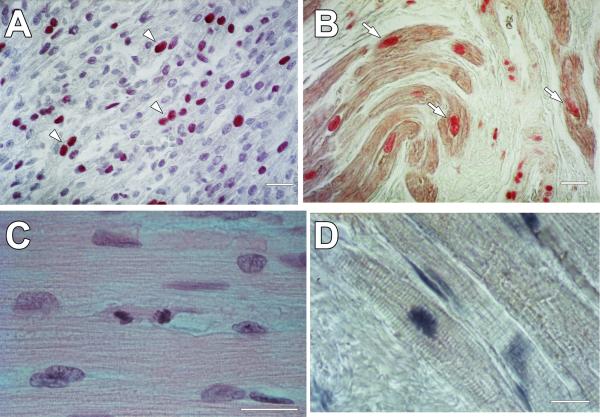
Several reports have suggested that ventricular myocytes in humans reenter the cell cycle and synthesize DNA.1,2,16,23,24 But whether DNA replication leads to the formation of ploidy, karyokinesis in the absence of cytokinesis, or to karyokinesis followed by cytokinesis has been a matter of intense debate. Although morphometric studies have shown that myocyte regeneration occurs in the severely hypertrophied heart with no changes in the proportion of mononucleated and binucleated cells,1,16,19 mitotic figures in myocytes have been difficult to identify. The lack of a direct documentation of dividing myocytes has been raised frequently as a critical argument questioning the validity of these morphometric results. The first demonstration of the localization of the proliferating cell nuclear antigen (PCNA) in human fetal and adult cardiomyocytes and the identification of myocyte mitotic images in routine histology sections was obtained in the mid 1990s (Figure 2);25,26 the mitotic index of myocytes and interstitial cells was also measured, providing an estimate of this phenomenon.
Figure 2. Cycling and dividing cardiomyocytes.
A, PCNA is expressed in several nuclei (arrowheads) of human fetal myocardium collected at autopsy. B, Nuclear localization of PCNA in cardiomyocytes (arrows) of a human heart with ischemic cardiomyopathy. PCNA was detected by alkaline phosphatase streptavidin labeling and α-sarcomeric actin (α-SA) by immunoperoxidase. C, Dividing human myocyte located in the border zone of a patient who died as a result of an extensive myocardial infarct. Hematoxylin and eosin staining. D, Mitotic image in a myocyte of a human heart with ischemic cardiomyopathy. Myofibrils, α-SA (light brown); nuclei, hematoxylin (blue). Scale bars, 10 μm. Adapted from reference 25.
Subsequent studies with more sophisticated techniques have acquired evidence of replicating cardiomyocytes, and myocyte karyokinesis and cytokinesis.24,27-31 The implementation of immunolabeling and confocal microscopy has improved significantly image resolution and the accuracy of the quantitative data. While routine histology was unable to detect with certainty mitotic images in cardiomyocytes of normal hearts,25 the expression of cell cycle proteins, nuclear mitotic division and myocyte cell division were identified by immunolabeling and confocal microscopy.28-31
Whether the fraction of replicating myocytes or the myocyte mitotic index is evaluated, these snap-shot results can be combined with the number of cardiomyocytes present in the ventricle and an approximate length of the cell cycle and mitosis to appreciate the actual magnitude of the process.28-31 When this type of analysis is introduced, cardiomyocyte regeneration appears to be relatively high, suggesting that potentiation of this mechanism of cardiac recovery may have significant effects on the restoration of cardiac function following myocardial injury. However, the measured values are relatively small and it is difficult to extrapolate from these data the degree of myocyte formation occurring over time (Table 1). Although these factors have to be acknowledged, evidence accumulated in the last 15 years is consistent with the view that myocyte renewal is a relevant component of cardiac homeostasis and repair of the human heart.16,19
Myocyte Regeneration in Animals: The Controversy
The general opinion is that myocardial hypertrophy in the adult rodent heart is accomplished by enlargement of preexisting cardiomyocytes with no cell proliferation. Studies aiming at the identification and quantification of DNA replication in the overloaded myocardium have documented none, little DNA synthesis in myocyte nuclei possibly representing polyploidy,2,32 or significant DNA synthesis consistent with de novo formation of cardiomyocytes.1,33-36 Mitotic figures in myocytes have also been shown.37-39 Myocyte restoration occurs under a variety of experimental conditions characterized by a large and prolonged stress on the heart; they include myocardial infarction, non-occlusive coronary artery constriction, pressure overload, and aging.1,34-41 Similar findings have been found in larger animals.1,16,19
The impairment in cardiac performance appears to be critical for the activation of myocyte proliferation. Abnormal increases in diastolic wall stress promote myocyte proliferation, while changes in systolic stress have a lesser impact on this cellular process.37 The mechanism by which diastolic load triggers the formation of myocytes is currently unknown; however, alterations in ventricular compliance and sarcomere stretching have multiple effects on the synthesis and secretion of growth factors and cytokines implicated in cell viability, growth and contractile behavior.42 The activation of autocrine/paracrine signals may influence dramatically the fate of cardiomyocytes.
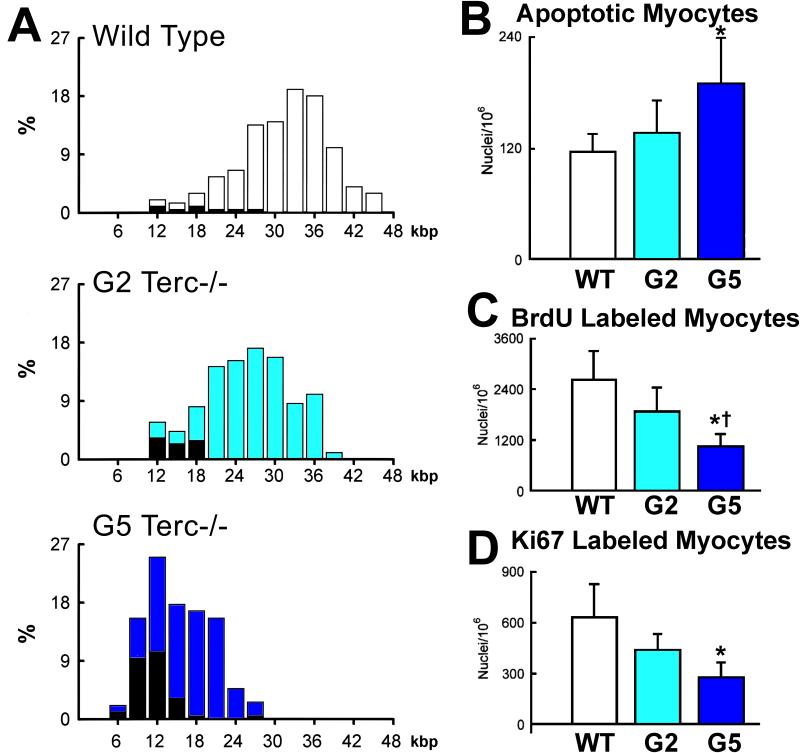
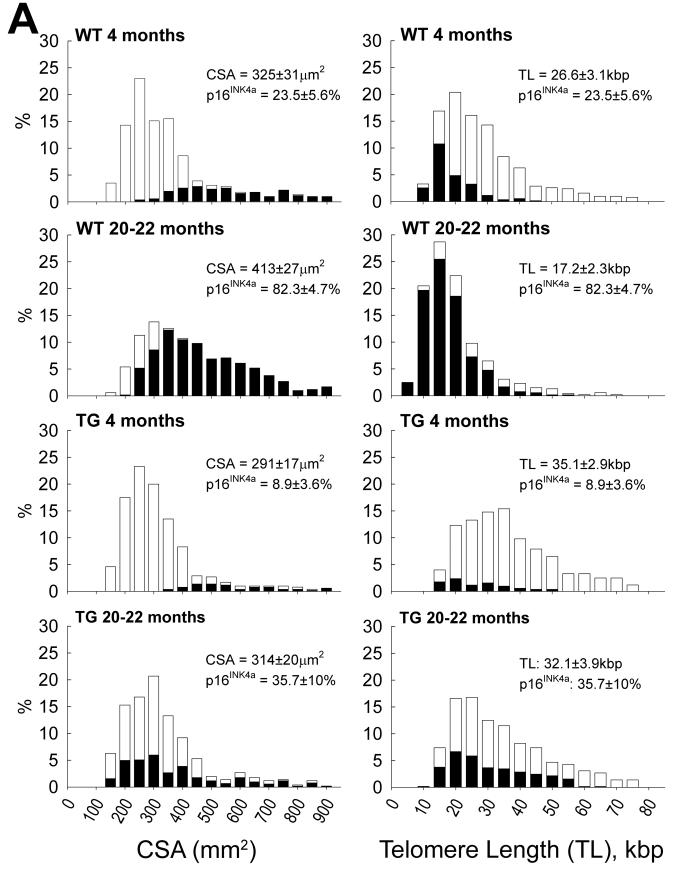
Lineage tracing studies in mice have been performed in an attempt to measure the rate of myocyte renewal in the adult and old heart.32,43 By this genetic approach, myocyte formation was found to be minimal at baseline increasing modestly with pathologic overloads. These results, however, are inconsistent with the magnitude of myocyte death occurring in the adult and aging mouse heart.38,44,45 The identification of cardiac troponin in the circulation of healthy mice provides additional evidence of a chronic loss of cardiomyocytes by the necrotic pathway.46 Myocyte regeneration has to occur for the heart to perform its function. If myocyte turnover is inconsequential, cardiomyocytes should share common characteristics. But this is not the case. Telomere attrition and the nuclear localization of p16INK4a and p53, indicative of cellular aging, are not uniformly distributed in cardiomyocytes.44,45 Similarly, apoptosis and the level of myocyte regeneration are conditioned by telomere length (Figure 3). Chronological age and cellular age do not coincide, but cellular age and cell death do coincide, although not all p16INK4a-positive myocytes undergo apoptosis or necrosis. Cardiac restricted overexpression of IGF-1 positively interferes with these negative variables of myocyte aging (Figure 4A-4C).45
Figure 3. Heterogeneity of the myocyte cell population in wild-type and Terc−/− mice at the second (G2) and fifth (G5) generation.
A, Frequency distribution of telomere length in wild-type, G2 Terc−/− and G5 Terc−/− mice (open bars). The corresponding values for each subclass of nuclei expressing p53 are shown by solid bars. B through D, Defects in the telomere-telomerase axis impinge upon myocyte apoptosis and proliferation. Results are means ± SD. * and †, P < 0.05 versus WT and G2, respectively. Adapted from reference 44.
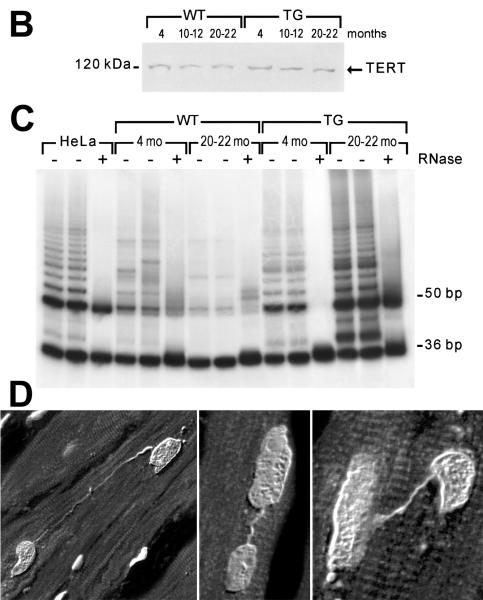
Figure 4. Telomere length and telomerase activity in cardiomyocytes.
A, Distribution of myocyte cross-sectional area (CSA) and telomere length. Aging in wild type mice results in a shift in the distribution of CSA to the right and telomere length to the left. The solid portion of the bars corresponds to p16INK4a-positive myocyte nuclei; they increase with age in both cases. In transgenic mice carrying IGF-1 under the control of the α-MHC promoter, these changes are attenuated. B, Catalytic subunit of telomerase (TERT) in myocyte nuclear lysates. C, Products of telomerase activity start at 50 bp and display a 6-bp periodicity. Myocytes treated with RNase (+) were used as negative control and HeLa cells as positive control. Adapted from reference 45. D, In failing human hearts, chromatin bridges, indicative of abortive mitosis, were detected between dividing myocyte nuclei, reflecting senescent cells unable to replicate (Courtesy of Dr. Shao-Min Yan and Prof. Alberto C. Beltrami, University of Udine, Italy).
Thus, the heart is composed of a heterogeneous myocyte population undergoing constant death and renewal, a possibility in harmony with the maintenance of a dynamic steady state of the myocardium. Myocyte formation is substantial and plays a role in tissue homeostasis and in the response to aging and hemodynamic stress. The intrinsic mechanisms regulating myocyte regeneration are necessary and sufficient to preserve the integrity of the adult organ.47 Myocyte growth, however, fails to meet the demands of the old heart and, as in humans,27 an aging myopathy develops.34,36,45 Similarly, the partial restoration of myocytes following ischemia or increases in pressure and/or volume loads cannot prevent cardiac dysfunction and HF in rodents and patients.
Cardiomyocytes Are Not Terminally Differentiated Cells
Based on the assumption that a pool of mature ventricular myocytes synthesizes DNA and divides, mitogenic stimulation has been attempted by interfering with inhibitors of the cell cycle or overexpressing components of the replicating machinery. The retinoblastoma protein (Rb) regulates the post-mitotic myocyte phenotype, but cardiac-specific conditional deletion of Rb in transgenic mice does not increase the number of proliferating cells.48 Double deletion of Rb and p130 are required to synergistically upregulate Myc, E2F-1, and G1 cyclin-dependent kinases and expand the compartment of myocytes in S-phase and mitosis (Table 1).48 Early findings suggesting that cyclin D downregulation is implicated in the acquisition of the permanent dormant state of cardiomyocytes have prompted the development of transgenic mice overexpressing cyclin D1, D2, or D3 under the control of the myocyte-restricted α-myosin heavy chain (α-MHC) promoter. However, only mice overexpressing cyclin D2 show an increase in 3H-thymidine labeling in myocytes after infarction, leading to a significant reduction in scar size.49 These findings led to the interpretation that cell proliferation can be induced in mature myocytes by overexpression of cyclin D2.
Along the same principle the growth factor neuregulin has been claimed to trigger myocyte regeneration physiologically and in the infarcted heart.50 The interaction of neuregulin with its tyrosine kinase receptor, ErbB4, may change the phenotypic properties of mature cardiomyocytes reactivating the cell cycle. This growth reserve, however, is confined to mononucleated cells which comprise less than 10% of the myocyte population. Similarly, periostin, which stimulates integrins located on the plasma membrane of cardiomyocytes and the phosphatidylinositol-3-OH kinase pathway, promotes reentry of mononucleated myocytes into the cell cycle.51 The relevance of periostin for cardiac hypertrophy has also been reported.52 Inhibition of p38 MAP kinase enhances myocyte renewal by reversing the post-mitotic state.53 A defect in anillin localization at the mid-body of dividing cells may be responsible for myocyte binucleation, ploidy and lack of cytokinesis.54 Blockade of p38 MAP kinase reverses the process and reinstitutes cell division.54 Similarly, the delivery of cyclin A2 appears to trigger myocyte proliferation.55
The conclusions reached in these mouse models are problematic. The examples of replicating myocytes given in these reports do not reflect the criteria dictated by the biology of the cell cycle. Adult myocytes measure ~20,000-25,000 μm3; if these cells reenter the cell cycle and divide, they have to acquire in mitosis a volume of ~40,000-50,000 μm3 before two daughter cells of ~20,000-25,000 μm3 each are formed. Replicating myocytes in rodents are small, rarely binucleated and vary in volume from 1,500 to 7,000 μm3 (unpublished data); when binucleated myocytes enter the cell cycle, both nuclei are in mitosis and in a rather synchronized manner.38
In humans, dividing myocytes are predominantly mononucleated; thus far, replicating binucleated myocytes were not detected in the female or male human heart. In both animals and humans, myocyte cytokinesis has been shown by the localization of aurora B kinase at the duplicated sets of telophase chromosomes and cleavage furrow of the dividing cells. The forced re-entry of post-mitotic myocytes into the cell cycle results in abortive mitosis and apoptosis.56 Overexpression of cell cycle genes favors the progression of cardiomyocytes from G0 to G1 and subsequently to the S-phase. But cells do not traverse the G2/M checkpoint and divide; the arrest in G2/M leads to cellular enlargement, senescence, and death.56
Hypertrophied, senescent myocytes attempt to proliferate but cannot complete successfully cell replication. These myocytes may generate chromosomal bridges (Figure 4D; Courtesy of Dr. Shao-Min Yan and Prof. Alberto C. Beltrami, University of Udine, Italy). Accumulation of G-rich single stranded DNA fragments, together with downregulation of the telomeric binding proteins TRF1 and TRF2, results in telomere erosion and formation of intercellular anaphase bridges. These cells possess properties that support the illegitimate rejoining of DNA broken ends: critical shortening of telomeres, telomere uncapping and foci of phosphorylated histone H2AX.57 Chromosomal instability precludes completion of mitosis.
Death of adult cardiomyocytes in response to mitogenic signals occurs following the intramyocardial delivery of adenoviruses carrying the cell cycle regulator E2F-1.56 Expression of E2F-1 induces G1 exit and massive apoptosis, a process viewed as a repressive control against the inappropriate cell cycle reentry of quiescent post-mitotic myocytes. The intramyocardial injection of E2F-1 in p53 null mice stimulates DNA synthesis and the accumulation of myocytes in G2/M; however, E2F-1 triggers apoptosis and rapid mortality of the animals. These findings are consistent with a cooperative role of p53 and the pocket protein family in maintaining myocyte quiescence in the adult heart.56
Cardiomyocyte Dedifferentiation and Replication
Changes in the phenotypic characteristics of cells contribute to the regenerative reserve of plants, invertebrates, teleost fishes, and amphibians. Cell dedifferentiation is a common phenomenon in plants.58 The main function of mesophyll cells in a leaf is to facilitate and carry out photosynthesis. By the process of dedifferentiation, mesophyll cells can reacquire a primitive state, and the plasticity of plant protoplasts is reminiscent of the totipotency of embryonic stem cells.58 Plant protoplasts form calli, a mass of unorganized parenchyma cells, from which roots grow and generate entire plants.
In vertebrates, mostly in amphibians and fishes, quiescent specialized cells can revert their phenotype and assume the properties of replicating progenitors.59 These progenitors multiply and differentiate restoring the lost anatomical structure. When the morphological and functional integrity is reinstated, progenitor cells lose their function. The repair mechanism originates from the formation of a mass of multipotent progenitors or different progenitors with restricted regeneration potential,60 i.e., the blastema, from which the entire structure is re-grown. The process that governs the replacement of anatomical parts is termed epimorphic regeneration. Conversely, stably committed mammalian cells are thought to be unable to dedifferentiate into stem cells. However, there are some exceptions; hepatocytes may revert their specific function, enter the cell cycle and proliferate following partial hepatectomy.61 Similarly, airway epithelial cells can dedifferentiate into basal primitive cells62 and exocrine pancreatic cells may change their intrinsic regulatory properties and form β-cells synthesizing insulin.63
An interesting model of spontaneous myocyte proliferation with reconstitution of the injured heart is found in lower vertebrates like zebrafish.64,65 In this organism, the myocardium retains an extraordinary capacity for regeneration throughout life, a process that results in minimal residual tissue scarring of the organ. The replicating cardiomyocytes, however, have an immature phenotype raising the question whether they constitute a pool of cells with the inherent ability to replicate or dedifferentiated cells which have reacquired the propensity to multiply. Additionally, whether the zebrafish heart contains a compartment of resident progenitors that acquire the cardiomyocyte lineage and contribute to cardiac repair remains to be determined. A similar phenomenon is observed in the neonatal mouse heart where the resection of the apical region can be efficiently restored by the ability that the spared cardiomyocytes have to reenter the cell cycle and divide, reconstituting the integrity of the organ, a characteristic which is lost rapidly with maturation.66 Additionally, activation of the IGF-1-IGF-1 receptor system at a later time point promotes myocyte formation.67
The definition of cardiomyocyte dedifferentiation in the adult mammalian heart has been based on the interpretation of electron micrographs; dedifferentiated cardiomyocytes are characterized by partial loss of contractile material and abnormal Z lines. The sarcoplasmic reticulum and T-tubules are decreased while the number of mitochondria is increased, in combination with areas of glycogen accumulation (Table 1).68 Moreover, the enhanced expression of several fetal genes has been described to provide a more precise characterization of the process of conversion of post-mitotic myocytes into immature cells.4,69 Recently, the distribution of the actin depolymerizing protein destrin was analyzed. Destrin was not detected in control hearts but was strongly visible in the perinuclear region of myocytes bordering the infarcted human myocardium; these myocytes also expressed the stem cell marker Runx1. Runx1 is a transcription factor that regulates the differentiation of HSCs into mature blood cells.69 The mRNA level of the surface antigen c-kit, which is shared by HSCs, CSCs and lung stem cells,70-72 was upregulated only in culture preparations of dedifferentiated myocytes.69
Although these are interesting findings supporting the notion that adult cardiomyocytes retain a degree of plasticity which was not anticipated previously, there are several caveats that need to be discussed. Similar ultrastructural alterations have been described with myocyte degeneration.73 In fact, dedifferentiated myocytes were interpreted initially as damaged cells in areas of hibernating myocardium with a phenotype consistent with a “prelude” to cell death.68
The electron microscopic characterization of dedifferentiated cardiomyocytes mimics closely the changes observed following muscle unloading and cellular atrophy.74 Dedifferentiation has been claimed to result in the acquisition of a fetal neonatal program, but no quantitative data have been obtained to demonstrate that the alteration in the proportion of myofibrils and mitochondria with cell dedifferentiation reflects the cytoplasmic composition of fetal-neonatal myocytes.75 The notion that neonatal myocytes are immature cells lacking sarcomere striation4,69 is incorrect (Figure 5A). Well oriented myofibrils with intact sarcomeres are present in the fetal heart and the undifferentiated cytoplasm is rapidly replaced with myofibrillar structures and mitochondria shortly after birth in rodents.75 If the re-expression of the fetal program, postulated but not proven, pertains to dedifferentiated cardiomyocytes, protein synthesis needs to be shown by light and electron microscopic autoradiography (Figure 5B and 5C).75-77 Cells undergoing degenerative changes can be distinguished from dedifferentiating myocytes experiencing active protein synthesis.
Figure 5. Protein synthesis in developing or hypertrophying cardiomyocytes.
A, Phase contrast micrographs of fetal atrial (upper left) and ventricular (upper right) myocardium. Myofibrils are located predominantly at the subsarcolemmal region. The lower two panels illustrate by light microscopic autoradiography 3H-leucine incorporation (silver grains) indicative of ongoing protein synthesis within the atrial (left) and ventricular (right) fetal myocardium. Scale bars, 10 μm. B, Electron microscopic autoradiographs of developing fetal ventricular cardiomyocytes. Silver grains (3H-leucine) are located over contractile filaments and mitochondria, and are frequently associated with the periphery of the cells. Scale bars, 100 nm. C, Autoradiographs of transverse sections of LV myocardium after sham-operation (upper panel) or following constriction of the abdominal aorta (lower panel). The number of silver grains (3H-leucine) per high power field is 43% higher in the hypertrophied heart. Scale bars, 10 μm. Adapted from references 75 (panels A and B) and 76 (panel C).
Braun and colleagues have extended this original hypothesis and prospected that myocyte dedifferentiation constitutes a fundamental mechanism able to reinstitute the replicating process in post-mitotic myocytes. Following this molecular reprogramming, cardiomyocytes can divide and contribute to the homeostatic control of the organ and be responsible for the restoration of the structural and functional integrity of the myocardium following injury. Oncostatin M (OCM), through the activation of the OCM receptors in cardiomyocytes, converts adult myocytes into fetal-neonatal cells.69
Myocytes with the dedifferentiated cell phenotype were detected in idiopathic dilated cardiomyopathy (IDC) and in the border zone of infarcted human hearts. The expression of OCM was increased in these pathologic hearts and in a mouse model of dilated cardiomyopathy. However, these findings were not associated with indication of myocyte multiplication. Similarly, adult myocytes in culture exposed to OCM for 6 days acquired dedifferentiated properties but cell division was not shown, although adult myocytes have been reported to divide in vitro.78 Cell cycle progression was illustrated only in cultures of neonatal myocytes following exposure to OCM and stimulation with FGF-2. But neonatal myocytes have high telomerase activity and divide spontaneously in vitro.79 Moreover, the in vivo administration of OCM produced cellular changes consistent with myocyte dedifferentiation but myocyte replication was not apparent.
Myocyte dedifferentiation was not appreciated in human studies of myocardial aging, IDC, myocardial infarction and aortic stenosis.27-31 Myocyte mitotic images were identified, together with a significant increase in number of replicating cardiomyocytes expressing the cell cycle proteins Ki67 and MCM5. Large foci of newly-formed small cardiomyocytes were found in the absence of morphological changes of cell dedifferentiation.30 Clusters of immature cardiomyocytes were also occasionally seen within the necrotic infarcted myocardium.31 Analogous observations have been made experimentally following myocardial infarction, coronary artery constriction, myocardial aging and pacing-induced heart failure.34,36,37,80
Whether embryonic and fetal myocytes reverse to an immature phenotype assuming stem cell characteristics has been tested. Mice with EGFP-tagged c-kit-positive cells were used. Myocytes collected from fetal hearts at E16-E18 were cultured for a period of 2-3 days and the expression of EGFP in dividing myocytes was determined;79 102 myocytes in mitosis were examined and none showed EGFP labeling. Similarly, non-dividing fetal myocytes expressing different levels of sarcomeric proteins were all EGFP-negative. Additionally, c-kit-positive cells had a volume of ~115 μm3, while the size of cardiomyocytes varied from 600 to 1,100 μm3. These findings suggest that myocytes failed to reacquire a stem/progenitor cell fate.
In summary, the question whether the evidence provided supports the notion that post-mitotic cardiomyocytes have the ability to reprogram themselves, alter the complex, tightly organized composition of the cytoplasm and obtain a replicative cell phenotype cannot be positively answered. Similarly, whether upregulation of the inflammatory cytokine OCM has to be considered critical in the initiation and regulation of myocyte division is questionable. Adult myocytes exposed to OCM increase significantly in size and lose sarcomeric structures,69 a phenomenon that is inconsistent with the acquisition of fetal-neonatal characteristics. Fetal-neonatal myocytes have a volume of ~1,000-1,500 μm3 while the volume of adult cardiomyocytes is ~20,000-25,000 μm3. Cell volume should decrease dramatically for myocytes to acquire the properties of proliferating fetal-neonatal cells. There are no examples of replicating myocytes in rodents with a volume of ~20,000 μm3 or larger.38 Thus, the possibility that c-kit-positive CSCs represent dedifferentiated multiplying myocytes re-expressing the stem cell antigen c-kit77 is not supported by the majority of published observations.
Cardiomyogenesis and Bone Marrow Progenitor Cells
Despite the controversy that permeates the field, it is now generally accepted that myocyte renewal occurs in the adult heart and the debate is confined to the magnitude of the process rather than to the process itself. The recognition that myocytes are replaced continuously has put forward the challenging question concerning the cell(s) responsible for myocyte renewal. Over the last decade, contrasting results have been obtained on the contribution of HSCs and, more in general, bone marrow progenitor cells (BMPCs) to myocyte regeneration. If HSCs-BMPCs are directly implicated in the replacement of cardiomyocytes, this process requires stem cell transdifferentiation.
This biological process belongs to the class of cell transformations defined as metaplasia; metaplasia includes cases in which stem cells of one tissue acquire the cell phenotype of another tissue. Prenatally, undifferentiated cells undergo a progressive restriction of developmental options and this mechanism of embryonic specification was thought to be irreversible in adult life. This notion, however, has been challenged by several examples of transition from one cell type to another and from one cell lineage to another cell lineage.82 The ability of adult stem cells to generate cells beyond their own tissue boundary has been defined as cellular plasticity. Currently, the terms plasticity and transdifferentiation are used as synonyms.
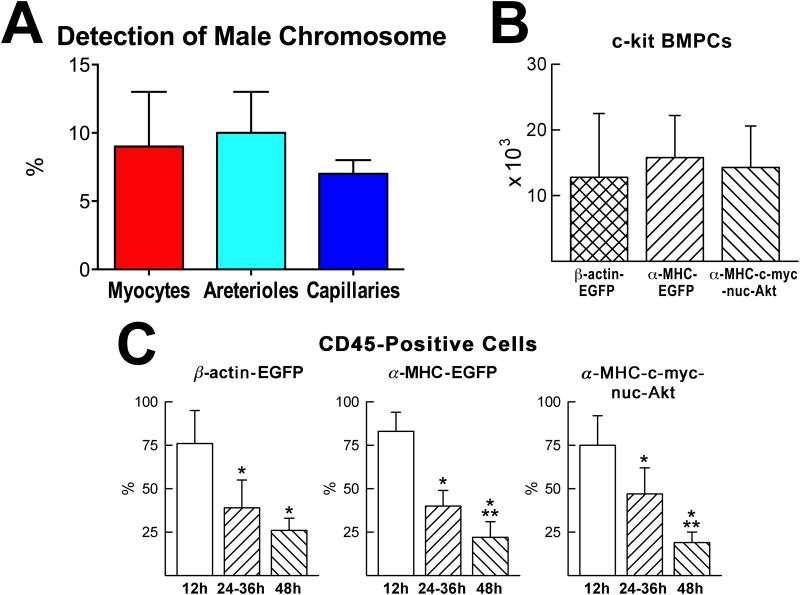
The concept of HSC-BMPC transdifferentiation was based on the identification of male cells in female human hearts transplanted in male recipients.83 In these cases of sex-mismatched cardiac transplant, the female heart in a male host showed a significant number of Y-chromosome positive myocytes and coronary vessels. Although discrepancies exist among laboratories in terms of the degree of cardiac chimerism, these results suggested that male cells can colonize the female heart and differentiate into myocytes and vascular structures (Figure 6A). The presence of male cells in the female heart was consistent with the contention that stem-like cells migrate to the cardiac allograft and give rise to the three main cardiac cell lineages: cardiomyocytes, endothelial cells, and smooth muscle cells.
Figure 6. Stem cell homing and myocardial regeneration.
A, Detection of host male cells in human female hearts transplanted in male recipients. B, Number of BMPCs present in the recipient infarcted mouse myocardium 48 hours after BMPC injection. C, The fraction of CD45-positive BMPCs decreases from 12 to 48 hours following cell implantation. Results are mean±SD. *P<0.05 vs. 12 h; **P<0.05 vs. 24–36 h. Adapted from reference 88.
To date, the c-kit-positive HSC-BMPC is the most versatile stem cell capable of breaking the law of tissue fidelity. The documentation in 2001 that c-kit-positive HSCs-BMPCs commit to cardiac cell lineages, replace the infarcted myocardium, and improve ventricular function70 has divided the scientific community initiating a debate that continues inexorably today (Table 1). These early results argued in favor of the differentiation of the injected cells into the myogenic and vascular cell phenotypes as the mechanism of the myocardial recovery after infarction. Subsequent studies have confirmed the original observations.84-90
C-kit-positive HSCs-BMPCs constitute the class of hematopoietic progenitors that has been at the center of the controversy of stem cell plasticity. However, CD34-positive cells, CD133-positive cells, endothelial progenitor cells (EPCs), and bone marrow side population cells (BMSP) have all been shown to transdifferentiate to a certain degree.91-94 Similarly, bone marrow mesenchymal stromal cells (MSCs) can acquire the cardiomyocyte lineage, although their principal function appears to be mediated by the release of growth factors and cytokines with subsequent activation of resident CSCs.95 Recently, c-kit-positive cortical bone-derived stem cells (CBSCs) have been shown to generate myocytes and coronary vessels.96 CBSCs differentiate into myocytes and activate indirectly the formation of coronary arterioles and capillaries by secreting proangiogenic factors that stimulate endogenous neovascularization. Thus, CD34-positive cells, CD133-positive cells, EPCs, BMSP, MSCs and CBSCs can create in variable proportions new myocardium; however, paracrine signals originated by the delivered cells may be as relevant as transdifferentiation.
It is difficult to recognize the variables that condition a negative versus a positive result and provide the reader with an explanation for the discrepancy among laboratories.
Initially, the data in favor of the plasticity of c-kit-positive HSCs-BMPCs were challenged based on the protocol employed for the recognition of the injected cells and characterization of the differentiated progeny.97,98 The identification of cardiomyocytes derived from HSC-BMPC transdifferentiation70,84 was criticized as being the product of unspecific staining and autofluorescence artifacts. This comment was triggered by the erroneous interpretation of poor fixation of skeletal muscle;99 the uneven distribution of green fluorescence in sections of skeletal muscle was considered equivalent to the fluorescence resulting from immunolabeling of EGFP-positive cells. The autofluorescence generated by the cross-linking of skeletal muscle proteins during aldehyde fixation has nothing in common with the detection of EGFP with anti-GFP; the intensity of the fluorescent signal is 20-30-fold stronger than background fluorescence.86,100 More importantly, a methodology was introduced in which immunolabeling of proteins was obtained in the absence of autofluorescence inherent in tissue sections of formalin-fixed myocardium. Myocardial regeneration promoted by c-kit-positive HSCs-BMPCs was confirmed strengthening the original findings.88
In a comparable manner, methodological artifacts were raised as the source of negative results. The use of frozen myocardial sections for light and confocal microscopy97,98 was criticized as having serious problems related to the poor quality of the preparation, inaccuracy of immunolabeling and inadequate microscopic resolution.86,88 Major difficulties exist in the recognition of small newly formed structures, and cardiomyocytes derived from bone marrow cell transdifferentiation have often a diameter of 3-5 μm and a volume ~500 μm3. The infarct is rarely preserved in frozen sections and the regenerated cells are nearly 100% restricted to the injured myocardium. Technical limitations were combined with unrealistic statements which fueled the controversy. To justify some unusual results, a 100% degree of success in the injection of cells in the mouse heart was claimed.97 Moreover, a mortality rate of 8% with infarcts affecting 60% of the mouse left ventricle was reported. In spite of the perfect care that patients have in the most sophisticated medical centers, a 46% infarct results in intractable cardiogenic shock. Rodents are not different, although they can survive slightly larger infarcts. The hearts analyzed for the presence of cardiac regeneration were not the same studied functionally or with routine histology.97,98 Whether coronary ligation was unsuccessful or a small or large infarct was obtained was not determined and negative claims were based on only two animals97 that were supposedly properly infarcted and injected with cells comparable to those used in the earlier positive study.70
A double transgenic mouse model for genetic lineage mapping was introduced to determine whether c-kit-positive BMPCs transdifferentiate and generate cardiomyocytes after infarction.101 In this model, all cardiomyocytes express β-galactosidase but, following a pulse of 4-OH-tamoxifen, myocytes convert the expression of β-galactosidase to EGFP as a result of Cre-mediated DNA recombination. The changes in the proportion of β-galactosidase and EGFP positive cardiomyocytes was employed to monitor the origin of myocytes from: a) BMPCs (myocytes negative for β-galactosidase and EGFP); b) endogenous progenitors (myocytes positive for β-galactosidase only); or c) pre-existing cardiomyocytes (myocytes positive for EGFP only).101
In this study, a 40-50% infarct size resulted in no increase in end-diastolic pressure, and systolic pressure was higher in infarcted than in sham-operated animals. Moreover, the measurements of chamber volume were highly problematic and inconsistent with stroke volume and cardiac output in this model.102 Apparently, the injected BMPCs activated an unknown pool of resident cells, which led to myocyte formation in the infarct border zone; these myocytes were β-galactosidase-positive only.101 A major effort was then made to exclude that the administered c-kit-positive BMPCs, or the resident c-kit-positive CSCs had any role in the stimulation of cardiomyogenesis. The injected BMPCs disappeared rapidly, being undetectable by 4 weeks; myocyte formation was not seen at 2 weeks but was evident at 8 weeks.101
Myocardial infarction and the delivery of c-kit-positive BMPCs led to a 2.7-fold increase in the number of regenerated cardiomyocytes in the infarct border zone, but this remarkable growth response had no impact on infarct dimension or cardiac performance. The high level of myocyte formation was consistent with the rather striking increase in the percentage of BrdU labeled cardiomyocytes during the first week after myocardial infarction. However, there was no increase in myocyte regeneration at two weeks; both sets of data cannot be correct. Moreover, the dramatic increase in Nkx.2.5, GATA4, and Nkx2.5-GATA4 positive cells in the infarct border zone at 1 week can hardly be reconciled with the lack of newly formed myocytes at 2 weeks.101
Another confusing factor is the absence of information concerning whether the injected c-kit-positive BMPCs engrafted within the infarcted myocardium. Although an extravagant number of BMPCs (6 × 105) was administered, it can be assumed that only a very low fraction homed to the intact myocardium bordering the infarct. The large majority of cells would be expected to accumulate at the interface between the infarct and the spared tissue where the regenerative response commonly occurs. Importantly, newly formed cardiomyocytes in the infarct border zone and within the necrotic myocardium are small, often in mitosis, and are easily distinguishable from preexisting cells.70,86,88 Unexpectedly, the new myocytes were all fully differentiated and showed morphology identical to that of post-mitotic surviving cells. In the absence of β-galactosidase staining, it would be impossible to recognize preexisting EGFP-positive cardiomyocytes from the regenerated cells. It is difficult to reconcile biologically that only EGFP-negative cardiomyocytes in which somehow DNA recombination did not occur reentered the cell cycle and divided, while the EGFP-positive cells hypertrophied only.
The implementation of genetically engineered mice enabling lineage mapping studies is unquestionably valuable but, in this case, there are problems with either the mouse model or the protocols employed for the detection of the engraftment and lineage specification of the delivered cells. It is unfortunate that the authors did not attempt to reconcile their data with the results obtained in all previous studies whether in favor or against BMPC transdifferentiation. In all cases, rare myocytes, inflammatory cells, macrophages or vascular profiles derived from the c-kit-positive cells were found; the claim that no cells of bone marrow origin were present in the infarcted or surrounding myocardium101 is in contrast with all published reports.70,86,88,89,97,98,103-105
To address the question concerning homing, engraftment and differentiation of c-kit-positive BMPCs, and demonstrate reproducibility of results, four laboratories with complementary expertise undertook a series of joined experiments.88 BMPCs for myocardial regeneration were obtained from three transgenic mice. In the first, EGFP was driven by the ubiquitous β-actin promoter; in the second, EGFP was driven by the cardiac-specific α-myosin-heavy-chain (α-MHC) promoter; and in the third, a c-myc-tagged nuclear-targeted-Akt transgene was driven by the α-MHC-promoter. With the first category of BMPCs (β-actin-EGFP), all cardiac cells formed by BMPC differentiation were expected to express EGFP; with the second category of BMPCs (α-MHC-EGFP), only myocytes formed by BMC differentiation were expected to express EGFP; and with the third category of BMPCs (α-MHC-c-myc-tagged-nuc-Akt), only myocytes formed by BMC differentiation were expected to express c-myc in their nuclei. In all cases, male BMPCs were injected in female infarcted mice so that cell genotyping would allow the distinction between resident female cardiac cells and newly generated male cells.
By this approach, it was demonstrated that c-kit-positive BMPCs engraft in proximity to the infarcted myocardium and differentiate into cells of the cardiogenic lineage, forming functionally-competent cardiomyocytes and vascular structures; this process is associated with the loss of bone marrow cell epitopes (Figure 6B and 6C). Cell fusion was also excluded by documenting a diploid DNA content together with one set only of sex chromosomes in nuclei of newly formed myocytes, and vascular cells. Importantly, cardiac repair attenuated ventricular remodeling and the deterioration in cardiac function that occurs after infarction. Thus, adult c-kit-positive BMPCs implanted in the infarcted heart lose the hematopoietic fate and form de novo myocardium. These genetic studies88 argue strongly against the negative results recently reported.101
Why myocardial regeneration mediated by HSC-BMPC transdifferentiation has been caught in the controversy remains unexplained. The discrepancy may be dictated by differences in the methodology employed and the complexity related to the identification of small developing myocytes, often searched for with tools that lack the necessary resolution required for this analysis. The localization of the Y-chromosome in newly formed cells is more sensitive than the detection of the fluorescent tag, which leads to underestimation of cell plasticity.106 There is no evidence that the entire transdifferentiated progeny retains EGFP or that the level of EGFP in the regenerated cells is higher than background autofluorescence. The expansion of the small pool of engrafted donor cells carrying EGFP may affect transgene expression even when driven by a ubiquitously active promoter.106 The contrasting results, however, raise the possibility that distinct classes of c-kit-positive HSCs-BMPCs may have been tested and only a selective subset of bone marrow cells retains the ability to transdifferentiate and acquire the cardiomyocyte lineage. Moreover, the molecular pathway that controls the change in the phenotypic properties of HSCs-BMPCs remains to be defined. The identification of this determinant of HSC-BMPC fate is currently under intense investigation in view of its biological significance and important clinical implications.
Cardiomyogenesis and Resident Cardiac Progenitor Cells
The new century has observed a dramatic change in our understanding of myocardial biology. Towards the end of the last century and the beginning of the new century, several reports have suggested that a pool of cardiomyocytes is cycling in the normal and pathologic human heart28-31 and similar observations were made in small and large animals following myocardial infarction, pressure overload, physiological aging or pacing-induced heart failure.25,34,36,40,41,45,80 This body of work has provided the fundamental information that has led to the identification of a compartment of resident CSCs in 2003.107 By employing the surface antigen c-kit, CSCs were first recognized and characterized in rats107 and subsequently in mice,35,108 dogs109 and, ultimately, in human beings.67,110,111 The c-kit-positive CSCs are self-renewing clonogenic and multipotent in vitro and in vivo meeting the criteria of bona fide tissue specific adult stem cells. Serial transplantation assay in vivo, together with different models of myocardial damage and HF, have shown that this primitive cell class is necessary and sufficient for the regeneration and repair of the damaged myocardium.47 The c-kit-positive CSCs are present in the left and right ventricle but tend to accumulate in the atria and apex, which correspond to anatomical areas exposed to low levels of hemodynamic stress.34,36,112
Several laboratories have made a major effort to define the distribution of this class of cardiac progenitors in the different anatomical regions of the human heart and in various pathological conditions (Table 1). CSCs are present in the outflow tract of the right ventricle in infants with tetralogy of Fallot113 and can be isolated and propagated in vitro from explanted hearts of pediatric patients with end stage HF.114 These progenitors are most abundant in the neonatal period and decrease rapidly in infants and children, losing in part their potency to reconstitute the injured myocardium.115,116 In the adult human heart, CSCs tend to accumulate in the subepicardium and, in the presence of severe ventricular dysfunction, may lose some of their differentiation properties and ability to reach functional competence.117-119 The right atrium is the most appropriate source for c-kit-positive CSCs,120 although no detectable differences in the growth behavior of human CSCs obtained from the four cardiac chambers have also been reported.121 The number of intact CSCs decreases with age, diabetes and chronic coronary artery disease,122 emphasizing the relevance that resident progenitors may have in the manifestations of the aging and diabetic myopathy, and ischemic HF. In a clever manner, adoptive transfer has been introduced to rejuvenate human CSCs and restore their impaired regenerative capacity.123,124 In this regard, multiple protocols have been developed to optimize the acquisition and expansion of human c-kit-positive CSCs for clinical use.125-130
In a comparable manner, several independent studies have identified, collected and expanded c-kit-positive CSCs in small and large animals and developed strategies aiming at the enhancement of their growth reserve and effectiveness in promoting cardiac repair following ischemic myocardial damage.131-140 Additionally, activation and commitment of endogenous c-kit-positive CSCs occur with pregnancy and dynamic exercise playing a role in the growth response of the myocardium.141,142 An increase in maternal cortisol during pregnancy stimulates c-kit-positive cells and results in an increase in heart weight and ventricular wall thickness.143
The recognition that CSCs reside in the adult heart has raised the question whether the CSC pool is organ specific or derives from colonization of HSCs from the bone marrow to the myocardium. If the CSCs are not a subpopulation of HSCs, they may be present early during development and be responsible for cardiomyogenesis in the embryonic and fetal heart, a role that they may continue to have postnatally144 and in adulthood.24,27 Conversely, if these primitive cells represent a HSC subset, they are located in the hematopoietic system, and continuously translocate to the developing heart, where they assume specific functions, leading to the formation of the mature cardiac phenotype. To address this issue, the entire live embryo, including the yolk sac, has been cultured utilizing a mouse model with genetically-tagged CSCs and analyzed by two-photon microscopy.79 EGFP-positive cells were present in the yolk sac but did not translocate to the heart. CSCs were detected in the heart tube where they showed morphogenetic movements only, supporting the notion that prenatal cardiac development is controlled by growth and differentiation of CSCs, which are responsible for the formation of the myocyte progeny present at birth.79 Although homing of non-cardiac EGFP-positive cells to the myocardium was not identified with certainty, it cannot be excluded that the bone marrow contributes partly to the growth of the embryonic and fetal heart, a mechanism consistent with the ability of HSCs to acquire the cardiomyocyte fate.16,19 With maturation, CSCs progressively lose their primitive state, contractile proteins accumulate and when the adult cell phenotype is acquired cell division is suppressed.
Stem cells are stored in niches that constitute the microenvironment in which stem cells are maintained in a quiescent state.145 After activation, stem cells replicate and migrate out of the niches to sites of cell replacement where they differentiate and acquire the adult phenotype. Niche homeostasis is controlled by stem cell division, which preserves the proportion of primitive and committed cells within the parenchyma.145 In the niches, stem cells are connected to the supporting cells which anchor stem cells to the niche and modulate growth signals from the surrounding tissue.145,146 Stem cells divide rarely and their growth kinetics and localization can be assessed by the label-retaining assay; the long-term label-retaining property of a cell documents its stemness, while the progressive dilution of the label identifies the formed progeny.147,148
Based on this premise, long-term BrdU-positive CSCs have been identified mostly in the atria and apex of the adult mouse heart.34-36,112 Clusters of uncommitted CSCs are surrounded by fibronectin and are connected by junctional complexes, made by connexin 43 and N-cadherin, to post-mitotic cardiomyocytes and fibroblasts. Connexins are gap junction channel proteins that mediate passage of small molecules involved in cell-to-cell communication. Cadherins are calcium-dependent transmembrane adhesion molecules, which have a dual function: they anchor stem cells to the microenvironment and promote a cross-talk between stem cells and between stem cells and the supporting cells. Additionally, CSCs divide symmetrically and asymmetrically in vitro and in vivo and their fate is dictated by the distribution of Numb and α-adaptin and the activity of the Notch receptor.71,112,144,149 Cardiac niches create the necessary, permissive milieu for the long-term residence, survival, and growth of CSCs.
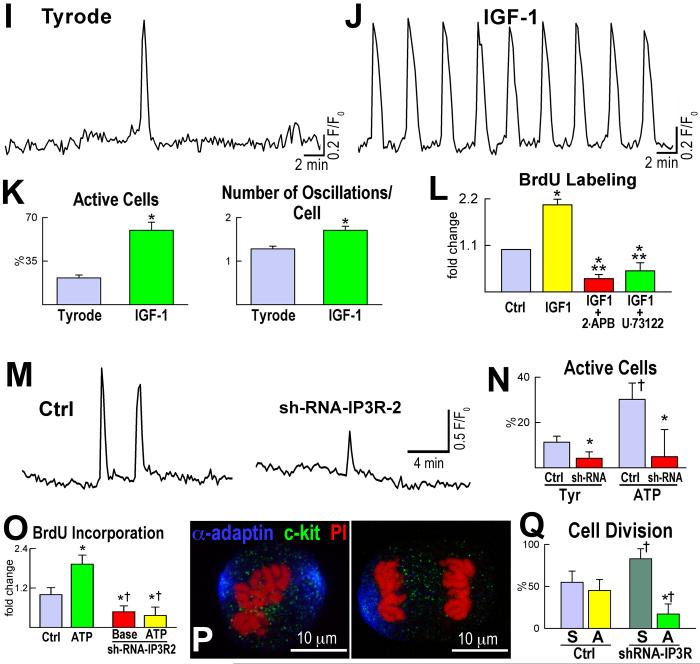
The identification of the mechanisms responsible for the activation and lineage specification of CSCs has been a difficult challenge. Ca2+ has two fundamental functions in the heart: it activates growth processes and modulates the mechanical behavior of cardiomyocytes.150 Spontaneous Ca2+ oscillations occur in adult human CSCs and in mouse embryonic-fetal and adult CSCs, and this process promotes their entry into the cell cycle (Figure 7).79,151 Similarly, Ca2+ oscillations control the progression into the cell cycle of ESCs and favor proliferation and differentiation of MSCs.152,153 The regulation of Ca2+ in human and mouse CSCs does not involve cell-to-cell communication and the interstitial milieu; Ca2+ oscillations take place independently151 from coupling with cardiomyocytes, or from the presence of extracellular Ca2+. Cytosolic Ca2+ plays a crucial role in CSC growth, and induction of Ca2+ oscillations in human CSCs, prior to their intramyocardial delivery in vivo, is coupled with enhanced cell engraftment within the infarcted myocardium, increased cell expansion and potentiation of myocyte regeneration.151
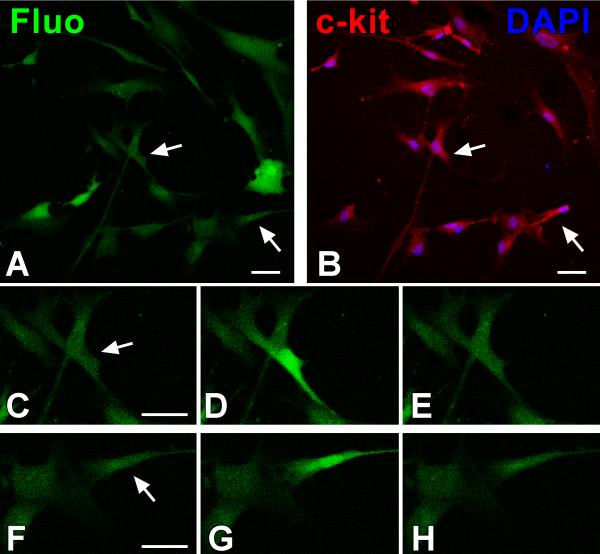
Figure 7. Intracellular Ca2+ in c-kit-positive human cardiac progenitor cells (hCPCs).
A through H, hCPCs loaded with the Ca2+ sensitive dye Fluo-3 (Fluo, green) (A) were monitored for intracellular Ca2+ oscillations and subsequently fixed and stained (B) with DAPI (blue) and c-kit antibody (red). The c-kit receptor was detected in cells (arrows) experiencing Ca2+ oscillations (C through E and F through H). Scale bars: 40 μm. I, Cytosolic Ca2+ level in a single hCPC in Tyrode solution showing a single Ca2+ elevation over a period of 33 min. J, Intracellular Ca2+ in a single hCPC exposed to the mitogen IGF-1. K, Frequency of Ca2+ oscillations in hCPCs at baseline (Tyrode solution) and following exposure to IGF-1. *P<0.05 versus Tyrode. L, Proliferation of hCPCs in the presence of IGF-1 alone or in combination with the Ca2+ oscillation inhibitors 2-APB or U-73122. *P<0.05 vs. Ctrl, ** P<0.05 vs. IGF-1. Adopted from reference 210. M, Ca2+ oscillations of c-kit-positive fetal mouse CPCs at baseline (Ctrl) and after downregulation of IP3 receptor type-2 by sh-RNA (sh-RNA-IP3-R2). N, The percentage of CPCs displaying Ca2+ oscillations in Tyrode or following exposure to ATP is attenuated in sh-RNA-IP3-R2 (sh-RNA). *,†P<0.05 vs. Ctrl and ATP alone, respectively. O, ATP-induced Ca2+ oscillations in CPCs favor cell replication. This effect is abrogated with reduced expression of IP3 receptor type-2 by sh-RNA. *,†P<0.05 vs. Ctrl and ATP alone, respectively. Baseline (Base): CSCs infected with control vector. P, Symmetric (left) and asymmetric (right) division of fetal CPCs determined by the localization of the cell fate protein α-adaptin (blue). c-kit, green; chromosomes: PI, red. Q, Reduced IP3 receptor expression inhibits asymmetric cell division. *,†P<0.05 vs. symmetric division (S) and Ctrl, respectively. Asymmetric division (A). Adapted from references 151 (panels A through L) and 79 (panels M through Q).
The growth promoting effects of c-kit-positive CSCs and ESCs are both initiated by spontaneous oscillations in intracellular Ca2+ through the activation of inositol-1,4,5-triphosphate receptors, which condition CSC asymmetric division and myocyte lineage specification.79,151 The differentiation of CSCs into mature myocytes may involve the loss of T-type Ca2+ channels, which is associated with withdrawal of amplifying myocytes from the cell cycle and binucleation, Ca2+ influx via L-type Ca2+ channels (LTCC) and transient receptor potential (TRP) channels. Increases in LTCC and TRP current activate NFAT signaling and myocyte hypertrophy.154-156
Collectively, significant information has been acquired as a result of the effort made by multiple groups in defining the properties of c-kit-positive human and non human CSCs. In vivo protocols have provided strong evidence concerning the ability of these cells to form functionally-competent cardiomyocytes and coronary vessels integrated with the recipient myocardium.71,107,109-111,157,158 Human CSCs can divide by asymmetric and symmetric chromatid segregation111,159 and CSCs inheriting only the mother DNA have an unprecedented capacity to repair the infarcted myocardium experimentally, emphasizing the clinical relevance of carefully defined biological processes. Gain and loss of function assays have shown that scattered myocyte loss activates the endogenous regenerative process by which c-kit-positive CSCs restore the structural and functional integrity of the injured heart.37 Based on high-throughput transcriptional profiling, c-kit-positive CSCs represent the most primitive cell population in the myocardium and their molecular signature is distinct from c-kit-positive HSCs.160
Conclusions
In summary, cardiomyocytes derive from transdifferentiation of HSCs-BMPCs and/or activation and lineage specification of resident CSCs. The data in favor of myocyte dedifferentiation are inconclusive and far from proving the validity of this mechanism of cell replication. Similarly, the possibility that post-mitotic myocytes can reenter the cell cycle and divide has little basis. As described previously in several review articles,16-19 in addition to c-kit-positive CSCs, other progenitor classes have been identified in the adult heart and their ability to form myocytes in vitro and in vivo has been carefully shown. Despite the importance of these other categories of primitive cells, the c-kit-positive CSC has been chosen here for discussion in view of the current controversy and the attention it has received in the scientific community worldwide. Laboratories in different continents have been able to isolate and propagate c-kit-positive CSCs from animals and humans and document their relevance in pre-clinical studies. Although the translation of this significant body of work to patients is in its infancy,18 it is reasonable to prospect clinical trials aiming at the recognition whether the delivery of autologous CSCs interferes with the unfavorable evolution of advanced HF.
The phase during which the existence and significance of c-kit-positive CSCs were questioned is ending and the collaborative effort of several laboratories will unquestionably promote the implementation of this discovery in human beings. Complex animal models cannot be taken and interpreted at face value ignoring the extraordinary progress that has been made concerning our understanding of myocardial biology. There is no organ in our organism in which stem cells have been identified and shown to have no fundamental importance in regulating cell homeostasis and regeneration. Problematic, often un-interpretable experimental results cannot condition the inclusion of CSCs into the management of human HF.
Recently, a lineage tracing study in the mouse has disputed the implications that c-kit-positive CSCs have in the regulation of myocyte renewal in the adult heart.161 The knock in strategy employed has led to the loss of one allele of the c-kit gene and this may have affected the function of the c-kit receptor and the ability of CSCs to proliferate and form a myocyte progeny. Defects in c-kit receptor function may be organ specific and the myocardium could have been more severely influenced than the bone marrow and the lung. The discrepancy between the number of cardiomyocytes and the number of alveolar epithelial cells derived from c-kit-positive cells in this transgenic mouse model clearly indicates that this may be actually the case.161 Additionally, given the substantial evidence supporting a role of c-kit-positive CSCs in myocardial homeostasis, repair after injury and aging, the new results are surprising.161 Rectifying the bases of the differences in findings with this new mouse model versus those of our group and others is clearly important to the field.
Over the last 25 years, a large number of transgenic mice have been generated and this remarkable effort has advanced our knowledge of the multiple signaling pathways of myocyte growth. Very disappointing advances, however, have been made in the elucidation of the etiology of human HF and its treatment. The translation of basic information to patient care has been an occasional event created predominantly by the ingenuity and curiosity of isolated investigators. This is true for the implications that myocyte death, myocyte regeneration, and exogenous and endogenous stem cells have in the progression and future treatment of HF. However, there is still a strong debate in the field regarding the robustness of the regenerative response of the adult myocardium (Table 1) and the mechanisms by which new myocytes are formed. It is difficult to predict at present when a general consensus on the extent and origin of myocytes will be achieved in the scientific community, but an optimistic view can be prospected since several laboratories are engaged in resolving this biological problem which has critical implications in the etiology and severity of human HF.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by NIH and AHA grants.
Nonstandard Abbreviations and Acronyms
- HSCs
hematopoietic stem cells
- CSCs
cardiac stem cells
- HF
heart failure
- PCNA
proliferating cell nuclear antigen
- Rb
retinoblastoma protein
- OCM
oncostatin M
- IDC
idiopathic dilated cardiomyopathy
- BMPCs
bone marrow progenitor cells
- EPCs
endothelial progenitor cells
- BMSP
bone marrow-derived side population cells
- MSCs
mesenchymal stromal cells
- CBSCs
cortical bone-derived stem cells
- α-MHC
α-myosin heavy chain
- ESCs
embryonic stem cells
- iPS cells
induced pluripotent stem cells
- ER
endoplasmic reticulum
- LTCC
L-type Ca2+ channels
- TRP channels
transient receptor potential channels
- α-sarcomeric actin
α-SA
- LV
left ventricle
- PI
propidium iodide
- CSA
myocyte cross-sectional area
- hCPCs
human cardiac progenitor cells
Footnotes
Disclosures
None.
References
- 1.Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83:1–1. doi: 10.1161/01.res.83.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res. 1998;83:15–15. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 3.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–326. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun T, Martire A. Cardiac stem cells: paradigm shift or broken promise? A view from developmental biology. Trends Biotechnol. 2007;25:441–441. doi: 10.1016/j.tibtech.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Yi BA, Wernet O, Chien KR. Pregenerative medicine: developmental paradigms in the biology of cardiovascular regeneration. J Clin Invest. 2010;120:20–20. doi: 10.1172/JCI40820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez LR, Schocket AL, Stanford RE, Claman HN, Kohler PF. Gastrointestinal involvement in leukocytoclastic vasculitis and polyarteritis nodosa. J Rheumatol. 1980;7:677–677. [PubMed] [Google Scholar]
- 7.Maude GH. Bone marrow infarction in sickle cell anemia. Blood. 1984;63:243. [PubMed] [Google Scholar]
- 8.Watanabe K, Abe H, Mishima T, Ogura G, Suzuki T. Polyangitis overlap syndrome: a fatal case combined with adult Henoch-Schönlein purpura and polyarteritis nodosa. Pathol Int. 2003;53:569–569. doi: 10.1046/j.1440-1827.2003.01515.x. [DOI] [PubMed] [Google Scholar]
- 9.Leong FT, Freeman LJ. Acute renal infarction. JR Soc Med. 2005;98:121–121. doi: 10.1258/jrsm.98.3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berland T, Oldenburg WA. Acute mesenteric ischemia. Curr Gastroenterol Rep. 2008;10:341–341. doi: 10.1007/s11894-008-0065-0. [DOI] [PubMed] [Google Scholar]
- 11.Leri A, Kajstura J, Anversa P. Cardiac Regeneration and Aging. In: Rosenthal N, Harvey RP, editors. Heart Development and Regeneration. II. London: 2010. pp. 951–980. [Google Scholar]
- 12.Gersh BJ, Simari RD, Behfar A, Terzic CM, Terzic A. Cardiac cell repair therapy: a clinical perspective. Mayo Clin Proc. 2009;84:876–876. doi: 10.4065/84.10.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beohar N, Rapp J, Pandya S, Losordo DW. Rebuilding the damaged heart: the potential of cytokines and growth factors in the treatment of ischemic heart disease. J Am Coll Cardiol. 2010;56:1287–1287. doi: 10.1016/j.jacc.2010.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohsin S, Siddiqi S, Collins B, Sussman MA. Empowering adult stem cells for myocardial regeneration. Circ Res. 2011;109:1415–1415. doi: 10.1161/CIRCRESAHA.111.243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–923. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res. 2011;109:941–941. doi: 10.1161/CIRCRESAHA.111.243154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Anversa P, Leri A. Innate regeneration in the aging heart: healing from within. Mayo Clin Proc. 2013;88:871–871. doi: 10.1016/j.mayocp.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–810. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anversa P, Kajstura J, Rota M, Leri A. Regenerating new heart with stem cells. J Clin Invest. 2013;123:62–62. doi: 10.1172/JCI63068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Januzzi JL, Jr, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J. 2012;33:2265–2265. doi: 10.1093/eurheartj/ehs191. [DOI] [PubMed] [Google Scholar]
- 21.Dorn GW., 2nd Molecular mechanisms that differentiate apoptosis from programmed necrosis. Toxicol Pathol. 2013;41:227–227. doi: 10.1177/0192623312466961. [DOI] [PubMed] [Google Scholar]
- 22.Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Røsjø H, Šaltytė Benth J, Maggioni A, Domanski MJ, Rouleau JL, Sabatine MS, Braunwald E, PEACE Investigators Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol. 2013;61:1240–1240. doi: 10.1016/j.jacc.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Wohlschlaeger J, Levkau B, Brockhoff G, Schmitz KJ, von Winterfeld M, Takeda A, Takeda N, Stypmann J, Vahlhaus C, Schmid C, Pomjanski N, Böcking A, Baba HA. Hemodynamic support by left ventricular assist devices reduces cardiomyocyte DNA content in the failing human heart. Circulation. 2010;121:989–989. doi: 10.1161/CIRCULATIONAHA.108.808071. [DOI] [PubMed] [Google Scholar]
- 24.Kajstura J, Urbanek K, Perl S, Hosoda T, Zheng H, Ogórek B, Ferreira-Martins J, Goichberg P, Rondon-Clavo C, Sanada F, D'Amario D, Rota M, Del Monte F, Orlic D, Tisdale J, Leri A, Anversa P. Cardiomyogenesis in the adult human heart. Circ Res. 2010;107:305–305. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Quaini F, Cigola E, Lagrasta C, Saccani G, Quaini E, Rossi C, Olivetti G, Anversa P. End-stage cardiac failure in humans is coupled with the induction of proliferating cell nuclear antigen and nuclear mitotic division in ventricular myocytes. Circ Res. 1994;75:1050–1050. doi: 10.1161/01.res.75.6.1050. [DOI] [PubMed] [Google Scholar]
- 26.Beltrami CA, Di Loreto C, Finato N, Rocco M, Artico D, Cigola E, Gambert SR, Olivetti G, Kajstura J, Anversa P. Proliferating cell nuclear antigen (PCNA), DNA synthesis and mitosis in myocytes following cardiac transplantation in man. J Mol Cell Cardiol. 1997;29:2789–2789. doi: 10.1006/jmcc.1997.0514. [DOI] [PubMed] [Google Scholar]
- 27.Kajstura J, Gurusamy N, Ogórek B, Goichberg P, Clavo-Rondon C, Hosoda T, D'Amario D, Bardelli S, Beltrami AP, Cesselli D, Bussani R, del Monte F, Quaini F, Rota M, Beltrami CA, Buchholz BA, Leri A, Anversa P. Myocyte turnover in the aging human heart. Circ Res. 2010;107:1374–1374. doi: 10.1161/CIRCRESAHA.110.231498. [DOI] [PubMed] [Google Scholar]
- 28.Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci USA. 1998;95:8801–8801. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1750. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 30.Urbanek K, Quaini F, Tasca G, Torella D, Castaldo C, Nadal-Ginard B, Leri A, Kajstura J, Quaini E, Anversa P. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci USA. 2003;100:10440–10440. doi: 10.1073/pnas.1832855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci USA. 2005;102:8692–8692. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–433. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capasso JM, Bruno S, Cheng W, Li P, Rodgers R, Darzynkiewicz Z, Anversa P. Ventricular loading is coupled with DNA synthesis in adult cardiac myocytes after acute and chronic myocardial infarction in rats. Circ Res. 1992;71:1379–1379. doi: 10.1161/01.res.71.6.1379. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Müller P, Esposito G, Bearzi C, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, Leri A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–597. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 35.Hosoda T, D'Amario D, Cabral-Da-Silva MC, Zheng H, Padin-Iruegas ME, Ogorek B, Ferreira-Martins J, Yasuzawa-Amano S, Amano K, Ide-Iwata N, Cheng W, Rota M, Urbanek K, Kajstura J, Anversa P, Leri A. Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci USA. 2009;106:17169–17169. doi: 10.1073/pnas.0903089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanada F, Kim J, Czarna A, Chan NY, Signore S, Ogórek B, Isobe K, Wybieralska E, Borghetti G, Pesapane A, Sorrentino A, Mangano E, Cappetta D, Mangiaracina C, Ricciardi M, Cimini M, Ifedigbo E, Perrella MA, Goichberg P, Choi AM, Kajstura J, Hosoda T, Rota M, Anversa P, Leri A. c-Kit-positive cardiac stem cells nested in hypoxic niches are activated by stem cell factor reversing the aging myopathy. Circ Res. 2014;114:41–41. doi: 10.1161/CIRCRESAHA.114.302500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kajstura J, Zhang X, Reiss K, Szoke E, Li P, Lagrasta C, Cheng W, Darzynkiewicz Z, Olivetti G, Anversa P. Myocyte cellular hyperplasia and myocyte cellular hypertrophy contribute to chronic ventricular remodeling in coronary artery narrowing-induced cardiomyopathy in rats. Circ Res. 1994;74:383–383. doi: 10.1161/01.res.74.3.383. [DOI] [PubMed] [Google Scholar]
- 38.Limana F, Urbanek K, Chimenti S, Quaini F, Leri A, Kajstura J, Nadal-Ginard B, Izumo S, Anversa P. bcl-2 overexpression promotes myocyte proliferation. Proc Natl Acad Sci USA. 2002;99:6257–6257. doi: 10.1073/pnas.092672899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haubner BJ, Adamowicz-Brice M, Khadayate S, Tiefenthaler V, Metzler B, Aitman T, Penninger JM. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging (Albany NY) 2012;4:966–966. doi: 10.18632/aging.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olivetti G, Ricci R, Anversa P. Hyperplasia of myocyte nuclei in long-term cardiac hypertrophy in rats. J Clin Invest. 1987;80:1818–1818. doi: 10.1172/JCI113278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Capasso JM. Hypertensive cardiomyopathy. Myocyte nuclei hyperplasia in the mammalian rat heart. J Clin Invest. 1990;85:994–994. doi: 10.1172/JCI114576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Force T, Michael A, Kilter H, Haq S. Stretch-activated pathways and left ventricular remodeling. J Card Fail. 2002;8:S351–S358. doi: 10.1054/jcaf.2002.129272. [DOI] [PubMed] [Google Scholar]
- 43.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–970. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leri A, Franco S, Zacheo A, Barlucchi L, Chimenti S, Limana F, Nadal-Ginard B, Kajstura J, Anversa P, Blasco MA. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 2003;22:131–131. doi: 10.1093/emboj/cdg013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–514. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 46.Torzewski M, Wenzel P, Kleinert H, Becker C, El-Masri J, Wiese E, Brandt M, Pautz A, Twardowski L, Schmitt E, Münzel T, Reifenberg K. Chronic inflammatory cardiomyopathy of interferon γ-overexpressing transgenic mice is mediated by tumor necrosis factor-α. Am J Pathol. 2012;180:73–73. doi: 10.1016/j.ajpath.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, Henning BJ, Stirparo GG, Papait R, Scarfò M, Agosti V, Viglietto G, Condorelli G, Indolfi C, Ottolenghi S, Torella D, Nadal-Ginard B. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827–827. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 48.Sdek P, Zhao P, Wang Y, Huang CJ, Ko CY, Butler PC, Weiss JN, Maclellan WR. Rb and p130 control cell cycle gene silencing to maintain the postmitotic phenotype in cardiac myocytes. J Cell Biol. 2011;194:407–407. doi: 10.1083/jcb.201012049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hassink RJ, Pasumarthi KB, Nakajima H, Rubart M, Soonpaa MH, de la Rivière AB, Doevendans PA, Field LJ. Cardiomyocyte cell cycle activation improves cardiac function after myocardial infarction. Cardiovasc Res. 2008;78:18–18. doi: 10.1093/cvr/cvm101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–257. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 51.Kühn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–962. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 52.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–313. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–1175. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engel FB, Schebesta M, Keating MT. Anillin localization defect in cardiomyocyte binucleation. J Mol Cell Cardiol. 2006;41:601–601. doi: 10.1016/j.yjmcc.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 55.Shapiro SD, Ranjan AK, Kawase Y, Cheng RK, Kara RJ, Bhattacharya R, Guzman-Martinez G, Sanz J, Garcia MJ, Chaudhry HW. Cyclin A2 induces cardiac regeneration after myocardial infarction through cytokinesis of adult cardiomyocytes. Sci Transl Med. 2014;6:224ra27. doi: 10.1126/scitranslmed.3007668. [DOI] [PubMed] [Google Scholar]
- 56.Agah R, Kirshenbaum LA, Abdellatif M, Truong LD, Chakraborty S, Michael LH, Schneider MD. Adenoviral delivery of E2F-1 directs cell cycle reentry and p53-independent apoptosis in postmitotic adult myocardium in vivo. J Clin Invest. 1997;100:2722–2722. doi: 10.1172/JCI119817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murnane JP. Telomere dysfunction and chromosome instability. Mutat Res. 2012;730:28–28. doi: 10.1016/j.mrfmmm.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grafi G. How cells dedifferentiate: a lesson from plants. Dev Biol. 2004;268:1–1. doi: 10.1016/j.ydbio.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 59.Poss KD. Getting to the heart of regeneration in zebrafish. Semin Cell Dev Biol. 2007;18:36–36. doi: 10.1016/j.semcdb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 60.Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, Tanaka EM. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:60–60. doi: 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]
- 61.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. J Hepatol. 2012;57:692–692. doi: 10.1016/j.jhep.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 62.Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, Medoff BD, Rajagopal J. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–218. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–627. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–601. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kikuchi K, Poss KD. Cardiac regenerative capacity and mechanisms. Annu Rev Cell Dev Biol. 2012;28:719–719. doi: 10.1146/annurev-cellbio-101011-155739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 201;331:1078–1078. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naqvi N, Li M, Calvert JW, Tejada T, Lambert JP, Wu J, Kesteven SH, Holman SR, Matsuda T, Lovelock JD, Howard WW, Iismaa SE, Chan AY, Crawford BH, Wagner MB, Martin DI, Lefer DJ, Graham RM, Husain A. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell. 2014;157:795–795. doi: 10.1016/j.cell.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ausma J, Litjens N, Lenders MH, Duimel H, Mast F, Wouters L, Ramaekers F, Allessie M, Borgers M. Time course of atrial fibrillation-induced cellular structural remodeling in atria of the goat. J Mol Cell Cardiol. 2001;33:2083–2083. doi: 10.1006/jmcc.2001.1472. [DOI] [PubMed] [Google Scholar]
- 69.Kubin T, Pöling J, Kostin S, Gajawada P, Hein S, Rees W, Wietelmann A, Tanaka M, Lörchner H, Schimanski S, Szibor M, Warnecke H, Braun T. Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell. 2011;9:420–420. doi: 10.1016/j.stem.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 70.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–701. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 71.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–14068. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kajstura J, Rota M, Hall SR, Hosoda T, D'Amario D, Sanada F, Zheng H, Ogórek B, Rondon-Clavo C, Ferreira-Martins J, Matsuda A, Arranto C, Goichberg P, Giordano G, Haley KJ, Bardelli S, Rayatzadeh H, Liu X, Quaini F, Liao R, Leri A, Perrella MA, Loscalzo J, Anversa P. Evidence for human lung stem cells. N Engl J Med. 2011;364:1795–1795. doi: 10.1056/NEJMoa1101324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Elsässer A, Schlepper M, Klövekorn WP, Cai WJ, Zimmermann R, Müller KD, Strasser R, Kostin S, Gagel C, Münkel B, Schaper W, Schaper J. Hibernating myocardium: an incomplete adaptation to ischemia. Circulation. 1997;96:2920–2920. doi: 10.1161/01.cir.96.9.2920. [DOI] [PubMed] [Google Scholar]
- 74.Tomanek RJ, Cooper G., 4th Morphological changes in the mechanically unloaded myocardial cell. Anat Rec. 1981;200:271–271. doi: 10.1002/ar.1092000305. [DOI] [PubMed] [Google Scholar]
- 75.Anversa P, Vitali-Mazza L, Loud AV. Morphometric and autoradiographic study of developing ventricular and atrial myocardium in fetal rats. Lab Invest. 1975;33:696–696. [PubMed] [Google Scholar]
- 76.Anversa P, Vitali-Mazza L, Gandolfi A, Loud AV. Morphometry and autoradiography of early hypertrophic changes in the ventricular myocardium of adult rat. A light microscopic study. Lab Invest. 1975;33:125–125. [PubMed] [Google Scholar]
- 77.Anversa P, Loud AV, Vitali-Mazza L. Morphometry and autoradiography of early hypertrophic changes in the ventricular myocardium of adult rat: an electron microscopic study. Lab Invest. 1976;35:475–475. [PubMed] [Google Scholar]
- 78.Zhang Y, Li TS, Lee ST, Wawrowsky KA, Cheng K, Galang G, Malliaras K, Abraham MR, Wang C, Marbán E. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLoS One. 2010;5:e12559. doi: 10.1371/journal.pone.0012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferreira-Martins J, Ogórek B, Cappetta D, Matsuda A, Signore S, D'Amario D, Kostyla J, Steadman E, Ide-Iwata N, Sanada F, Iaffaldano G, Ottolenghi S, Hosoda T, Leri A, Kajstura J, Anversa P, Rota M. Cardiomyogenesis in the developing heart is regulated by c-kit-positive cardiac stem cells. Circ Res. 2012;110:701–701. doi: 10.1161/CIRCRESAHA.111.259507. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Leri A, Barlucchi L, Limana F, Deptala A, Darzynkiewicz Z, Hintze TH, Kajstura J, Nadal-Ginard B, Anversa P. Telomerase expression and activity are coupled with myocyte proliferation and preservation of telomeric length in the failing heart. Proc Natl Acad Sci USA. 2001;98:8626–8626. doi: 10.1073/pnas.151013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morrisey EE. Rewind to recover: dedifferentiation after cardiac injury. Cell Stem Cell. 2011;9:387–387. doi: 10.1016/j.stem.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leri A, Kajstura J, Anversa P. Identity deception: not a crime for a stem cell. Physiology (Bethesda) 2005;20:162–162. doi: 10.1152/physiol.00005.2005. [DOI] [PubMed] [Google Scholar]
- 83.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–5. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 84.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10344. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuramochi Y, Fukazawa R, Migita M, Hayakawa J, Hayashida M, Uchikoba Y, Fukumi D, Shimada T, Ogawa S. Cardiomyocyte regeneration from circulating bone marrow cells in mice. Pediatr Res. 2003;54:319–319. doi: 10.1203/01.PDR.0000078275.14079.77. [DOI] [PubMed] [Google Scholar]
- 86.Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, Kasahara H, Zias E, Bonafé M, Nadal-Ginard B, Torella D, Nascimbene A, Quaini F, Urbanek K, Leri A, Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127–127. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 87.Lagostena L, Avitabile D, De Falco E, Orlandi A, Grassi F, Iachininoto MG, Ragone G, Fucile S, Pompilio G, Eusebi F, Pesce M, Capogrossi MC. Electrophysiological properties of mouse bone marrow c-kit+ cells co-cultured onto neonatal cardiac myocytes. Cardiovasc Res. 2005;66:482–482. doi: 10.1016/j.cardiores.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 88.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104:17783–17783. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li TS, Hayashi M, Ito H, Furutani A, Murata T, Matsuzaki M, Hamano K. Regeneration of infarcted myocardium by intramyocardial implantation of ex vivo transforming growth factor-beta-preprogrammed bone marrow stem cells. Circulation. 2005;111:2438–2438. doi: 10.1161/01.CIR.0000167553.49133.81. [DOI] [PubMed] [Google Scholar]
- 90.Xaymardan M, Cimini M, Fazel S, Weisel RD, Lu WY, Martin U, Harvey RP, Li RK. c-Kit function is necessary for in vitro myogenic differentiation of bone marrow hematopoietic cells. Stem Cells. 2009;27:1911–1911. doi: 10.1002/stem.106. [DOI] [PubMed] [Google Scholar]
- 91.Yoon YS, Wecker A, Heyd L, Park JS, Tkebuchava T, Kusano K, Hanley A, Scadova H, Qin G, Cha DH, Johnson KL, Aikawa R, Asahara T, Losordo DW. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326–326. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2163. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 93.Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, Sadamoto K, Horii M, Matsumoto T, Murasawa S, Shibata T, Suehiro S, Asahara T. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–1311. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 94.Yoon J, Choi SC, Park CY, Choi JH, Kim YI, Shim WJ, Lim DS. Bone marrow-derived side population cells are capable of functional cardiomyogenic differentiation. Mol Cells. 2008;25:216–216. [PubMed] [Google Scholar]
- 95.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–913. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duran JM, Makarewich CA, Sharp TE, Starosta T, Zhu F, Hoffman NE, Chiba Y, Madesh M, Berretta RM, Kubo H, Houser SR. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circ Res. 2013;113:539–539. doi: 10.1161/CIRCRESAHA.113.301202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–668. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 98.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–664. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 99.Jackson KA, Snyder DS, Goodell MA. Skeletal muscle fiber-specific green autofluorescence: potential for stem cell engraftment artifacts. Stem Cells. 2004;22:180–180. doi: 10.1634/stemcells.22-2-180. [DOI] [PubMed] [Google Scholar]
- 100.Anversa P, Leri A, Rota M, Hosoda T, Bearzi C, Urbanek K, Kajstura J, Bolli R. Concise review: stem cells, myocardial regeneration, and methodological artifacts. Stem Cells. 2007;25:589–589. doi: 10.1634/stemcells.2006-0623. [DOI] [PubMed] [Google Scholar]
- 101.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–389. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pacher P, Ngayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3:1422–1422. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1395. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1865. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fazel SS, Chen L, Angoulvant D, Li SH, Weisel RD, Keating A, Li RK. Activation of c-kit is necessary for mobilization of reparative bone marrow progenitor cells in response to cardiac injury. FASEB J. 2008;22:930–930. doi: 10.1096/fj.07-8636com. [DOI] [PubMed] [Google Scholar]
- 106.Theise ND, Krause DS, Sharkis S. Comment on little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2003;299:1317. doi: 10.1126/science.1078412. [DOI] [PubMed] [Google Scholar]
- 107.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–763. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 108.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–663. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 109.Linke A, Müller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Böhm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102:8966–8966. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.D'Amario D, Cabral-Da-Silva MC, Zheng H, Fiorini C, Goichberg P, Steadman E, Ferreira-Martins J, Sanada F, Piccoli M, Cappetta D, D'Alessandro DA, Michler RE, Hosoda T, Anastasia L, Rota M, Leri A, Anversa P, Kajstura J. Insulin-like growth factor-1 receptor identifies a pool of human cardiac stem cells with superior therapeutic potential for myocardial regeneration. Circ Res. 2011;108:1467–1467. doi: 10.1161/CIRCRESAHA.111.240648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Kajstura J, Bai Y, Cappetta D, Kim J, Arranto C, Sanada F, D'Amario D, Matsuda A, Bardelli S, Ferreira-Martins J, Hosoda T, Leri A, Rota M, Loscalzo J, Anversa P. Tracking chromatid segregation to identify human cardiac stem cells that regenerate extensively the infarcted myocardium. Circ Res. 2012;111:894–894. doi: 10.1161/CIRCRESAHA.112.273649. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 112.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103:9226–9226. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sukhacheva TV, Chudinovskikh YA, Eremeeva MV, Serov RA, Samsonova MV, Chernyaev AL, Kim AI, Bockeria LA. Resident cardiomyocyte precursor stem cells in the myocardium of infants of the first two years of life with tetralogy of Fallot. Bull Exp Biol Med. 2012;154:158–158. doi: 10.1007/s10517-012-1898-y. [DOI] [PubMed] [Google Scholar]
- 114.Steele A, Boucek RJ, Jr, Jacobs JP, Steele P, Asante-Korang A, Chamizo W, Steele J, Chai PJ, Quintessenza JA. Heart cells with regenerative potential from pediatric patients with end stage heart failure: a translatable method to enrich and propagate. Stem Cells Int. 2012;2012:452102. doi: 10.1155/2012/452102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mishra R, Vijayan K, Colletti EJ, Harrington DA, Matthiesen TS, Simpson D, Goh SK, Walker BL, Almeida-Porada G, Wang D, Backer CL, Dudley SC, Jr, Wold LE, Kaushal S. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation. 2011;123:364–364. doi: 10.1161/CIRCULATIONAHA.110.971622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Simpson DL, Mishra R, Sharma S, Goh SK, Deshmukh S, Kaushal S. A strong regenerative ability of cardiac stem cells derived from neonatal hearts. Circulation. 2012;126:S46–S53. doi: 10.1161/CIRCULATIONAHA.111.084699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Castaldo C, Di Meglio F, Nurzynska D, Romano G, Maiello C, Bancone C, Müller P, Böhm M, Cotrufo M, Montagnani S. CD117-positive cells in adult human heart are localized in the subepicardium, and their activation is associated with laminin-1 and alpha6 integrin expression. Stem Cells. 2008;26:1723–1723. doi: 10.1634/stemcells.2007-0732. [DOI] [PubMed] [Google Scholar]
- 118.Di Meglio F, Castaldo C, Nurzynska D, Romano V, Miraglia R, Bancone C, Langella G, Vosa C, Montagnani S. Epithelial-mesenchymal transition of epicardial mesothelium is a source of cardiac CD117-positive stem cells in adult human heart. J Mol Cell Cardiol. 2010;49:719–719. doi: 10.1016/j.yjmcc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 119.Nurzynska D, Di Meglio F, Romano V, Miraglia R, Sacco AM, Latino F, Bancone C, Della Corte A, Maiello C, Amarelli C, Montagnani S, Castaldo C. Cardiac primitive cells become committed to a cardiac fate in adult human heart with chronic ischemic disease but fail to acquire mature phenotype: genetic and phenotypic study. Basic Res Cardiol. 2013;108:320. doi: 10.1007/s00395-012-0320-2. [DOI] [PubMed] [Google Scholar]
- 120.Itzhaki-Alfia A, Leor J, Raanani E, Sternik L, Spiegelstein D, Netser S, Holbova R, Pevsner-Fischer M, Lavee J, Barbash IM. Patient characteristics and cell source determine the number of isolated human cardiac progenitor cells. Circulation. 2009;120:2559–2559. doi: 10.1161/CIRCULATIONAHA.109.849588. [DOI] [PubMed] [Google Scholar]
- 121.Torella D, Ellison GM, Karakikes I, Nadal-Ginard B. Resident cardiac stem cells. Cell Mol Life Sci. 2007;64:661–661. doi: 10.1007/s00018-007-6519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hu S, Yan G, He W, Liu Z, Xu H, Ma G. The influence of disease and age on human cardiac stem cells. Ann Clin Biochem. 2014;51:582–582. doi: 10.1177/0004563213511065. [DOI] [PubMed] [Google Scholar]
- 123.Mohsin S, Khan M, Toko H, Bailey B, Cottage CT, Wallach K, Nag D, Lee A, Siddiqi S, Lan F, Fischer KM, Gude N, Quijada P, Avitabile D, Truffa S, Collins B, Dembitsky W, Wu JC, Sussman MA. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J Am Coll Cardiol. 2012;60:1278–1278. doi: 10.1016/j.jacc.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mohsin S, Khan M, Nguyen J, Alkatib M, Siddiqi S, Hariharan N, Wallach K, Monsanto M, Gude N, Dembitsky W, Sussman MA. Rejuvenation of human cardiac progenitor cells with Pim-1 kinase. Circ Res. 2013;113:1169–1169. doi: 10.1161/CIRCRESAHA.113.302302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.He JQ, Vu DM, Hunt G, Chugh A, Bhatnagar A, Bolli R. Human cardiac stem cells isolated from atrial appendages stably express c-kit. PLoS One. 2011;6:e27719. doi: 10.1371/journal.pone.0027719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cai C, Teng L, Vu D, He JQ, Guo Y, Li Q, Tang XL, Rokosh G, Bhatnagar A, Bolli R. The heme oxygenase 1 inducer (CoPP) protects human cardiac stem cells against apoptosis through activation of the extracellular signal-regulated kinase (ERK)/NRF2 signaling pathway and cytokine release. J Biol Chem. 2012;287:33720–33720. doi: 10.1074/jbc.M112.385542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Choi SH, Jung SY, Suh W, Baek SH, Kwon SM. Establishment of isolation and expansion protocols for human cardiac C-kit-positive progenitor cells for stem cell therapy. Transplant Proc. 2013;45:420–420. doi: 10.1016/j.transproceed.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 128.Choi SH, Jung SY, Yoo SM, Asahara T, Suh W, Kwon SM, Baek SH. Amine-enriched surface modification facilitates expansion, attachment, and maintenance of human cardiac-derived c-kit positive progenitor cells. Int J Cardiol. 2013;168:100–100. doi: 10.1016/j.ijcard.2012.09.065. [DOI] [PubMed] [Google Scholar]
- 129.Choi SH, Jung SY, Yoo SY, Yoo SM, Kim da Y, Kang S, Baek SH, Kwon SM. Regulation of ROS-independent ERK signaling rescues replicative cellular senescence in ex vivo expanded human c-kit-positive cardiac progenitor cells. Int J Cardiol. 2013;169:73–73. doi: 10.1016/j.ijcard.2013.08.076. [DOI] [PubMed] [Google Scholar]
- 130.Kryukov O, Ruvinov E, Cohen S. Three-Dimensional Perfusion Cultivation of Human Cardiac-Derived Progenitors Facilitates Their Expansion While Maintaining Progenitor State. Tissue Eng Part C Methods. 2014 doi: 10.1089/ten.TEC.2013.0528. [DOI] [PubMed] [Google Scholar]
- 131.Fransioli J, Bailey B, Gude NA, Cottage CT, Muraski JA, Emmanuel G, Wu W, Alvarez R, Rubio M, Ottolenghi S, Schaefer E, Sussman MA. Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem Cells. 2008;26:1315–1315. doi: 10.1634/stemcells.2007-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009;120:2077–2077. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang J, Huang C, Wu P, Yang J, Song T, Chen Y, Fan X, Wang T. Differentiation induction of cardiac c-kit positive cells from rat heart into sinus node-like cells by 5-azacytidine. Tissue Cell. 2011;43:67–67. doi: 10.1016/j.tice.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 134.Kurazumi H, Kubo M, Ohshima M, Yamamoto Y, Takemoto Y, Suzuki R, Ikenaga S, Mikamo A, Udo K, Hamano K, Li TS. The effects of mechanical stress on the growth, differentiation, and paracrine factor production of cardiac stem cells. PLoS One. 2011;6:e28890. doi: 10.1371/journal.pone.0028890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Seshadri G, Che PL, Boopathy AV, Davis ME. Characterization of superoxide dismutases in cardiac progenitor cells demonstrates a critical role for manganese superoxide dismutase. Stem Cells Dev. 2012;21:3136–3136. doi: 10.1089/scd.2012.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Y, Luther K. Genetically manipulated progenitor/stem cells restore function to the infarcted heart via the SDF-1α/CXCR4 signaling pathway. Prog Mol Biol Transl Sci. 2012;111:265–265. doi: 10.1016/B978-0-12-398459-3.00012-5. [DOI] [PubMed] [Google Scholar]
- 137.Di Felice V, Zummo G. Stem cell populations in the heart and the role of Isl1 positive cells. Eur J Histochem. 2013;57:e14. doi: 10.4081/ejh.2013.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Konstandin MH, Toko H, Gastelum GM, Quijada P, De La Torre A, Quintana M, Collins B, Din S, Avitabile D, Völkers M, Gude N, Fässler R, Sussman MA. Fibronectin is essential for reparative cardiac progenitor cell response after myocardial infarction. Circ Res. 2013;113:115–115. doi: 10.1161/CIRCRESAHA.113.301152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Saravanakumar M, Devaraj H. Distribution and homing pattern of c-kit+ Sca-1+ CXCR4+ resident cardiac stem cells in neonatal, postnatal, and adult mouse heart. Cardiovasc Pathol. 2013;22:257–257. doi: 10.1016/j.carpath.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 140.Chen Z, Pan X, Yao Y, Yan F, Chen L, Huang R, Ma G. Regulation of c-kit+ progenitor cells by stromal cell derived factor-1α in adult murine heart. Heart Lung Circ. 2014;23:75–75. doi: 10.1016/j.hlc.2013.05.652. [DOI] [PubMed] [Google Scholar]
- 141.Xiao J, Li J, Xu T, Lv D, Shen B, Song Y, Xu J. Pregnancy-induced physiological hypertrophy protects against cardiac ischemia-reperfusion injury. Int J Clin Exp Pathol. 2013;7:229–229. [PMC free article] [PubMed] [Google Scholar]
- 142.Xiao J, Xu T, Li J, Lv D, Chen P, Zhou Q, Xu J. Exercise-induced physiological hypertrophy initiates activation of cardiac progenitor cells. Int J Clin Exp Pathol. 2014;7:663–663. [PMC free article] [PubMed] [Google Scholar]
- 143.Feng X, Reini SA, Richards E, Wood CE, Keller-Wood M. Cortisol stimulates proliferation and apoptosis in the late gestation fetal heart: differential effects of mineralocorticoid and glucocorticoid receptors. Am J Physiol Regul Integr Comp Physiol. 2013;305:R343–R350. doi: 10.1152/ajpregu.00112.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Urbanek K, Cabral-da-Silva MC, Ide-Iwata N, Maestroni S, Delucchi F, Zheng H, Ferreira-Martins J, Ogórek B, D'Amario D, Bauer M, Zerbini G, Rota M, Hosoda T, Liao R, Anversa P, Kajstura J, Leri A. Inhibition of notch1-dependent cardiomyogenesis leads to a dilated myopathy in the neonatal heart. Circ Res. 2010;107:429–429. doi: 10.1161/CIRCRESAHA.110.218487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–327. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–19. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 147.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–359. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849–849. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 149.Kechad A, Jolicoeur C, Tufford A, Mattar P, Chow RW, Harris WA, Cayouette M. Numb is required for the production of terminal asymmetric cell divisions in the developing mouse retina. J Neurosci. 2012;32:17197–17197. doi: 10.1523/JNEUROSCI.4127-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Houser SR, Molkentin JD. Does contractile Ca2+ control calcineurin-NFAT signaling and pathological hypertrophy in cardiac myocytes? Sci Signal. 2008;1:pe31. doi: 10.1126/scisignal.125pe31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ferreira-Martins J, Rondon-Clavo C, Tugal D, Korn JA, Rizzi R, Padin-Iruegas ME, Ottolenghi S, De Angelis A, Urbanek K, Ide-Iwata N, D'Amario D, Hosoda T, Leri A, Kajstura J, Anversa P, Rota M. Spontaneous calcium oscillations regulate human cardiac progenitor cell growth. Circ Res. 2009;105:764–764. doi: 10.1161/CIRCRESAHA.109.206698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kapur N, Mignery GA, Banach K. Cell cycle-dependent calcium oscillations in mouse embryonic stem cells. Am J Physiol Cell Physiol. 2007;292:C1510–C1518. doi: 10.1152/ajpcell.00181.2006. [DOI] [PubMed] [Google Scholar]
- 153.Kawano S, Otsu K, Kuruma A, Shoji S, Yanagida E, Muto Y, Yoshikawa F, Hirayama Y, Mikoshiba K, Furuichi T. ATP autocrine/paracrine signaling induces calcium oscillations and NFAT activation in human mesenchymal stem cells. Cell Calcium. 2006;39:313–313. doi: 10.1016/j.ceca.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 154.Gao H, Wang F, Wang W, Makarewich CA, Zhang H, Kubo H, Berretta RM, Barr LA, Molkentin JD, Houser SR. Ca(2+) influx through L-type Ca(2+) channels and transient receptor potential channels activates pathological hypertrophy signaling. J Mol Cell Cardiol. 2012;53:657–657. doi: 10.1016/j.yjmcc.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Goonasekera SA, Hammer K, Auger-Messier M, Bodi I, Chen X, Zhang H, Reiken S, Elrod JW, Correll RN, York AJ, Sargent MA, Hofmann F, Moosmang S, Marks AR, Houser SR, Bers DM, Molkentin JD. Decreased cardiac L-type Ca2+ channel activity induces hypertrophy and heart failure in mice. J Clin Invest. 2012;122:280–280. doi: 10.1172/JCI58227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang F, Gao H, Kubo H, Fan X, Zhang H, Berretta R, Chen X, Sharp T, Starosta T, Makarewich C, Li Y, Molkentin JD, Houser SR. T-type Ca2+ channels regulate the exit of cardiac myocytes from the cell cycle after birth. J Mol Cell Cardiol. 2013;62:122–122. doi: 10.1016/j.yjmcc.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tillmanns J, Rota M, Hosoda T, Misao Y, Esposito G, Gonzalez A, Vitale S, Parolin C, Yasuzawa-Amano S, Muraski J, De Angelis A, Lecapitaine N, Siggins RW, Loredo M, Bearzi C, Bolli R, Urbanek K, Leri A, Kajstura J, Anversa P. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci USA. 2008;105:1668–1668. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, Jneid H, Rota M, Leri A, Kajstura J. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128:122–122. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sundararaman B, Avitabile D, Konstandin MH, Cottage CT, Gude N, Sussman MA. Asymmetric chromatid segregation in cardiac progenitor cells is enhanced by Pim-1 kinase. Circ Res. 2012;110:1169–1169. doi: 10.1161/CIRCRESAHA.112.267716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dey D, Han L, Bauer M, Sanada F, Oikonomopoulos A, Hosoda T, Unno K, De Almeida P, Leri A, Wu JC. Dissecting the molecular relationship among various cardiogenic progenitor cells. Circ Res. 2013;112:1253–1253. doi: 10.1161/CIRCRESAHA.112.300779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marbán E, Molkentin JD. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.