Abstract
Objective
An audit of neonatal care services provided by clinical training centres was undertaken to identify areas requiring improvement as part of wider efforts to improve newborn survival in Kenya.
Design
Cross-sectional study using indicators based on prior work in Kenya. Statistical analyses were descriptive with adjustment for clustering of data.
Setting
Neonatal units of 22 public hospitals.
Patients
Neonates aged <7 days.
Main outcome measures
Quality of care was assessed in terms of availability of basic resources (principally equipment and drugs) and audit of case records for documentation of patient assessment and treatment at admission.
Results
All hospitals had oxygen, 19/22 had resuscitation and phototherapy equipment, but some key resources were missing—for example kangaroo care was available in 14/22. Out of 1249 records, 56.9% (95% CI 36.2% to 77.6%) had a standard neonatal admission form. A median score of 0 out of 3 for symptoms of severe illness (IQR 0–3) and a median score of 6 out of 8 for signs of severe illness (IQR 4–7) were documented. Maternal HIV status was documented in 674/1249 (54%, 95% CI 41.9% to 66.1%) cases. Drug doses exceeded recommendations by >20% in prescriptions for penicillin (11.6%, 95% CI 3.4% to 32.8%) and gentamicin (18.5%, 95% CI 13.4% to 25%), respectively.
Conclusions
Basic resources are generally available, but there are deficiencies in key areas. Poor documentation limits the use of routine data for quality improvement. Significant opportunities exist for improvement in service delivery and adherence to guidelines in hospitals providing professional training.
Keywords: Neonatology, Health services research, Measurement, Evidence Based Medicine, Data Collection
What is already known on this topic.
In Kenya, 60% of infant deaths and 40% of all under-5 deaths occur in the neonatal period.
Clinical training facilities play a key role in effective delivery of essential neonatal interventions at a national scale.
A previous study on a small number of Kenyan public hospitals suggested significant problems in provision of neonatal services.
What this study adds.
These are the most comprehensive data until now on routine neonatal care in a low-income African country.
There is some improvement in the availability of basic resources and routine clinical practices.
Errors in prescribing and provision of supportive care and poor data remain a challenge undermining practice and health service monitoring.
Background
It is estimated that between 1990 and 2009, 79 million neonatal deaths occurred worldwide. Over 98% of these occurred in low-income and middle-income countries.1 In 2009, there were 42 013 neonatal deaths in Kenya,1 and as child mortality falls, the proportion of under-5 mortality due to neonatal deaths is rising. The most recent estimates indicate that 60% of infant deaths and 40% of all under-5 deaths occurred in the neonatal period,2 with this high neonatal mortality being a major reason why Kenya is not on track to achieve its fourth Millennium Development Goal target. The Ministry of Health has, therefore, started to prioritise interventions and investments to promote newborn (and maternal) health,3 basing its strategy on the essential newborn care package, including a number of low-cost, high-impact interventions.4 5
In Kenya, health workers receive limited preservice instruction on neonatal care in their basic training, gaining most practical experience during clinical placements or internship in hospitals recognised as ‘internship training centres’. The knowledge and skills gained in such centres will likely therefore determine whether essential neonatal interventions are effectively delivered at a national scale. Unfortunately, a previous study on a small number of Kenyan public hospitals suggested significant problems in provision of neonatal services.6 7 Therefore, in a partnership with Kenya's Ministry of Health, an assessment of neonatal care services provided by internship training centres was undertaken to identify areas requiring improvement as part of wider efforts to improve newborn and child survival.
Methods
Indicators
Indicators were based on prior work identifying national and international priorities8 and adapted from previous studies in Kenyan hospitals.6 7 They focused on the Donabedian domains of structure (resources) and process.6 7 9 For structure, we checked for availability of core resources (see table 1) and if a recommended standard admission record form (newborn admission record, NAR) was in use. For process, we audited documentation of three key symptoms and eight key clinical signs of severe illness that are prioritised nationally,10 as they are associated with requirement for hospitalisation or referral.11 Dosages of prescribed antibiotics (as recorded on the treatment sheets) at admission were compared against those recommended in national guidelines. A margin of error of 20% above (overdose) and below (underdose) recommendations was allowed. Prescriptions of intravenous fluids and feeds were assessed in the same manner. Evidence of monitoring of vital signs, weight and fluids was defined as the presence of a chart(s) in which these were recorded at intervals. Mortality was the main outcome assessed.
Table 1.
Availability of essential newborn care resources
| Resources (n=22 hospitals) | Present n (%) |
|---|---|
| Ward organisation | |
| Most seriously ill babies are cared for in a section near nursing station | 18 (82) |
| Isolation area in neonatal unit* | 11 (50) |
| Hand hygiene | |
| Sink, clean running water and soap† | 18 (82) |
| Alcohol hand rub | 10 (46) |
| Emergency care | |
| Defined area for emergencies | 13 (59) |
| Suction equipment working (n=20)‡ | 19 (95) |
| Bag valve mask set working (n=20)‡ | 19 (95) |
| Oxygen from any source available and working | 22 (100) |
| Working pulse oximeter | 4 (18) |
| Routine care | |
| Vitamin K (n=21)§ | 18 (86) |
| All babies adequately warmed | 22 (100) |
| Special care/sick babies | |
| Working¶ phototherapy equipment (n=21)§ | 19 (91) |
| Benzylpenicillin | 22 (100) |
| Gentamicin | 18 (82) |
| Phenorbabitone injection | 17 (77) |
| Kangaroo Mother Care (in any form) | 14 (64)** |
| Paediatric burettes | 12 (55) |
| Laboratory tests | |
| Blood glucose | 22 (100) |
| Full haemogram | 22 (100) |
| Bilirubin | 19 (86) |
| Blood culture | 10 (45) |
Resource availability was assessed by direct observation (including checking drug stocks) by the researcher in the neonatal unit rather than by interviewing staff.
*Any of the following: designated isolation cot/incubator or a separate isolation room for separating sick (infected) babies from healthy ones.
†All three available.
‡These equipment (working or not) were available in 20/22 hospitals.
§Data missing for one hospital.
¶Working means the lights would turn on; irradiance was not measured.
**5/14 had a designated space for providing kangaroo care.
Study design and population
This was a cluster survey of public hospitals providing internship training and the population of interest was admitted neonates aged <7 days.
Sample size and sampling
At the time of survey, 40 public hospitals were recognised as providing internship training. The hospitals included were purposefully selected by the Ministry of Health to complement additional evaluation exercises, to ensure reasonable regional representation and with a sample size fixed at 22 sites because of budgetary constraints. Process of care was assessed by examining case records of admissions to the hospital area designated as the ‘newborn unit’. The number of cases per hospital was calculated to enable reporting of an observed proportion of 50% correct care across all hospitals with a precision (95% CI) of ±5%. To achieve this, assuming a coefficient of variation of 0.2 to account for clustering,12 we aimed to retrieve 60 case records per facility. Records were identified from the register of admissions starting from 31 May 2012 and going back through the register until 60 records were retrieved ensuring selection of records of those who had already been discharged or died.
Data collection
Data collection was done over 4 weeks in July 2012. Survey staff comprised 22 Ministry of Health employees (nurses, records officers or clinical officers) with one drawn from each selected hospital. Staff underwent 1 week of training that included a pilot survey in a non-study hospital. Staff were subsequently divided into five teams (4–5 per team) that each visited 4–5 hospitals for 3–4 days.
Resource availability was assessed by walking through the neonatal units using a standard checklist.13 Availability was classified as universal (available in all 22 hospitals), mostly available (17–21), moderate (11–16) and low availability (0–10). Process of care and outcome data were entered directly into laptops using a data capture tool specifically designed for the survey in REDCap (Research Electronic Data Capture, a secure web-based application designed for research studies).14 A handbook of standard operating procedures was used in training and by all teams to guide all data entry.
Data were examined for errors in real time (in REDCap) and at the end of each day using STATA V.12 check files. Corrections were made by referring back to the source document under the supervision of the team leader. The clean data files from all sites were then uploaded into a central server.
Statistical analysis
Availability of resources is presented as frequencies (and percentages) across the 22 hospitals. For process indicators, results are descriptive and where proportions were computed, the 95% CIs are adjusted for clustering at hospital level. Summary scores of symptoms and signs were constructed by allocating a score of 0 or 1 to each symptom or sign documented and summing these scores for each case record. We computed a median score and IQR for each hospital. To summarise across hospitals, the median of these median scores with the range across the 22 hospitals is reported. Outcomes are presented as mortality by birth weight.
Results
Hospital characteristics
We surveyed 22 hospitals of which 10 were administratively recognised as high-volume hospitals. The median number of deliveries per hospital in the month prior to the survey was 292 but ranged from 112 to 747. All hospitals had a neonatal unit and on the day of the survey, the median number of neonates in the units was 11 (range 1–47). Sixteen hospitals had a single paediatrician, six had two.
Essential newborn care resources
At least one working source of oxygen was universally available (table 1). Bag valve masks, sinks, clean running water and soap were mostly available but paediatric intravenous fluid giving sets and a defined area for providing emergency care were moderately available. There was only low availability of alcohol hand rub or an area designated for kangaroo care. Stratification of resource availability by hospital category (normal/high volume) did not suggest any major differences (data not shown) except for the ability to undertake blood culture; available in 8/10 high-volume hospitals and 2/12 low-volume hospitals.
Patient characteristics
A total of 1249 case records were examined (table 2). The most well-documented characteristic was mode of delivery with only 7% (82/1249) missing data, while the least documented was gestation by dates; 45% (561/1249) missing.
Table 2.
Patient characteristics at admission of those included in process of care evaluation
| Characteristics | Pooled data | Hospital-specific estimates | |||
|---|---|---|---|---|---|
| n | % | 95% CI* | Median % | Range % | |
| Sex (n=1088/1249, 87%)† | |||||
| Female | 484 | 45 | 40 to 49 | 45 | 30–64 |
| Male | 604 | 56 | 51 to 60 | 55 | 36–70 |
| Birth weight (n=1165/1249, 93%)† | |||||
| ELBW (<1000 g) | 17 | 1.5 | 0.8 to 2.3 | 0.8 | 0–7.1 |
| VLBW (1000–<1500 g) | 118 | 10 | 6.9 to 15 | 7.1 | 1.7–46 |
| LBW (1500–<2500 g) | 370 | 32 | 29 to 35 | 33 | 19–46 |
| Normal (2500–<4000 g) | 607 | 52 | 48 to 57 | 54 | 21–68 |
| LGA (≥4000 g) | 53 | 4.6 | 3.3 to 63 | 3.6 | 0–13 |
| Documented gestation by dates (n=688/1249, 55%)† | |||||
| Preterm | 339 | 49 | 43 to 56 | 49 | 30–100 |
| Term | 344 | 50 | 44 to 56 | 50 | 0–68 |
| Postdates | 5 | 0.7 | 0.3 to 1.9 | 0 | 0–4 |
| Mode of delivery (n=1167/1249, 93%)† | |||||
| SVD | 767 | 66 | 60 to 71 | 66 | 42–96 |
| Assisted vaginal | 2 | 0.2 | 0.0004 to 0.7 | 0 | 0–1.8 |
| Breech | 26 | 2.2 | 1.2 to 4.3 | 0.8 | 0–11 |
| Caesarean | 372 | 32 | 27 to 38 | 32 | 3.9–51 |
| Born before arrival (n=1055/1249, 84%)† | 158 | 15 | 11 to 20 | 13 | 2.2–58 |
*Adjusted for clustering at hospital level.†n refers to the numerator equal to number of cases with data out of the total 1249 cases; the value for n becomes the item-specific denominator for each section.
ELBW, extremely low birth weight; LBW, low birth weight; LGA, large for gestational age; VLBW, very low birth weight; SVD, spontaneous vaginal delivery.
Available data showed 55% were male (604/1088, 95% CI 51% to 60%), 52% (607/1165, 95% CI 48% to 57%) had normal birth weight (2500–<4000 g) while one-third (32%, 370/1165, 95% CI 29% to 35%) were low birth weight (1500–<2500 g). There were similar numbers of preterm babies (<37 weeks gestation) 49% (339/688, 95% CI 43% to 56%) and term babies 50% (344/688, 95% CI 44% to 56%). Overall, most neonatal admissions followed spontaneous vaginal delivery, that is, 66% (767/1167, 95% CI 60% to 71%), but in individual hospitals caesarean section was as high as 51%.
Three conditions accounted for the majority of disease episodes at admission: birth asphyxia 36% (446/1249, 95% CI 27% to 44%), prematurity/low birth weight 32% 396/1249, (95% CI 27% to 37%) and neonatal sepsis 19% (238/1249, 95% CI 14% to 25%). There were considerable overlaps in these three diagnoses (figure 1). Congenital anomalies were uncommon 8% (98/1249, 95% CI 2% to 14%), while the least commonly documented were meningitis, meconium aspiration and jaundice together accounting for <5%.
Figure 1.
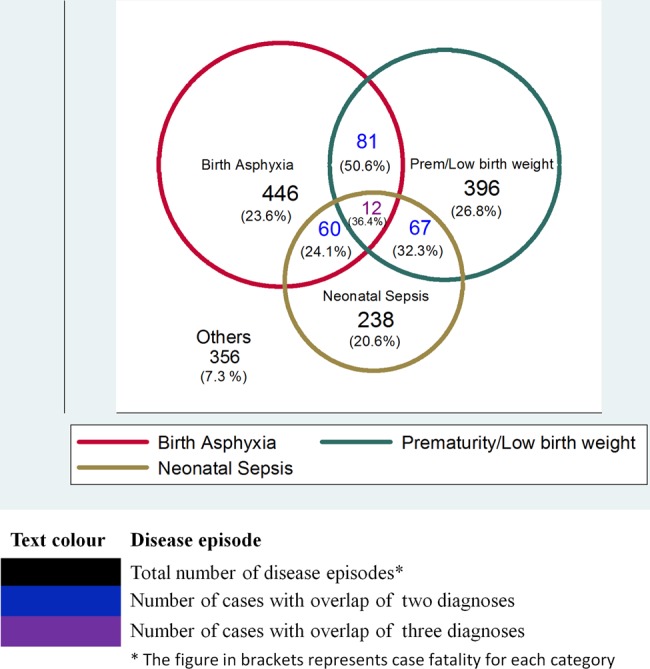
Top three disease episodes.
Assessment
Overall, 57% (711/1249, 95% CI 36.2% to 77.6%) of the case files had an NAR form, but across hospitals ranged from 0% to 100% (table 3). Symptoms of severe illness were poorly documented with a median score of 0/3 overall (IQR 0–3), but six hospitals achieved a score of 3/3 (table 3) and all of them had the NAR in use. Signs of severe illness were better documented with an overall median of 6/8 signs documented (IQR 4–7). HIV exposure status was documented in only 54% (674/1249, 95% CI 41.9% to 66.1%). Among these, 12% of mothers were HIV positive (82/674, 95% CI 7.5% to 19.6%) but only two-thirds of their babies (54/82, 65.9%, 95% CI 46.9% to 80.8%) had a prescription for antiretroviral drugs in the case notes.
Table 3.
Assessment, treatment and monitoring
| Pooled estimates | Hospital-specific estimates | ||||
|---|---|---|---|---|---|
| Indicator | n | % | Cluster adjusted 95% CI |
Median %* | Range %† |
| 1. NAR used (n=1249) | 711 | 57 | 36 to 78 | 75 | 0–100 |
| 2. Symptoms and signs | |||||
| Symptom score (maximum 3)‡ | 0 | 0–3§ | – | 0 | 0–3 |
| Sign score (maximum 8)¶ | 6 | 4–7§ | – | 5.8 | 0–8 |
| 3. Maternal HIV status | |||||
| Status documented | 674 | 54 | 42 to 66 | 64 | 0–93 |
| Positive | 83/674 | 12 | 8 to 20 | 9.1 | 0–56 |
| ARV for PMTCT prescribed for baby | 54/82 | 66 | 47 to 81 | – | – |
| 4. Antibiotic prescription | |||||
| Benzylpenicillin dosage (n=778) | |||||
| Appropriate | 649 | 83 | 66 to 93 | 93 | 0–100 |
| Overdose | 90 | 12 | 3.4 to 33 | 4.2 | 0–100** |
| Gentamicin dosage (n=761) | |||||
| Appropriate | 473 | 62 | 51 to 73 | 67 | 7.7–91 |
| Overdose | 141 | 19 | 13 to 25 | 20 | 1.9–67 |
| 5. Supportive care | |||||
| Vitamin K prescribed (n=1213) | 843 | 70 | 57 to 80 | 73 | 10–100 |
| Appropriate dose of intravenous fluids (n=473)†† | 290 | 61 | 52 to 71 | 57 | 0–90 |
| Appropriate amount of feeds (n=109)†† | 55 | 51 | 26 to 75 | 14 | 0–100 |
| 6. Medical review and monitoring | |||||
| Reviewed >24 h after admission (n=922) | 20 | 2.2 | 1 to 4.8 | 0.8 | 0–21 |
| Vital signs charted (n=1248) | 970 | 78 | 68 to 87 | 85 | 8.3–100 |
| Weight charted (n=1249) | 510 | 41 | 25 to 56 | 39 | 0–100 |
| Fluids monitored (n=559)‡‡ | 193 | 35 | 19 to 55 | 19 | 0–100 |
*Median of individual hospital median scores.
†Range of individual hospital median scores.
‡Difficulty feeding, convulsions and fits.
§IQR.
¶Temperature, bulging fontanelle, suck reflex/ability to feed, muscle tone, respiratory rate, severe indrawing, grunting and cyanosis.
**In the facility with 100% overdose, all penicillin doses were double the recommended.
††Treatment sheet with either intravenous fluids or supplementary feeds only for the first 24 h of life.
‡‡Five hundred and fifty-nine neonates had a fluid prescription on any day of life (473 of these on the first day).
ARV, antiretroviral; NAR, newborn admission record; PMTCT, preventing mother-to-child transmission.
Treatment
About one in 10 of benzylpenicillin prescriptions was overdose (11.6%, 90/778, 95% CI 3.4% to 32.8%) (table 3) in contrast to almost one in five (18.5%, 141/761, 95% CI 13.4% to 25%) for gentamicin. Out of these 141 gentamicin overdose prescriptions, 87 (61.7%, 95% CI 50% to 72.4%) were 50% greater than the recommended dose. Birth weight was documented in 78 out of these 87 and the majority (68/78, 87.2%) were low birth weight (<2500 g).
Supportive care, medical review and monitoring
From the pooled data, 70% (843/1213, 95% CI 56.9% to 79.7%) had Vitamin K prescribed (table 3) with two hospitals having 100% prescription rates. For those on intravenous fluids only on the first day of life, the pooled estimate had 61.3% (290/473, 95% CI 51.7% to 70.8%) with an appropriate volume. For those on feeds only, about one-half of the pooled estimates (51%, 95% CI 26% to 75%) had an appropriate volume. Time of first clinician doctors or paramedics known as clinical officers review after admission could be determined in 74% (922/1249), out of these 42% (383/922, 95% CI 27% to 58%) were seen within 6 h and 2% (20/922, 95 CI 1% to 5%) had no documented clinician review within the first 24 h. In general monitoring of weight, vital signs and fluids were poorly documented (table 3).
Outcomes
Outcome by birth weight data was missing in 15% (184/1249) of records but where available the overall crude mortality was 17% (180/1065, 95% CI 11% to 24%) (table 4).The largest absolute number of deaths was among the normal birth weight (n=62). However, the highest case fatality was in the extremely low birthweight category (<1000 g) at 88% (14/16, 95% CI 58% to 97%). At individual hospital level, the highest mortality rate for newborn unit admissions within the sample of 60 cases was 46%. Of deaths, 61% (101/166, 95% CI 50% to 80%) occurred within the first 24 h after admission (table 4).
Table 4.
Mortality by birth weight; extremely low birth weight (ELBW, <1000 g), very low birth weight (VLBW, 1000–<1500 g), low birth weight (LBW, 1500–<2500 g), normal (2500–<4000 g) and large for gestational age (LGA, ≥4000 g)
| Mortality | Time to death Time to death in days* | ||||
|---|---|---|---|---|---|
| Birth weight | Pooled estimates | Hospital-specific estimates | |||
| n (%) | 95% CI | Median (%) | Range (%) | Median (range) | |
| ELBW (n=16) | 14 (88) | 58 to 97 | 100 | 0–100 | 1 (<1–54) |
| VLBW (n=100) | 51 (51) | 32 to 70 | 33 | 0–100 | 1 (<1–15) |
| LBW (n=340) | 49 (14) | 9 to 22 | 13 | 0–47 | 1 (<1–93) |
| Normal (n=559) | 62 (11) | 6.5 to 18 | 7.7 | 0–50 | 1 (<1–14) |
| LGA (n=50) | 4 (8) | 3.6 to 17 | 0 | 0–33 | 1.5 (<1–20) |
| Total (n=1065)† | 180 (17) | 11 to 24 | 16.4 | 0–46‡ | 1 (<1–93) |
*Time to death from admission.
†In 15% of cases (184/1249), it was not possible to determine the outcome by birth weight (either due to missing outcome or birth weight data).
‡Four hospitals recorded no mortality, possibly representing a ‘retrieval’ bias in obtaining mortality files.
Discussion
As neonatal mortality declines below 30/1000 (Kenya currently 31/1000), interventions delivered at facility level become increasingly important to achieve further declines.15 Each of the hospitals surveyed had a specific neonatal unit. They varied in size having between 2 and 15 working incubators and from 0 to 46 cots. Six hospitals were not able to allocate even one nurse specifically to their newborn unit.
All newborn units had at least one working source of oxygen and almost all were able to provide basic equipment for resuscitation and phototherapy. Key resources were missing in some hospitals, for instance, alcohol hand rub, bag valve mask sets and Kangaroo Mother Care (KMC). Although KMC is recommended for stable babies in national guidelines as it may reduce mortality and risk of sepsis and hypothermia,16 its implementation requires significant resources, including staff time. These resources are often not available likely explaining the challenges hospitals face in translating this policy into practice.
Resource limitations undermine the provision of basic neonatal care although there is improvement compared with a previous local survey.7 In that survey, half the hospitals did not offer phototherapy and less than half had phenorbabitone injection.7 Similar concerns have also been noted in Tanzania and Ghana,17 18 Central Asia and Eastern Europe19 and Bangladesh.20 However, the specific nature of resource challenges differs across place underscoring the need for local knowledge such as from this survey to help plan improvement efforts.
Patient records provide a means to document and communicate information about patients and their care.21 They are also a vital source of data on workload, morbidity and mortality. Previous works on quality of neonatal care in Kenya and other countries have been severely limited by poor availability of records.6 17 We retrieved a total of 1249 records but in some sites faced difficulties identifying admission records. Indeed, in four hospitals, no records of deaths were found raising the possibility that our results are affected by a form of response (or retrieval) bias. Such missing data undermine accurate reporting of patient characteristics and outcomes at scale. Despite this, our data remain the best current description of quality of routine neonatal service delivery for a country with over 1.25 million births per year.
Of concern is that almost 20% of gentamicin prescriptions were for an overdose and most were in preterm/low birthweight newborns with doses <50% above that recommended. Gentamicin is potentially ototoxic and nephrotoxic,22 23 and drug level monitoring is not possible in any of the hospitals surveyed. Considerable variation in errors across hospitals is of great interest; for instance, the range of penicillin and gentamicin overdoses was 0%–100% and 1.9%–66.7%, respectively. Although standard guidelines are now more widely available in Kenya than previously,10 guidelines by themselves are insufficient to change practice. Thus, although there is evidence that neonatal prescribing is improving,6 9 additional interventions that include regular assessment of quality of care may be required to promote good practices among early career clinicians at scale.12
The data available on outcomes are limited by missing data on birth weight, sex and particularly gestation. Available data suggest that many deaths occur early in admission and indicate very high case fatality in the extremely low birthweight babies; this may be a reflection of the lack of resources for more advanced care. However, the largest number of deaths occurred in normal weight births, suggesting significant opportunities to improve outcomes through improved basic perinatal and neonatal care. Better routine data in future may allow for analysis of the effect of quality of care on outcomes.
Limitations
These results may not be representative of all hospital care for neonates in Kenya. Data are cross-sectional, from internship centres and based on observation of resources and record retrieval and review. Clinical practice in these hospitals is supervised by a paediatrician and thus may overestimate quality of care if results are generalised to the many hospitals with no paediatrician. In addition, we focused only on aspects of quality of care directly linked to implementation of guidelines. However, these are the most comprehensive data until now on routine neonatal care in a low-income African country.
Conclusion
This audit shows improvement in the availability of basic resources and routine clinical practices. However, errors in prescribing and provision of supportive care and poor data remain a challenge undermining practice and health service monitoring. These data may indicate that such problems are more widespread in the region and we argue for specific efforts to promote quality care and monitor service delivery at scale as part of efforts to reduce newborn mortality.
Acknowledgments
We thank the director of medical services in the Ministry of Health who gave permission for conducting the study in the government hospitals, the medical superintendents of the hospitals for providing access and all the research assistants and hospital staff who were essential to data collection. This work is also published with the permission of the director of KEMRI.
Footnotes
Collaborators: The SIRCLE/Ministry of Health Hospital Survey Group included: Koigi Kamau; Francis Kimani; John Masasabi; Wycliffe Mogoa; Simon Mueke; Stephen B. Mwinga; Elesban Kihuba; Arnold Njagi; Isaac Odongo; and Jim Todd. All these authors contributed to the design of the survey and data collection tools. Data collection was led by JA, DG, EK, RK and SBM. All authors reviewed the draft manuscript and approved the final manuscript.
Contributors: The roles of the contributors were as follows: JA, AW, RN, FW and ME conceptualised the study. JA took primary responsibility for and conducted the analyses and drafted the initial manuscript with support from ME.
Funding: JA's contribution to this work was made possible by the Kenyan Ministry of Health and a grant from the Consortium for National Health Research (Kenya) to the SIRCLE Collaboration. ME has been supported by funds from The Wellcome Trust (#076827 and #097170). Additional funds from a Wellcome Trust Strategic Award (#084538) and a Wellcome Trust core grant awarded to the KEMRI-Wellcome Trust Research Programme (#092654) made this work possible. These grants supplemented salary support from the Ministry of Health (Kenya, to RN) and the University of Nairobi (to AW and FW). The Wellcome Trust and other funders had no role in developing this manuscript nor in the decision to submit for publication.
Competing interests: None.
Ethics approval: Scientific and ethical approval was obtained from the KEMRI ethics review committee. The Ministry of Health provided permission to conduct the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Collaborators: Koigi Kamau, Francis Kimani, John Masasabi, Wycliffe Mogoa, Simon Mueke, Stephen B. Mwinga, Elesban Kihuba, Arnold Njagi, Isaac Odongo, and Jim Todd
References
- 1.Oestergaard M, Inoue M, Yoshida S, et al. Neonatal mortality levels for 193 countries in 2009 with trends since 1990: a systematic analysis of progress, projections, and priorities. PLoS Med 2011;8:e1001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kenya National Bureau of Statistics (KNBS) and ICF Macro. Infant and child mortality. In: Kenya Demographic and Health Survey 2008–09. http://dhsprogram.com/pubs/pdf/FR229/FR229.pdf. [Google Scholar]
- 3.Ministry of Health, Government of Kenya. The Kenya Health Sector Strategic and Investment Plan-KHSSP July 2012-June 2017 2013.
- 4.WHO. Pregnancy, childbirth, postpartum and newborn care: a guide for essential practice. 2009. [PubMed]
- 5.The Partnership for Maternal, Newborn & Child Health. A Global Review of the Key Interventions Related to Reproductive, Maternal, Newborn and Child Health (RMNCH) 2011.
- 6.Gathara D, Opiyo N, Wagai J, et al. Quality of hospital care for sick newborns and severely malnourished children in Kenya: a two-year descriptive study in 8 hospitals. BMC Health Serv Res 2011;11:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Opondo C, Ntoburi S, Wagai J, et al. Are hospitals prepared to support newborn survival?—an evaluation of eight first-referral level hospitals in Kenya. Tropical Med Int Health 2009;14:1165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ntoburi S, Hutchings A, Sanderson C, et al. Development of paediatric quality of inpatient care indicators for low-income countries—a Delphi study. BMC Pediatr 2010;10:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.English M, Esamai F, Wasunna A, et al. Assessment of inpatient paediatric care in first referral level hospitals in 13 districts in Kenya. Lancet 2004;363:1948–53. [DOI] [PubMed] [Google Scholar]
- 10.Ministry of Medical Services, Government of Kenya. Basic Paediatric Protocols-Revised 2010.
- 11.Opiyo N, English M. What clinical signs best identify severe illness in young infants aged 0–59 days in developing countries? A systematic review. Arch Dis Child 2011;96:1052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayieko P, Ntoburi S, Wagai J, et al. A multifaceted intervention to implement guidelines and improve admission paediatric care in Kenyan district hospitals: a cluster randomized trial. PLoS Med 2011:8:e1001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.English M, Ntoburi S, Wagai J, et al. An intervention to improve paediatric and newborn care in Kenyan district hospitals: understanding the context. Implement Sci 2009;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milner KM, Duke T, Bucens I. Reducing newborn mortality in the Asia–Pacific region: quality hospital services and community-based care. J Paediatr Child Health 2013;49:511–18. [DOI] [PubMed] [Google Scholar]
- 16.Conde-Agudelo A, Belizán J, Diaz-Rossello J. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev 2011;16(3):CD002771. [DOI] [PubMed] [Google Scholar]
- 17.Mbwele B, Reddy E, Reyburn H. A rapid assessment of the quality of neonatal healthcare in Kilimanjaro region, northeast Tanzania. BMC Pediatr 2012;12:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vesel L, Manu A, Lohela TJ, et al. Quality of newborn care: a health facility assessment in rural Ghana using survey, vignette and surveillance data. BMJ Open 2013;3:e002326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamburlini G, Siupsinskas G, Bacci A, et al. Quality of maternal and neonatal care in Albania, Turkmenistan and Kazakhstan: a systematic, standard-based, participatory assessment. PLoS ONE 2011;6:e28763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoque D, Rahman M, Billah S, et al. An assessment of the quality of care for children in eighteen randomly selected district and sub-district hospitals in Bangladesh. BMC Pediatr 2012;12:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann R, Williams J. Standards in medical record keeping. Clin Med 2003;3:329–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao SC, Srinivasjois R, Hagan R, et al. One dose per day compared to multiple doses per day of gentamicin for treatment of suspected or proven sepsis in neonates. Cochrane Database Syst Rev 2011;(11):CD005091. [DOI] [PubMed] [Google Scholar]
- 23.Krishnamoorthy S, Nair A, Furness J, et al. Gentamicin use in neonates: should we have a change of practice? Scott Med J 2013;58:241–5. [DOI] [PubMed] [Google Scholar]


