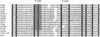Abstract
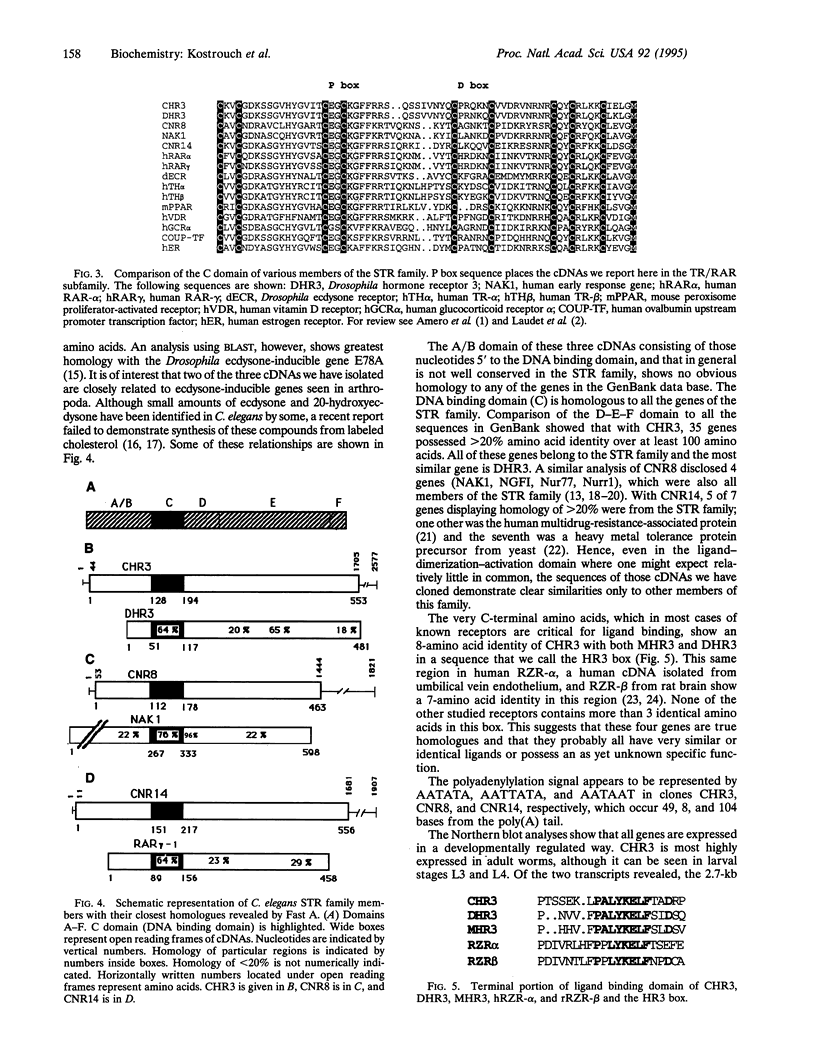
The large family of steroid/thyroid hormone receptor (STR) genes has been extensively studied in vertebrates and insects but little information is available on it in more primitive organisms. All members possess a DNA binding domain of zinc fingers of the C2, C2 type. We have used the polymerase chain reaction with degenerate oligonucleotide primers covering this region to clone three distinct members of this family from the nematode Caenorhabditis elegans. All three belong to the retinoic acid receptor (RAR), thyroid hormone receptor subfamily of genes. The cDNA of one of these clones shows such a high homology to DHR3, an early ecdysone response gene found in Drosophila, and MHR3, identified in Manduca sexta, that we have termed it CHR3. Furthermore, the C-terminal portion of the deduced protein sequence shows a box containing eight identical amino acids among CHR3, DHR3, and MHR3 suggesting an identical specific ligand for these proteins. CNR8 shows homology to NAK1, and CNR14 has homology to both the RAR-gamma 1 gene and to another ecdysone response gene, E78A. Neither of the latter two cDNAs is a clear homologue of any known gene and each is distinctive. All of these genes are expressed varyingly in both larval and adult stages of nematode development as shown by Northern blot analyses. These data demonstrate that the STR family of genes is represented in a nematode whose ancestor appeared well before the branching that gave rise to the Arthropoda and Chordata.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amero S. A., Kretsinger R. H., Moncrief N. D., Yamamoto K. R., Pearson W. R. The origin of nuclear receptor proteins: a single precursor distinct from other transcription factors. Mol Endocrinol. 1992 Jan;6(1):3–7. doi: 10.1210/mend.6.1.1738368. [DOI] [PubMed] [Google Scholar]
- Becker-André M., André E., DeLamarter J. F. Identification of nuclear receptor mRNAs by RT-PCR amplification of conserved zinc-finger motif sequences. Biochem Biophys Res Commun. 1993 Aug 16;194(3):1371–1379. doi: 10.1006/bbrc.1993.1976. [DOI] [PubMed] [Google Scholar]
- Carlberg C., Hooft van Huijsduijnen R., Staple J. K., DeLamarter J. F., Becker-André M. RZRs, a new family of retinoid-related orphan receptors that function as both monomers and homodimers. Mol Endocrinol. 1994 Jun;8(6):757–770. doi: 10.1210/mend.8.6.7935491. [DOI] [PubMed] [Google Scholar]
- Chan S. M., Xu N., Niemeyer C. C., Bone J. R., Flytzanis C. N. SpCOUP-TF: a sea urchin member of the steroid/thyroid hormone receptor family. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10568–10572. doi: 10.1073/pnas.89.22.10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Davis I. J., Hazel T. G., Lau L. F. Transcriptional activation by Nur77, a growth factor-inducible member of the steroid hormone receptor superfamily. Mol Endocrinol. 1991 Jun;5(6):854–859. doi: 10.1210/mend-5-6-854. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Samuels H. H. Interactions among a subfamily of nuclear hormone receptors: the regulatory zipper model. Mol Endocrinol. 1990 Sep;4(9):1293–1301. doi: 10.1210/mend-4-9-1293. [DOI] [PubMed] [Google Scholar]
- Freedman L. P. Anatomy of the steroid receptor zinc finger region. Endocr Rev. 1992 May;13(2):129–145. doi: 10.1210/edrv-13-2-129. [DOI] [PubMed] [Google Scholar]
- Green S., Kumar V., Theulaz I., Wahli W., Chambon P. The N-terminal DNA-binding 'zinc finger' of the oestrogen and glucocorticoid receptors determines target gene specificity. EMBO J. 1988 Oct;7(10):3037–3044. doi: 10.1002/j.1460-2075.1988.tb03168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle M. R., Segraves W. A., Hogness D. S. DHR3: a Drosophila steroid receptor homolog. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6167–6171. doi: 10.1073/pnas.89.13.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987 Jun 19;49(6):753–761. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krust A., Kastner P., Petkovich M., Zelent A., Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudet V., Hänni C., Coll J., Catzeflis F., Stéhelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992 Mar;11(3):1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. W., Conneely O. M., DeMayo F. J., O'Malley B. W. Identification of a new brain-specific transcription factor, NURR1. Mol Endocrinol. 1992 Dec;6(12):2129–2135. doi: 10.1210/mend.6.12.1491694. [DOI] [PubMed] [Google Scholar]
- Mercer J. G., Munn A. E., Rees H. H. Caenorhabditis elegans: occurrence and metabolism of ecdysteroids in adults and dauer larvae. Comp Biochem Physiol B. 1988;90(2):261–267. doi: 10.1016/0305-0491(88)90070-3. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988 May;1(3):183–188. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- Nakai A., Kartha S., Sakurai A., Toback F. G., DeGroot L. J. A human early response gene homologous to murine nur77 and rat NGFI-B, and related to the nuclear receptor superfamily. Mol Endocrinol. 1990 Oct;4(10):1438–1443. doi: 10.1210/mend-4-10-1438. [DOI] [PubMed] [Google Scholar]
- Ortiz D. F., Kreppel L., Speiser D. M., Scheel G., McDonald G., Ow D. W. Heavy metal tolerance in the fission yeast requires an ATP-binding cassette-type vacuolar membrane transporter. EMBO J. 1992 Oct;11(10):3491–3499. doi: 10.1002/j.1460-2075.1992.tb05431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palli S. R., Hiruma K., Riddiford L. M. An ecdysteroid-inducible Manduca gene similar to the Drosophila DHR3 gene, a member of the steroid hormone receptor superfamily. Dev Biol. 1992 Apr;150(2):306–318. doi: 10.1016/0012-1606(92)90244-b. [DOI] [PubMed] [Google Scholar]
- Stone B. L., Thummel C. S. The Drosophila 78C early late puff contains E78, an ecdysone-inducible gene that encodes a novel member of the nuclear hormone receptor superfamily. Cell. 1993 Oct 22;75(2):307–320. doi: 10.1016/0092-8674(93)80072-m. [DOI] [PubMed] [Google Scholar]
- Truss M., Beato M. Steroid hormone receptors: interaction with deoxyribonucleic acid and transcription factors. Endocr Rev. 1993 Aug;14(4):459–479. doi: 10.1210/edrv-14-4-459. [DOI] [PubMed] [Google Scholar]
- Waterston R., Martin C., Craxton M., Huynh C., Coulson A., Hillier L., Durbin R., Green P., Shownkeen R., Halloran N. A survey of expressed genes in Caenorhabditis elegans. Nat Genet. 1992 May;1(2):114–123. doi: 10.1038/ng0592-114. [DOI] [PubMed] [Google Scholar]