Abstract
Background
Women have an unexplained worse outcome after myocardial infarction (MI) compared with men in many studies. Depressive symptoms predict adverse post-MI outcomes and are more prevalent among women than men. We examined whether depressive symptoms contribute to women’s worse outcomes after MI.
Methods and Results
In a prospective multicenter study (PREMIER), 2411 (807 women) MI patients were enrolled. Depressive symptoms were assessed with the Patient Health Questionnaire. Outcomes included 1-year rehospitalization, presence of angina using the Seattle Angina Questionnaire, and 2-year mortality. Multivariable analyses were used to evaluate the association between sex and these outcomes, adjusting for clinical characteristics. The depressive symptoms score was added to the models to evaluate whether it attenuated the association between sex and outcomes. Depressive symptoms were more prevalent in women compared with men (29% versus 18.8%, P<0.001). After adjusting for demographic factors, comorbidities, and MI severity, women had a mildly higher risk of rehospitalization (hazard ratio, 1.20; 95% CI, 1.04 to 1.40), angina (odds ratio, 1.32; 95% CI, 1.00 to 1.75), and mortality (hazard ratio, 1.27; 95% CI, 0.98 to 1.64). After adding depressive symptoms to the multivariable models, the relationship further declined toward the null, particularly for rehospitalization (hazard ratio, 1.14; 95% CI, 0.98 to 1.34) and angina (odds ratio, 1.22; 95% CI, 0.91 to 1.63), whereas there was little change in the estimate for mortality (hazard ratio, 1.24; 95% CI, 0.95 to 1.62). Depressive symptoms were significantly associated with each of the study outcomes with a similar magnitude of effect in both women and men.
Conclusions
A higher prevalence of depressive symptoms in women modestly contributes to their higher rates of rehospitalization and angina compared with men but not mortality after MI. Our results support the recent recommendations of improving recognition of depressive symptoms after MI.
Keywords: sex, depression, myocardial infarction, women
Despite recent advances in prevention, diagnosis, and treatment, coronary heart disease (CHD) claims the lives of more than half a million women every year in the United States.1 Women are protected against coronary atherosclerosis compared with men until their later years of life.2,3 However, once they develop an acute myocardial infarction (MI), women have a higher mortality than men, a finding that is seen in several age strata with the exception of the oldest patients.1,4 In several2,5,6 but not all7,8 studies, the worse outcome in women persists after adjusting for age, medical history, clinical severity, hospital treatment, and cardiac procedures. The reasons for the worse outcomes of women are poorly understood. Because the higher risk in women is not explained by conventional risk factors and treatments, alternative explanations must be sought.
The presence of depressive symptoms is independently associated with higher cardiac and all-cause mortality, rehospitalization, and worse functional status including angina and quality of life after MI.9–12 Women, especially younger women, are uniquely prone to depression in the peri- and post-MI period, with a prevalence of depression almost twice that of men.13 Thus, it is possible that depressive symptoms may account for some of the excess risk observed in women recovering from a MI. To date, there has been no prospective study examining whether the higher prevalence of depressive symptoms among women contributes to observed sex disparities in survival and health status outcomes after MI. The purpose of this prospective study was to examine the extent to which depressive symptoms, assessed at the time of initial MI hospitalization, accounted for the higher rates of post-MI adverse outcomes in women.
Methods
Participants and Data Collection
Between January 2003 and June 2004, 2498 MI patients from 19 US hospitals participated in the Prospective Registry Evaluating Outcomes After Myocardial Iinfaction: Events and Recovery (PREMIER) study. The methodology of this study has been described previously.14 Patients were aged ≥18 years and had a MI confirmed by elevated biomarkers of myocardial injury (troponin or creatine kinase MB fraction) and supporting evidence of a MI (eg, prolonged ischemic signs/symptoms, electrocardiographic ST changes). Eligible patients either presented directly to the enrolling institution or were transferred within the first 24 hours of symptoms. Incarcerated patients or those with elevated cardiac enzymes as a complication of elective coronary revascularization were not eligible. Of 10 911 patients screened for inclusion in PREMIER, 3953 patients were eligible and 2498 (63%) were enrolled into PREMIER. Few clinically significant differences between the total patients screened and those enrolled into PREMIER were observed. Patients consenting to enroll in PREMIER were younger and more often male and white. They were also more likely to have ST-elevation MI (STEMI) and prior angiography and percutaneous intervention.14
As described previously, comprehensive data were collected from inpatient chart abstractions, baseline interviews administered by trained personnel within 24 to 72 hours of admission, and follow-up telephone interviews at 1 year after discharge by a centralized follow-up center. Patient interviews were completed at 1-year post-MI. We continued to obtain data on vital status. At the time of this analysis, 2-year vital status was available on all patients. The Institutional Review Board at each participating institution approved the study, and all patients provided informed consent.
Measurement of Depressive Symptoms
Depressive symptoms were assessed at the time of MI hospitalization (baseline interview) using the 9-question Primary Care Evaluation of Mental Disorders Brief Patient Health Questionnaire (PHQ).15,16 In the PHQ, patients indicate, for each of 9 depressive symptoms, whether the symptom has bothered them “not at all,” “several days,” “more than half the days,” or “nearly every day” during the previous 2 weeks. Each PHQ question yields a score of 0 to 3 and the total score ranges from 0 to 27. Consistent with previous studies,11,17 we classified patients to have depressive symptoms if their PHQ score was ≥10 corresponding to a level of at least moderate depressive symptoms and representing the minimum number of symptoms required for the diagnosis of major depression.18 The PHQ has a sensitivity of 88% and a specificity of 88% for major depression when a cutoff of ≥10 is used.15,16 Previous history of depression was defined as receiving medications or counseling for depression.
Outcome Variables
Outcome measures were (1) all-cause rehospitalization at 1 year, (2) presence of angina at 1 year, and (3) all-cause mortality at 2 years. In the follow-up interview of 1-year survivors, patients were queried whether they were readmitted to a hospital after their index MI admission, and if they were, to provide the hospitalization date. In addition, as a measure of health status, presence of angina was assessed at baseline and at the 1-year follow-up interview using the Seattle Angina Questionnaire (SAQ), which measures symptom burden over the preceding 4 weeks.19 All SAQ domains have been validated and the SAQ predicts mortality and subsequent cardiac events in patients with CHD.19,20 Because the therapeutic goal is for post-MI patients to be free of angina and because of an extremely skewed distribution of 1-year SAQ angina frequency scores (80% of patients were angina-free at 1 year), we examined angina as a dichotomous variable (presence or absence of angina, as defined by SAQ score <100 and =100, respectively, at 1 year).11,19 Lastly, vital status at 2 years after discharge was obtained from contacts with family members and the Social Security Death Master File.
Data Analysis
Depressive symptoms were examined both as a continuous variable (PHQ depression score) and as a dichotomous variable (presence of at least moderate depressive symptoms using the standard cutoff of PHQ ≥10). Bivariate analyses were conducted to examine the unadjusted association of demographic and clinical characteristics between men and women separately and also according to depressive symptoms status (PHQ ≥10) using t-tests for continuous variables and χ2 or Fisher exact tests for categorical variables, as appropriate. Unadjusted rates of all-cause rehospitalization and all-cause mortality between men and women are reported as Kaplan-Meier estimates.
Time-to-event outcomes of 1-year rehospitalization and 2-year mortality were modeled using multivariable shared frailty proportional hazards regression, which accounts for clustering within site. These models include a frailty parameter for each hospital that describes unexplained heterogeneity in survival rates across the hospitals. The proportional hazards assumption was tested for each model and verified using Schoenfeld residuals. For the dichotomous outcome of presence of angina, we used multivariable hierarchical logistic regression models, which accounted for variation within site.
To assess the independent effect of sex on outcomes, multivariable modeling was conducted in several sequential steps: (1) sex, (2) addition of age, and (3) fully adjusted multivariable model. Covariates in multivariable models included traditional CHD prognostic variables that are known to influence CHD outcome and/or covariates that differed significantly by sex on a bivariate basis (P<0.05). These covariates in the fully adjusted multivariable models were patient demographics (age, race), medical history (congestive heart failure, diabetes, hypertension, lung disease, smoking, previous history of CHD), and type of MI (STEMI versus non–ST-Elevation MI [NSTEMI]). In the multivariable model of 1-year angina, patients’ baseline angina frequency, as a continuous variable, was also included as a covariate. As a final step for all models, we added PHQ depression score. The extent to which adjustment for the PHQ depression score attenuated the corresponding coefficient for sex toward 1 (no effect) was used as a measure of the degree to which depressive symptoms attenuate the observed differences in outcome between men and women. Logistic regression model fit and discrimination were assessed using the Hosmer and Lemeshow goodness-of-fit test and the c statistic, respectively.
We conducted 3 sets of secondary analyses. First, because the degree of depressive symptoms may be influenced by antidepressants and previous history of depression, we adjusted for antidepressant use at baseline and previous history of depression as sensitivity analyses. Second, as another sensitivity analysis, because education, marital status, left ventricular ejection fraction, and MI quality of care may differ according to sex and depressive symptoms, we adjusted for marital status, education, left ventricular ejection fraction, and coronary artery bypass grafting, percutaneous intervention, or cardiac catheterization received during hospitalization, percent of quality indicators received as defined by the Centers for Medicare and Medicaid Services and the Joint Commission on Accreditation of Healthcare Organizations21,22 and number of quality indicators patients were eligible for. Third, we also examined depressive symptoms’ effect on each outcome using the methods and covariates described above stratified by sex. Additionally, we tested a first-level interaction term between sex and depressive symptoms for each outcome. None of the interactions were significant.
Finally, we evaluated potential bias from missing 1-year outcome data due to patients who were lost to follow-up or refused the 1-year interview. The study sample of 2411 patients all had 2-year mortality data; 2318 (96%) had rehospitalization data and 1957 (81%) had 1-year angina data. In the overall sample including these patients (but excluding patients who were deceased or too ill to be interview at 1-year follow-up) we calculated a propensity score of having a missing 1-year interview using logistic regression. Predictor variables used in the propensity score analysis that were not included in the Table were economic burden, living at home, ischemic symptoms on arrival, shock, pulmonary embolism, history of cocaine and alcohol abuse, physical function, taking part in decision making, instructions received by patient on whom to call for emergency/any questions, lipid evaluation, cardiac rehabilitation, and length of stay in hospital. The propensity score was the probability of a person with given characteristics having a missing 1-year interview. Predictor variables included variables in the Table. The reciprocal of this score was then used as a weight in the analyses, resulting in higher weight given to observations from patients that are similar to those with missing outcome data. All tests for statistical significance were 2-tailed with an α level of 0.05. All analyses were conducted using SAS software, release 9.1 (SAS Institute, Cary, NC) and R version 2.1.1.23 The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Table.
Comparison of Baseline Characteristics Between Men and Women
| Characteristic | Male (N=1604) | Female (N=807) | P |
|---|---|---|---|
| Sociodemographic factors | |||
| Age, years | 59.1±12.5 | 63.6±13.5 | <0.001 |
| Black race | 288 (18.1) | 237 (29.5) | <0.001 |
| Married | 1082 (68.5) | 345 (43.5) | <0.001 |
| Working full or part time | 814 (51.6) | 236 (29.6) | <0.001 |
| Greater than high school education | 802 (50.9) | 310 (39.5) | <0.001 |
| Avoid getting health care due to cost | 268 (17.1) | 162 (20.5) | 0.05 |
| Primary medical insurance | |||
| Medicare | 533 (34.6) | 376 (48.5) | <0.001 |
| Medicaid | 118 (7.7) | 126 (16.3) | <0.001 |
| Self-pay/none | 207 (13.5) | 82 (10.6) | 0.04 |
| Medical history | |||
| History of angina pectoris | 278 (17.3) | 129 (16.0) | 0.41 |
| Presence of angina by SAQ frequency score | 854 (53.5) | 416 (51.7%) | 0.41 |
| History of MI | 344 (21.4) | 165 (20.4) | 0.57 |
| History of congestive heart failure | 167 (10.4) | 115 (14.3) | 0.006 |
| History of hypertension | 949 (59.2) | 576 (71.4) | <0.001 |
| History of diabetes | 408 (25.4) | 273 (33.8) | <0.001 |
| History of hypercholesterolemia | 793 (49.4) | 383 (47.5) | 0.36 |
| History of chronic renal failure | 151 (9.4) | 91 (11.3) | 0.15 |
| History of cerebrovascular accident | 93 (5.8) | 61 (7.6) | 0.09 |
| Smoking | 563 (35.5) | 258 (32.3) | 0.12 |
| History of PCI | 305 (19.0) | 123 (15.2) | 0.02 |
| History of CABG | 229 (14.3) | 74 (9.2) | <0.001 |
| Prior coronary heart disease (MI, PCI, or CABG) | 520 (32.4) | 233 (28.9) | 0.08 |
| History of chronic lung disease | 188 (11.7) | 126 (15.6) | 0.007 |
| History of coronary stenosis | 258 (16.1) | 115 (14.3) | 0.24 |
| History of coronary angiography | 1445 (90.1) | 676 (83.8) | <0.001 |
| Family history of coronary artery disease | 547 (34.1) | 269 (33.3) | 0.71 |
| Clinical characteristics at admission | |||
| Systolic blood pressure, mm Hg | |||
| >120 | 1119 (69.8) | 566 (70.1) | 0.28 |
| 90 to 120 | 402 (25.1) | 188 (23.3) | |
| <90 | 83 (5.2) | 53 (6.6) | |
| Heart rate >100 beats/min | 201 (12.5) | 140 (17.3) | 0.001 |
| Myocardial infarction diagnosis | |||
| STEMI | 760 (47.4) | 306 (37.9) | <0.001 |
| NSTEMI | 844 (52.6) | 501 (62.1) | |
| Ejection fraction <40 | 415 (25.9) | 210 (26.1) | 0.93 |
| PHQ depression score | 5.2±5.3 | 6.8±5.8 | <0.001 |
| In-hospital events | |||
| Atrial fibrillation/flutter | 131 (8.2) | 77 (9.5) | 0.26 |
| Congestive heart failure | 121 (7.5) | 75 (9.3) | 0.14 |
| Cerebrovascular accident | 14 (0.9) | 8 (1.0) | 0.77 |
| Re-infarction | 19 (1.2) | 6 (0.7) | 0.31 |
| QOC | |||
| Diagnostic cardiac catheterization | 666 (41.5) | 350 (43.4) | 0.39 |
| Received aspirin at arrival | 1525 (97.4) | 749 (96.5) | 0.24 |
| Received aspirin at discharge | 1476 (95.3) | 705 (90.9) | <0.001 |
| Received ACE inhibitor for LVSD at discharge | 306 (83.6) | 137 (76.1) | 0.04 |
| Received smoking cessation instructions | 452 (74.3) | 186 (68.9) | 0.09 |
| Received β-blocker at discharge | 1403 (93.1) | 683 (90.3) | 0.02 |
| Received β-blocker at arrival | 1357 (92.7) | 664 (92.4) | 0.78 |
| Received reperfusion for STEMI/LBBB | 581 (72.3) | 244 (64.6) | 0.007 |
| Received timely reperfusion for STEMI/LBBB | 359 (67.1) | 147 (64.8) | 0.53 |
| No. of QOC-eligible indicators received | 5.2±1.3 | 5.1±1.3 | 0.002 |
| Percentage of eligible QOC indicators received | 88.8±15.8 | 86.1±18.4 | <0.001 |
Data are presented as mean±SD or n (%). PCI indicates percutaneous intervention; CABG, coronary artery bypass grafting; QOC, quality of care; ACE, angiotensin-converting enzyme; LVSD, left ventricular systolic dysfunction; LBBB, left bundle-branch block.
Results
Study Population
Of the 2498 patients enrolled in the PREMIER registry, 17 did not survive the index hospitalization and were excluded from the analysis. In addition, 70 patients were excluded from the 2 Veterans Health Administration hospital sites because all but 1 of the patients enrolled from these hospitals were men. Thus, 2411 patients (807 women) were included in these analyses.
There were substantial differences in baseline characteristics between women and men (Table). Compared with men, women were older, had lower household income, and were more often black (all P<0.001). Women were less likely than men to be married, to have completed high school, or to be employed. Compared with men, women also had more comorbid conditions, including diabetes, hypertension, congestive heart failure, and chronic lung disease, but were less likely to have STEMI, prior coronary artery bypass graft, and percutaneous intervention. There was no association between sex and left ventricular ejection fraction, and in-hospital events such as atrial fibrillation, reinfarction, or congestive heart failure. Women had lower rates of coronary revascularization and received a lower percentage of eligible quality-of-care indicators compared to men.
Presence of depressive symptoms, as defined by a PHQ score ≥10, was common: 22.3% of the patients had depressive symptoms. The prevalence of depressive symptoms was higher in women compared with men (29% versus 18.8%, P<0.001). Examination of depressive symptoms as a continuous variable (mean PHQ scores) by sex demonstrated that women had significantly higher PHQ scores (6.8±5.8 versus 5.2±5.3; t=6.68; P<0.001), indicating more depressive symptoms (Table). The sex differences in patient characteristics, comorbid conditions, clinical severity of MI, and in-hospital events were similar among those with and without depressive symptoms (data not shown).
Only 19% of patients with depressive symptoms were discharged on antidepressants. Women with depressive symptoms were more likely to be discharged on antidepressants compared with men with depressive symptoms (24.8% versus 14.8%; P=0.015). Women also tended to have more often than men a previous history of depression (32.2% versus 23.7% for women and men with depressive symptoms, respectively; P=0.06).
Association Between Sex and Outcomes
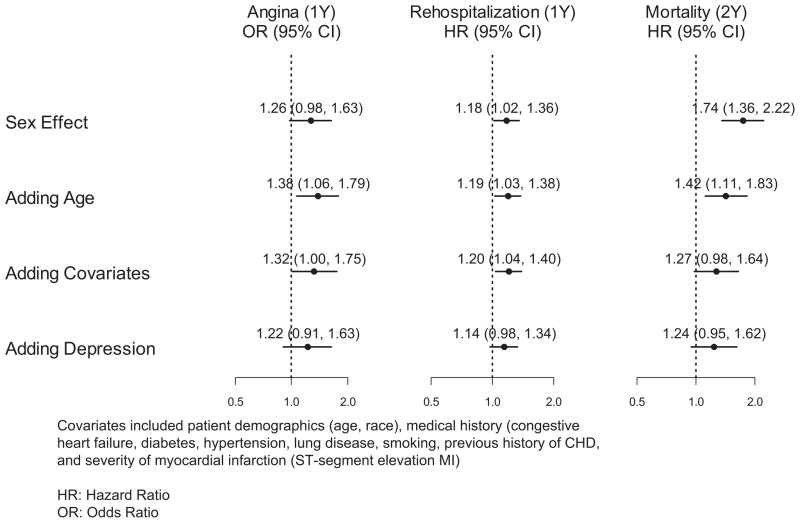
During the 1-year follow-up, 297 women and 526 men were rehospitalized. The rehospitalization rate was significantly higher in women than in men (43% versus 38%; unadjusted hazard ratio [HR], 1.18; 95% CI, 1.02 to 1.36). After adjusting for age, women continued to experience a significantly higher hazard for rehospitalization compared to men (age-adjusted HR, 1.19; 95% CI, 1.03 to 1.38) (Figure 1). After adjusting for patient demographics including age, medical history, and severity of MI, women had a 20% higher hazard for rehospitalization (adjusted HR, 1.20; 95% CI, 1.04 to 1.40). With further adjustment for depressive symptoms, sex was no longer significantly associated with rehospitalization, with an absolute risk reduction of 6% following the addition of depressive symptoms to the model (adjusted HR, 1.14; 95% CI, 0.98 to 1.34) (Figure 2).
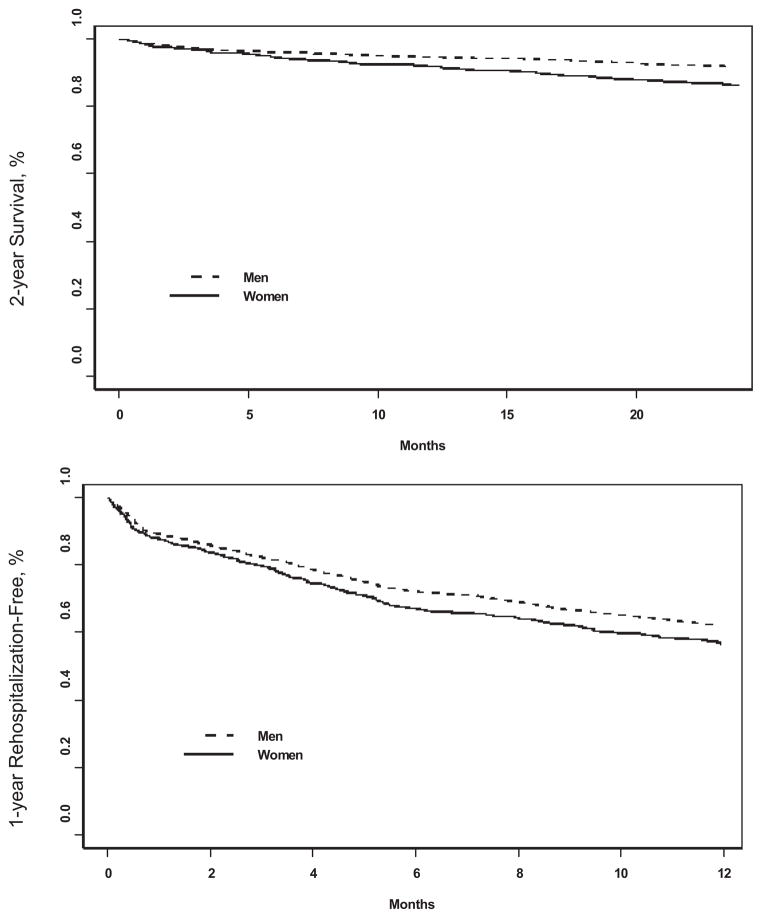
Figure 1.
Kaplan–Meier curves for age-adjusted rehospitalization and mortality after MI.
Figure 2.
Sex and outcomes after MI, with incremental risk-adjustment models. HRs and ORs for women as compared with men are shown.
During the follow-up, 120 women and 140 men died. Women had a higher mortality rate compared with men (15.0% versus 8.8%; HR, 1.74; 95% CI, 1.36 to 2.22). The difference decreased after age adjustment (HR, 1.42; 95% CI, 1.11 to 1.83). After further adjusting for race, medical history, and severity of MI as above, the sex difference in mortality diminished further (HR, 1.27; 95% CI, 0.98 to 1.64) (Figures 1 and 2). When depressive symptoms were added to the model, the magnitude of the HR remained practically unchanged (adjusted HR, 1.24; 95% CI, 0.95 to 1.62) (Figure 2).
Although in unadjusted analysis there was no significant difference in angina at 1 year between women and men (22.4% versus 18.6%; odds ratio [OR], 1.26; 95% CI, 0.98 to 1.63), after adjustment for age, the sex difference became significant, with women having a 38% higher odds of angina compared to men (OR, 1.38; 95% CI, 1.06 to 1.79). There was a 6% absolute risk reduction in angina after adjustment for patient demographics, medical history, and severity of MI; women continued to experience significantly higher odds of angina (OR, 1.32; 95% CI, 1.00 to 1.75). With further adjustment for depressive symptoms, however, there was a 10% absolute risk reduction, and sex was no longer significantly associated with the presence of angina at 1 year (OR, 1.22; 95% CI, 0.91 to 1.63) (Figure 2).
All results remained unchanged after adding antidepressant use at discharge and previous history of depression to the model. In addition, all results remained unchanged after adjusting for marital status, education, left ventricular ejection fraction, revascularization (coronary artery bypass grafting, percutaneous intervention), coronary catheterization, and Joint Commission on Accreditation of Healthcare Organizations/Centers for Medicare and Medicaid Services quality of care for MI. The results of logistic regression analyses using propensity scores were also similar to the primary analyses, suggesting no observable bias due to patients lost to follow-up.
In the population as whole, depressive symptoms (per point increase in PHQ score) were significantly associated with mortality (crude and adjusted HRs, 1.04 and 1.02, respectively), rehospitalization (crude and adjusted HRs, 1.03 and 1.02, respectively), and angina (crude and adjusted OR 1.09 and 1.05, respectively) (all P<0.05). In additional analyses, we stratified by sex and examined the interaction between sex and depressive symptoms for each study outcome. Depressive symptoms were significantly and similarly associated with each of the study outcomes in both men and women. The adjusted HRs for mortality for women and men were 1.01 and 1.04, respectively (per PHQ score point increase), and for rehospitalization, they were 1.03 and 1.02, respectively (all P<0.05). For presence of angina, the corresponding adjusted ORs were 1.04 and 1.05 (all P<0.05) for women and men, respectively. There was no significant interaction between sex and depressive symptoms for any of the outcomes, confirming that the associations were of similar magnitude according to sex.
Discussion
In this prospective study of post-MI patients, depressive symptoms modestly accounted for women’s worse outcome after MI. Consistent with previous studies, women had a higher risk of adverse outcomes post-MI, especially rehospitalization and angina. Women also had a higher prevalence of depressive symptoms post-MI compared with men.13,24 Depressive symptoms predicted worse outcomes in a similar magnitude in men and women, over and above that of other prognostic factors. However, after adjusting for age, patient demographics, comorbidities, severity of MI, and quality of care, depressive symptoms only modestly accounted for the remaining higher risk in post-MI women for rehospitalization and angina compared with men. They accounted for 6% higher risk of rehospitalization and 10% higher risk of angina in women compared with men, respectively. There was no attenuation of mortality risk following adjustment of depressive symptoms because most of the sex difference in mortality was already accounted for by age, patient comorbidities, demographic characteristics, and MI clinical severity indicators.
Comparison With Previous Studies
The results of our study expand previous literature in several important ways. First, despite the intense research focus on MI in women in the past 10 to 15 years, the vulnerability of women toward adverse outcomes after MI remains only partially explained by age, CHD risk factors, and clinical characteristics.5 Whether depressive symptoms play a role in the worse outcomes of women was never specifically addressed before, despite the fact that depressive symptoms are independent risk factors for outcomes after MI and are present in nearly 1 in 3 post-MI women. In the only study with information on this subject, a secondary analysis of 283 women and 613 men pooled from 2 separate studies, the relationship between sex and outcome was less marked after controlling for depression for all of the cardiac end points.24 However, the only outcome variable for which there was a significant sex difference (MI recurrences) remained significant after taking depression into account, suggesting that at least in that study, depression did not account for sex differences in prognosis. Consistent with this previous information and using a prospective national MI registry, we found that after adjusting for a comprehensive set of covariates such as patient demographics, comorbidities, clinical severity of MI, and quality of care, depressive symptoms modestly affected women’s worse outcomes after MI.
Second, depressive symptoms contributed ≈10% higher absolute risk of angina in women, after adjusting for clinical and quality-of-care factors. Although this effect may not seem large, angina symptoms affect women’s survival rates, functional status, quality of life, and health-related costs.25,26 Thus, this modest contribution of depressive symptoms to women’s angina may be clinically important. Third, previous studies have rarely stratified effects of depression on outcomes in MI patients by sex, perhaps due to the small number of women included in several of these studies. In the only study addressing this question,24 depression was found to be a significant independent predictor of 1-year cardiac mortality in both women and men hospitalized with MI, with a similar magnitude of effect by sex. Our study demonstrates that depressive symptoms at the time of hospitalization for MI predict outcomes in men and women in a similar fashion. Taken together, our study results suggest that after MI, depressive symptoms are more commonly present in women; they predict adverse outcomes in both men and women, and drive some of the higher rate of unfavorable outcomes in women post-MI.
Clinical Implications
Depressive symptoms are common, adversely affect prognosis, and can be effectively recognized and treated in cardiac patients.27–29 However, the recognition and treatment of depressive symptoms in real-world MI practice appear dismal despite the recent emphasis on depression screening and treatment.1,30 In our study, consistent with the previous literature,31 only a small fraction of depressed patients (19%) were discharged on antidepressants. Although there is a critical need to determine whether treatment of depression can reduce adverse outcomes after MI, data from clinical trials are limited. The only large trial to examine this question to date, the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study, did not demonstrate a benefit on mortality and reinfarction by using cognitive-behavioral therapy in post-MI depressed patients.29 Current data indicate that selective serotonin reuptake inhibitor antidepressants are safe in patients with CHD.27–29 Although recognition and treatment of depression in cardiac patients is advocated,30 its utility in improving depression and cardiac outcomes is still debated32 and needs evaluation of novel approaches.
Study Limitations
Some issues should be considered in the interpretation of this study. First, instead of obtaining a clinical diagnosis of major depression, we measured depressive symptoms because PHQ administration is a more feasible strategy in a setting of an acute MI than a structured clinical interview for major depression. We chose to use a PHQ cutoff of 10 to define the presence of at least moderate depressive symptoms in our study, consistent with previous literature on depression in CHD patients.11,17 Although evaluation of depression in the setting of an acute event has limitations, previous studies have shown that depressive symptoms without a clinical diagnosis of major depression, either transient, persistent or new after MI are independently associated with worse outcomes.10 Second, the end point of rehospitalization and angina was by self-report, and we were not able to independently adjudicate the cause of death or of rehospitalization. Although this could theoretically lead to bias in the data collection through incomplete or inaccurate patient recall, this bias is more likely to affect studies in which the link between depressive symptoms and functional status is assessed cross-sectionally. In our study, we focused on the impact of depressive symptoms on prospective longitudinal changes in outcomes and we took into account the baseline level of reported angina in the analysis. In addition, our employment of centrally trained interviewers and the use of standardized interviews should minimize the impact of this potential bias. Furthermore, angina assessed through self-report does not necessarily indicate an ischemic event. However, angina was assessed by using the Seattle Angina Questionnaire which has been validated in CHD patients.19,20 In addition, subjective symptoms, part of the quality-of-life construct, are an important outcome to consider. We were able to capture a comprehensive range of outcomes post-MI, including death, presence of angina, and rehospitalization, and include a diverse, prospectively collected national sample, which increases generalizability. Third, rehospitalization data were available only on patients who survived to the follow-up interview, and results may be biased because of censoring of rehospitalization at date of death if a death occurred before rehospitalization or because of inaccuracy in patient recall. Fourth, our sample size was not large enough to allow age stratification. Future studies should specifically examine the impact of depressive symptoms on the outcome of younger women with MI, a group characterized by remarkably higher prevalence of depressive symptoms13 and higher mortality rates compared with men.4,5 Finally, because this is an observational study, unmeasured confounding is always a potential limitation. Nonetheless, a wide array of known variables was available for risk adjustment, and we are not aware of any important unmeasured confounders that could affect our results.
Conclusions
Women have a higher prevalence of depressive symptoms after MI compared with men. However, a higher prevalence of depressive symptoms in women after MI only modestly contributes to their worse outcomes compared with men. Other demographic and clinical factors explain most of the sex differences in these outcomes, particularly mortality. Depressive symptoms, nonetheless, predict adverse outcomes in both men and women after MI, with a similar magnitude of effect. Thus, our results support the recent recommendations of improving recognition of depressive symptoms after MI in both men and women.
Acknowledgments
Sources of Funding
Cardiovascular Therapeutics and Cardiovascular Outcomes funded the PREMIER data collection and analysis. This study was also supported by the Emory University General Clinical Research Center (NIH MO1-RR00039) and National Institutes of Health grant K12RR17643. Dr Parashar is supported by Mentored Clinical Scientist Development Award 1K23RR023171. Dr Vaccarino is supported by grant K24HL077506. Ms Reid is supported through National Institutes of Health grant P50 HL077113. Ms Reid had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Disclosures
None.
References
- 1.American Heart Association. 2006 Heart and Stroke Statistical Update. Dallas, Tex: American Heart Association; 2006. [Google Scholar]
- 2.Vaccarino V, Krumholz HM, Yarzebski J, Gore JM, Goldberg RJ. Sex differences in 2-year mortality after hospital discharge for myocardial infarction. Ann Intern Med. 2001;134:173–181. doi: 10.7326/0003-4819-134-3-200102060-00007. [DOI] [PubMed] [Google Scholar]
- 3.Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, Van de Werf F, Aylward P, Topol EJ, Califf RM. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. N Engl J Med. 1999;341:226–232. doi: 10.1056/NEJM199907223410402. [DOI] [PubMed] [Google Scholar]
- 4.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. N Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 5.Vaccarino V, Mallik S. Gender differences in outcome of acute myocardial infarction. In: Legato ML, editor. Principles of Gender-Specific Medicine. Vol. 2. San Diego, Calif: Academic Press; 2004. pp. 224–233. [Google Scholar]
- 6.Wenger NK. Coronary heart disease: the female heart is vulnerable. Prog Cardiovasc Dis. 2003;46:199–229. doi: 10.1016/j.pcad.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Heer T, Schiele R, Schneider S, Gitt AK, Wienbergen H, Gottwik M, Gieseler U, Voigtländer T, Hauptmann KE, Wagner S, Senges J. Gender differences in acute myocardial infarction in the era of reperfusion (the MITRA registry) Am J Cardiol. 2002;89:511–517. doi: 10.1016/s0002-9149(01)02289-5. [DOI] [PubMed] [Google Scholar]
- 8.Heer T, Gitt AK, Juenger C, Schiele R, Wienbergen H, Towae F, Gottwitz M, Zahn R, Zeymer U, Senges J. Gender differences in acute non-ST-segment elevation myocardial infarction. Am J Cardiol. 2006;98:160–166. doi: 10.1016/j.amjcard.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 9.Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction: impact on 6-month survival. JAMA. 1993;270:1819–1825. [PubMed] [Google Scholar]
- 10.Parashar S, Rumsfeld JS, Spertus JA, Reid KJ, Wenger NK, Krumholz HM, Amin A, Weintraub WS, Lichtman J, Dawood N, Vaccarino V. Time course of depression and outcome of myocardial infarction. Arch Intern Med. 2006;166:2035–2043. doi: 10.1001/archinte.166.18.2035. [DOI] [PubMed] [Google Scholar]
- 11.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Melle J, de Jonge P, Spijkerman T, Tijssen J, Ormel J, van Veldhuisen D, van den Brink R, van den Berg M. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66:814–822. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 13.Mallik S, Spertus JA, Reid KJ, Krumholz HM, Rumsfeld JS, Weintraub WS, Agarwal P, Santra M, Bidyasar S, Lichtman JH, Wenger NK, Vaccarino V. Depressive symptoms after acute myocardial infarction: evidence for highest rates in younger women. Arch Intern Med. 2006;166:876–883. doi: 10.1001/archinte.166.8.876. [DOI] [PubMed] [Google Scholar]
- 14.Spertus JA, Peterson ED, Rumsfeld JS, Jones PG, Decker C, Krumholz H. The PREMIER (Prospective Registry Evaluating Myocardial Infarction: Event and Recovery) registry: evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151:589–597. doi: 10.1016/j.ahj.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 16.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whooley MA. Depression and cardiovascular disease: healing the broken-hearted. JAMA. 2006;295:2874–2881. doi: 10.1001/jama.295.24.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Psychiatric Association Committee on Nomenclature and Statistics. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 19.Spertus JA, Winders JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 20.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43–49. doi: 10.1161/01.cir.0000020688.24874.90. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Medicare and Medicaid Services Hospital Quality Measures. Available at: http://www.cms.hhs.gov/HospitalQualityInits/10_HospitalQualityMeasures.asp#TopOfPage.
- 22.The Centers for Medicare and Medicaid Services and the Joint Commission on Accreditation of Healthcare Organizations. Current Specification Manual for National Hospital Quality Measures. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement?current+NHQM+Manual.htm.
- 23.R version 2.1.1 R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- 24.Frasure-Smith N, Lesperance F, Juneau M, Talajic M, Bourassa MG. Gender, depression and one-year prognosis after myocardial infarction. Psychosom Med. 1999;61:26–37. doi: 10.1097/00006842-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Hemingway H, McCallum A, Shipley M, Manderbacka K, Martikainen P, Keskimaki I. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA. 2006;295:1404–1411. doi: 10.1001/jama.295.12.1404. [DOI] [PubMed] [Google Scholar]
- 26.Sheps DS, Kaufmann PG, Sheffield D, Light KC, McMahon RP, Bonsall R, Maixner W, Carney RM, Freedland KE, Cohen JD, Goldberg AD, Ketterer MW, Raczynski JM, Pepine CJ. Sex differences in chest pain in patients with documented coronary artery disease and exercise-induced ischemia: results from the PIMI study. Am Heart J. 2001;142:864–871. doi: 10.1067/mhj.2001.119133. [DOI] [PubMed] [Google Scholar]
- 27.Lesperance F, Frasure-Smith N, Koszycki D, Laliberte MA, van Zyl LT, Baker B, Swenson JR, Ghatavi K, Abramson BL, Dorian P, Guertin MC. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: The Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) Trial. JAMA. 2007;297:367–379. doi: 10.1001/jama.297.4.367. [DOI] [PubMed] [Google Scholar]
- 28.Glassman AH. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 29.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N. Enhancing recovery in Coronary Heart Disease Patients Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289:3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 30.Lichtman JH, Bigger JT, Jr, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lespérance F, Mark DB, Sheps DS, Taylor CB, Froelicher ES. Depression and coronary heart disease: recommendations for screening, referral and treatment. A science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research. Circulation. 2008;118:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 31.Fielding R. Depression and acute myocardial infarction: a review and reinterpretation. Soc Sci Med. 1991;32:1017–1028. doi: 10.1016/0277-9536(91)90159-a. [DOI] [PubMed] [Google Scholar]
- 32.Thombs BD, de Jonge P, Coyne JC, Whooley MA, Frasure-Smith N, Mitchell AJ, Zuidersma M, Eze-Nliam C, Lima BB, Smith CG, Soderlund K, Ziegelstein RC. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA. 2008;300:2161–2171. doi: 10.1001/jama.2008.667. [DOI] [PubMed] [Google Scholar]