Abstract
Background
Tourette syndrome (TS) is a disorder of chronic motor and vocal tics that begins in childhood.
Methods
A systematic Medline search was conducted to identify existing anatomical imaging studies in persons with TS.
Results
Thirty studies were identified, and their methods and findings were reviewed. Findings of reduced caudate volumes across the life span and thinning of sensorimotor cortices that is proportional with tic severity in children with TS implicate these regions in the genesis of tics. Hypertrophy of limbic and prefrontal cortices and a smaller corpus callosum accompany fewer symptoms in children with TS, likely representing an activity-dependent plasticity within these regions that help to modulate tic severity.
Conclusion
Although existing studies differ with respect to sample size, gender composition, quality of clinical characterization, pulse sequences, and methods of image analysis, the preponderance of evidence suggests that disturbances in the development of the motor portions of cortical–subcortical circuits likely predispose to the development TS and that neuroplastic changes in control systems of the brain help to modulate the severity of symptom expression. These findings from cross-sectional studies require confirmation in more representative populations within longitudinal studies.
Keywords: Anatomical imaging, CNS vulnerability, Plasticity, Tourette syndrome
Tourette syndrome (TS) is defined by the presence of motor and vocal tics for more than one year [1]. Tics are brief, involuntary, or semivoluntary movements and sounds that often occur in bouts. Although numerous family genetic and twin studies have demonstrated a strong genetic basis for TS, the precise mode of familial transmission is unclear, and the unambiguous identification of vulnerability genes has proved elusive [2,3]. Symptoms typically begin as simple tics between 3 and 8 years of age, most commonly with eye blinking, facial grimacing, or throat clearing [4]. Individuals with TS frequently experience their tics as responses to a crescendo of sensory discomfort that precedes tic behaviors [5]. Tics often attenuate in severity during or after puberty [6], and they fully remit in more than 40% of children with TS by the age of 18 years [7].
The attenuation or remission of tic symptoms during adolescence, and the general improvement in the capacity for self-regulation of thought, emotion, and behaviour during normal adolescence [8], suggests that the attenuation in tic symptoms may be a consequence of normal developmental processes in adolescents who have TS. Brain activation becomes more focused within frontal cortical regions during inhibitory tasks in healthy adolescents [9–11], perhaps as a consequence of synaptic pruning, cortical thinning, and the myelination of white matter in the adolescent brain, all of which occur simultaneously with improving impulse control [12–16]. These changes probably support more efficient neural processing within the prefrontal cortices that subserve inhibitory control, which imaging studies suggest also improves the suppression of tics in persons with TS.
Herein we provide a systematic review of findings derived from anatomical imaging studies in persons with TS. Anatomical imaging reveals regional disturbances in the morphological features of the brain in persons with TS, as well as the correlations of morpholgical deviations with clinical characteristics. Many of these anatomical correlates provide evidence for the presence of neuroplastic responses that likely help to compensate for the presence of the symptoms of TS and comorbid illnesses. Important sampling characteristics of the population being studied, however, such as its age and gender distribution, as well as the prevalence of comorbid illnesses and use of medication, seem to contribute to some of the inconsistencies in findings across studies.
Methods
We have elected to limit this review to anatomical magnetic resonance imaging (MRI) studies of TS. This imaging modality provides excellent tissue contrast at high spatial resolution, thus permitting precise measurement of even of small structures in the brain. We performed a Medline search with the following search terms: (anatomical MR OR MRI OR Neuroimaging) AND (TS OR Tourette syndrome OR tics OR tic disorder) (n=408). We selected for review all original studies that used anatomical MRI to study at least 10 persons with TS in papers published by June 2009 (n=30). Findings were categorized by the brain region that was the focus of the report (Table 1A–E. Finally, we assessed differences in study design, image quality, and image processing that may have accounted for inconsistencies in findings across studies.
Table 1.
Main findings of the anatomical MRI-studies (n=27) in persons with TS for various brain regions: (a) Subcortical, (b) Corpus Callosum, (c) Cortex, and (d) Limbic Regions and (e) Whole-Brain Analyses
| Year | Area of interest | Correction for brain size | Diagnosis | Male/female | Age (±S.D.) | Controls; age (±S.D.) | Method | Main findings | |
|---|---|---|---|---|---|---|---|---|---|
| A. Subcortical regions | |||||||||
| Peterson et al. [28] | 1993 | BG | Brain size index | TS (n=14) | 11/3 | 31.8 years (8.5) | n=14; 32.4 years (8.8) | VOI | Volume of the left lenticular nucleus ↓ (P<.25) in TS |
| Singer et al. [29] | 1993 | BG | Area of the 5 largest intracranial slices × slice thickness | TS (n=19); TS+ADHD (n=18) | 14/5; 15/3 | 11.8 years (range 7–16); 11.1 years (range 9–13) | n=18; 9.8 years (range 6–15) | VOI | Boys with TS showed trend toward a left smaller putamen (P<.08) |
| Hyde et al. [32] | 1995 | BG | Total brain volume | 10 mono-zygotic twin pairs with TS | 16/4 | 16.3 years (range 9–31) | No controls | VOI | Caudate nucleus volumes ↓ in the more severely affected twin (6%; P<.01) |
| Castellanos et al. [34] | 1996 | BG and anterior frontal region | Total brain volume | TS+ADHD (n=14); ADHD (26) | 14/0; 26/0 | 10.4 years (1.9); 10.7 years (1.9) | n=31; 10.9 years (1.9) | VOI | Rightward asymmetry of putamen reversed in the ADHD and TS+ADHD group (P<.009) |
| Zimmerman et al. [35] | 2000 | BG and ventricles | Area of the 5 largest intracranial slices×slice thickness | TS (n=19) | 0/19 | 11.0 years (range 7–15) | n=21; 10.7 years (range 8–15) | VOI | No robust differences between girls with TS and controls |
| Peterson et al. [33] | 2003 | BG | WBV | TS (n=154) | 115/39 | 18.7 years (range 6–63) | n=130; 21.0 years (range 6–63) | VOI | Caudate nucleus ↓ in the TS group, independent of age (P=.01); lenticular nucleus ↓ in TS adults (P<.02) |
| Amat et al. [37] | 2006 | BG, cortex, cerebellum | Not relevant | TS (n=62); ADHD (n=45); OCD (n=28) | 78/22 | 11.4 years (2,6) | n=32; 10.0 years (1.7) | Radiological evaluation of proton density and T2 w Images; brain regions determined by VOI | Higher proportion of subcortical hyperintensities in children with neuropsychiatric disorders (OR=6.9), no difference with respect to cerebral hyperintensities |
| Lee et al. [43] | 2006 | Thalamus | WBV | TS (n=18); comorbidity any Axis 1 exclusion criteria | 18/0 | 9.3 years (2.3); | n=16; 10.0 years (1.8) | VOI | Left thalamus ↑ in TS (P=.002); group difference in overall brain size (P=.013) and in IQ (P=.019) |
| Wang et al. [44] | 2007 | BG and thalamus | Total cerebral volumes and transformation | TS (n=13); chronic tic disorder (n=2) | 10/5 | 33.4 years (11.0) | n=15; 33.1 years (11.6) | VOI of the BG, large deformation high-dimensional brain mapping | No significant group differences in volume or shape of the BG (caudate, putamen, globus pallidus) and the thalamus |
| Makki et al. [45] | 2008 | BG and thalamus | DTI/VBM | TS (n=23) | 19/4 | 11.8 years (3.3) | n=35; 13.1 years (3.2) | DTI and VOI tracing and VBM | Decreased anisotropy right thalamus (P=.025) and increased water diffusity bilaterally putamen (P=.027); left caudate volume ↓ (P=.011) and bilateral thalamus volume ↓ (left P=.011; right P=.006) |
| B. Cortex | |||||||||
| Peterson et al. [47] | 2001 | Cortical regions, ventricles | WBV | TS (n=155) | 114/41 | 18.7 years (13.4) | n=131; 20.8 years (13.4) | Parcellation of cerebral subdivisions | TS subjects dorsal prefrontal volumes ↑ (P<.0004) and parieto-occipital regions (P<.002), but inferior occipital volumes ↓ (P<.03). |
| Fredericksen et al. [50] | 2002 | Frontal volume, white/gray matter composition | Total frontal volume | TS (n=11); TS+ADHD (n=14); ADHD (n=12) | 11/0; 14/0; 12/0 | 10.7 years (2.2); 11.7 years (2.4); 10.6 years (1.7) | n=26; 10.6 years (2.7) | Semiautomated normalization into Talairach space | TS larger proportion of white matter in the right frontal lobe (P<.01), ADHD reduced frontal volume (P<.05). |
| Kates et al. [51] | 2002 | 4 Frontal regions, deep white matter | WBV | TS (n=13); ADHD (n=13) | 13/0; 13/0 | 9.9 years (1.1); 9.4 years (1.2) | n=13; 10.0 years (1.5) | Frontal subparcellation protocol determining gray and white matter | Decrease of deep white matter in the left frontal lobe in TS (P=.02). |
| Hong et al. [52] | 2002 | Cerebral and cerebellar volumes | Total brain volume | TS (n=19) | 19/0 | 9.7 years (2.7) | n=17; 9.8 years (1.9) | Semiautomated stereotactic-based parcellation method | TS had larger frontal lobe white matter (P=.038), smaller right (3.3%) and larger left frontal lobe gray matter (3.3%) |
| Sowell et al. [54] | 2008 | Cortical thickness | Coregistration | TS (n=25) | 18/7 | 12.4 years (range 7–18) | n=35; 12.3 years (range 7–21) | Cortical pattern matching | Cortical thinning in frontal and parietal lobes in TS; thinning in sensorimotor regions correlated positively with tic symptoms. |
| Amat et al. [37] | 2006 | BG, cortex, cerebellum | Not relevant | TS (n=62); ADHD (n=45); OCD (n=28) | 78/22 | 11.4 years (2,6) | n=32; 10.0 years (1.7) | Radiological evaluation of Proton density and T2-weighted images; brain regions determined by VOI | Higher proportion of subcortical hyperintensities in children with neuropsychiatric disorders (OR=6.9), no difference with respect to cerebral hyperintensities |
| Lee et al. [43] | 2006 | Thalamus | WBV | TS (n=18); comorbidity any Axis 1 exclusion criteria | 18/0 | 9.3 years (2.3); | n=16; 10.0 years (1.8) | VOI | Left thalamus ↑ in TS (P=.002); group difference in overall brain size (P=.013) and in IQ (P=.019) |
| Wang et al. [44] | 2007 | BG and thalamus | Total cerebral volumes and transformation | TS (n=13); chronic tic disorder (n=2) | 10/5 | 33.4 years (11.0) | n=15; 33.1 years (11.6) | VOI of the BG, large deformation high-dimensional brain mapping | No significant group differences in volume or shape of the BG (caudate, putamen, globus pallidus) and the thalamus |
| Makki et al. [45] | 2008 | BG and thalamus | DTI/VBM | TS (n=23) | 19/4 | 11.8 years (3.3) | n=35; 13.1 years (3.2) | DTI and VOI tracing and VBM | Decreased anisotropy right thalamus (P=.025) and increased water diffusity bilaterally putamen (P=.027); Left caudate volume ↓ (P=.011) and bilateral thalamus volume ↓ (left P=.011; right P=.006) |
| C. CC | |||||||||
| Peterson et al. [55] | 1994 | CC | Midsagittal head area | TS (n=14) | 11/3 | 31.8 years (8.5) | n=14; 32.4 years (8.8) | ROI | CC reduced by 18% in the TS group (P<.006) |
| Baumgardner et al. [57] | 1996 | CC | Intracranial area | TS (n=16); TS+ADHD (n=21); ADHD (n=13) | 13/3; 19/2; 13/0 | 12.6 years (2.2); 11.2 years (1.6); 11.3 years (1.4) | n=27; 10.8 years (2.6) | ROI | Compared with HCs, the rostral body of the callosum was 17% ↑ in the TS group (P<.007); TS+ADHD: intermediate CC size; Pure ADHD: ↓ CC (P<.004). |
| Moriarty et al. [56] | 1997 | CC and BG | Brain size index | TS (n=17) | 11/6 | 35.0 years (range 17–62) | n=8; 33.0 years (range 20–45) | ROI+VOI | TS group had ↑ CC and loss of asymmetry in caudate nucleus. |
| Kim et al. [65] | 2003 | CSP | WBV | TS children (n=97); TS adults (n=43) | 77/20 | 11.2 years (2.3) | n=17; 9.8 years (1.9) | Presence and size of CSP rated on an ordinal scale | TS predictor for CSP grade, ↓ CSP (P<.03 in children; P<.05 in adults); ↓ CSP was correlated with higher ADHD symptom severity for inattention (P<.002) and hyperactivity/impulsivity (P<.003) in the TS group |
| Plessen et al. [60] | 2004 | CC | WBV | TS (n=158) | 117/41 | 18.5 years (13.3) | n=121; 19.7 years (12.6) | ROI | CC ↓ in the TS group (P<.005); interaction with age: TS children having ↓ CCs and TS adults ↑ CCs. |
| Plessen et al. [61] | 2006 | CC | DTI local measurement | TS (n=20) | 20/0 | 13.6 years (1.9) | n=20; 13.4 years (2.4) | DTI analysis of CC subdivisions | TS decreased FA values in all subdivisions of the CC (P<.009) |
| D. Limbic regions | |||||||||
| Peterson et al. [68] | 2007 | Amygdala and hippocampus | WBV and coregistration | TS (n=154) | 115/39 | 18.7 years (13.4) | n=128; 20.2 years (13.2) | VOI and surface matching analyses | Overall volumes amygdala and hippocampus ↑ in TS group (P=.006) |
| Ludolph et al. [69] | 2008 | Amygdala | WBV | TS (n=17) | 17/0 | 11.7 years (2.0); | n=17; 12.6 years (2.1) | VOI | Proportion of the left sided amygdala/whole brain ↓ in TS group (P=.03), correlated inversely with DSM-IV ADHD criteria (P=.027) |
| E. Whole-brain analyses | |||||||||
| Ludolph et al. [71] | 2006 | Whole brain with focus on BG | Normalized to VBM template (children) | TS (n=14) | 14/0 | 12.5 years | n=15; 13.4 years | VBM | Locally ↑ gray matter volumes bilaterally ventral putamen and decreases left hippocampal gyrus (P<.001) |
| Garreaux et al. [72] | 2006 | Whole brain with focus on Midbrain and BG | Normalized to VBM template | TS (n=31) | 25/7 | 32.0 years (10.5) | n=31; 32.0 years (11.0) | VBM | Locally ↑ in left midbrain gray matter volume (FDR corrected P=.03) |
| Martino et al. [79] | 2008 | Whole brain with focus on BG | Normalized to VBM template | TS, Anti-basal ganglia antibodies (ABGA)+ (n=9): TS ABGA- (n=13) | 4/5; 7/6 | 34.3 years (11.4); 29.5 years (14.2) | No controls | VBM and DTI (voxel-based FA maps) | No differences in morphometry or water diffusity in adult patients with TS that were ABGA+ or ABGA– |
| Thomalla et al. [74] | 2009 | Whole brain with focus on white matter | Normalized to VBM template | TS (n=15) | 13/2 | 34.5 years (8.9) | n=15;34.6 y (9.1) | VBM in DTI | Bilateral FA in WM post-and precentral gyrus, left supplementary motor area and right thalamus; inverse correlation with tic severity |
| Müller-Vahl et al. [73] | 2009 | Whole brain | Normalized to VBM template | TS (n=19) | 19/0 | 30.4 years (range 18–60) | n=20; 31.7 years (range 18–65) | VBM and MTI | Locally ↓ gray matter volumes prefrontal areas, the anterior cingulate gyrus; white matter ↓ right inferior frontal gyrus, left superior frontal gyrus, anterior CC |
| Makki et al. [75] | 2009 | Whole brain with focus on frontostriatal connections | Normalized to MNI template images | TS (n=18) | 14/4 | 11.3 years (2.4) | n=20;12.2 years (4.1) | Whole-brain analyses with fiber tracking | Connections between caudate nucleus and anterior dorsolateral cortex ↓ |
“↑” = Increased volume; “↓” = Decreased volume; ADHD = Attention-deficit/hyperactivity disorder; BG = Basal ganglia; CC = Corpus callosum; CSP = Cavum septi pellucidi; CT = Chronic tic disorder; DLPF = Dorsolateral prefrontal; DTI = Diffusion Tensor Imaging; FDR = False discovery rate; HC = Healthy control; MNI = Montreal Neurological Institute; MTI= Magnetization transfer imaging; ROI = Region of interest measurements; TS = Tourette syndrome; VBM = Voxel-based morphometry; VOI = Volume of interest measurement; WBV = Whole brain volume; WM = White matter.
Results
Subcortical regions
Basal ganglia
Several lines of evidence suggest that the basal ganglia are a likely site for the generation of tics. Motor portions of the basal ganglia control the velocity and direction of movement, and a loss of behavioral control for scaling of those two features of otherwise normal movements is innate to tics [17]. The stereotyped, repetitive nature of tics are also akin to motor or vocal habits [18], which derive primarily from the striatum [19]. Children and adults with TS are impaired in striatum-based habit learning, even though their declarative memory is normal [20]. Animal studies have shown that stereotyped behaviors arise from the basal ganglia following the application of stimulants [21] or dopamine receptor agonists [22], and lesions to the basal ganglia in humans can either produce or exacerbate tic-like behaviors [23–25]. Finally, an autoimmune response mis-directed toward the basal ganglia may produce a sudden onset of tic disorder in a minority of patients [26,27].
Early MRI studies that measured the volume of the different portions of the basal ganglia reported findings that were sometimes complementary but often contradictory (Table 1A). The first volumetric imaging study found reduced volumes of the left lenticular nucleus (putamen and globus pallidus combined) in 14 adults with TS compared with 14 controls [28]. Another study reported a trend toward reduced volumes of the lenticular nucleus in 37 boys with TS compared with control children [29]. Both studies reported evidence for a reduced or reversed (right larger than left) asymmetry of the lenticular nucleus, which usually is larger on the left compared to the right side [30,31], suggesting that cerebral asymmetries may be altered in persons with TS. A subsequent study of 10 monozygotic twin pairs who were discordant across co-twins for the severity of tic symptoms reported that the caudate nuclei of the more severely affected co-twin were smaller than the less severely affected twin [32]. The absence of a control group of singletons, however, precluded assessing whether volumes of the caudate were reduced in both co-twins relative to singletons.
The largest anatomical MRI study of the basal ganglia thus far [33] reported reduced volumes of the caudate nucleus in 154 children and adults with TS and 130 healthy control subjects. Moreover, those in the TS group who were taking typical neuroleptics (13%) had significantly larger volumes of their caudate (P=.03) and globus pallidus (P<.001) nuclei, and a trend toward a larger putamen (P=.09) compared with those not taking neuroleptics. Comorbidity with attention-deficit/hyperactivity disorder (ADHD) did not influence the main findings in the TS group, whereas children with TS who also had comorbid obsessive–compulsive disorder (OCD) had significantly smaller putamen volumes compared with children who had TS only (and those with TS only had normal putamen volumes). Findings from this study were consistent with the reduced caudate volumes reported in the twin study [32], but this study demonstrated in addition that caudate volumes are reduced in TS singletons compared to volumes in a control group. This study also reported smaller volumes of the putamen and globus pallidus in adults with TS compared with control adults, consistent with findings in the earliest studies of the basal ganglia in adults with TS [28]. Finally, findings from this large study did not support the earlier preliminary findings of deviant patterns of asymmetry that were detected previously in images of poorer quality in much smaller anatomical studies of TS [28,29].
The findings from these prior studies taken together suggest that discrepant reports of basal ganglia volumes derive from differences in sampling of study participants, image quality, and image processing across studies. Differences in basal ganglia findings, for example, seem at least in part to have been the consequence of differing ages of participants across studies with findings differing depending on whether the studies were of adults [28] or children with TS [29,34,35]. In addition, the quality of the images and the methods used to measure basal ganglia volumes in more recent studies have been substantially better than those in the earlier studies, likely producing more precise measures and more reproducible findings. The largest study also rated the degree of motion artifact present in each scan on a six-point ordinal scale to ensure that the quality of images was comparable across diagnostic groups. Finally, more recent studies have used highly detailed and rigorously operationalized protocols for the manual tracing of basal ganglia nuclei to help ensure the accuracy and consistency of measurements throughout the time the images were processed.
The clinical relevance of the findings in the largest basal ganglia imaging study was demonstrated in a prospective follow-up study in which 43 of the children with TS were assessed for tic and OCD symptoms when they were young adults, on average, 7.5 years after their MRI scan. Tic severity in young adulthood (mean age 18.7±1.7 years) correlated inversely with volumes of the caudate nucleus in childhood (P=.004), whereas caudate volumes did not correlate with tic severity at the age of initial scanning [36]. This is to our knowledge the first study to report the predictive value of imaging measures in childhood for outcome in young adulthood within a neuropsychiatric population.
Further imaging evidence implicating the basal ganglia in the pathophysiology of TS included an MRI study reporting a higher proportion of subcortical hyperintensities (on T2-weighted images) in 100 children with a diagnosis of TS, ADHD, or OCD compared with 32 healthy control children [37]. Autoimmune processes following infection with Group A beta-hemolytic Streptococcus may unmask a disposition for TS in a minority of persons developing the disorder [38]. This hypothesized impairment of the basal ganglia through streptococcus-antibodies may derive from an autoimmune reaction of the vascular bed in this region of the brain, which in turn may appear as subcortical hyperintensities or volumetric abnormalities on MR images [27,39].
The presence of significantly smaller caudate nuclei in both children and adults suggests that hypoplasia of the caudate nucleus is a trait morphological abnormality in persons with TS. Caudate volumes did not correlate with tic severity at the time of scan, although caudate volumes in childhood did correlate inversely with the severity of tic and OCD symptoms in adulthood [36], after the flux of symptoms during early adolescence development settled down. This prediction of symptom severity in adulthood by caudate volumes in childhood suggests further that hypoplasia of the caudate nucleus represents a trait morphological disturbance in TS.
Thalamus
Although not part of the basal ganglia, the thalamus is an important component of the densely interconnected cortical–subcortical circuits in which the basal ganglia reside. The thalamus therefore may play an important role in the pathogenesis of TS. Indeed, lesions to the thalamus have been reported to attenuate the severity of tic symptoms [40–42]. Few imaging studies, however, have examined the thalamus, and then in only relatively small samples. The left thalamus was reported larger in 18 medication-naïve boys with pure TS compared to 16 controls, after adjusting for a significant group difference in overall brain size and IQ [43]. In contrast, no significant group differences were detected in the thalamus or basal ganglia (caudate, putamen, and globus pallidus) in a study of 15 adults with TS or chronic tic disorder compared with 15 healthy controls [44]. In addition, fractional anisotropy, a measure of the degree of tissue organization, was reduced in the right thalamus of 23 children with TS compared with 35 control children [45]. If this index of tissue organization is a marker of neuronal organization in projections of the thalamus to the cortex, then reduced thalamocortical connectivity could produce less cortical inhibition and thus facilitate the generation of tics. Thalamic volumes thus warrant further study in larger samples of persons with TS.
Cortical gray matter and white matter
Interactions of the frontal cortex and striatum guide and regulate behavior [19,46]. Considerable evidence suggests that the cortical portion of these connections is involved in regulating the severity of tic symptoms. Regional volumes of the dorsal prefrontal and parietal cortex were significantly larger in 155 children and adults with TS compared with 131 controls. This enlargement was detected uniquely in the children with TS, whereas volumes were significantly smaller in adults with TS compared with control adults [47]. Moreover, larger volumes of both the orbitofrontal and parietal regions were associated with significantly fewer tic symptoms, suggesting that these cortical abnormalities may serve a compensatory function in persons with TS. The hypertrophy may increase the capacity to regulate the severity of tic symptoms, a possibility supported by the prior demonstration of prominent activations in prefrontal, frontotemporal, and parietal cortices during the willful suppression of tics [48]. Consistent with this interpretation is the extensive documentation of adaptive anatomical changes in neural tissue in response to experiential demand in cell culture, animal models, and human imaging studies, an adaptation that is termed neural plasticity [49].
These anatomical findings stem from a cross-sectional study of clinically identified individuals, and rather than demonstrating a developmental trajectory in the same population, the diametrically opposite findings of larger prefrontal and parietal cortices in children with TS and smaller volumes in adults with TS suggested that the sampling frame of the study included two different subpopulations of persons within TS: one, a child sample that well represented children in the general population who have TS and whose symptoms in most instances will either attenuate or remit entirely with time, and two, a severely affected adult population whose symptoms on average neither attenuated nor remitted during adolescence and who therefore represented only a minority of those in the general population who have a lifetime history of TS. Therefore, the smaller frontal and parietal volumes detected in the adults of this sample, as well as the inverse correlations of volumes with symptom severity were interpreted as likely representing markers for more persistent and more severe illness in persons who have TS.
Several subsequent, smaller studies have reported abnormalities of white and gray matter in children with TS. A higher proportion of white matter was measured in the right frontal lobe in 11 boys with TS compared with 26 controls [50]. The same group also reported a decrease in volumes of the deep white matter in the left hemisphere in 13 boys with TS compared to 13 boys with ADHD and 13 controls [51]. A third study reported more frontal lobe white matter, less right and larger left frontal lobe gray matter, and smaller total brain volumes in 19 boys with TS compared with 17 controls [52]. Together, these findings support the hypothesis that the ongoing need to suppress tics in children with TS may alter development of the frontal cortex [53].
The importance of motor and sensorimotor cortices in generating and monitoring both normal and abnormal movements implicates those regions in the pathogenesis of TS. Indeed, these cortices are the origin and target regions for the motor pathways within cortical–subcortical circuits that have been implicated in producing tics. One imaging study reported significant thinning of sensorimotor cortices in 25 children with TS compared with 35 sex- and age-matched controls [54]. The thinning was prominent in ventral portions of the motor and sensorimotor cortices, the portions of the motor and sensorimotor homunculi that are known from preclinical studies to control muscles of the face, mouth, tongue, and larynx. More severe tic symptoms accompanied thinner cortices in these regions, suggesting the presence of a dose-response relationship between the degree of cortical thinning and the severity of symptoms and providing compelling evidence that these cortices may be involved in the genesis of tic behaviors.
Commissural pathways
The corpus callosum (CC) is the massive commissural pathway providing axonal connectivity across the cerebral hemispheres. It has long been of interest to imaging studies of TS, probably because of the early reports of reduced anatomical brain asymmetry of the basal ganglia that suggested the presence of altered cerebral asymmetries in persons with TS, and those aberrant asymmetries might be evident in morphological features of the commissural pathway that connects to two hemispheres of the brain. Early studies of the size of the CC, however, yielded inconsistent findings (Table 1C). The midsagittal cross-sectional area of the CC in one study was smaller in 14 young adults with TS compared with the areas in a control group [55], but it was larger in the TS group in another study of 17 adults with TS and eight controls [56]. Another study reported that 16 children with pure TS had on average a larger CC, whereas children with comorbid TS+ADHD (n=21) had an intermediate size of the CC, and finally a pure ADHD group (n=13) had a smaller CC compared with 21 healthy control children [57]. Moreover, the absence of differences in size of the CC across diagnostic groups in a study involving 10 girls with TS, nine girls with comorbid TS-ADHD, and a control group of 22 girls was interpreted as indicating that abnormalities of the CC are restricted to boys [58]. However, these studies failed to realign MR images to a true sagittal midline prior to measuring the size of CC, which added to inter-individual variance and may have created systematic differences in measurement across groups [59].
The largest study thus far of CC size in 158 persons with TS compared to 121 controls [60] realigned images to a true sagittal midline. The size of the midsagittal CC was smaller in children with TS but larger in adults with TS, compared with healthy age-matched controls. The size of the CC correlated positively with the severity of motor tics, indicating that the smaller CC in children may have moderated the severity of tics. Furthermore, CC size correlated inversely with volumes of the prefrontal cortex in both the TS and control groups, although the magnitudes of these inverse correlations were significantly higher in the TS group. This exaggerated correlation may indicate the presence of an adaptive or compensatory process in the brains of persons with TS, given that smaller CCs and larger prefrontal cortices were both associated with less severe tic symptoms. Furthermore, another study reported that 20 boys with TS had lower anisotropy measures in their CCs measured with diffusion tensor imaging (DTI) compared to 20 controls, also suggesting the presence of reduced interhemispheric connectivity in persons with TS [61]. We note that although the motivation for examining the CC in TS was early evidence for reduced anatomical asymmetry of the basal ganglia, subsequent empirical studies focusing on anatomical and functional brain lateralization in TS [33,62,63] have not supported the hypothesized disturbances in anatomical or functional lateralization of the brain in persons with TS [64].
The cavum septum pellucidum is a brain structure that is intimately related to the development of the CC and limbic system during early development of the brain. A significantly smaller cavum septum has been demonstrated in both children and adults with TS [65]. Because development of the cavum septum ends during fetal or early postnatal life, a smaller cavum in children and adults with TS is thought to represent a marker for an enduring early developmental vulnerability in persons with TS.
Limbic system
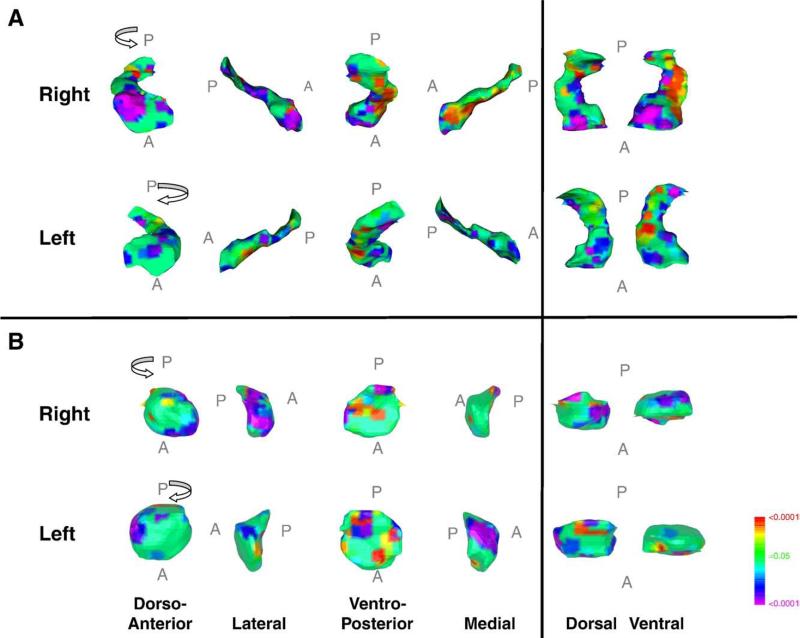
Few anatomical studies have focused on limbic brain regions in persons with TS, even though problems with emotional regulation [66] and affective illness [67] co-occur frequently in this population. Limbic regions, however, were examined using anatomical MRI in 154 children and adults with TS and 128 controls. Children with TS had larger overall hippocampus and amygdala volumes compared with control children, whereas adults with TS had smaller hippocampus and amygdala volumes compared to same-age controls [68]. Finer-grained surface analyses localized the volumetric differences in the children with TS mainly to the head and to the medial surface of the hippocampus and to the dorsal and ventral surfaces of the amygdala. Tic severity correlated inversely with the volumetric deviations in these subregions and thus suggested that the hypertrophy of hippocampus and amygdala was adaptive and compensatory (Fig. 1). The intimate anatomical connections and functional interactions of the hippocampus and the amygdala with prefrontal and parietal cortices further support their roles as adaptive in persons with TS, presumably supporting cortical efforts of tic suppression, or possibly compensating for previously documented striatum-based impairments in habit learning in persons with TS [20].
Fig. 1.
Symptom correlates in surface morphologic features of hippocampus and amygdala in persons with TS. Analyses include 154 persons with TS (mean age 18.7±13.4 years). The right and left hippocampus and amygdala are shown in their dorso-anterior, lateral, ventero-posterior, medial, dorsal and ventral views. The statistical model included tic severity and age as covariates. The color bar indicates the color coding for P values for the correlations of current motor and phonic symptom severity (total current score as measured with the Yale Global Tic Severity Scale) with measures of local volumes (indentations or protrusions) at each voxel along the surface of the hippocampus or amygdala. In the hippocampus, inverse correlations of tic severity with local volume were detected over the head and medial surface in all participants bilaterally, suggesting the possible compensatory functions of the larger hippocampus. The larger sizes of these regions in the TS groups compared with controls [68] were carried by the disproportionate number of children in the sample. Adapted from Arch Gen Psychiatry 64 (2007) 1281–1291.
In contrast to these findings of amygdala and hippocampus hypertrophy, however, a smaller subsequent study reported reduced amygdala volumes in 17 children and adolescents with TS compared with 17 controls [69]. No significant correlations of volume with tic severity were detected. The discrepancy of these findings with those of the first study, however, could be the consequence of including in the latter study a higher proportion of children with comorbid ADHD and using less reliable methods for region definition.
Whole-brain analyses
Recent studies have used automated procedures termed “voxel-based morphometry” (VBM) to compare TS and control groups in various indices of local volume compression or expansion in gray and white matter throughout the entire brain simultaneously [70]. One study detected evidence for locally increased gray matter volumes bilaterally in the ventral putamen and decreased volumes of the left hippocampal gyrus in 14 boys with TS and 15 controls [71]. Another study reported decreased gray matter volumes in the left midbrain of 31 adults with TS compared to 31 controls [72]. Another VBM study of 19 male adults with TS compared with 20 age-matched controls [73] reported decreases in gray matter volumes of prefrontal, anterior cingulate, sensorimotor, the primary sensory cortices and the left caudate nucleus. Reductions in white matter volumes in the TS group were localized in the right inferior frontal gyrus, left middle frontal gyrus, and left sensorimotor area. Finally, a preliminary study of nine adults with TS who were positive for anti-basal ganglia antibodies (ABGAs) did not differ in VBM-defined gray matter volumes compared with 13 adults with TS who were negative for ABGAs [79], suggesting that persons who have a putative autoimmune etiology for their tics may not have prominent underlying disturbances in brain structure.
The normalization of DTI images to a template brain permits exploration of group differences in white matter characteristics throughout the entire brain. One recent whole-brain DTI study of 15 unmedicated adults with TS and 15 healthy controls [74] reported increased connectivity [as measured with fractional anisotropy (FA)] in white matter bilaterally underlying the pre- and post-central gyri, the left sensorimotor area, and thalamus. Tractography confirmed that voxels with higher FA were part of the primary sensory and motor pathways. Moreover, FA values correlated with fewer tic symptoms, consistent with the previously reported thinning of sensorimotor cortices in children with TS [54]. Finally, a second whole-brain DTI study used probabilistic fiber tracking to investigate the anatomical connectivity of fronto-striato-thalamic circuits in 18 children with TS and 12 age-matched control children [75]. Connectivity in children with TS was reduced between the caudate nucleus and anterior dorsolateral frontal cortex. This reduced connectivity, if confirmed in larger studies, is consistent with the previously reported abnormalities in basal ganglia volumes in persons with TS [33].
Discussion
Findings from anatomical imaging studies have helped to improve our understanding of the pathophysiology of TS and the neurobiological determinants of the clinical course of this disorder. First, the basal ganglia and motor cortex portions of cortical–subcortical circuits are recognized as the likely origin of tic behaviors, with a smaller caudate nucleus present in children [32,33,45] and adults [33], and a thinning of the sensorimotor and motor cortices documented in children [54]. The size of the caudate, moreover, seems to predict the severity of tics in early adulthood [36]. Second, several portions of the brain, including the prefrontal cortex [47,50,51], CC [60], amygdala, and hippocampus [68] have been proposed to constitute a network of neural adaptation to the presence of tics, a network in which compensatory changes help to suppress or modulate the severity of tic symptoms. Evidence for this network relies on developmental interpretations of cross-sectional data, however, and should therefore be regarded with caution and as preliminary. In addition, these findings suggesting the presence of neural plasticity and compensation have not been replicated in all studies, likely as a consequence of differences in study design that include differences in sample sizes, demographic characteristics, and rates of comorbid illnesses, as well as differences in the acquisition and processing of the images. We will discuss details of these characteristics of the reviewed studies to understand, first, how findings in anatomical studies may derive from factors other than those of the underlying neurobiology of TS, and second, how those factors may produce divergent findings across studies.
Characteristics of existing studies
Clinical characterization
Gender
The male/female ratio in clinically identified samples of TS is approximately 4:1 [76]. Consequently, girls with TS generally have been underrepresented in anatomical imaging studies. Many studies have included only boys [34,43,50–52,61,69,71], whereas two have been restricted to girls [35,58]. Although the largest studies have included both genders, girls have always been included in lower proportion, ranging from 1.8 [56] to 6.4 [57] (mean proportion 3.5, S.D.=1.2):1 in favor of males. The typical gender ratio in imaging studies thus corresponds roughly to the ratio expected in a representative clinical sample. Although the main effect of gender typically has been included in statistical models, the effects of gender-by-group interactions on regional brain volumes have only been rarely assessed [29,33,47]. Current knowledge of the neuroanatomical basis of TS is thus largely founded on studies of males with this disorder. Understanding gender-based differences in the brains of children and adults with TS therefore remains an important and under-explored area of study [77].
Comorbid disorders
Because nearly all patients in clinical samples of patients with TS have co-occurring psychiatric disorders [76], analyses have typically controlled for their potential confounding effects. In early anatomical studies in TS, however, sample descriptions were often incomplete and unstandardized. The most frequent comorbidities, and the ones that usually have constituted subgroups of sufficient size for statistical analysis, have been OCD and ADHD [78]. Most studies have accounted for the presence of comorbid disorders by testing their effects in statistical models. In addition, analyses restricted to all individuals with “pure” TS and the control group helped to ensure that the primary findings did not derive from the presence of the comorbid illnesses [33,47,60]. Other studies have included contrast groups (such as ADHD only) or separate groups, consisting of participants with comorbid disorders (e.g., TS +ADHD), to map the specific anatomical deviations in a particular subgroup [50,57,58]. This approach, unfortunately, has usually produced small groups and reduced overall statistical power, thereby risking Type II (false negative) statistical errors.
Medication
Medications sometimes can have prominent effects on brain structure that cannot easily be distinguished from the primary effects of the disorder itself. Some investigators have repeated analyses after excluding all persons taking a particular medication [33]. Other studies have been restricted to children who were medication-naïve [43,69,71] or to persons who were medication-free for a specified period of time prior to scanning [31,50,55]. This approach, however, may favor persons with milder forms of TS and therefore may produce nonrepresentative samples that have less prominent abnormalities in brain structure.
Data acquisition
Differing image acquisitions
Data from various MRI acquisitions have been explored to identify anatomical disturbances in patients with TS. Most anatomical studies, however, have reported results from analyses of T1-weighted images (n=29). Although earlier studies [28,29] of basal ganglia volumes acquired proton density-weighted images in addition to the T1-weighted data, more recent studies have analyzed only high resolution, T1-weighted data [33,35,43–45]. Six studies explored abnormalities in brain connectivity using DTI data [45,61,73–75,79], and one study evaluated hyperintensities using T2- and proton density-weighted images [37]. These divergent image acquisitions differ dramatically in the information they provide about brain structure and thereby undoubtedly account for at least some of the variability in findings reported across anatomical imaging studies of TS.
Movement-related artifacts
Anatomical MRIs are especially susceptible to movement artifacts, and tics often make lying still difficult during image acquisition. This difficulty may disproportionately degrade the quality of imaging data and consequently alter the perceptible boundaries of brain subregions in the TS group, which in turn may create artificial group differences in morphological analyses. Only a few existing studies, however, have addressed strategies to minimize artifacts during scanning [69], and even fewer have invoked systematic procedures to ensure comparable image quality across groups [33].
Image quality
Image quality has differed considerably across studies. Technological advances have produced progressive improvements in the spatial resolution and signal-to-noise ratios of the images. Voxels are larger at lower resolution and therefore are more likely to cross regional boundaries, making them more difficult to discriminate from each other. Improvements in image quality therefore have permitted more precise and reliable anatomical measures over time.
Data analysis
Image analysis
Variability in the methods of image analysis has likely contributed to variability in findings reported across studies. First, most but not all studies have relied on manual tracing of the boundaries of brain subregions. Manual tracing is a precise method to define anatomical subregions, but it depends on designing and implementing valid and reliable tracing protocols [80]. Second, the ways in which reliability measures have been calculated have varied across studies. How frequently reliability has been measured during a study and, therefore, how stable the measures were assured to be across time, has also varied [28,33,34,55–57,68,69]. Third, not all studies have indicated that region definitions were conducted blind to group membership and other clinical characteristics, which is of utmost importance for unbiased and valid findings. Fourth, flipping the images randomly across the midline to blind investigators to the hemisphere being measured has been instituted only rarely [33,68].
In contrast to manual tracing to define and measure brain regions, other methods have compared diagnostic groups across the entire brain or across the surfaces of anatomic subregions. Two methods that have been used in a limited number of TS imaging studies are VBM [71–74,79] and tensor-based morphometry [44,54,68]. These methods both coregister all images into a common space. This registration requires a nonlinear warping of individual brains into a standardized template brain so that a voxel in one brain overlaps, to the greatest extent possible, the corresponding voxel in another brain. VBM and tensor-based morphometry both assume that voxels in this common space represent precisely the same cube of brain tissue across participants of the study. An individual's normal anatomical variability, together with variability in patients caused by the presence of a neuropsychiatric disorder, may produce systematic differences in these correspondences, especially in small sub-regions of the brain.
Unlike VBM, surface morphometry examines anatomical features of a single brain region that has already been delineated manually. Differences in local volumes at the surface of that brain region presumably represent variations in underlying cellularity. Compared with VBM, surface morphometry minimizes the spatial variability of corresponding voxels across groups of individuals, because this method usually focuses on one particular region of the brain [81]. We provide an example for this approach (Fig. 1) that illustrates how surface analyses help to localize volumetric differences to distinct anatomical subregions of that structure. Moreover, correlations of these local volumes with measures of symptom severity help in interpreting the pathophysiological significance of the anatomic deviations in patients with TS.
Correcting for brain size
Anatomical studies have differed in how they correct for scaling effects in the brain and for individual differences in overall brain size. Body size is a strong predictor of brain size, and brain size is a strong predictor of the volumes of individual subregions of the brain. An analysis of MRI data must account for these scaling effects. Anatomical studies of patients with TS have controlled for scaling either by introducing whole brain volume (WBV) as a covariate in the statistical model or by performing a statistical analysis on the ratios of the volumes of specific regions to the WBV. The use of ratios has the considerable disadvantage, however, of including measurement error in the volumes of both the subregion and the WBV (the numerator and the denominator of the ratio), thereby multiplying measurement error and reducing the statistical power of group comparisons [82]. Both the ratio and WBV have the disadvantage of not being readily suitable for addressing scaling effects if the average WBVs differ between the TS and control groups [43,52], as correcting for scaling effects in that case would risk erroneously eliminating a real difference in the size of brain subregions when they are compared across groups in statistical analyses. The preferred covariate in that instance to address overall scaling effects is usually body size rather than WBV.
Neural plasticity in persons with TS
Evidence for the presence of activity-dependent plasticity in the brains of persons with TS that help to regulate or compensate for the presence of tics is based primarily on cross-sectional imaging studies. The diametrically opposite findings that have been commonly reported in children and adults with TS—such as the presence of larger prefrontal cortex, parietal cortex, and hippocampus volumes in TS children but smaller volumes of those same brain regions in TS adults compared with healthy controls—could be interpreted as suggesting that TS is a neurodegenerative disorder. These group differences, however, seem more likely to derive from a systematic ascertainment bias in adults with TS, in that recruiting into an imaging study clinically identified adults whose tics are persistent and severe preferentially includes a relatively rare subsample of all persons who have a lifetime history of TS, those with unremitting illness. Therefore, rather than interpreting smaller regional volumes in the adults as representing a degenerative disease, the smaller volumes are more sensibly interpreted as contributing either to the persistence of illness into adulthood or to more severe symptoms overall, or both. The significant inverse correlations of volumes with severity in both children and adults with TS support the latter interpretation more than the former.
The cross-sectional and correlational design of the studies that have reported larger cortical and hippocampal volumes in persons with TS makes impossible any definitive interpretation of the causes of the larger volumes. Indeed, developmental interpretations of cross-sectional findings are notoriously hazardous because the inclusion of participants with differing ages risks the introduction of differing ascertainment biases and differing patterns of cooccurring illnesses into the various age groups of the study's participants [83–85]. Moreover, formulating correct causal interpretations of developmental changes is a challenge even in longitudinal studies. If regional hyper-trophy is confirmed in a longitudinal study, for example, determining whether the hypertrophy represents the cause of tic symptoms or the compensation for them, or even whether it simply represents the effects of chronic illness, will be difficult or impossible to discern conclusively [86]. Combining longitudinal studies with the appropriate randomized clinical trials (e.g., trials of cognitive or behavioral exercises that are designed to activate and enlarge prefrontal cortices) in the future may provide the experimental control that is necessary to determine whether hypertrophy is truly compensatory or merely an epiphenomenon associated with the presence of tics. Alternatively, imaging studies of unaffected family members could help to disentangle the brain abnormalities that represent trait disturbances and underlying vulnerabilities from those that represent the effects of neural compensation or chronic illness [86].
Future directions for anatomical imaging studies in persons with TS
Small sample sizes and the lack of rigorous protocols for data collection and data analysis were responsible in part for the limited reproducibility of findings from the early imaging studies of TS. Subsequent studies, however, have used much more rigorous and more reliable methods for region definition in images of considerably better quality and in much larger samples of participants, producing findings that have been more reproducible across studies and more internally consistent across brain regions. Several improvements in study design may further help to overcome the limitations of cross-sectional imaging data acquired in clinical settings. Longitudinal studies in younger individuals with TS or in unaffected family members, for example, may help to disentangle causes from compensatory effects and the effects of chronic illness. Furthermore, studies increasingly will combine neuropsychological measures with imaging data to help constrain interpretation of the functional and behavioral consequences of imaging findings. Moreover, the combination of several MRI modalities, including anatomical MRI, functional MRI, DTI, spectroscopy, and even concurrent electroencephalographic recordings, will aid the pathophysiological interpretation of imaging findings in TS. They will supply complementary views of brain structure and function that together will be more helpful than any of the modalities can be alone when interpreting how abnormalities in brain structure, function, connectivity, and metabolism conspire to produce a given behavioral phenotype in persons with TS. In addition, the use of scanners that have higher field strengths and a greater number of radiofrequency channels will permit acquisition of higher quality datasets in briefer scanning times, which will be especially helpful for acquiring data that are free of motion artifact in children with TS [87]. Finally, rapid and continuing advances in the methods of image processing will likely begin to bridge the gap from research to clinical applications in the coming years. Individual MRI scans may be compared against probabilistic atlases from other persons who have the same disorder, an approach that may help to confirm or refute a given provisional diagnosis.
Supplementary Material
Footnotes
This work was supported in part by National Institute of Mental Health grants (MHK02-74677, MH59139, and MH068318), the Suzanne Crosby Murphy Endowment at Columbia University College of Physicians and Surgeons, and by a grant from the MoodNet, Health Authorities of West Norway.
References
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed American Psychiatric Association; 1994. [Google Scholar]
- 2.Pauls DL. An update on the genetics of Gilles de la Tourette syndrome. J Psychosom Res. 2003;55:7–12. doi: 10.1016/s0022-3999(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 3.Keen-Kim D, Freimer NB. Genetics and epidemiology of Tourette syndrome. J Child Neurol. 2006;21:665–71. doi: 10.1177/08830738060210081101. [DOI] [PubMed] [Google Scholar]
- 4.Leckman JF. Tourette's syndrome. Lancet. 2002;360:1577–86. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- 5.Leckman JF, Walker DE, Cohen DJ. Premonitory urges in Tourette's syndrome. Am J Psychiatry. 1993;150:98–102. doi: 10.1176/ajp.150.1.98. [DOI] [PubMed] [Google Scholar]
- 6.Pappert EJ, Goetz CG, Louis ED, Blasucci L, Leurgans S. Objective assessments of longitudinal outcome in Gilles de la Tourette's syndrome. Neurology. 2003;61:936–40. doi: 10.1212/01.wnl.0000086370.10186.7c. [DOI] [PubMed] [Google Scholar]
- 7.Leckman JF, Zhang H, Vitale A, Lahnin F, Lynch K, Bondi C, et al. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102:14–9. doi: 10.1542/peds.102.1.14. [DOI] [PubMed] [Google Scholar]
- 8.Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–78. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- 10.Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, et al. A developmental fmri study of self-regulatory control. Hum Brain Mapp. 2006;27:848–63. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–93. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huttenlocher PR. Synapse elimination and plasticity in developing human cerebral cortex. Am J Ment Defic. 1984;88:488–96. [PubMed] [Google Scholar]
- 13.Giedd JN, Blumenthal J, Jeffries N, Castellanos F, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal mri study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 14.Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–9. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 15.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–15. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 16.Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–54. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- 17.Peterson BS, Leckman JF, Arnstein A, Anderson GM, Staib L, Gore JC, et al. Neuroanatomical cicuitry. In: Leckman JF, Cohen DJ, editors. Tourette's syndrome-tics, obsessions, compulsions. John Wiley and Sons; New York: 1999. pp. 230–60. [Google Scholar]
- 18.Saka E, Graybiel AM. Pathophysiology of Tourette's syndrome: striatal pathways revisited. Brain Dev. 2003;25(Suppl 1):S15–9. doi: 10.1016/s0387-7604(03)90002-7. [DOI] [PubMed] [Google Scholar]
- 19.Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–87. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 20.Marsh R, Alexander GM, Packard MG, Zhu H, Wingard JC, Quackenbush G, et al. Habit learning in Tourette syndrome: a translational neuroscience approach to a developmental psychopathology. Arch Gen Psychiatry. 2004;61:1259–68. doi: 10.1001/archpsyc.61.12.1259. [DOI] [PubMed] [Google Scholar]
- 21.Kelley AE, Lang CG, Gauthier AM. Induction of oral stereotypy following amphetamine microinjection into a discrete subregion of the striatum. Psychopharmacology (Berl) 1988;95:556–9. doi: 10.1007/BF00172976. [DOI] [PubMed] [Google Scholar]
- 22.Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nat Neurosci. 2000;3:377–83. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- 23.Dale RC, Church AJ, Heyman I. Striatal encephalitis after varicella zoster infection complicated by tourettism. Mov Disord. 2003;18:1554–6. doi: 10.1002/mds.10610. [DOI] [PubMed] [Google Scholar]
- 24.Gomis M, Puente V, Pont-Sunyer C, Oliveras C, Roquer J. Adult onset simple phonic tic after caudate stroke. Mov Disord. 2008;23:765–6. doi: 10.1002/mds.21955. [DOI] [PubMed] [Google Scholar]
- 25.Peterson BS, Bronen RA, Duncan CC. Three cases of symptom change in Tourette's syndrome and obsessive-compulsive disorder associated with paediatric cerebral malignancies. J Neurol Neurosurg Psychiatry. 1996;61:497–505. doi: 10.1136/jnnp.61.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Church AJ, Dale RC, Lees AJ, Giovannoni G, Robertson MM. Tourette's syndrome: a cross sectional study to examine the pandas hypothesis. J Neurol Neurosurg Psychiatry. 2003;74:602–7. doi: 10.1136/jnnp.74.5.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson BS, Leckman JF, Tucker D, Scahill L, Staib L, Zhang H, et al. Preliminary findings of antistreptococcal antibody titers and basal ganglia volumes in tic, obsessive–compulsive, and attention-deficit/hyperactivity disorders. Arch Gen Psychiatry. 2000;57:364–72. doi: 10.1001/archpsyc.57.4.364. [DOI] [PubMed] [Google Scholar]
- 28.Peterson BS, Riddle MA, Cohen DJ, Katz LD, Smith JC, Hardin MT, et al. Reduced basal ganglia volumes in Tourette's syndrome using three-dimensional reconstruction techniques from magnetic resonance images. Neurology. 1993;43:941–9. doi: 10.1212/wnl.43.5.941. [DOI] [PubMed] [Google Scholar]
- 29.Singer H, Reiss A, Brown J, Aylward E, Shih B, Chee E, et al. Volumetric mri changes in basal ganglia of children with Tourette's syndrome. Neurology. 1993;43:950–6. doi: 10.1212/wnl.43.5.950. [DOI] [PubMed] [Google Scholar]
- 30.Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 31.Peterson BS, Riddle M, Cohen DJ, Katz L, Smith J, Leckman JF. Human basal ganglia volume asymmetries on magnetic resonance images. Magn Reson Imaging. 1993;11:493–8. doi: 10.1016/0730-725x(93)90468-s. [DOI] [PubMed] [Google Scholar]
- 32.Hyde T, Stacey M, Coppola R, Handel S, Rickler K, Weinberger D. Cerebral morphometric abnormalities in Tourette's syndrome: a quantitative MRI study of monozygotic twins. Neurology. 1995;45:1176–82. doi: 10.1212/wnl.45.6.1176. [DOI] [PubMed] [Google Scholar]
- 33.Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, et al. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60:415–24. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- 34.Castellanos F, Giedd J, Hamburger S, Marsh W, Rapoport J. Brain morphometry in Tourette's syndrome: the influence of comorbid attention-deficit/hyperactivity disorder. Neurology. 1996;47:1581–3. doi: 10.1212/wnl.47.6.1581. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerman AM, Abrams MT, Giuliano JD, Denckla MB, Singer HS. Subcortical volumes in girls with Tourette syndrome: support for a gender effect. Neurology. 2000;54:2224–9. doi: 10.1212/wnl.54.12.2224. [DOI] [PubMed] [Google Scholar]
- 36.Bloch M, Michael H, Leckman JF, James F, Zhu H, Hongtu Peterson BS, Bradley S. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65:1253–8. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amat JA, Bronen RA, Saluja S, Sato N, Zhu H, Gorman DA, et al. Increased number of subcortical hyperintensities on mri in children and adolescents with Tourette's syndrome, obsessive-compulsive disorder, and attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163:1106–8. doi: 10.1176/appi.ajp.163.6.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoekstra PJ, Kallenberg CG, Korf J, Minderaa RB. Is Tourette's syndrome an autoimmune disease? Mol Psychiatry. 2002;7:437–45. doi: 10.1038/sj.mp.4000972. [DOI] [PubMed] [Google Scholar]
- 39.Giedd JN, Rapoport JL, Kruesi MJ, Parker C, Schapiro MB, Allen AJ, et al. Sydenham's chorea: magnetic resonance imaging of the basal ganglia. Neurology. 1995;45:2199–202. doi: 10.1212/wnl.45.12.2199. [DOI] [PubMed] [Google Scholar]
- 40.Rauch SL, Baer L, Cosgrove GR, Jenike MA. Neurosurgical treatment of Tourette's syndrome: a critical review. Compr Psychiatry. 1995;36:141–56. doi: 10.1016/s0010-440x(95)90109-4. [DOI] [PubMed] [Google Scholar]
- 41.Hassler R, Dieckmann G. Stereotaxic treatment of tics and inarticulate cries or coprolalia considered as motor obsessional phenomena in Gilles de la Tourette's disease. Rev Neurol (Paris) 1970;123:89–100. [PubMed] [Google Scholar]
- 42.Rickards H, Wood C, Cavanna AE. Hassler and Dieckmann's seminal paper on stereotactic thalamotomy for Gilles de la Tourette syndrome: translation and critical reappraisal. Mov Disord. 2008;23:1966–72. doi: 10.1002/mds.22238. [DOI] [PubMed] [Google Scholar]
- 43.Lee JS, Yoo SS, Cho SY, Ock SM, Lim MK, Panych LP. Abnormal thalamic volume in treatment-naive boys with Tourette syndrome. Acta Psychiatr Scand. 2006;113:64–7. doi: 10.1111/j.1600-0447.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Lee DY, Bailey E, Hartlein JM, Gado MH, Miller MI, et al. Validity of large-deformation high dimensional brain mapping of the basal ganglia in adults with Tourette syndrome. Psychiatry Res. 2007;154:181–90. doi: 10.1016/j.pscychresns.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makki MI, Behen M, Bhatt A, Wilson B, Chugani HT. Microstructural abnormalities of striatum and thalamus in children with Tourette syndrome. Mov Disord. 2008;23:2349–56. doi: 10.1002/mds.22264. [DOI] [PubMed] [Google Scholar]
- 46.Rueda MR, Posner MI, Rothbart MK. The development of executive attention: contributions to the emergence of self-regulation. Dev Neuropsychol. 2005;28:573–94. doi: 10.1207/s15326942dn2802_2. [DOI] [PubMed] [Google Scholar]
- 47.Peterson BS, Staib L, Scahill L, Zhang H, Anderson C, Leckman JF, et al. Regional brain and ventricular volumes in Tourette syndrome. Arch Gen Psychiatry. 2001;58:427–40. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- 48.Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55:326–33. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- 49.Munte TF, Altenmuller E, Jancke L. The musician's brain as a model of neuroplasticity. Nature Reviews Neuroscience. 2002;3:473–8. doi: 10.1038/nrn843. [DOI] [PubMed] [Google Scholar]
- 50.Fredericksen K, Cutting L, Kates W, Mostofsky S, Singer H, Cooper K, et al. Disproportionate increases of white matter in right frontal lobe in Tourette syndrome. Neurology. 2002;58:85–9. doi: 10.1212/wnl.58.1.85. [DOI] [PubMed] [Google Scholar]
- 51.Kates WR, Frederikse M, Mostofsky SH, Folley BS, Cooper K, Mazur-Hopkins P, et al. Mri parcellation of the frontal lobe in boys with attention deficit hyperactivity disorder or Tourette syndrome. Psychiatry Res. 2002;116:63–81. doi: 10.1016/s0925-4927(02)00066-5. [DOI] [PubMed] [Google Scholar]
- 52.Hong KE, Ock SM, Kang MH, Kim CE, Bae JN, Lim MK, et al. The segmented regional volumes of the cerebrum and cerebellum in boys with Tourette syndrome. J Korean Med Sci. 2002;17:530–6. doi: 10.3346/jkms.2002.17.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marsh R, Gerber AJ, Peterson BS. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2008;47:1233–51. doi: 10.1097/CHI.0b013e318185e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sowell ER, Kan E, Yoshii J, Thompson PM, Bansal R, Xu D, et al. Thinning of sensorimotor cortices in children with Tourette syndrome. Nat Neurosci. 2008;11:637–9. doi: 10.1038/nn.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peterson BS, Leckman J, Duncan J, Wetzles R, Riddle M, Hardin M, et al. Corpus callosum morphology from magnetic resonance images in Tourette's syndrome. Psychiatry Res. 1994;55:85–99. doi: 10.1016/0925-4927(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 56.Moriarty J, Varma A, Stevens J, Fish M, Trimble M, Robertson M. A volumetric mri study of Gilles de la Tourette's syndrome. Neurology. 1997;49:410–5. doi: 10.1212/wnl.49.2.410. [DOI] [PubMed] [Google Scholar]
- 57.Baumgardner TL, Singer HS, Denckla MB, Rubin MA, Abrams MT, Colli MJ, et al. Corpus callosum morphology in children with Tourette syndrome and attention deficit hyperactivity disorder. Neurology. 1996;47:477–82. doi: 10.1212/wnl.47.2.477. [DOI] [PubMed] [Google Scholar]
- 58.Mostofsky S, Wendlandt J, Cutting L, Denckla M, Singer H. Corpus callosum measurements in girls with Tourette syndrome. Neurology. 1999;53:1345–7. doi: 10.1212/wnl.53.6.1345. [DOI] [PubMed] [Google Scholar]
- 59.Rauch R, Jinkins J. Variability of corpus callosal area measurements from midsagittal mr images: effect of subject placement within the scanner. AJNR Am J Neuroradiol. 1996;17:27–8. [PMC free article] [PubMed] [Google Scholar]
- 60.Plessen KJ, Wentzel-Larsen T, Hugdahl K, Feineigle P, Klein J, Staib LH, et al. Altered interhemispheric connectivity in individuals with Tourette's disorder. Am J Psychiatry. 2004;161:2028–37. doi: 10.1176/appi.ajp.161.11.2028. [DOI] [PubMed] [Google Scholar]
- 61.Plessen KJ, Grüner R, Lundervold A, Hirsch JG, Xu D, Bansal R, et al. Reduced white matter connectivity in the corpus callosum of children with Tourette syndrome. J Child Psychol Psychiatry. 2006;47:1013–22. doi: 10.1111/j.1469-7610.2006.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Margolis A, Donkervoort M, Kinsbourne M, Peterson BS. Interhemispheric connectivity and executive functioning in adults with Tourette syndrome. Neuropsychology. 2006;20:66–76. doi: 10.1037/0894-4105.20.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plessen KJ, Lundervold A, Grüner R, Hammar A, Lundervold A, Peterson BS, et al. Functional brain asymmetry, attentional modulation, and interhemispheric transfer in boys with Tourette syndrome. Neuropsychologia. 2007;45:767–74. doi: 10.1016/j.neuropsychologia.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Witelson SF. Clinical neurology as data for basic neuroscience: Tourette's syndrome and the human motor system. Neurology. 1993;43:859–61. doi: 10.1212/wnl.43.5.859. [DOI] [PubMed] [Google Scholar]
- 65.Kim KJ, Peterson BS. Cavum septi pellucidi in Tourette syndrome. Biol Psychiatry. 2003;54:76–85. doi: 10.1016/s0006-3223(02)01830-9. [DOI] [PubMed] [Google Scholar]
- 66.Budman CL, Bruun RD, Park KS, Olson ME. Rage attacks in children and adolescents with Tourette's disorder: a pilot study. J Clin Psychiatry. 1998;59:576–80. doi: 10.4088/jcp.v59n1103. [DOI] [PubMed] [Google Scholar]
- 67.Robertson MM. Mood disorders and Gilles de la Tourette's syndrome: an update on prevalence, etiology, comorbidity, clinical associations, and implications. J Psychosom Res. 2006;61:349–58. doi: 10.1016/j.jpsychores.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 68.Peterson BS, Choi HA, Hao X, Amat JA, Zhu H, Whiteman R, et al. Morphologic features of the amygdala and hippocampus in children and adults with Tourette syndrome. Arch Gen Psychiatry. 2007;64:1281–91. doi: 10.1001/archpsyc.64.11.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ludolph AG, Pinkhardt EH, Tebartz van Elst L, Libal G, Ludolph AC, Fegert JM, et al. Are amygdalar volume alterations in children with Tourette syndrome due to adhd comorbidity? Dev Med Child Neurol. 2008;50:524–9. doi: 10.1111/j.1469-8749.2008.03014.x. [DOI] [PubMed] [Google Scholar]
- 70.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston K, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 71.Ludolph AG, Juengling FD, Libal G, Ludolph AC, Fegert JM, Kassubek J. Grey-matter abnormalities in boys with Tourette syndrome: magnetic resonance imaging study using optimised voxel-based morphometry. Br J Psychiatry. 2006;188:484–5. doi: 10.1192/bjp.bp.105.008813. [DOI] [PubMed] [Google Scholar]
- 72.Garraux G, Goldfine A, Bohlhalter S, Lerner A, Hanakawa T, Hallett M. Increased midbrain gray matter in Tourette's syndrome. Ann Neurol. 2006;59:381–5. doi: 10.1002/ana.20765. [DOI] [PubMed] [Google Scholar]
- 73.Müller-Vahl KR, Kaufmann J, Grosskreutz J, Dengler R, Emrich HM, Peschel T. Prefrontal and anterior cingulate cortex abnormalities in Tourette syndrome: evidence from voxel-based morphometry and magnetization transfer imaging. BMC Neurosci. 2009;10:47. doi: 10.1186/1471-2202-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomalla G, Siebner HR, Jonas M, Baumer T, Biermann-Ruben K, Hummel F, et al. Structural changes in the somatosensory system correlate with tic severity in Gilles de la Tourette syndrome. Brain. 2009;132:765–77. doi: 10.1093/brain/awn339. [DOI] [PubMed] [Google Scholar]
- 75.Makki MI, Govindan RM, Wilson BJ, Behen ME, Chugani HT. Altered fronto-striato-thalamic connectivity in children with Tourette syndrome assessed with diffusion tensor mri and probabilistic fiber tracking. J Child Neurol. 2009;24:669–78. doi: 10.1177/0883073808327838. [DOI] [PubMed] [Google Scholar]
- 76.Freeman RD, Fast DK, Burd L, Kerbeshian J, Robertson MM, Sandor P. An international perspective on Tourette syndrome: selected findings from 3,500 individuals in 22 countries. Dev Med Child Neurol. 2000;42:436–47. doi: 10.1017/s0012162200000839. [DOI] [PubMed] [Google Scholar]
- 77.Alexander GM, Peterson BS. Testing the prenatal hormone hypothesis of tic-related disorders: gender identity and gender role behavior. Dev Psychopathol. 2004;16:407–20. doi: 10.1017/s095457940404458x. [DOI] [PubMed] [Google Scholar]
- 78.Plessen KJ, Royal JM, Peterson BS. Neuroimaging of tic disorders with co-existing attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2007;16(Suppl 1):60–70. doi: 10.1007/s00787-007-1008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martino D, Draganski B, Cavanna A, Church A, Defazio G, Robertson MM, et al. Anti-basal ganglia antibodies and Tourette's syndrome: a voxel-based morphometry and diffusion tensor imaging study in an adult population. J Neurol Neurosurg Psychiatry. 2008;79:820–2. doi: 10.1136/jnnp.2007.136689. [DOI] [PubMed] [Google Scholar]
- 80.Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of mri measurement of the amygdala and hippocampus in children with fragile × syndrome. Psychiatry Res. 1997;75:31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- 81.Bansal R, Gerber AJ, Peterson BS. Brain morphometry using anatomical magnetic resonance imaging. J Am Acad Child Adolesc Psychiatry. 2008;47:619–21. doi: 10.1097/CHI.0b013e31816c54ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arndt S, Cohen G, Alliger RJ, Swayze VW, Andreasen NC. Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Res. 1991;40:79–89. doi: 10.1016/0925-4927(91)90031-k. [DOI] [PubMed] [Google Scholar]
- 83.Kurlan R, Como PG, Miller B, Palumbo D, Deeley C, Andresen EM, et al. The behavioral spectrum of tic disorders: a community-based study. Neurology. 2002;59:414–20. doi: 10.1212/wnl.59.3.414. [DOI] [PubMed] [Google Scholar]
- 84.Spencer T, Biederman J, Harding M, O'Donnell D, Wilens T, Faraone S, et al. Disentangling the overlap between Tourette's disorder and ADHD. J Child Psychol Psychiatry. 1998;39:1037–44. [PubMed] [Google Scholar]
- 85.Termine C, Balottin U, Rossi G, Maisano F, Salini S, Nardo RD, et al. Psychopathology in children and adolescents with Tourette's syndrome: a controlled study. Brain Dev. 2005 doi: 10.1016/j.braindev.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 86.Peterson BS. Conceptual, methodological, and statistical challenges in brain imaging studies of developmentally based psychopathologies. Development and Psychopathology. 2008;15:811–32. doi: 10.1017/s0954579403000385. [DOI] [PubMed] [Google Scholar]
- 87.Blamire AM. The technology of mri-the next 10 years? Br J Radiol. 2008;81:601–17. doi: 10.1259/bjr/96872829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.