Abstract
Resistant hypertension continues to pose a major challenge to clinicians worldwide and has serious implications for patients who are at increased risk of cardiovascular morbidity and mortality with this diagnosis. Pharmacological therapy for resistant hypertension follows guidelines-based regimens although there is surprisingly scant evidence for beneficial outcomes using additional drug treatment after three antihypertensives have failed to achieve target blood pressure. Recently there has been considerable interest in the use of endoluminal renal denervation as an interventional technique to achieve renal nerve ablation and lower blood pressure. Although initial clinical trials of renal denervation in patients with resistant hypertension demonstrated encouraging office blood pressure reduction, a large randomised control trial (Symplicity HTN-3) with a sham-control limb, failed to meet its primary efficacy end point. The trial however was subject to a number of flaws which must be taken into consideration in interpreting the final results. Moreover a substantial body of evidence from non-randomised smaller trials does suggest that renal denervation may have an important role in the management of hypertension and other disease states characterised by overactivation of the sympathetic nervous system. The Joint UK Societies does not recommend the use of renal denervation for treatment of resistant hypertension in routine clinical practice but remains committed to supporting research activity in this field. A number of research strategies are identified and much that can be improved upon to ensure better design and conduct of future randomised studies.
Scope of the consensus statement
Resistant hypertension (RHTN) is increasingly recognised as an important subset of uncontrolled hypertension that carries substantial additional risk for cardiovascular disease.1 2 Wide-ranging prevalence for RHTN has been reported which varies depending on which cohorts have been examined. However, it seems likely that the overall prevalence is around 8–10% of all patients with hypertension and that improved treatment of this group would lead to considerable reduction in cardiovascular morbidity and mortality making it a high priority hypertension subset.3 4
Renal denervation (RDN) therapy has recently become available as a treatment for proven RHTN, albeit with a clinical effectiveness evidence base limited to studies lacking blinded end points or sham controls and mostly with small numbers of patients.5–7 Following the announcement by Medtronic on 9 January 2014 that the Symplicity hypertension (HTN)-3 trial had failed to meet its primary efficacy end point, the Joint UK Societies released an interim statement recommending a temporary moratorium on RDN procedures undertaken as a standard of care in the UK while awaiting formal reporting and peer-reviewed publication of the study results.8 9 This updated statement was prepared following the recent publication of the Symplicity HTN-3 study—a prospective, single-blind, randomised, sham-controlled trial that took place in the USA.10 In this document we consider the RDN landscape that set the stage for the Symplicity HTN-3 study and thereafter review the study design and results. A number of limitations affecting the execution of the study are now apparent that provide important lessons for future studies of RDN. Importantly, given the conflicting evidence regarding the role of RDN, it is too early to use the Symplicity HTN-3 outcome as a rationale to abandon this novel therapeutic development. Here we also address critical issues that must be overcome if RDN is to progress as a therapy for RHTN or other forms of hypertension.
Symplicity HTN-3 background
The Symplicity HTN-3 study was conceived and designed as a regulatory study for US approval of RDN therapy as part of an ongoing multistage global clinical evaluation programme for radiofrequency (RF) RDN initiated by Medtronic.11 The study design allowed the investigators to address numerous concerns that had arisen in light of the initial studies reporting RDN as an efficacious and safe therapy for RHTN (see table 1).
Table 1.
Criticisms of clinical renal denervation (RDN) data to date
| Criticism | Problem | Potential solutions |
|---|---|---|
| Trial design | Non-blinded design Lack of sham control |
Double-blind RDN versus sham procedure with best medical therapy10 |
| Patient selection and management | No per protocol exclusion of secondary causes of hypertension | Mandatory per protocol exclusion of secondary causes of hypertension |
| Ensure adherence to medication Per protocol managed lifestyle changes |
Plasma/urine assay of medications/metabolites±directly observed therapy before qualifying BP measurement Optimal 24-h urinary sodium excretion |
|
| Non-optimised antihypertensive medications Heterogeneity of patient suitability rates between RDN centres12 13 |
Guidelines-based treatment regimens with add-on spironolactone and/or α-blockade Referral to hypertension specialist centres |
|
| Ambulatory BP | Unnecessary inclusion of pseudoresistant patients with hypertension5
6 and potential overestimation of effect on office BP Less reduction in ambulatory BP parameters compared with office BP14 |
Use of ambulatory BP as entry and outcome criteria15 16 |
| Human renal nerve anatomy uncertain Renovascular safety |
Optimal sites for RDN yet to be defined Incomplete characterisation of renovascular injury periprocedurally and in the long term17 Reporting of only >60% stenoses6 No histological safety data from humans Requirement for concurrent, periprocedural antiplatelet therapy to be determined17 |
Targeting of renal nerves depends also on energy modality used18 Further use of optical coherence tomography17 Postprocedure CT angiography or magnetic resonance angiography (MRA) at 6–12 months19 Further studies needed—may vary with energy modality used for RDN |
| Predictors of response Heterogeneity of BP response |
Only baseline, office SBP reliably predicts response across studies5
6
20 Inability to distinguish between procedural failure or non-response Wide variation in response to RDN raises questions about role of renal nerves in hypertension21 |
Autonomic function testing (where available) to be included in clinical studies and registries5
22
23 Research into ontable assessment of efficacy of RDN24 25 Research focusing on renal nerve physiology in hypertension and role of RDN26 27 |
| Durability of response and end points | 6-month office BP as primary end point5 6 20 | Major adverse events as primary end points |
| No data greater than 3 years post procedure published28 | Studies and registries to extend to several years |
BP, blood pressure; SBP, systolic BP.
Principal among these concerns were the fact that existing studies had failed to include blinded end points and, in particular, there was no mandated use of ambulatory blood pressure monitoring (ABPM) as a means to diagnose RHTN and to monitor response to the therapy. Furthermore, although Symplicity HTN-2 was a randomised controlled study, there was no sham control procedure which has been recorded as important in the proper evaluation of novel interventional procedures.29 30 Despite being a novel interventional procedure, there have been very few adverse reports pertaining to safety of the procedure, or long-term harmful sequelae although this may simply reflect numbers of procedures performed to date and lack of true long-term follow-up. Case reports of renal artery stenosis following denervation are a reminder of the importance of long-term scrutiny for renovascular disorder following RDN and careful surveillance of biochemical renal function.31 32
Other concerns over published RDN data sets included lack of specialist management of patients with RHTN (diagnosis and treatment, adequacy of per-protocol exclusion of secondary HTN), and that adherence to antihypertensive medication prior to enrolment and during the course of studies had not been assessed.33 34 Concomitantly, hypertension specialists in a number of European centres had also started to investigate the utility of RDN in patients with RHTN and have reported that the number of patients suitable for RDN (after specialist assessment and medication adjustment) would be very limited.12 35 36 Furthermore the high responder rates of 80% or more patients exhibiting office systolic blood pressure (SBP) reduction of >10 mm Hg in earlier trials were not replicable in a number of smaller studies which showed less striking blood pressure (BP) lowering and substantial heterogeneity of BP responses to RDN.37–39
Symplicity HTN-3 study design and results
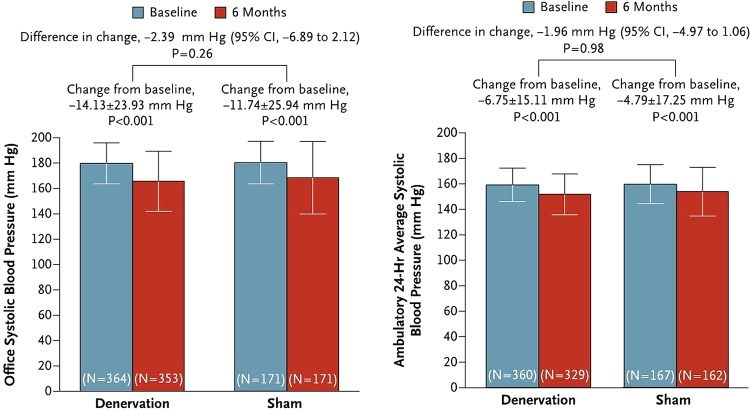
The Symplicity HTN-3 study was a prospective, single-blind, randomised, sham-controlled trial in which patients with severe RHTN (office BP >160 mm Hg and 24-h mean SBP >135 mm Hg) were randomly assigned in a 2:1 ratio to undergo RDN or a sham procedure.11 Before randomisation, patients were intended to be receiving a stable antihypertensive regimen involving maximally tolerated doses of at least three drugs, including a diuretic. The primary efficacy end point was the change in office SBP at 6 months; a secondary efficacy end point was the change in mean 24-h ambulatory SBP. In total 535 subjects were randomised of whom 364 received RDN therapy and 171 had a sham procedure. The investigators ensured adequate blinding of study participants such that they were unaware whether they had received RDN therapy or sham control (renal angiogram) and BP assessors were blinded to the study group assignments. Although the primary safety end point was met, with a major adverse event rate of only 1.4% (set against an objective performance goal of <9.8%), the study failed to meet its primary and secondary efficacy end points with no statistically significant difference in either office or ABPM BP lowering between the RDN treatment and sham control arms (figure 1). Substantial variation in BP responses was noted in both study groups and subgroup analyses indicated that RDN therapy might work better in patients of non-African-American ethnicity, patients below the age of 65 years and those with preserved renal function (estimated glomerular filtration rate >60 mL/min).10
Figure 1.
Symplicity HTN-3 results of primary and secondary efficacy end points.
Symplicity HTN-3: study limitations and lessons for future trials of RDN
The Symplicity HTN-3 study improved upon Symplicity HTN-1 and HTN-2 in having a blinded secondary end point (ABPM) and a sham control group. However, following publication of the results and presentation in international meetings, a number of findings have come to light that indicate that there are important lessons to be learnt about the optimal design and execution of such studies.
Medications stability
The study design mandated each enrolled patient was on a maximum tolerated dose or maximum Food and Drug Administration (FDA)-approved dose of at least three antihypertensive agents from different classes, for at least 2 weeks prior to enrolment. Although there was an emphasis on maintaining a constant therapeutic regimen throughout the study, as many as 20% of patients were not on stable medications within 6 weeks of inclusion.40 Given that attainment of full effect of diuretics (including aldosterone antagonists) is known to take up to 8 weeks,41 it is quite possible that some of the BP changes in both study limbs were due to late effects of the enrolment medication regimen.
There were also substantial differences in use of aldosterone antagonists (22.5/28.7%) and vasodilators (36.8/45%) between the RDN/sham limbs at baseline, respectively, and a preponderance of African-Americans, who are more likely to respond to these drug classes, in the sham group (29.2% sham vs 24.8% RDN). Indeed African-American patients demonstrated a striking SBP reduction of 17.8 mm Hg in the sham arm versus 15.5 mm Hg in the RDN limb.
Approximately 40% of patients in both arms required medications changes during the study which is unusual.40 The exact reasons for and the disposition of the medication changes are yet to be clarified. It is noteworthy that there were eight points of investigator/subject contact during the study with close scrutiny of medications and BP at every point. It is possible that medication compliance was artificially enhanced in this study (the Hawthorne effect). Of note, although patients were asked to maintain a medication diary, adherence to antihypertensive medication was not formally tested by directly observed therapy or by urine antihypertensive drug assay in this study. It is also unfortunate that long-term follow-up of the sham control population is already confounded by high cross-over to active therapy with RDN which will negatively impact on the opportunity to determine if the sham treatment alone had long-term implications for BP control.
RDN procedures
Attention has already focused on the large number of sites in Symplicity HTN-3 with 111 interventionists who performed at least one RDN procedure (34% did only a single procedure) and with only 26 operators undertaking more than five procedures.42 Although procedures were proctored (mostly by non-clinical staff), this does not imply that optimal denervation was achieved.
In fact in this study the mean total number of ablation attempts was 11.2±2.8 per patient with a mean of 9.2±2.0 per protocol 120 s ablations. In the Global Symplicity Registry (GSR), ambulatory SBP reduced by 10.3 mm Hg (in patients with office BP >160 mm Hg and ambulatory BP >135 mm Hg) compared with a reduction in ambulatory SBP of only 6.8 mm Hg in the RDN group and 4.8 mm Hg in the sham group in Symplicity HTN-3.43 In the GSR the mean number of bilateral ablations was 13.5±4.1, and the mean number of 120 s bilateral ablations was 11.3±3.4. In HTN-3 it was shown that with an increasing number of RF ablations there was a greater reduction in BP; in 40 patients who underwent RDN who had more than 13 total ablation attempts there was a 10.3 mm Hg reduction in ambulatory SBP, matching the effect size in the GSR.40
In Symplicity HTN-3 only a small proportion of patients had per-protocol RF ablation in all four quadrants of both renal arteries.40 Disappointingly, but not surprisingly, matched cohort analysis has indicated that delivery of ablations in all four quadrants of the renal artery was also associated consistently with the greatest reductions in office, home and ambulatory SBP (−24.3, −9.0 and −10.3 mm Hg, respectively) but this only applied in 19 patients out of 364 patients undergoing RDN.40
Evidence that suggests RDN may be beneficial for RHTN
Data from numerous animal models have previously suggested an important role for the renal sympathetic nervous system (SNS) in the initiation and perpetuation of hypertension.44 In addition, a number of human studies had demonstrated the importance of renal nerve signalling in hypertension prior to the publication of the Symplicity HTN-1 and HTN-2 studies. These data have been reviewed extensively elsewhere.45 Data from a number of smaller studies have indicated efficacy for RDN in BP lowering and improving other aspects of cardiovascular function and merit consideration as follows.
Improvement in ABPM parameters
While Symplicity HTN-1 and HTN-2 provided data for efficacy and safety of RDN, the lack of ABPM in either inclusion criteria or monitoring of BP in response to RDN has been criticised. However, non-randomised data supporting sizeable effects of RDN on office and ABPM parameters have now been published in a study of 346 patients with true RHTN (n=303) and pseudoresistant HTN (n=43).14 Early and sustained significant reductions in office SBP and diastolic blood pressure (DBP) were shown at 3 months, 6 months and 12 months follow-up (office SBP: −21.5/−23.7/−27.3 mm Hg, office DBP: −8.9/−9.5/−11.7 mm Hg), respectively. There was a significant reduction with RDN in 24 h SBP (−10.1/−10.2/−11.7 mm Hg,) and diastolic blood pressure (DBP) (−4.8/−4.9/−7.4 mm Hg) at 3 months, 6 months and 12 months, respectively, as well as reduction in BP variability in those patients with true RHTN. In patients with pseudoresistant hypertension there was no effect on ABPM post denervation arguing strongly against a sham effect. The GSR, presented at the American College of Cardiology meeting (March 2014), demonstrated a reduction of 10.3 mm Hg in mean 24 h SBP in 52 patients with treatment-RHTN taking three or more antihypertensives at maximally tolerated doses with an office BP >160 mm Hg.43
RDN enables regression of LV hypertrophy
Reduction in LV hypertrophy and improvement in cardiac function has been demonstrated in patients with RHTN following RDN.46 Intriguingly it has been proposed that improvement in cardiac function and LV hypertrophy regression was independent of the magnitude of BP reduction, suggesting suppression of neurohumoural mechanisms. It must be noted however that this was a prospective, non-randomised, open-label unipolar RF denervation single-centre study in a small number of patients without sham control. More recently catheter based RDN was demonstrated to significantly reduce BP and LV mass and improve EF in patients with RHTN using cardiac MRI.47 Once again this prospective multicentre study of only 72 patients was non-randomised and with open-label use of RDN. Importantly, however, the magnitude of LV mass reduction seen was less than that noted in echocardiographic studies of RDN but still comparable with that seen in patients with hypertension treated with pharmacotherapy.48 Although a profound placebo effect or greater compliance with medication might have influenced results, to some extent the reduction in LV mass occurred independently of BP reduction which raises the tantalising prospect that downregulation of central cardiac (but not vasomotor) sympathetic outflow resulting from RDN may have a direct role to play in modulating hypertensive heart disease. The potential of RDN as a treatment for heart failure warrants further study.
Other possible cardiovascular benefits of RDN
RDN reduces heart rate in humans and may have beneficial effects in cardiac rhythm disturbance including reduction in burden of atrial fibrillation and possibly ventricular arrhythmias as well.49 50 RDN was shown to reduce arterial stiffness and central haemodynamics which are important prognostic markers and this too merits further investigation.51 In two small studies, investigators have shown that RDN improves health-related quality of life and that this improvement was not necessarily related to the magnitude of BP reduction.52 53
Current state of RDN therapy in the UK
When RDN therapy first became available as a treatment for RHTN, there was rapid uptake of the procedure in a number of European Union territories where a Conformité Européene mark had been established for various competing technologies and this led to over 10 000 RDN procedures being undertaken worldwide with regrettably little in the way of data capture with the exception of several investigators who have been pioneers in the field. In contrast, UK uptake has been much slower with only a handful of centres offering the procedure with responder rates that have been markedly heterogeneous and less encouraging on the whole.22 54 55
At one point in 2013 there were more than 60 companies with technologies in development for RDN including first and second generation RF ablation catheters, ultrasound catheters, radiotherapy catheters, cryotherapy and chemical ablation technologies.18 Following the Symplicity HTN-3 announcement a number of companies have announced their withdrawal from the field of device therapy for hypertension including such large entities as Covidien. However, several of the major devices manufactures including Boston Scientific, Medtronic and St Jude Medical, having suspended clinical trial activity within pre-existing programmes, have announced their intention to commit to the field of renal sympathectomy. They are using the outputs from Symplicity HTN-3 as an opportunity to improve design of their future clinical trials.
The Joint UK Societies updated perspective
After reviewing the evidence from the Symplicity HTN-1 and HTN-2 studies, the Joint UK Societies produced guidance for the use of RDN in the UK in 2011 which was summarised in an electronically published summary consensus statement.56 The National Institute for Health and Care Excellence subsequently produced interim guidance for RDN that was very much in line with our initial guidance.57 In order to protect patients from overenthusiastic and/or inappropriate application of the procedure, it was mandated that patients only be treated with RDN in the context of multidisciplinary assessment in centres of excellence with Hypertension Specialists leading on case management. The procedure, which would be costly for the UK's National Health Service, was to be funded by a novel process of ‘Commissioning Through Evaluation’ designed to ensure capture of data from all procedures in a national registry, which would serve as a clinical and academic resource.58 Subsequently other expert group statements have also been published from the European Society of Hypertension, European Society of Cardiology, Cardiovascular and Interventional Radiology Society of Europe and an International expert Consensus Statement all supporting the use of RDN therapy as a treatment option for RHTN where routine measures to control BP have failed.59–62
Following close upon the heels of the Medtronic Symplicity HTN-3 press announcement, the Joint UK Societies produced an interim statement calling for a temporary moratorium on UK use of RDN therapy in anticipation of the formal publication and presentation of the data.9 Even allowing for debate over the interpretation of the outcomes of the Symplicity HTN-3 study, there is still a lack of evidence to support the routine use of RDN as a standard of care in RHTN. Hence, we recommend the current moratorium on use of this procedure in the UK remains in place until such time as there is Level 1 evidence in favour of use of RDN. This view is in line with the published evidence and is supported by a recent meta-analysis suggesting that more large-scale randomised controlled trials in RDN are needed before this treatment should be considered in routine clinical practice.63
Importantly, the Joint UK Societies recognise that a substantial amount of (Level II or lesser) evidence does suggest clinical utility for RDN therapy in some patients and thus we feel that the therapy should not be abandoned in light of the Symplicity HTN-3 study result. Indeed, this study has continued to disseminate outputs that are proving to be helpful in our learning of how better to manage future studies of RDN. It will be essential for device manufacturers, multidisciplinary clinicians and researchers to collaborate closely to effectively define the path for future studies. Key learning points from the Symplicity HTN-3 study are summarised in table 2.
Table 2.
Key lessons from Symplicity HTN-3
| Lesson | Recommendations for the future |
|---|---|
| Hypertension specialists were not involved in most centres Antihypertensive medications stability is critical
|
Routine use of multidisciplinary teams led by accredited hypertension specialist Hypertension specialists should design the clinical trials in conjunction with scientists and interventionalists Stable medication regimen for at least 8 weeks prior to study entry or use of optimised medical regimen with washout period of 4 weeks prior to baseline if feasible and forced titration during study (does not necessarily apply to trials of RHTN) Maintain stable medications throughout study per protocol: strict criteria for clinically necessary medication changes |
| Heterogeneous study population differed from prior trials of RDN with more African-Americans Procedural factors
|
Study subjects should reflect the population of resistant hypertensives—it is entirely appropriate to recruit all ethnicities as black ethnicity is a risk factor for RHTN Adequate proctoring for inexperienced operators Ensure delivery of adequate ablations per artery Ensure 4 quadrant delivery of ablation Assume learning curve of at least 10 procedures with each RDN system |
RDN, renal denervation; RHTN, Resistant Hypertension.
The UK remains committed to leading, supporting and participating in well-designed randomised controlled studies to assess the future role of RDN in RHTN. As such the moratorium does not apply to clinical trials of RDN therapy. It is important that alternative energy modalities, such as ultrasound and chemical ablation technologies should be investigated along with second and third generation RF catheters in appropriately designed clinical studies with blinded end points to include ABPM. In RHTN it is vital that careful evaluation of drug adherence is included as a prerequisite to study entry unless severe medication intolerance has been diagnosed. Recently mass spectrometry drug measurement in urine has demonstrated that many patients are not adherent with their therapy.64 The application of similar measures in future studies will robustly confirm resistance and allow us to detect non-concordance. It is also important that patients should not be enrolled in studies within 8 weeks of the last change in treatment.
Critical areas to address to determine the role of RDN as a therapy for RHTN
The key areas that future research in RDN must address are summarised in table 1. It is remarkable that to date there is no agreement on the precise anatomy of the human sympathetic nerves and in particular the variation in their trajectories relative to the renal arteries. In a small study of nine renal arteries harvested from five human autopsies, it was reported that the majority of renal nerves lie within 2 mm distance from the lumen of the renal artery.65 However, the fixation methodology used has been criticised and other workers have suggested that in humans, renal nerve distribution varies between the proximal-distal segments of the renal artery and that the 50th and 75th centiles of distance of nerves in the proximal segment are 2.84 and 4.67 mm, respectively.66 Clearly, precise localisation of human renal nerves has crucial implications for the application of RDN and it is important to bear in mind that different ablation technologies (eg, RF energy, ultrasound energy, chemical ablation) offer varying depths of renal sympathectomy and it remains to be proven which is most effective for human use.
Since renal nerves spiral around the artery, it is also clear that denervation in all four quadrants of the renal artery is necessary. This may be achieved through the use of multielectrode RF ablation catheters and alternative means of sympathectomy including ultrasound and chemical ablation techniques. Ultrasound energy may offer benefit by avoiding endothelial damage and deeper penetration of heating energy than RF. Chemical ablation techniques with alcohol or guanethidine also offer potential advantage in obviating endothelial damage although it remains unclear whether or not dissemination of neurotoxin beyond the periadventitial layer could be an undesired effect of such therapy.18 Determining which energy modality and ablation therapy is best to achieve near complete renal nerve ablation in the absence of damage to the renal artery lumen (and off-target effects) remains a priority.
At present there is no marker of procedural success to inform operators that RDN therapy has been successfully delivered although in a number of studies it has been demonstrated that the higher the starting SBP, the greater the response to RDN. Preliminary data from humans indicate there could be a role for high frequency electrical field stimulation of the renal arteries.24 In a canine model, intralumenal renal artery electrical stimulation (to activate renal nerves) was shown to increase BP, serum catecholamines and heart rate variability, presumably through activation of renal afferent reflexes; these effects were attenuated following RDN.25 Currently there are no established biomarkers of renal nerve injury (a neural ‘troponin’ would be ideal) and early plasma markers of renal injury have not translated as trustworthy markers. Thus, interventionists remain uninformed as to whether or not the RDN procedure they have undertaken has achieved its intended purpose.
Ideally treatment with RDN would be limited to those patients most likely to respond to the therapy. If a response rate is defined as a reduction in office SBP of 10 mm Hg or more, a number of non-randomised and non-blinded studies have indicated responder rates in excess of 80%. However real world experience of RDN suggests true responder rates are much more variable22 39 54 55 and as such it might be ideal to phenotype patients with hypertension to better predict responders/non-responders. Biochemical and ethnic phenotyping is already in place in the National Institute for Health and Care Excellence/British Hypertension Society guideline for antihypertensive pharmacotherapy where patients with low renin status (black ethnicity) are offered calcium channel blockers as first-line therapy and those with high renin status (Caucasian ethnicity) receive ACE/angiotensin receptor blocker drugs instead.67 Given the complexity of SNS signalling and how this related to changes in vasomotor tone, and the lack of easy applicable tests for large-scale determination of renal sympathetic overactivation, it seems unlikely that hypertension phenotyping for SNS upregulation will evolve into mainstream clinical practice.
One approach that merits further study is the use of non-invasive techniques to assess baroreflex sensitivity (BRS) as impaired BRS was shown to be strongly predictive of response to RDN in patients with RHTN (although it did not predict the magnitude of BP reduction).23 RDN was found to consistently improve BRS in all patients studied regardless of heterogeneous effects on BP.22 Multivariate logistic regression analysis showed lower levels of BRS to be the strongest predictor of response to RDN and independent of all other variables.23 Another small study has demonstrated a novel potential role for elevated plasma levels of angiogenic markers such as soluble fms-like tyrosine kinase-1, intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 as predictors of response to RDN.68
Conclusion
Despite the negative results from Symplicity HTN-3, it is too early to decide that RDN therapy is a failed treatment strategy for RHTN. Sufficient encouraging data for RDN as a means to treat hypertension have emerged in a number of animal models of hypertension as well as some human studies to attest to its potential as an effective treatment for at least a subsection of patients. Furthermore, studies have indicated that RDN may attenuate hypertensive heart disease, decrease arrhythmia burden and reduce arterial stiffness in addition to lowering BP. Heterogeneity in BP responses do suggest that RDN is not a treatment for all-comers with RHTN and that careful patient selection will need to be augmented by better understanding of who might best respond.
It is now the time to focus on improved study design and meticulous execution of clinical trials with careful proctoring of interventionalists who are unfamiliar with the procedure as there is undoubtedly a learning curve with all novel procedures no matter how simple they may seem from the outset. Although there is unmet need for improved treatment strategies for patients with RHTN, it is also quite possible that chronic RHTN, with all the attendant circulatory maladaptation and arterial stiffening that ensues, may not represent the best target group of patients who would derive most benefit from RDN. It remains to be proven whether or not patients with milder forms of hypertension of shorter duration could be the most appropriate population for this therapy—once again high quality clinical studies are mandated particularly as the therapy becomes less expensive in the future. Until we have better evidence of the clinical efficacy in well conducted trials the Joint UK Societies recommend continuing the moratorium on RDN in routine UK healthcare. The UK is ready to contribute to further study the place of this therapy in the management of RHTN. With the current uncertainties, it would not be reasonable to expect commissioners to meet the costs of treatment in newly designed postmarketing studies. Industry will need to meet the costs of industry-sponsored studies although we hope that commissioners will support the clinical costs of National Institute for Health Research-funded studies.
Footnotes
Contributors: MDL conceived, designed and drafted this article, has undertaken multiple revisions of the text and takes full responsibility for this article. All other authors have reviewed and contributed to revisions of the manuscript.
Competing interests: MDL is on the speaker bureau for St Jude Medical and has participated in advisory boards for St Jude Medical.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 2012;125:1635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierdomenico SD, Lapenna D, Bucci A, et al. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens 2005;18:1422–8. [DOI] [PubMed] [Google Scholar]
- 3.Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011;57:1076–80. [DOI] [PubMed] [Google Scholar]
- 4.de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension 2011;57:898–902. [DOI] [PubMed] [Google Scholar]
- 5.Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009;373:1275–81. [DOI] [PubMed] [Google Scholar]
- 6.Symplicity HTNI, Esler MD, Krum H, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010;376:1903–9. [DOI] [PubMed] [Google Scholar]
- 7.Symplicity HTNI. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011;57:911–17. [DOI] [PubMed] [Google Scholar]
- 8.Medtronic Announces U.S. Renal Denervation Pivotal Trial Fails to Meet Primary Efficacy Endpoint While Meeting Primary Safety Endpoint [press release]. 09/01/2014 2014.
- 9.Caulfield M, de Belder M, Cleveland T, et al. The Joint UK Societies Working Group on Renal Denervation. Initial response to the Medtronic Symplicity HTN3 announcement 2014. http://www.bhsoc.org/files/2913/9084/2931/The_Joint_UK_Societies_Working_Group_on_Renal_Denervation_Statement_on_Symplicity_HTN3_pre-final.pdf
- 10.Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014;370:1393–401. [DOI] [PubMed] [Google Scholar]
- 11.Catheter-Based Renal Denervation for Resistant Hypertension: Rationale and Design of the SYMPLICITY HTN-3 Trial 2012.
- 12.Savard S, Frank M, Bobrie G, et al. Eligibility for renal denervation in patients with resistant hypertension: when enthusiasm meets reality in real-life patients. J Am Coll Cardiol 2012;60:2422–4. [DOI] [PubMed] [Google Scholar]
- 13.Verloop WL, Vink EE, Voskuil M, et al. Eligibility for percutaneous renal denervation: the importance of a systematic screening. J Hypertens 2013;31:1662–8. [DOI] [PubMed] [Google Scholar]
- 14.Mahfoud F, Ukena C, Schmieder RE, et al. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation 2013;128:132–40. [DOI] [PubMed] [Google Scholar]
- 15.Ott C, Mahfoud F, Schmid A, et al. Renal denervation in moderate treatment-resistant hypertension. J Am Coll Cardiol 2013;62:1880–6. [DOI] [PubMed] [Google Scholar]
- 16.Kaltenbach B, Franke J, Bertog SC, et al. Renal sympathetic denervation as second-line therapy in mild resistant hypertension: a pilot study. Catheter Cardiovasc Interv 2013;81:335–9. [DOI] [PubMed] [Google Scholar]
- 17.Templin C, Jaguszewski M, Ghadri JR, et al. Vascular lesions induced by renal nerve ablation as assessed by optical coherence tomography: pre- and post-procedural comparison with the Simplicity catheter system and the EnligHTN multi-electrode renal denervation catheter. Eur Heart J 2013;34:2141–8, 8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapil V, Jain AK, Lobo MD. Renal sympathetic denervation—a review of applications in current practice. Intervent Cardiol Rev 2014;9:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persu A, Sapoval M, Azizi M, et al. Renal artery stenosis following renal denervation: a matter of concern. J Hypertens 2014;32:2101–5. [DOI] [PubMed] [Google Scholar]
- 20.Worthley SG, Tsioufis CP, Worthley MI, et al. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J 2013;34:2132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fink GD, Osborn JW. Renal nerves: time for reassessment of their role in hypertension? Am J Hypertens 2014;27:1245–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart EC, McBryde FD, Burchell AE, et al. Translational examination of changes in baroreflex function after renal denervation in hypertensive rats and humans. Hypertension 2013;62:533–41. [DOI] [PubMed] [Google Scholar]
- 23.Zuern CS, Eick C, Rizas KD, et al. Impaired cardiac baroreflex sensitivity predicts response to renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol 2013;62:2124–30. [DOI] [PubMed] [Google Scholar]
- 24.Pokushalov E, Romanov A, Corbucci G, et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol 2012;60:1163–70. [DOI] [PubMed] [Google Scholar]
- 25.Chinushi M, Izumi D, Iijima K, et al. Blood pressure and autonomic responses to electrical stimulation of the renal arterial nerves before and after ablation of the renal artery. Hypertension 2013;61:450–6. [DOI] [PubMed] [Google Scholar]
- 26.Henegar JR, Zhang Y, Rama RD, et al. Catheter-based radiorefrequency renal denervation lowers blood pressure in obese hypertensive dogs. Am J Hypertens 2014;27:1285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linz D, Mahfoud F, Linz B, et al. Effect of obstructive respiratory events on blood pressure and renal perfusion in a pig model for sleep apnea. Am J Hypertens 2014;27:1293–300. [DOI] [PubMed] [Google Scholar]
- 28.Krum H, Schlaich MP, Sobotka PA, et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet 2014;383:622–9. [DOI] [PubMed] [Google Scholar]
- 29.Leon MB, Kornowski R, Downey WE, et al. A blinded, randomized, placebo-controlled trial of percutaneous laser myocardial revascularization to improve angina symptoms in patients with severe coronary disease. J Am Coll Cardiol 2005;46:1812–19. [DOI] [PubMed] [Google Scholar]
- 30.Serruys PW. The scientific power of a “sham arm”? EuroIntervention 2014;9:1129–31. [DOI] [PubMed] [Google Scholar]
- 31.Kaltenbach B, Id D, Franke JC, et al. Renal artery stenosis after renal sympathetic denervation. J Am Coll Cardiol 2012;60:2694–5. [DOI] [PubMed] [Google Scholar]
- 32.Vonend O, Antoch G, Rump LC, et al. Secondary rise in blood pressure after renal denervation. Lancet 2012;380:778. [DOI] [PubMed] [Google Scholar]
- 33.Persu A, Renkin J, Thijs L, et al. Renal denervation: ultima ratio or standard in treatment-resistant hypertension. Hypertension 2012;60:596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapil V, Lobo MD. . Clinical Considerations and the Potential for Renal Denervation. Endovascular Today [Internet] 2013; 10:[1–7 pp.]. http://evtoday.com/pdfs/et1013_F3_Lobo.pdf
- 35.Persu A, Jin Y, Baelen M, et al. Eligibility for renal denervation: experience at 11 European expert centers. Hypertension 2014;63:1319–25. [DOI] [PubMed] [Google Scholar]
- 36.Hayek SS, Abdou MH, Demoss BD, et al. Prevalence of resistant hypertension and eligibility for catheter-based renal denervation in hypertensive outpatients. Am J Hypertens 2013;26:1452–8. [DOI] [PubMed] [Google Scholar]
- 37.Ezzahti M, Moelker A, Friesema EC, et al. Blood pressure and neurohormonal responses to renal nerve ablation in treatment-resistant hypertension. J Hypertens 2014;32:135–41. [DOI] [PubMed] [Google Scholar]
- 38.Fadl Elmula FE, Hoffmann P, Larstorp AC, et al. Adjusted drug treatment is superior to renal sympathetic denervation in patients with true treatment-resistant hypertension. Hypertension 2014;63:991–9. [DOI] [PubMed] [Google Scholar]
- 39.Persu A, Jin Y, Azizi M, et al. Blood pressure changes after renal denervation at 10 European expert centers. J Hum Hypertens 2014;28:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, et al. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. European Heart Journal Published Online First: 16 Nov 2014 dx.doi.org/10.1093/eurheartj/ehu441 [DOI] [PMC free article] [PubMed]
- 41.Roos JC, Boer P, Koomans HA, et al. Haemodynamic and hormonal changes during acute and chronic diuretic treatment in essential hypertension. Eur J Clin Pharmacol 1981;19:107–12. [DOI] [PubMed] [Google Scholar]
- 42.Luscher TF, Mahfoud F. Renal nerve ablation after SYMPLICITY HTN-3: confused at the higher level? Eur Heart J 2014;35:1706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Böhm M, Mahfoud F, Ukena C, et al. Effect of renal denervation in a real world population of patients with uncontrolled hypertension-the Global SYMPLICITY Registry. ACC Washington DC 2014; Late Breaking Clinical Trials. March 2014, DC, USA. [Google Scholar]
- 44.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 1997;77:75–197. [DOI] [PubMed] [Google Scholar]
- 45.Esler M. The sympathetic system and hypertension. Am J Hypertens 2000;13:99s–105s. [DOI] [PubMed] [Google Scholar]
- 46.Schirmer SH, Sayed MM, Reil JC, et al. Improvements in left ventricular hypertrophy and diastolic function following renal denervation: effects beyond blood pressure and heart rate reduction. J Am Coll Cardiol 2014;63:1916–23. [DOI] [PubMed] [Google Scholar]
- 47.Mahfoud F, Urban D, Teller D, et al. Effect of renal denervation on left ventricular mass and function in patients with resistant hypertension: data from a multi-centre cardiovascular magnetic resonance imaging trial. Eur Heart J 2014;35:2224–31. [DOI] [PubMed] [Google Scholar]
- 48.Fagard RH, Celis H, Thijs L, et al. Regression of left ventricular mass by antihypertensive treatment: a meta-analysis of randomized comparative studies. Hypertension 2009;54:1084–91. [DOI] [PubMed] [Google Scholar]
- 49.Ukena C, Mahfoud F, Spies A, et al. Effects of renal sympathetic denervation on heart rate and atrioventricular conduction in patients with resistant hypertension. Int J Cardiol 2013;167:2846–51. [DOI] [PubMed] [Google Scholar]
- 50.Ukena C, Mahfoud F, Linz D, et al. Potential role of renal sympathetic denervation for the treatment of cardiac arrhythmias. EuroIntervention 2013;9(Suppl R):R110–16. [DOI] [PubMed] [Google Scholar]
- 51.Brandt MC, Reda S, Mahfoud F, et al. Effects of renal sympathetic denervation on arterial stiffness and central hemodynamics in patients with resistant hypertension. J Am Coll Cardiol 2012;60:1956–65. [DOI] [PubMed] [Google Scholar]
- 52.Lambert GW, Hering D, Esler MD, et al. Health-related quality of life after renal denervation in patients with treatment-resistant hypertension. Hypertension 2012;60:1479–84. [DOI] [PubMed] [Google Scholar]
- 53.Dorr O, Liebetrau C, Mollmann H, et al. Influence of renal sympathetic denervation on quality of life. J Interv Cardiol 2013;26:536–41. [DOI] [PubMed] [Google Scholar]
- 54.Chan K, Ng F, Saxena M, et al. Renal Denervation in Resistant Hypertension—a prospective case series. J Hum Hypertens 2012;26:616.22971800 [Google Scholar]
- 55.Dasgupta I, Taylor AH, Watkin R, et al. Real world experience of renal denervation in the United Kingdom-a two centre study. J Hum Hypertens 2013;27:646. [Google Scholar]
- 56.Caulfield M, de Belder M, Cleveland T, et al. 2011. Joint UK Societies’ Consensus Summary Statement on Renal Denervation for Resistant Hypertension. http://www.bhsoc.org/docs/Joint-UK-Societies-Summary-on-Renal-Denervation.pdf.
- 57.Excellence NIfHaC. Percutaneous transluminal radiofrequency sympathetic denervation of the renal artery for resistant hypertension 2012. http://www.nice.org.uk/guidance/ipg418
- 58.NHS E.2013. NHS England invites specialised services providers to take part in its innovative new programme ‘Commissioning through Evaluation’. http://www.england.nhs.uk/2013/09/26/com-through-eval/
- 59.Schmieder RE, Redon J, Grassi G, et al. ESH position paper: renal denervation—an interventional therapy of resistant hypertension. J Hypertens 2012;30:837–41. [DOI] [PubMed] [Google Scholar]
- 60.Mahfoud F, Luscher TF, Andersson B, et al. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J 2013;34:2149–57. [DOI] [PubMed] [Google Scholar]
- 61.Moss J, Vorwerk D, Belli AM, et al. Cardiovascular and Interventional Radiological Society of Europe (CIRSE) position statement on renal denervation for resistant hypertension. Cardiovasc Intervent Radiol 2014;37:11–12. [DOI] [PubMed] [Google Scholar]
- 62.Schlaich MP, Schmieder RE, Bakris G, et al. International expert consensus statement: Percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol 2013;62:2031–45. [DOI] [PubMed] [Google Scholar]
- 63.Davis MI, Filion KB, Zhang D, et al. Effectiveness of renal denervation therapy for resistant hypertension: a systematic review and meta-analysis. J Am Coll Cardiol 2013;62:231–41. [DOI] [PubMed] [Google Scholar]
- 64.Tomaszewski M, White C, Patel P, et al. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart 2014; 100:855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Atherton DS, Deep NL, Mendelsohn FO. Micro-anatomy of the renal sympathetic nervous system: a human postmortem histologic study. Clin Anat 2012;25:628–33. [DOI] [PubMed] [Google Scholar]
- 66.Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol 2014;64:635–43. [DOI] [PubMed] [Google Scholar]
- 67.Excellence NIfHaC. Hypertension: Clinical management of primary hypertension in adults. 2011. http://www.nice.org.uk/guidance/cg127
- 68.Dorr O, Liebetrau C, Mollmann H, et al. Soluble fms-like tyrosine kinase-1 and endothelial adhesion molecules (intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1) as predictive markers for blood pressure reduction after renal sympathetic denervation. Hypertension 2014;63:984–90. [DOI] [PubMed] [Google Scholar]