Abstract
Astrocytes have been shown to protect neurons from delayed neuronal death and increase their survival in cerebral ischemia. One of the main mechanisms of astrocyte protection is rapid removal of excess glutamate from synaptic sites by astrocytic plasma membrane glutamate transporters such as GLT-1/EAAT-2, reducing excitotoxicity. Astrocytic mitochondrial function is essential for normal GLT-1 function. Manipulating astrocytic mitochondrial and GLT-1 function is thus an important strategy to enhance neuronal survival and improve outcome following cerebral ischemia. Increasing evidence supports the involvement of microRNAs (miRNA), some of them being astrocyte-enriched, in the regulation of cerebral ischemia. This chapter will first update the information about astrocytes, GLT-1, astrocytic mitochondria, and delayed neuronal death. Then we will focus on two recently reported astrocyte-enriched miRNAs (miR-181 and miR-29 families), their effects on astrocytic mitochondria and GLT-1 as well as on outcome after cerebral ischemia.
Keywords: Astrocyte, glutamate transporter, GLT-1, microRNA, miR-181, miR-29, cerebral ischemia, delayed neuronal death
1. Introduction
Cerebral ischemia is a key pathological event in several disease states; stroke, cardiac arrest and resuscitation, and head trauma being the most common ones. Stroke is one of the leading causes of death worldwide and the leading cause of long-term neurological disability. Although many clinical stroke trials have been completed, the only efficacious treatment identified to date is thrombolysis (Blakeley and Llinas, 2007). Similarly, the cerebral injury resulting from cardiac arrest and resuscitation leads to death and neurological impairment, but clinically this has only been effectively treated with hypothermia (Bernard et al., 2002; THACAS, 2002). Lack of consideration of the role of astrocytes, the importance of cell-cell interactions, the complex interplay among signaling pathways, and poorly defined treatment windows for specific targets are thought to be factors in the clinical failure of many potential neuroprotective strategies.
Astrocytes play many key roles both in normal and pathological central nervous system (CNS) functioning. Astrocytes are central to potassium homeostasis, neurotransmitter uptake, synapse formation and regulation of the blood brain barrier. Astrocytes are the most abundant brain cell type, and in addition to multiple important homeostatic roles, they are essential to CNS development, helping to organize the structural architecture of the brain and communication pathways, and modulating neuronal plasticity (for recent reviews see Sofroniew and Vinters (2010); Barreto et al. (2011); Clarke and Barres (2013)). The importance of astrocytes for neuroprotection after cerebral ischemia has been reviewed by our group and others recently (Sofroniew and Vinters, 2010; Barreto et al., 2011).
MicroRNAs (miRNAs) are a novel and abundant class of 19- to 22-nucleotide noncoding RNAs that control gene expression primarily at the post-transcriptional level. The most common mechanism is for miRNAs to bind to messenger RNAs (mRNAs), known as their targets, based on sequence complementarity and direct the degradation or repression of translation of the targeting mRNAs. Recent evidence increasingly supports a role for miRNAs in response to cerebral ischemia, as we have reviewed recently (Ouyang et al., 2013b; Ouyang et al., 2013a). The fast post-transcriptional effect of miRNAs, and their ability to simultaneously coordinate regulation of many target genes, suggests that miRNAs may have greater therapeutic potential as candidates for the treatment of stroke than therapies targeting a single gene by direct transcriptional control. Numerous miRNAs are expressed in a cell-specific manner and some are enriched in astrocytes (Smirnova et al., 2005; Ouyang et al., 2013d).
In this chapter we will summarize the central role of astrocytes in cerebral ischemia focusing on astrocytic glutamate transporters and then explore the novel regulation of astrocytes by astrocyte-enriched miRNAs.
2. Delayed neuronal death
The concept of “delayed neuronal death” was described with the development of forebrain ischemia (Kirino, 1982). Brief global cerebral ischemia, as seen with cardiac arrest and resuscitation, and modeled in rodents as forebrain ischemia or four vessel occlusion, causes delayed loss of neurons in cornu ammonis 1 (CA1) pyramidal neurons, a slow process taking 4–7 days before the final morphologic outcome (see middle panel in Fig. 1A) is observed. Later the concept was extended to focal ischemia referring to neuronal death in the peri-ischemic (penumbral) area (Liu et al., 1999). In focal cerebral ischemia, modeled commonly in rodents as transient middle cerebral artery occlusion, the neurons die within hours in the center of the ischemic territory (ischemic core) forming the initial area of infarction while the neurons in the adjacent penumbra area may either survive via induction of pro-survival signaling pathways, or die at a later period of reperfusion (also called delayed neuronal death) via initiation of cell death pathways (Ferrer and Planas, 2003).
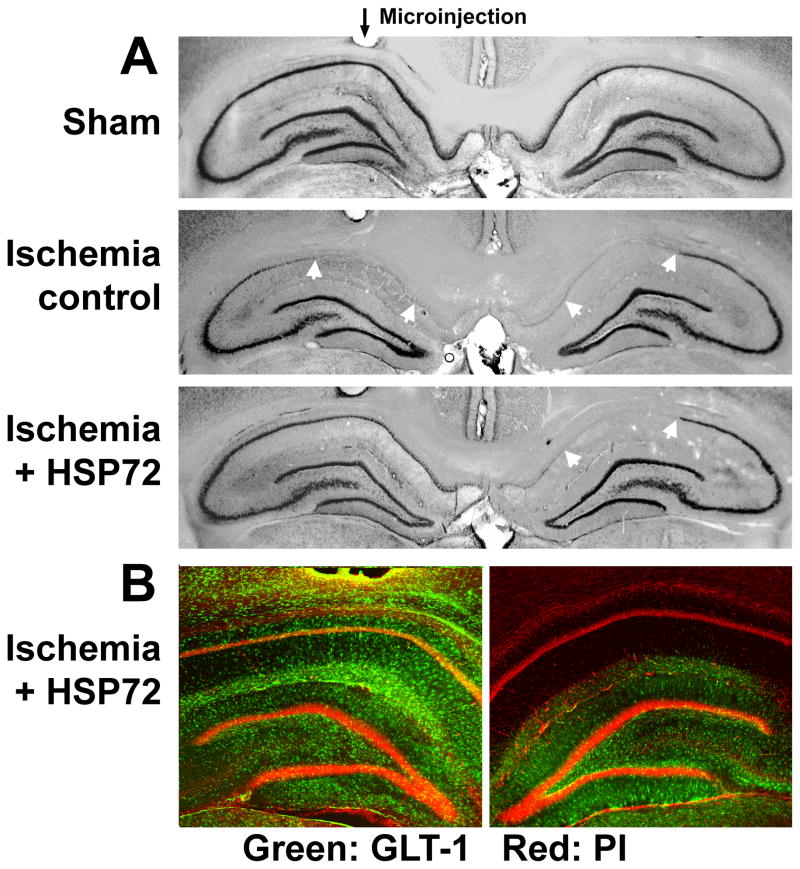
Fig. 1.
Targeted overexpression of HSP72 in astrocytes reduces delayed CA1 neuronal death and preserves GLT-1 expression. A: GFAPp-HSP72 or control DNA was injected stereotaxically just above CA1 on one side (black arrow for microinjection track) two days before forebrain ischemia. Selective loss of CA1 hippocampal neurons (between white arrows in middle and lower panels) was observed at seven days reperfusion by cresyl violet staining. The loss of CA1 hippocampal neurons was significantly reduced on the HSP72 injected side compared with the noninjected side (lower panel) or the control DNA injected hippocampus (middle panel). B: GLT-1 immunoreactivity was significantly greater after GFAPp-HSP72 injection compared with control after 10 min ischemia and 5 hour reperfusion. Propidium iodide (PI) labels cell nuclei. Modified from Figs. 2 and 3 in Xu et al. (2010).
The form of delayed neuronal death is generally believed to be programmed cell death or apoptosis (Nitatori et al., 1995; Kirino, 2000). The mechanisms of delayed neuronal death are complex but one of the key mechanisms is prolonged excess release of glutamate, which activates neurotransmitter receptor ion channels and induces intracellular calcium overload (Choi, 1996). Excessive increases in mitochondrial matrix calcium alter the function and permeability of mitochondria and finally lead to opening of the mitochondrial permeability transition pore (Ouyang et al., 1997), causing the release of cytochrome c (Ouyang et al., 1999) and other proapoptotic factors into the cytoplasm. The released cytochrome c activates caspase-3, one of the executioner caspases to initiate apoptotic cell death (for a recent review, see Fig. 1a in Ouyang and Giffard (2012)).
3. Astrocytes and their vulnerability to ischemia
For decades, astrocytes have been considered to be non-excitable support cells that are relatively resistant to ischemic injury. This view has changed radically during the past twenty years. Multiple essential functions are performed by astrocytes in normal brain. Astrocytes are dynamically involved in synaptic transmission, metabolic and ionic homeostasis, inflammatory response, antioxidant defense, trophic support of neurons, as well as in the establishment and maintenance of the blood brain barrier (Kettenmann and Ransom, 2005). Advances in our understanding of astrocytes include new observations about their structure, organization, and function. Hippocampal astrocytes occupy exclusive, non-overlapping territories (Bushong et al., 2002). This pattern develops in the early postnatal period in parallel with neuronal and vascular territories; each astrocyte interfaces with the microvasculature and thousands of synapses (Bushong et al., 2004). That astrocytes both respond to signals from and signal actively to neurons, endothelium, and microglia is now well accepted (for reviews see Ransom et al. (2003); Volterra and Meldolesi (2005)).
It has often been said that neurons are the cells most vulnerable to ischemia. This view is based primarily on observations that astrocytes in culture show greater resistance than neurons to some ischemia-like insults (Goldberg and Choi, 1993; Xu et al., 2001) and that brief forebrain ischemia apparently results in selective loss of neurons. However, vulnerability of cultured astrocytes is markedly increased by acidic conditions relevant to ischemic injury (Giffard et al., 1990; Bondarenko and Chesler, 2001). Several laboratories have pointed out that glial cells may be injured more readily than previously thought, sometimes before neuronal damage is obvious (Petito et al., 1998; Liu et al., 1999; Zhao et al., 2003b).
Astrocytic processes were fragmented and mitochondria inhibited after 15 min exposure to acidic conditions in hippocampal slice cultures (Hulse et al., 2001). Astrocytic demise was found to precede delayed neuronal death after focal ischemia and astrocytic glial fibrillary acidic protein (GFAP) mRNA declined more quickly than neuronal glucose transporter 3 (GLUT3) mRNA in the ischemic core (Liu et al., 1999). Astrocytic demise was also found early after traumatic brain injury (Zhao et al., 2003b). In intact brain astrocytes are highly metabolically active, while astrocytes in the absence of neurons have few energy demands. We demonstrated that providing astrocytes a transportable substrate, aspartate, markedly increased their vulnerability to ischemia (Voloboueva et al., 2013). In addition, loss of glutamate transport activity and immunoreactivity for the astrocyte-specific plasma membrane glutamate transporter-1 (GLT-1) in astrocytes occurs at early reperfusion times following forebrain ischemia, hours to days before the death of CA1 neurons (Ouyang et al., 2007).
4. Astrocytic plasma membrane glutamate transporters
Extracellular glutamate is maintained at low levels, and rapid removal of glutamate from the extracellular space is required for the survival and normal function of neurons. To date, five brain plasma membrane glutamate transporters have been cloned, excitatory amino acid transporters 1–5 (EAAT1–5), of which GLT-1/EAAT2 and GLAST/EAAT1 are expressed in astrocytes. Although glutamate transporters are expressed by all CNS cell types, astrocytes are primarily responsible for glutamate uptake (Anderson and Swanson, 2000). Neuronal vulnerability to glutamate is 100-fold greater in astrocyte-poor cultures than in cultures with abundant astrocytes (Rosenberg and Aizenman, 1989), demonstrating that uptake by astrocytes in particular is key to neuronal survival. Reduction or loss of astroglial transporters EAAT2/GLT-1 or EAAT1/GLAST, but not the neuronal subtype EAAT3/EAAC1 (excitatory amino acid carrier 1), led to a tonic increase in extracellular glutamate concentration and subsequent neurodegeneration (Rothstein et al., 1996; Mitani and Tanaka, 2003) and increased ischemic injury after focal cerebral ischemia in rats (Watase et al., 1998; Fukamachi et al., 2001; Rao et al., 2001). GLT-1 appears to be especially important in the hippocampus, because mice lacking GLT-1 showed spontaneous seizures, selective hippocampal neurodegeneration, and exacerbation of acute cortical injury (Tanaka et al., 1997), whereas mice deficient in GLAST were more susceptible to cerebellar injury (Watase et al., 1998). We will focus on GLT-1 in the rest of this chapter.
Ceftriaxone treatment, which induces GLT-1 expression, reduces CA1 delayed neuronal death in hippocampal slice culture and in transient forebrain ischemia (Ouyang et al., 2007). Selective overexpression of GLT-1 in astrocytes provided neuroprotection from moderate hypoxia-ischemia (Watase et al., 1998). Targeted over-expression of GLT-1 reduces ischemic brain injury in a rat model of focal ischemia (Harvey et al., 2011).
5. Oxidative stress and GLT-1
Oxidative stress and inhibition of glutamate transport has been suggested to form a vicious circle in ischemia whereby increased oxidative stress impairs glutamate transport, which further exacerbates excitotoxicity which leads to more oxidative stress (Trotti et al., 1998). There is evidence that several different oxidants, including peroxide, superoxide, and treatment with amyloid β peptide, can reduce glutamate transport activity of GLT-1(Harris et al., 1996; Trotti et al., 1998; Lauderback et al., 2001). Several glutamate transporters, including GLT-1, have redox sensitive sulfhydryl regulation of activity (Trotti et al., 1997), but strong oxidants such as peroxynitrite can inhibit glutamate transport in a way that is not fully reversed by dithiothreitol, suggesting reaction with other sites such as histidine or lysine may also occur.
Work by Weiss and colleagues (Rao et al., 2003) showed that motor neurons only died after glutamate transport activity was lost in the astrocytes surrounding the neurons. This work suggested that oxidative stress eventually compromised astrocyte function, which then precipitated neuronal death. Oxidative stress has also been shown to downregulate astrocytic glutamate uptake in ammonia-induced toxicity (Jayakumar et al., 2006). Oxidative stress is known to contribute to damage and misfolding of proteins, including glutamate transporters (Lauderback et al., 2001). In CA1 after transient forebrain ischemia, increased generation of reactive oxygen species (ROS) compromises astrocyte function, which in turn compromises the ability of the CA1 pyramidal neurons to survive (Ouyang et al., 2007).
6. Mitochondria and GLT-1
Mitochondria play a central role in normal neuronal cell function by controlling cellular energy metabolism and producing ROS, but also as a central regulator of cell death via release of apoptotic factors into the cytosol (Murphy et al., 1999; Beal, 2000; Kroemer and Reed, 2000; Ravagnan et al., 2002). The mitochondrial respiratory chain, especially complexes I and III, is the major site of ROS production (Lin and Beal, 2006). Glutamate uptake in the CNS by high-affinity sodium dependent transporters such as GLT-1 requires large amounts of energy, largely adenosine triphosphate (ATP) produced from mitochondrial respiration. It may be noted that glycolytically/glycogenolytically derived ATP may play an important role for maintenance of glutamate uptake capacity in astrocytes since glycolytic enzymes, the sodium-potassium ATPase and the glutamate transporters are tightly coupled (Voloboueva et al., 2007; Parpura et al., 2012). Oxygen deprivation or mitochondrial inhibition cause reductions of ATP in brain or cultured astrocytes, and produce reductions in glutamate transport capacity (Longuemare et al., 1994; Swanson et al., 1994; Ouyang et al., 2007). In the extreme case of ATP depletion, as occurs during severe ischemia, all of these membrane gradients collapse. This results not only in cessation of uptake, but also efflux of glutamate via uptake reversal (Szatkowski et al., 1990).
7. Astrocytic mitochondria as targets for protection
Several studies in the literature suggest that neuronal mitochondria and astrocytic mitochondria respond differently to ischemic stress. Reichert et al. (2001) found that although co-cultured neurons and astrocytes subjected to combined oxygen glucose deprivation both showed marked loss of mitochondrial membrane potential, the astrocytes were able to recover over a period of more than an hour of reperfusion, while the neurons did not. Bambrick and coworkers have also pointed out differences between neuronal and astrocytic mitochondria and suggested that astrocyte mitochondria might be a potential target for treatment (Bambrick et al., 2004). Mitochondrial dysfunction has been implicated in the selective vulnerability of CA1 cells after ischemia, because cell death in this region after global ischemia is inhibited by cyclosporin A, a known inhibitor of the calcium-induced mitochondrial permeability transition in brain mitochondria (Uchino et al., 1995). The observation that cyclosporin A can protect mitochondria from calcium insult in astrocytes, but not in neurons (Bambrick et al., 2006; Kahraman et al., 2011), suggests that astrocyte mitochondria are central to protection by this drug in vivo. Methods to protect astrocyte mitochondria are described next.
7.1. Molecular chaperones
Originally molecular chaperones were defined as functionally related groups of proteins that assist in the folding or unfolding of proteins, the sequestration of denatured proteins, and the assembly or disassembly of multiprotein complexes. Recently, a more complex, role of these chaperones has been recognized, that of functioning as a chaperone network, contributing to organelle interactions. Chaperones act within and between organelles to integrate and support essential cellular functions. (Ouyang and Giffard, 2012). The heat shock protein 70 kDa family (HSP70) is the most extensively studied ATP-dependent chaperone family, and includes a cytosolic form HSP73 (also HSC70), an inducible cytosolic form HSP72, a mitochondrial form HSP75/GRP75/mortalin, and an endoplasmic reticulum (ER) form, GRP78/BIP. HSP70 family members that are involved in ER-mitochondria crosstalk and calcium transfer during cerebral ischemia (Ouyang and Giffard, 2012).
Work from our lab and several others has demonstrated neuroprotection from ischemic brain injury with overexpression of chaperones and co-chaperones, both in animal stroke models and in cultured brain cells (for a recent review, see Ouyang and Giffard (2013)). Our group has shown that selective overexpression of HSP72 in astrocytes reduces death of CA1 neurons (Fig. 1A). HSP72 was genetically targeted for expression in astrocytes using the astrocyte-specific human GFAP promoter (Xu et al., 2010). Protection was accompanied by preservation of astrocytic GLT-1 (Fig. 1B) and mitochondrial respiratory complexes I and IV activities, and reduced oxidative stress in the CA1 region (Xu et al., 2010).
7.2. Superoxide dismutase 2
Superoxide dismutases (SODs) comprise a family of metal-containing proteins that catalyze dismutation of superoxide radicals. Among the SOD family members, SOD1/ CuZn-SOD is a copper and zinc-containing homodimer, primarily localized in the cytoplasm; SOD2/Mn-SOD is a manganese-containing enzyme exclusively localized in mitochondria; and SOD3/EC-SOD is a copper- and zinc-containing tetramer, present largely in the extracellular space (Zelko et al., 2002). SOD overexpression provides direct neuroprotection, glioprotection, and protection of the blood brain barrier (Jung et al., 2009). Reduction of SOD2 activity was shown to increase neuronal death induced by transient cerebral ischemia (Chan, 2005). We have shown that targeting SOD2 overexpression to hippocampal astrocytes improves the survival of CA1 neurons and reduces ROS production and GLT-1 loss after transient forebrain ischemia (Xu et al., 2010).
7.3. BCL2 family
The BCL2 protein family, central regulator of life/death decisions in cells (Adams and Cory, 2007) largely influences cell death through regulating mitochondrial membrane integrity and function. The BCL2 protein family is classified into 3 subgroups according to structural homology (BCL2 homology [BH] domains): the pro-survival proteins of the BCL2 family (BCL2, BCL-xL, BCL-w, MCL1, and A1), the multi-domain pro-apoptotic proteins BAX and BAK and the BH3-only pro-apoptotic proteins (BIM, PUMA, BID, BAD, BIK, BMF, HRK, NOXA). In response to diverse intracellular and extracellular signals, the cell’s decision to undergo apoptosis is determined by interactions between these three groups within the BCL2 protein family.
We and others have reported that overexpressing prosurvival BCL2 family members protects against cerebral ischemia in vivo (Kitagawa et al., 1998; Zhao et al., 2003a) and in vitro (Xu et al., 1999). Neuroprotection involved maintaining mitochondrial function (for review see Ouyang and Giffard (2004)). Decreased BCL2 and increased BAX and BH3-only proteins were reported in CA1 neurons after global ischemia (Martinez et al., 2007). After global ischemia PUMA (p53-upregulated modulator of apoptosis) is upregulated in CA1 neurons, localizes to mitochondria, and binds BCL-xL and BAX (Niizuma et al., 2009). Selective CA1 injury induced by proteasomal inhibition was strongly reduced in PUMA knockout mice (Bonner et al., 2010; Tsuchiya et al., 2011). Interestingly anti-apoptotic protein BCL2 also exists in mitochondrial associated membranes (Fig. 2B in Ouyang et al. (2013a)), a site of mitochondria and ER interaction, and affects ER and mitochondrial calcium homeostasis (Foyouzi-Youssefi et al., 2000).
In summary we propose a mechanism of selective astrocyte dysfunction as illustrated in Fig. 2. Differences in mitochondrial response lead to greater production of ROS in astrocytes, which leads to greater energetic compromise, oxidative damage, and loss of GLT-1 function. The impaired astrocytes may be unable to carry out many of their normal functions, such as glutamate uptake, antioxidant defense, and regulation of extracellular ions, which eventually leads to neurotoxicity and delayed neuronal death. Protecting astrocytic mitochondrial function by increasing expression of HSP70 family members, SOD2, or anti-apoptotic members of the BCL2 family protects mitochondria to better maintain energy production and reduces ROS, maintaining GLT-1 function. By decreasing extracellular glutamate GLT-1 keeps CA1 neurons alive.
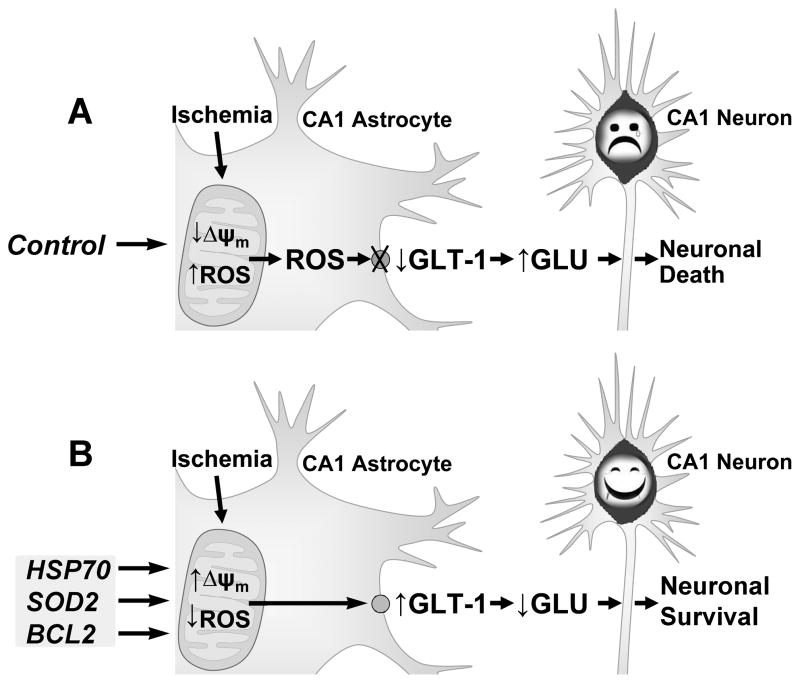
Fig. 2.
Proposed mechanism of astrocyte contribution to delayed neuronal death. A) Transient forebrain ischemia selectively decreases mitochondrial membrane potential (Δψm) and increases ROS in CA1 astrocytes. The greater production of ROS leads to astrocyte impairment including oxidative damage of GLT-1 on the astrocyte membrane. The loss of GLT-1 contributes to the increase of extracellular glutamate and excitotoxicity of the pyramidal neurons. B) By protection of astrocytic mitochondria using HSP72 or SOD2 or anti-apoptotic members of the BCL2 family, astrocytic GLT-1 is preserved and extracellular glutamate is decreased, which results in survival of CA1 neurons. Modified from Fig. 6 in Ouyang et al. (2007).
Despite the importance of GLT-1 in both physiological (Lopez-Bayghen and Ortega, 2011) and pathological (Kim et al., 2011) conditions, relatively little is known about the regulation of GLT-1 expression, especially by miRNAs. Global disruption of astroglial miRNA biogenesis through selective deletion of Dicer in cerebellar astrocytes significantly reduces GLT-1 expression (Tao et al., 2011). Recently a neuron-specific miRNA-mediated regulation of GLT-1 protein expression has been described. Neuronal exosomes containing miRNA 124a regulate astrocytic GLT-1 expression by transfer from neuron into astrocytes (Morel et al., 2013). However the results in the paper indicate that up-regulation of GLT-1 protein expression by miR-124a is likely to be indirect. In the following section we will focus on astrocyte-enriched miRNAs and their possible role in regulating GLT-1.
8. Astrocyte-enriched miRNAs as potential targets for protection
We demonstrated recently that two brain-enriched miRNAs, miR-181a (Ouyang et al., 2012a) and miR-29a (Ouyang et al., 2013d), are involved in the regulation of outcome following cerebral ischemia. Interestingly, the literature and our experiments suggest that both the miR-181 and miR-29 families are astrocyte-enriched (Hutchison et al., 2013; Ouyang et al., 2013d).
8.1. miR-181 family
The miR-181 family consists of four mature members (miR-181a, miR-181b, miR-181c, and miR-181d) from three polycistronic miRNA genes – miR-181a-1/b-1, miR-181a-2/b-2, and miR-181c/d. The miR-181 family was reported first as an important regulator of immune cell development (Chen et al., 2004). These earlier studies found that the miR-181 family, especially miR-181a and miR-181b, are enriched in brain (Chen et al., 2004; Miska et al., 2004) and their aberrant expression has been associated with brain diseases. miR-181a and miR-181b are reduced in human gliomas and glioma cell lines and expression is negatively correlated with tumor grade (Shi et al., 2008). miR-181a sensitizes human malignant glioma cells to radiation by targeting anti-apoptotic protein BCL2 (Chen et al., 2010). It was found recently that expression of the miR-181 family was strongly enriched in cultured astrocytes compared to neurons derived from neural stem cells (Hutchison et al., 2013). miR-181 family members were expressed at significantly higher levels in adult cortex relative to embryonic telencephalon, an early developmental stage prior to astrogenesis.
It was demonstrated by our group that miR-181a increased in vulnerable regions such as the ischemic core in focal ischemia (Ouyang et al., 2012b) or the hippocampal CA1 region after global ischemia (Moon et al., 2013), and decreased in the ischemia-resistant areas, the penumbra and hippocampal dentate gyrus, respectively. Antagomir to miR-181a reduced miR-181a levels in the brain and reduced infarct size in focal ischemia (Ouyang et al., 2012b) and CA1 neuronal loss in global cerebral ischemia (Moon et al., 2013). Anti-miR-181a reduced miR-181a levels in the brain and reduced infarct size in focal (Fig. 1A) (Ouyang et al., 2012b) and CA1 neuronal loss in global cerebral ischemia (Moon et al., 2013). Furthermore, transfecting primary cultures with miR-181a inhibitor led to protection of astrocytes, but not neurons from ischemia-like stresses (Ouyang et al., 2012a; Moon et al., 2013).
Recently, miR-181a was shown to directly target molecular chaperone GRP78 (Ouyang et al., 2012b), anti-apoptotic members of the BCL2 family BCL2 and myeloid cell leukemia (MCL) 1 (Ouyang et al., 2012a), and X-linked inhibitor of apoptosis (XIAP) (Hutchison et al., 2013) as well as some target proteins involved in controlling mitochondrial function, redox state, and inflammatory pathways (for recent reviews see Ouyang and Giffard (2013); Ouyang et al. (2013b)). Although miR-181a antagomir reduces miR-181a levels and significantly inhibits the decrease of GLT-1 after forebrain ischemia, miR-181a does not directly target GLT-1 (Moon et al., 2013). One interesting observation is the difference in effect of reducing miR-181a in different cell types. Reducing miR-181a increased BCL2 as one of the targets and increased survival of primary astrocytes (Ouyang et al., 2012b) while it failed to significantly change levels of BCL2 and did not change outcome in primary neurons after ischemia-like injury in vivo (Moon et al., 2013).
Some evidence supports the concept that not only mature miRNA but also pri-/pre-miRNA functions in target recognition and repression (Chen, 2013). One relevant observation is that although miR-181a-2/b-2 and miR-181a-1/b-1 produce identical mature miR-181a and miR-181b, deletion of miR-181a-1/b-1, but not miR-181a-2/b-2, selectively inhibits tumor transformation induced by Notch oncogenes (Fragoso et al., 2012). Because of the different effects of miR-181a it is interesting to further assess which miR-181a gene(s) (miR-181a-1/ or a-2) is expressed in brain cells and if pri/pre-miR-181a are also involved in cell type specific response in astrocytes compared to neurons.
8.2. miR-29 family
The miR-29 family consists of three members (a, b, and c) that map to two distinct genomic loci in clusters (Fig. 1A in Ouyang et al. (2013d)): miR-29 a/b-1 on chromosome 6 on mouse and 7 in human, and miR-29c/b-2 on chromosome 1 in both mouse and human. It has been demonstrated that miR-29a/b-1 is developmentally regulated in mouse brain with the highest expression observed in adults (Hebert et al., 2008; Kole et al., 2011). During brain development miR-29 was found more strongly expressed in astrocytes than neurons using primary cultures (Smirnova et al., 2005). The investigators used an equimolar mixture of the miR-29b and miR-29c isoforms as a probe for Northern blot analysis of miRNA expression in mouse brain development. Recently, using qPCR we compared postnatal brain, primary neuron, and astrocyte cultures, and showed the strongly astrocytic expression of miR-29a (Ouyang et al., 2013d). While miR-29a increased in brain, astrocytes, and neurons with development, at each time point levels of miR-29 in cultured astrocytes were 20–40 times higher than in cultured neurons, and levels in brain tissue were about 1/2 that seen in cultured astrocytes. This suggests that the increase of miR-29a in the brain with development may largely reflect increases in astrocyte miR-29a.
Interestingly miR-29a changed in the opposite direction compared to miR-181a after transient forebrain ischemia, showing a decrease in CA1 and increase in dentate gyrus area (Ouyang et al., 2013d). The protective effect of miR-29a on CA1 delayed neuronal death was demonstrated after forebrain ischemia by overexpressing miR-29a (Ouyang et al., 2013d).
The study of miR-29 began primarily in cancer research and focused on its role in regulation of apoptotic pathways. However, a question that has stirred controversy for several years is whether miR-29 is pro-survival or pro-apoptotic (Pekarsky et al., 2006). While miR-29 expression is elevated in some cancers where it appears to function as an oncogene (Gebeshuber et al., 2009; Han et al., 2010), others have found miR-29 to have tumor suppressor functions (Pekarsky et al., 2006; Wang et al., 2008). This question is not only relevant in cancer research but is also important in ischemia research. While downregulation of miR-29 protected hearts against ischemia-reperfusion injury (Ye et al., 2011), upregulation of miR-29 protected neurons from apoptosis during neuronal maturation (Kole et al., 2011) and forebrain cerebral ischemia (Ouyang et al., 2013d). Luciferase target assays conducted in our lab indicate that the miR-29 targets both pro- and anti-apoptotic BCL2 family members (Ouyang et al., 2013c). The results strongly suggest that the reported pro-apoptotic and anti-apoptotic effects of miR-29 result from different targets of miR-29 being inhibited in different cells or under different physiological or pathological settings. A group has reported that miR-29b is activated during neuronal maturation and targets several pro-apoptotic genes, BIM, BMF, HRK, PUMA, and BAK in the BCL2 family (Kole et al., 2011). We found that miR-29a targets BH3-only protein PUMA and reduces neuronal vulnerability to forebrain ischemia (Ouyang et al., 2013d). In contrast, increasing miR-29b had the effect of promoting neuronal cell death in focal ischemia by inhibiting BCL-w, an anti-apoptotic member of the BCL2 protein family (Shi et al., 2012).
Knowing that the targets of miR-181a and miR-29a validated to date (GRP78, BCL2 family members) are ER and mitochondria related (Ouyang and Giffard, 2012; Ouyang and Giffard, 2013; Ouyang et al., 2013a), a common mechanism for these two miRNAs in regulating cerebral ischemia may involve manipulating mitochondrial function. Indeed, using primary cultured astrocytes we have found that miR-181a inhibitor and miR-29a mimic both preserve mitochondrial function, reduce ROS production and protect astrocytes from ischemia-like stress (Ouyang et al., 2012a; Ouyang et al., 2013d) (Fig. 3).
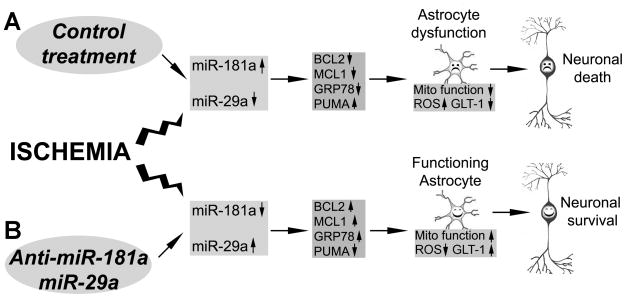
Fig. 3.
Protecting astrocytes using astrocyte-enriched miRNAs or their antagomirs improves neuronal survival after cerebral ischemia. A. Cerebral ischemia induces changes in astrocyte-specific miRNAs (increase in miR-181a and decrease in miR-29a) in vulnerable regions of the brain. They target specific proteins that disturb astrocyte mitochondrial function, increase production of ROS and damage GLT-1 in astrocytes, producing “unhappy” astrocytes and leading to delayed neuronal death. B. Treatment with miR-181a antagomir or miR-29a protects astrocyte mitochondria, reduces ROS and protects GLT-1, supporting neuronal survival. Modified from Fig. 3 in Ouyang et al. (2013c).
9. Future directions
Strategies to improve the neuron-supportive functions of astrocytes have been used successfully in animal and in vitro studies. We speculate that miRNAs may have greater therapeutic potential as candidates for the treatment of stroke than therapies targeting induction of a single gene because of their faster post-transcriptional effect and their ability to simultaneously regulate many target genes. Several astrocyte-enriched miRNAs such as miR-181a and miR-29a have been demonstrated to regulate focal and global cerebral ischemia in animal models by targeting several important cell death/survival pathways through specific mRNA targets (Fig. 3). While pretreatment has been tested in these early studies, investigating the effect of manipulating these astrocyte-enriched miRNAs after the onset of ischemia is an essential next step for translating these ideas toward clinical use. An increasing body of literature suggests that individual miRNAs may have modest effects on their target mRNAs, and several miRNAs may be required for larger effects. This raises the possibility that combination therapy with astrocyte-enriched miRNAs may be even more neuroprotective than a single miRNA. Several miRNAs are already in clinical trials in liver diseases, suggesting that formulation and administration will be possible in a new disease setting or for a new miRNA target. Work remains to be done to target different organs.
Acknowledgments
Grant sponsor: NIH; Grant numbers: NS084396, NS053898, NS080177. The authors thank William Magruder for help preparing the manuscript.
Footnotes
The authors have no conflicting financial interests.
References
- Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19:488–496. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Bambrick L, Kristian T, Fiskum G. Astrocyte mitochondrial mechanisms of ischemic brain injury and neuroprotection. Neurochemical research. 2004;29:601–608. doi: 10.1023/b:nere.0000014830.06376.e6. [DOI] [PubMed] [Google Scholar]
- Bambrick LL, Chandrasekaran K, Mehrabian Z, Wright C, Krueger BK, Fiskum G. Cyclosporin A increases mitochondrial calcium uptake capacity in cortical astrocytes but not cerebellar granule neurons. J Bioenerg Biomembr. 2006;38:43–47. doi: 10.1007/s10863-006-9004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto G, White RE, Ouyang Y, Xu L, Giffard RG. Astrocytes: targets for neuroprotection in stroke. Cent Nerv Syst Agents Med Chem. 2011;11:164–173. doi: 10.2174/187152411796011303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF. Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci. 2000;23:298–304. doi: 10.1016/s0166-2236(00)01584-8. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Blakeley JO, Llinas RH. Thrombolytic therapy for acute ischemic stroke. J Neurol Sci. 2007;261:55–62. doi: 10.1016/j.jns.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Bondarenko A, Chesler M. Rapid astrocyte death induced by transient hypoxia, acidosis, and extracellular ion shifts. Glia. 2001;34:134–142. doi: 10.1002/glia.1048. [DOI] [PubMed] [Google Scholar]
- Bonner HP, Concannon CG, Bonner C, Woods I, Ward MW, Prehn JH. Differential expression patterns of Puma and Hsp70 following proteasomal stress in the hippocampus are key determinants of neuronal vulnerability. J Neurochem. 2010;114:606–616. doi: 10.1111/j.1471-4159.2010.06790.x. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Ellisman MH. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Dev Neurosci. 2004;22:73–86. doi: 10.1016/j.ijdevneu.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH. Mitochondrial dysfunction and oxidative stress as determinants of cell death/survival in stroke. Ann N Y Acad Sci. 2005;1042:203–209. doi: 10.1196/annals.1338.022. [DOI] [PubMed] [Google Scholar]
- Chen CZ. An unsolved mystery: The target-recognizing RNA species of microRNA genes. Biochimie. 2013;95:1663–1676. doi: 10.1016/j.biochi.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science (New York, NY) 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- Chen G, Zhu W, Shi D, Lv L, Zhang C, Liu P, Hu W. MicroRNA-181a sensitizes human malignant glioma U87MG cells to radiation by targeting Bcl-2. Oncology reports. 2010;23:997–1003. doi: 10.3892/or_00000725. [DOI] [PubMed] [Google Scholar]
- Choi DW. Ischemia-induced neuronal apoptosis. Curr Opin Neurobiol. 1996;6:667–672. doi: 10.1016/s0959-4388(96)80101-2. [DOI] [PubMed] [Google Scholar]
- Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003;62:329–339. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- Foyouzi-Youssefi R, Arnaudeau S, Borner C, Kelley WL, Tschopp J, Lew DP, Demaurex N, Krause KH. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5723–5728. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso R, Mao T, Wang S, Schaffert S, Gong X, Yue S, Luong R, Min H, Yashiro-Ohtani Y, Davis M, Pear W, Chen CZ. Modulating the strength and threshold of NOTCH oncogenic signals by mir-181a-1/b-1. PLoS genetics. 2012;8:e1002855. doi: 10.1371/journal.pgen.1002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukamachi S, Furuta A, Ikeda T, Ikenoue T, Kaneoka T, Rothstein JD, Iwaki T. Altered expressions of glutamate transporter subtypes in rat model of neonatal cerebral hypoxia-ischemia. Brain Res Dev Brain Res. 2001;132:131–139. doi: 10.1016/s0165-3806(01)00303-0. [DOI] [PubMed] [Google Scholar]
- Gebeshuber CA, Zatloukal K, Martinez J. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO reports. 2009;10:400–405. doi: 10.1038/embor.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffard RG, Monyer H, Choi DW. Selective vulnerability of cultured cortical glia to injury by extracellular acidosis. Brain Res. 1990;530:138–141. doi: 10.1016/0006-8993(90)90670-7. [DOI] [PubMed] [Google Scholar]
- Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:3510–3524. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YC, Park CY, Bhagat G, Zhang J, Wang Y, Fan JB, Liu M, Zou Y, Weissman IL, Gu H. microRNA-29a induces aberrant self-renewal capacity in hematopoietic progenitors, biased myeloid development, and acute myeloid leukemia. The Journal of experimental medicine. 2010;207:475–489. doi: 10.1084/jem.20090831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ME, Wang Y, Pedigo NW, Hensley K, Butterfield DA, Carney JM. Amyloid β Peptide (25–35) Inhibits Na+-Dependent Glutamate Uptake in Rat Hippocampal Astrocyte Cultures. Journal of Neurochemistry. 1996;67:277–286. doi: 10.1046/j.1471-4159.1996.67010277.x. [DOI] [PubMed] [Google Scholar]
- Harvey BK, Airavaara M, Hinzman J, Wires EM, Chiocco MJ, Howard DB, Shen H, Gerhardt G, Hoffer BJ, Wang Y. Targeted over-expression of glutamate transporter 1 (GLT-1) reduces ischemic brain injury in a rat model of stroke. PLoS One. 2011;6:e22135. doi: 10.1371/journal.pone.0022135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulse RE, Winterfield J, Kunkler PE, Kraig RP. Astrocytic clasmatodendrosis in hippocampal organ culture. Glia. 2001;33:169–179. doi: 10.1002/1098-1136(200102)33:2<169::aid-glia1016>3.0.co;2-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison ER, Kawamoto EM, Taub DD, Lal A, Abdelmohsen K, Zhang Y, Wood WH, 3rd, Lehrmann E, Camandola S, Becker KG, Gorospe M, Mattson MP. Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes. Glia. 2013;61:1018–1028. doi: 10.1002/glia.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar AR, Panickar KS, Murthy Ch R, Norenberg MD. Oxidative stress and mitogen-activated protein kinase phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:4774–4784. doi: 10.1523/JNEUROSCI.0120-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JE, Kim GS, Narasimhan P, Song YS, Chan PH. Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:7003–7014. doi: 10.1523/JNEUROSCI.1110-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahraman S, Bambrick LL, Fiskum G. Effects of FK506 and cyclosporin a on calcium ionophore-induced mitochondrial depolarization and cytosolic calcium in astrocytes and neurons. J Neurosci Res. 2011;89:1973–1978. doi: 10.1002/jnr.22709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Ransom B. Neuroglia. New York: Oxford University Press; 2005. [Google Scholar]
- Kim K, Lee SG, Kegelman TP, Su ZZ, Das SK, Dash R, Dasgupta S, Barral PM, Hedvat M, Diaz P, Reed JC, Stebbins JL, Pellecchia M, Sarkar D, Fisher PB. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J Cell Physiol. 2011;226:2484–2493. doi: 10.1002/jcp.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death. Neuropathology. 2000;20(Suppl):S95–97. doi: 10.1046/j.1440-1789.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tsujimoto Y, Ohtsuki T, Kuwabara K, Matsushita K, Yang G, Tanabe H, Martinou JC, Hori M, Yanagihara T. Amelioration of hippocampal neuronal damage after global ischemia by neuronal overexpression of BCL-2 in transgenic mice. Stroke; a journal of cerebral circulation. 1998;29:2616–2621. doi: 10.1161/01.str.29.12.2616. [DOI] [PubMed] [Google Scholar]
- Kole AJ, Swahari V, Hammond SM, Deshmukh M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes & development. 2011;25:125–130. doi: 10.1101/gad.1975411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Abeta1–42. J Neurochem. 2001;78:413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu D, Smith CL, Barone FC, Ellison JA, Lysko PG, Li K, Simpson IA. Astrocytic demise precedes delayed neuronal death in focal ischemic rat brain. Brain Res Mol Brain Res. 1999;68:29–41. doi: 10.1016/s0169-328x(99)00063-7. [DOI] [PubMed] [Google Scholar]
- Longuemare MC, Hill MP, Swanson RA. Glycolysis can prevent non-synaptic excitatory amino acid release during hypoxia. Neuroreport. 1994;5:1789–1792. doi: 10.1097/00001756-199409080-00026. [DOI] [PubMed] [Google Scholar]
- Lopez-Bayghen E, Ortega A. Glial glutamate transporters: new actors in brain signaling. IUBMB Life. 2011;63:816–823. doi: 10.1002/iub.536. [DOI] [PubMed] [Google Scholar]
- Martinez G, Musumeci G, Loreto C, Carnazza ML. Immunohistochemical changes in vulnerable rat brain regions after reversible global brain ischaemia. J Mol Histol. 2007;38:295–302. doi: 10.1007/s10735-007-9102-9. [DOI] [PubMed] [Google Scholar]
- Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P, Constantine-Paton M, Horvitz HR. Microarray analysis of microRNA expression in the developing mammalian brain. Genome biology. 2004;5:R68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani A, Tanaka K. Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:7176–7182. doi: 10.1523/JNEUROSCI.23-18-07176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JM, Xu L, Giffard RG. Inhibition of microRNA-181 reduces forebrain ischemia induced neuronal loss. J Cereb Blood Flow Metab. 2013 doi: 10.1038/jcbfm.2013.157. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky S, Rothstein J, Yang Y. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. The Journal of biological chemistry. 2013;288:7105–7116. doi: 10.1074/jbc.M112.410944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AN, Fiskum G, Beal MF. Mitochondria in neurodegeneration: bioenergetic function in cell life and death. J Cereb Blood Flow Metab. 1999;19:231–245. doi: 10.1097/00004647-199903000-00001. [DOI] [PubMed] [Google Scholar]
- Niizuma K, Endo H, Nito C, Myer DJ, Chan PH. Potential role of PUMA in delayed death of hippocampal CA1 neurons after transient global cerebral ischemia. Stroke; a journal of cerebral circulation. 2009;40:618–625. doi: 10.1161/STROKEAHA.108.524447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitatori T, Sato N, Waguri S, Karasawa Y, Araki H, Shibanai K, Kominami E, Uchiyama Y. Delayed neuronal death in the CA1 pyramidal cell layer of the gerbil hippocampus following transient ischemia is apoptosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:1001–1011. doi: 10.1523/JNEUROSCI.15-02-01001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Giffard RG. Cellular neuroprotective mechanisms in cerebral ischemia: Bcl-2 family proteins and protection of mitochondrial function. Cell Calcium. 2004;36:303–311. doi: 10.1016/j.ceca.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Giffard RG. ER-Mitochondria Crosstalk during Cerebral Ischemia: Molecular Chaperones and ER-Mitochondrial Calcium Transfer. Int J Cell Biol. 2012;2012:493934. doi: 10.1155/2012/493934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Giffard RG. MicroRNAs Regulate the Chaperone Network in Cerebral Ischemia. Transl Stroke Res. 2013:1–11. doi: 10.1007/s12975-013-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Kuroda S, Kristian T, Siesjo BK. Release of mitochondrial aspartate aminotransferase (mAST) following transient focal cerebral ischemia suggests the opening of a mitochondrial permeability transition pore. Neuroscience Research Communications. 1997;20:167–173. [Google Scholar]
- Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Lu Y, Yue S, Giffard RG. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion. 2012a;12:213–219. doi: 10.1016/j.mito.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Stary CM, Yang GY, Giffard R. microRNAs: innovative targets for cerebral ischemia and stroke. Curr Drug Targets. 2013a;14:90–101. doi: 10.2174/138945013804806424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Stary CM, White RE, Voloboueva LA, Giffard RG. The use of microRNAs to modulate redox and immune response to stroke. Antiox Redox Signaling. 2013b doi: 10.1089/ars.2013.5757. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Xu L, Yue S, Liu S, Giffard RG. Neuroprotection by astrocytes in brain ischemia: importance of microRNAs. Neuroscience Letters. 2013c doi: 10.1016/j.neulet.2013.11.015. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Tan Y, Comb M, Liu CL, Martone ME, Siesjo BK, Hu BR. Survival- and death-promoting events after transient cerebral ischemia: phosphorylation of Akt, release of cytochrome C and Activation of caspase-like proteases. J Cereb Blood Flow Metab. 1999;19:1126–1135. doi: 10.1097/00004647-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Xu L, Lu Y, Sun X, Yue S, Xiong XX, Giffard RG. Astrocyte-enriched miR-29a targets PUMA and reduces neuronal vulnerability to forebrain ischemia. Glia. 2013d;61:1784–1794. doi: 10.1002/glia.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Lu Y, Yue S, Xu LJ, Xiong XX, White RE, Sun X, Giffard RG. miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiology of disease. 2012b;45:555–563. doi: 10.1016/j.nbd.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Heneka MT, Montana V, Oliet SHR, Schousboe A, Haydon PG, Stout RF, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A. Glial cells in (patho)physiology. Journal of Neurochemistry. 2012;121:4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarsky Y, Santanam U, Cimmino A, Palamarchuk A, Efanov A, Maximov V, Volinia S, Alder H, Liu CG, Rassenti L, Calin GA, Hagan JP, Kipps T, Croce CM. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer research. 2006;66:11590–11593. doi: 10.1158/0008-5472.CAN-06-3613. [DOI] [PubMed] [Google Scholar]
- Petito CK, Olarte JP, Roberts B, Nowak TS, Jr, Pulsinelli WA. Selective glial vulnerability following transient global ischemia in rat brain. J Neuropathol Exp Neurol. 1998;57:231–238. doi: 10.1097/00005072-199803000-00004. [DOI] [PubMed] [Google Scholar]
- Ransom B, Behar T, Nedergaard M. New roles for astrocytes (stars at last) Trends Neurosci. 2003;26:520–522. doi: 10.1016/j.tins.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Rao SD, Yin HZ, Weiss JH. Disruption of glial glutamate transport by reactive oxygen species produced in motor neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:2627–2633. doi: 10.1523/JNEUROSCI.23-07-02627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VL, Dogan A, Todd KG, Bowen KK, Kim BT, Rothstein JD, Dempsey RJ. Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:1876–1883. doi: 10.1523/JNEUROSCI.21-06-01876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravagnan L, Roumier T, Kroemer G. Mitochondria, the killer organelles and their weapons. J Cell Physiol. 2002;192:131–137. doi: 10.1002/jcp.10111. [DOI] [PubMed] [Google Scholar]
- Reichert SA, Kim-Han JS, Dugan LL. The mitochondrial permeability transition pore and nitric oxide synthase mediate early mitochondrial depolarization in astrocytes during oxygen-glucose deprivation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:6608–6616. doi: 10.1523/JNEUROSCI.21-17-06608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PA, Aizenman E. Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neuroscience Letters. 1989;103:162–168. doi: 10.1016/0304-3940(89)90569-7. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Shi G, Liu Y, Liu T, Yan W, Liu X, Wang Y, Shi J, Jia L. Upregulated miR-29b promotes neuronal cell death by inhibiting Bcl2L2 after ischemic brain injury. Exp Brain Res. 2012;216:225–230. doi: 10.1007/s00221-011-2925-3. [DOI] [PubMed] [Google Scholar]
- Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu Z, You Y. hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain Res. 2008;1236:185–193. doi: 10.1016/j.brainres.2008.07.085. [DOI] [PubMed] [Google Scholar]
- Smirnova L, Grafe A, Seiler A, Schumacher S, Nitsch R, Wulczyn FG. Regulation of miRNA expression during neural cell specification. The European journal of neuroscience. 2005;21:1469–1477. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Chen J, Graham SH. Glucose can fuel glutamate uptake in ischemic brain. J Cereb Blood Flow Metab. 1994;14:1–6. doi: 10.1038/jcbfm.1994.1. [DOI] [PubMed] [Google Scholar]
- Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348:443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science (New York, NY) 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tao J, Wu H, Lin Q, Wei W, Lu XH, Cantle JP, Ao Y, Olsen RW, Yang XW, Mody I, Sofroniew MV, Sun YE. Deletion of astroglial Dicer causes non-cell-autonomous neuronal dysfunction and degeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:8306–8319. doi: 10.1523/JNEUROSCI.0567-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THACAS. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci. 1998;19:328–334. doi: 10.1016/s0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- Trotti D, Rizzini BL, Rossi D, Haugeto O, Racagni G, Danbolt NC, Volterra A. Neuronal and glial glutamate transporters possess an SH-based redox regulatory mechanism. The European journal of neuroscience. 1997;9:1236–1243. doi: 10.1111/j.1460-9568.1997.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T, Bonner HP, Engel T, Woods I, Matsushima S, Ward MW, Taki W, Henshall DC, Concannon CG, Prehn JH. Bcl-2 homology domain 3-only proteins Puma and Bim mediate the vulnerability of CA1 hippocampal neurons to proteasome inhibition in vivo. The European journal of neuroscience. 2011;33:401–408. doi: 10.1111/j.1460-9568.2010.07538.x. [DOI] [PubMed] [Google Scholar]
- Uchino H, Elmer E, Uchino K, Lindvall O, Siesjo BK. Cyclosporin A dramatically ameliorates CA1 hippocampal damage following transient forebrain ischaemia in the rat. Acta Physiol Scand. 1995;155:469–471. doi: 10.1111/j.1748-1716.1995.tb09999.x. [DOI] [PubMed] [Google Scholar]
- Voloboueva LA, Suh SW, Swanson RA, Giffard RG. Inhibition of mitochondrial function in astrocytes: implications for neuroprotection. Journal of Neurochemistry. 2007;102:1383–1394. doi: 10.1111/j.1471-4159.2007.4634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloboueva LA, Emery JF, Sun X, Giffard RG. Inflammatory response of microglial BV-2 cells includes a glycolytic shift and is modulated by mitochondrial glucose-regulated protein 75/mortalin. FEBS Letters. 2013;587:756–762. doi: 10.1016/j.febslet.2013.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Wang H, Garzon R, Sun H, Ladner KJ, Singh R, Dahlman J, Cheng A, Hall BM, Qualman SJ, Chandler DS, Croce CM, Guttridge DC. NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer cell. 2008;14:369–381. doi: 10.1016/j.ccr.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S, Kawashima N, Hori S, Takimoto M, Wada K, Tanaka K. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. The European journal of neuroscience. 1998;10:976–988. doi: 10.1046/j.1460-9568.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- Xu L, Lee JE, Giffard RG. Overexpression of bcl-2, bcl-XL or hsp70 in murine cortical astrocytes reduces injury of co-cultured neurons. Neuroscience Letters. 1999;277:193–197. doi: 10.1016/s0304-3940(99)00882-4. [DOI] [PubMed] [Google Scholar]
- Xu L, Sapolsky RM, Giffard RG. Differential sensitivity of murine astrocytes and neurons from different brain regions to injury. Exp Neurol. 2001;169:416–424. doi: 10.1006/exnr.2001.7678. [DOI] [PubMed] [Google Scholar]
- Xu L, Emery JF, Ouyang YB, Voloboueva LA, Giffard RG. Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia. 2010;58:1042–1049. doi: 10.1002/glia.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Perez-Polo JR, Qian J, Birnbaum Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiological genomics. 2011;43:534–542. doi: 10.1152/physiolgenomics.00130.2010. [DOI] [PubMed] [Google Scholar]
- Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2003a;85:1026–1036. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- Zhao X, Ahram A, Berman RF, Muizelaar JP, Lyeth BG. Early loss of astrocytes after experimental traumatic brain injury. Glia. 2003b;44:140–152. doi: 10.1002/glia.10283. [DOI] [PubMed] [Google Scholar]