DELAYING PROGRESSION OF CKD
The following section describes key recommendations and guidance for people with CKD with respect to delaying progression of CKD. General lifestyle recommendations are provided as well as caveats given for those with diabetes. Cardiovascular risk reduction including management of hypertension, dyslipidemia, and hyperuricemia is further addressed. Unless otherwise stated, the guidance is intended to apply to adults with CKD.
For the practicing clinician, ideally working with a team of health-care professionals, it is important to institute general lifestyle modification practices in people with CKD so that they may gain the benefit of these in addition to more kidney-specific strategies. Often these general measures are overlooked or disregarded in people with CKD, thus their utility is underscored here.
3.1 PREVENTION OF CKD PROGRESSION
The management of progression of CKD is aimed at addressing a multiplicity of factors known to be associated with progression. There are general measures which have been shown to address cardiovascular health and CKD together, or each separately. Addressing CVD risk factors may indirectly and directly impact CKD progression. Strategies include general lifestyle measures which improve cardiovascular health, BP control, and interruption of the RAAS. In addition, control of other metabolic parameters such as blood sugar, uric acid, acidosis, and dyslipidemia may also be important. This section deals with management of BP, RAAS interruption, glycemic control and dietary/lifestyle manipulations which have been examined in the context of delaying progression of CKD.
BP and RAAS interruption
The following statements are excerpted and where necessary, condensed, from the KDIGO Clinical Practice Guideline for the Management of Blood Pressure in CKD.10
3.1.1: Individualize BP targets and agents according to age, coexistent cardiovascular disease and other comorbidities, risk of progression of CKD, presence or absence of retinopathy (in CKD patients with diabetes), and tolerance of treatment as described in the KDIGO 2012 Blood Pressure Guideline. (Not Graded)
3.1.2: Inquire about postural dizziness and check for postural hypotension regularly when treating CKD patients with BP-lowering drugs. (Not Graded)
3.1.3: Tailor BP treatment regimens in elderly patients with CKD by carefully considering age, comorbidities and other therapies, with gradual escalation of treatment and close attention to adverse events related to BP treatment, including electrolyte disorders, acute deterioration in kidney function, orthostatic hypotension and drug side effects. (Not Graded)
3.1.4: We recommend that in both diabetic and non-diabetic adults with CKD and urine albumin excretion <30 mg/24 hours (or equivalent*) whose office BP is consistently >140 mm Hg systolic or >90 mm Hg diastolic be treated with BP-lowering drugs to maintain a BP that is consistently ≤140 mm Hg systolic and ≤90 mm Hg diastolic. (1B)
3.1.5: We suggest that in both diabetic and non-diabetic adults with CKD and with urine albumin excretion of ≥30 mg/24 hours (or equivalent*) whose office BP is consistently >130 mm Hg systolic or >80 mm Hg diastolic be treated with BP-lowering drugs to maintain a BP that is consistently ≤130 mm Hg systolic and ≤80 mm Hg diastolic. (2D)
3.1.6: We suggest that an ARB or ACE-I be used in diabetic adults with CKD and urine albumin excretion 30-300 mg/24 hours (or equivalent*). (2D)
3.1.7: We recommend that an ARB or ACE-I be used in both diabetic and non-diabetic adults with CKD and urine albumin excretion >300 mg/24 hours (or equivalent*). (1B)
3.1.8: There is insufficient evidence to recommend combining an ACE-I with ARBs to prevent progression of CKD. (Not Graded)
3.1.9: We recommend that in children with CKD, BP-lowering treatment is started when BP is consistently above the 90th percentile for age, sex, and height. (1C)
3.1.10: We suggest that in children with CKD (particularly those with proteinuria), BP is lowered to consistently achieve systolic and diastolic readings less than or equal to the 50th percentile for age, sex, and height, unless achieving these targets is limited by signs or symptoms of hypotension. (2D)
3.1.11: We suggest that an ARB or ACE-I be used in children with CKD in whom treatment with BP-lowering drugs is indicated, irrespective of the level of proteinuria. (2D)
These statements are worded to maintain consistency with the KDIGO Clinical Practice Guideline for the Management of Blood Pressure in CKD,10 where the full rationale and evidence behind the statements may be found. In detailing BP targets, we recognize that we have not made recommendations or suggestions concerning lower limits of BP. The risks of overtreatment should be specifically considered when making decisions about BP lowering and this is encapsulated in the first two guideline statements.
CKD and risk of AKI
- 3.1.12: We recommend that all people with CKD are considered to be at increased risk of AKI. (1A)
- 3.1.12.1: In people with CKD, the recommendations detailed in the KDIGO AKI Guideline should be followed for management of those at risk of AKI during intercurrent illness, or when undergoing investigation and procedures that are likely to increase the risk of AKI. (Not Graded)
RATIONALE
Observational data suggest a strong association between pre-existing CKD and AKI. The appreciation that CKD patients may be more susceptible to AKI is the purpose of the above set of statements. However, methodological issues such as how CKD and AKI are defined in clinical studies and the statistical adjustments for non-uniformity of comorbidities among various studies may affect the validity of observed associations. These statements would be applicable in pediatrics, though the data are not available for this specific issue.
Evidence Base
CKD is designated as a risk factor for AKI because of the epidemiological association between the two.263, 264 A number of studies in a variety of settings report an association between pre-existing CKD and AKI.265, 266, 267, 268, 269, 270, 271 CKD is a potent predictor of acute decline in kidney function following exposure to radiocontrast,272 major surgery,273 and other medical conditions.274
Hsu et al.14 compared the pre-hospitalization MDRD GFR of 1764 adult members of the Kaiser Permanente Northern California health-care system who developed dialysis-requiring AKI during hospitalization with 600,820 individuals who did not. Compared with a reference baseline GFR of ≥60 ml/min/1.73 m2, a baseline GFR of 45–59 ml/min/1.73 m2 was associated with an adjusted odds ratio (OR) of in-hospital AKI of 1.66 (95% CI 1.40–1.97). For GFR values of 15–29 ml/min/1.73 m2, the adjusted OR for in-hospital AKI was 20.42 (95% CI 17.40–23.96). The presence of diabetes, hypertension, and proteinuria increased the likelihood of developing in-hospital AKI, with adjusted OR of 1.99 (95% CI 1.78–2.23), 1.55 (95% CI 1.37–1.76) and 2.84 (95% CI 2.52–3.19), respectively. The authors concluded that CKD is the main risk factor for AKI during hospitalization. A contrasting approach by Singh et al. defined AKI as dialysis-requiring acute renal failure.275 Because the clinical decision to dialyze a patient is frequently influenced by a higher overall SCr, presence of hemodialysis access, or consideration of inevitable progression to ESRD, this definition of AKI could bias toward capturing more AKI cases in CKD patients. Moreover, in patients with advanced CKD, the progression of CKD to ESRD may sometimes be difficult to separate from acute-on-chronic renal failure. A cohort study by Lafrance et al. followed a referred CKD population in British Columbia for a median of 19.4 months after achieving a GFR of ≤30 ml/min/1.73 m2. Forty-five percent had at least one episode of AKI.276 In another cohort study of 920,985 adults in Alberta, Canada with at least one outpatient measurement of SCr and proteinuria and not requiring chronic dialysis, risk of admission with AKI increased with heavier proteinuria and reduced GFR.16
International Relevance
The incidence of AKI in CKD populations may be different around the world, or have different etiologies. It is not yet clear what the recovery rates from AKI are in the CKD population, and how these vary around the world dependent on cause and duration of AKI.
Areas of Controversy, Confusion, or Non-consensus
Interpretation of published data examining the influence of pre-existing CKD on the increased likelihood of AKI is potentially confounded by a number of issues. These include the comorbidities associated with CKD, influenced by repeated exposure to various nephrotoxic insults or in-hospital errors,57, 277 or primarily due to the altered physiology in CKD. There are also methodological issues such as how CKD and AKI are defined in clinical studies and the varying statistical adjustments for comorbidities.
A further important issue to clarify is whether pre-existing CKD influences the outcome of AKI. Currently, there is no single biomarker that can differentiate ‘acute' from ‘chronic' kidney disease and help to address this issue. Several large observational and database studies report, surprisingly, lower in-hospital mortality in patients with AKI superimposed on CKD compared with controls.278, 279, 280, 281, 282, 283 Data from the Program to Improve Care in Acute Renal Disease (PICARD) reveal lower in-patient mortality and median length of stay in intensive-care unit (ICU) subjects with acute-on-chronic renal injury compared with non-CKD subjects with AKI, though the post-discharge dialysis rates were higher in subjects with pre-existing CKD.284
Clarification of Issues and Key Points
AKI is relatively common in CKD populations and impacts progression adversely. Clinicans should attempt to minimize avoidable episodes of AKI (see Chapter 4 for more details) as part of a holistic approach to delaying progression.
RESEARCH RECOMMENDATIONS
Prospectively designed clinical studies with a clear and uniform definition of CKD and AKI and adjusted for comorbidities are needed to determine the:
frequency of AKI events in a CKD population;
outcome of AKI in patients with CKD;
importance of proteinuria in addition to low GFR in the risk of AKI.
Protein intake
3.1.13: We suggest lowering protein intake to 0.8 g/kg/day in adults with diabetes (2C) or without diabetes (2B) and GFR <30 ml/min/ 1.73 m2 (GFR categories G4-G5), with appropriate education.
3.1.14: We suggest avoiding high protein intake (>1.3 g/kg/day) in adults with CKD at risk of progression. (2C)
RATIONALE
These statements are worded to reflect the potential benefits and dangers of varying dietary protein intake (DPI) in people with CKD. Excess dietary protein leads to the accumulation of uremic toxins, conversely insufficient protein intake may lead to loss of lean body mass, and malnutrition (the latter more frequent in the elderly). The benefits of dietary protein restriction include reduction of accumulation of metabolic waste products that may suppress the appetite and stimulate muscle protein wasting. The role of dietary protein restriction in slowing progression of CKD is more controversial and advanced CKD is associated with a protein wasting syndrome which is directly correlated with morbidity and mortality. Note that statements about reduction in dietary protein do not apply to pediatric populations given issues related to growth and nutrition.
Evidence Base
A number of systematic reviews and meta-analyses have pooled the available RCT data.285, 286, 287, 288, 289 Pedrini et al.288 compared a low-protein diet (LPD), defined as a DPI of 0.4 to 0.6 g/kg/day, with a usual diet (5 RCTs, N=1413) over a period of follow-up ranging between 18-36 months in people with non-diabetic CKD and GFR <55 ml/min/1.73 m2. Fouque et al.285 updated this analysis to include 8 RCTs in people with non-diabetic CKD (N=1524). DPI in their low-protein group was between 0.3-0.6 g/kg/day and follow-up ranged from 12-24 months (5 of 8 studies were in people with GFR categories G4-G5 (GFR <30 ml/min/1.73 m2). Roberston et al.289 compared diabetic subjects (8 studies in type 1 diabetes, N=322; 1 study in type 2 diabetes, N=263). DPI in the low-protein subjects was 0.3-0.8 g/kg/day and usual protein intake ranged from 1-2 g/kg/day. Mean follow-up ranged from 4.5 months to 4 years. In all studies, compliance with a low DPI was poor. There was no convincing or conclusive evidence that long-term protein restriction delayed the progression of CKD.
The largest RCT to date was the MDRD Study.227 The MDRD Study compared the effects of LPD and BP control on the progression of CKD in over 800 subjects split into 2 groups. Study A compared a DPI of 1.3 g/kg/day (usual protein intake) with 0.58 g/kg/day (LPD) in 585 subjects with a measured GFR of 25-55 ml/min/1.73 m2 and the actual DPIs were 1.11 and 0.73 g/kg/day, respectively. Study B randomized 255 patients with a measured GFR 13-24 ml/min/1.73 m2 to DPIs of 0.58 g/kg/day (LPD) or 0.28 g/kg/day supplemented by keto-aminoacids (denoted by VLPD-KA), actual DPIs were 0.69 and 0.46 g/kg/day, respectively. In each of the randomization groups ACE-Is were allowed and were used by 32-44% of patients. Mean follow-up was 2.2 years and the loss of GFR was estimated by the slope of 125I-iothalamate clearance measured over 2 years. There was no difference in GFR decline between groups in Study A and in Study B. Although there was a somewhat faster decline in GFR in the LPD group compared with the VLPD-KA group, this was not significant.
A follow-up study of the original MDRD Study followed those subjects recruited to Study B between 1989-1993 up until the year 2000. Median duration of follow-up until kidney failure, death, or administrative censoring was 3.2 years and median time to death was 10.6 years.290 The authors concluded that assignment to a very LPD did not delay progression to kidney failure, but appeared to increase the risk of death in the long-term. The chief limitation of this follow-up study was the lack of measurements of DPI and nutritional measurements during the course of the long-term follow-up period and it is therefore not known how many patients continued with the LPD or the VLPD-KA diets after the study concluded.
There is some evidence to suggest that higher protein diets above the recommended daily intake may accelerate renal functional decline in people with early CKD. In a study of 1624 women enrolled in the Nurses′ Health Study, Knight et al. described the effect of protein intake over an 11-year period in women with eGFR ≥80 ml/min/1.73 m2 (normal renal function) at baseline and those with eGFR 55-80 ml/min/1.73 m2.291 DPI was measured twice during the study period at intervals of 4 years using a semiquantitative food-frequency questionnaire that inquired about the average intake of specified foods and beverages during the previous year. In women with normal renal function at baseline high protein intake was not significantly associated with change in eGFR. However in those with eGFR 55-80 ml/min/1.73 m2 at baseline, protein intake was significantly associated with a change in eGFR of −1.69 ml/min per 1.73 m2 (95% CI, −2.93 to −0.45 ml/min per 1.73 m2) per 10 g increase in protein intake. The effect was greatest in those with the highest intake of non-dairy animal protein.
Dietary protein restriction of <0.80 g/kg/day appears to offer no advantage and any dietary protein restriction should include careful monitoring of clinical and biochemical markers of nutritional deficiencies. A high total protein intake, particularly high intake of non-dairy animal protein, may accelerate renal function decline in people with CKD and should be avoided.
International Relevance
Studies on protein restriction have not been widely tested in different ethnicities or within cultures with low baseline proten intake or purely vegetarian diets. Thus, the applicability of statements to all regions of the world is limited.
Implications for Clinical Practice and Public Policy
Clinicians should be aware of different sources of protein, and if lowering of protein is recommended, education and monitoring for malnutrition should be implemented. Appropriate dietary counseling for CKD patients may have health-care resource implications, although as part of a combined strategy to manage obesity, salt intake, and diabetes may be considered cost-effective on a population basis in certain countries. Avoidance of malnutrition is important.
Areas of Controversy, Confusion, or Non-consensus
Data are mixed on the value of protein restriction, the values which are achievable in general populations, and the level of GFR at which they should be instituted. Nonetheless, the Work Group felt that on balance there is enough data to support a reduction in dietary protein in selected individuals. It is important to avoid this advice in those with evidence of or at risk of malnutrition.
Pediatric Considerations
A Cochrane review addresses this issue in children292 by examining two RCTs with a total of 250 children to determine the effect of protein restriction on a number of variables. The RR of progression to ESRD in the low-protein restricted versus normal group was 1.12 (95% CI 0.54-2.33). At two years, progression of kidney disease was not significant as measured by change in CrCl: mean difference 1.47 ml/min/1.73 m2 (95% CI −1.19–4.14) or growth as measured by mean weight difference: −0.13 kg (95% CI −1.10–0.84) or mean height difference: −1.99 cm (95% CI −4.84–0.86). The conclusion of the authors was that a low-protein diet did not delay progression to kidney failure in children, but it may be detrimental to growth.
Glycemic control
Diabetes is the leading cause of CKD worldwide. Diabetic nephropathy occurs in 25–40% of patients with type 1 or type 2 diabetes within 20–25 years of disease onset and is an independent risk factor for early death due to CVD. The mortality rate in people with diabetes and urinary ACR >30 mg/g (>3 mg/mmol) is more than twice that in those with normal urinary albumin levels.
The National Kidney Foundation (NKF) KDOQI Clinical Practice Guideline for Diabetes and CKD293 has been updated in 2012. The first three recommendations below are reproduced verbatim from this guideline.
3.1.15: We recommend a target hemoglobin A1c (HbA1c) of ∼7.0% (53 mmol/mol) to prevent or delay progression of the microvascular complications of diabetes, including diabetic kidney disease. (1A)
3.1.16: We recommend not treating to an HbA1c target of <7.0% (<53 mmol/mol) in patients at risk of hypoglycemia. (1B)
3.1.17: We suggest that target HbA1c be extended above 7.0% (53 mmol/mol) in individuals with comorbidities or limited life expectancy and risk of hypoglycemia. (2C)
3.1.18: In people with CKD and diabetes, glycemic control should be part of a multifactorial intervention strategy addressing blood pressure control and cardiovascular risk, promoting the use of angiotensin-converting enzyme inhibition or angiotensin receptor blockade, statins, and antiplatelet therapy where clinically indicated. (Not Graded)
RATIONALE
These statements are included to reflect the current evidence that achieving a hemoglobin A1c (HbA1c) level of ∼7.0% (53 mmol/mol) is able to prevent the microvascular complications of diabetes, although recognizing that the major risk for patients attaining HbA1c levels <7.0% (<53 mmol/mol) is hypoglycemia, and that this risk will be higher in people with lower levels of kidney function.
Evidence Base
The evidence base for these statements is reviewed in the NKF KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update293 and will not be reiterated in full here. It should be noted that the evidence that intensive glycemic control reduces the microvascular complications of diabetes is based almost exclusively on prevention of development of albuminuria (ACR >30 mg/g or >3 mg/mmol) and prevention of increasing albuminuria. Evidence from the three most recent studies, Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE),294 Action to Control Cardiovascular Risk in Diabetes (ACCORD),295 and the Veterans Affairs Diabetes Trial (VADT),296 is summarized in Table 25.
Table 25. Intensive versus normal glycemic control and albuminuria outcome.
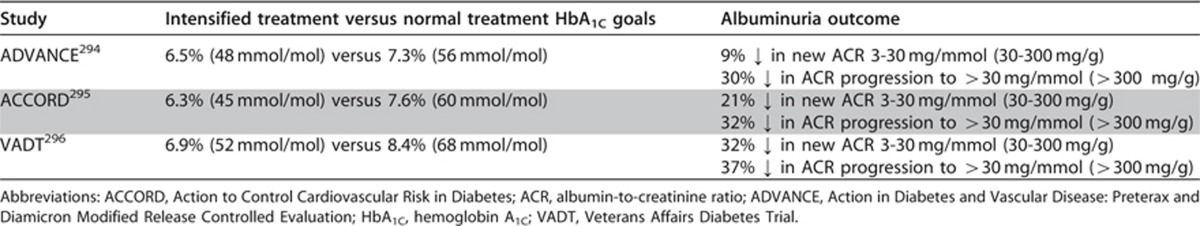
ADVANCE, ACCORD, or VADT did not show significant benefits of more intensive glycemic control on creatinine-based estimates of GFR. However, in the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) follow-up study, 1.4% of participants in the previously intensive treatment group and 3.6% of those in the previously conventional treatment group developed SCr concentrations >2.0 mg/dl (177 μmol/l) (P=0.01) and 0.6% versus 1.9% required kidney replacement therapy (P <0.03).297 For patients with type 2 diabetes, intensive treatment in the United Kingdom Prospective Diabetes Study (UKPDS) was associated with a 67% risk reduction for a doubling of plasma creatinine levels at 9 years (0.71% of the intensive group and 1.76% of the conventional group, P=0.027).298
International Relevance
The incidence and prevalence of diabetes is rising around the world and at rapid rates in developing countries. Glycemic control is therefore one of the most important strategies for delaying progression of CKD, irrespective of region or country. It is recognized that not all hypoglycemic strategies or treatments are available in all countries.
Implications for Clinical Practice and Public Policy
Practitioners should encourage glycemic control in all people with CKD and diabetes, including referral to education sessions and diabetic clinics where available. Public health policies and diabetic strategies should include screening in high-risk populations such as those with diabetes, as the presence of CKD would confer higher risk of adverse events, and could represent an opportunity for intensified intervention with large implications for health-care.
Areas of Controversy, Confusion, or Non-consensus
Recommendations on the use of specific medications for glycemic control (e.g., metformin and glyburide) remain variable depending on different perspectives. These are covered more fully in Chapter 4 on medication dosing and AKI.
Clarification of Issues and Key Points
Glycemic control improves outcomes in people with diabetes, with or without CKD. Those with diabetes and CKD have higher risk of adverse outcomes and therefore presumed higher benefit from control. Many agents are renally excreted and therefore adjustments to doses may be necessary as GFR declines or if patients are acutely unwell (see Chapter 4 on medication dosing).
Caveats in measuring glycemic control with HbA1C
HbA1Cmay not be reflective of glucose control in people with CKD who have reduced red cell life span, and thus should be interpreted with caution. Review of blood sugar daily logs may be more reliable.
Clinicians should be aware that HbA1C measurements that inform glycemic control are based on an assumed red cell life span of 90 days. In people with CKD the RBC life span is shortened, even if receiving erythropoiesis-stimulating agents (ESAs). As such, measurement may only reflect glycemic control over a shorter time period than the presumed 3 months and thus, HbA1C measurements may be falsely low. Awareness of this may alter clinicians' reliance on this measurement as a long-term measure.299, 300, 301, 302, 303, 304 Ongoing research intitiatives comparing HbA1C with glycated albumin using continuous glucose monitoring suggest that glycated albumin may provide a more reliable index of glycemic control in people with advanced CKD.
Pediatric Considerations
It is recommended that the Guidelines of the American Diabetes Association305 or similar national guidelines for diabetes management in children and adolescents, be reviewed to address issues related to management of diabetic children either with, or at risk of, CKD.
Salt intake
- 3.1.19: We recommend lowering salt intake to <90 mmol (<2 g) per day of sodium (corresponding to 5 g of sodium chloride) in adults, unless contraindicated (see rationale). (1C)
- 3.1.19.1: We recommend restriction of sodium intake for children with CKD who have hypertension (systolic and/or diastolic blood pressure >95th percentile) or prehypertension (systolic and/or diastolic blood pressure >90th percentile and <95th percentile), following the age-based Recommended Daily Intake. (1C)
- 3.1.19.2: We recommend supplemental free water and sodium supplements for children with CKD and polyuria to avoid chronic intravascular depletion and to promote optimal growth. (1C)
RATIONALE
In subjects with CKD, impaired excretion of sodium is often present. High sodium intake increases BP and proteinuria, induces glomerular hyperfiltration and blunts the response to RAAS blockade. Lowering salt intake not only reduces BP, but also lowers albuminuria. The importance of salt intake in the general management of CKD patients cannot be overemphasized, hence the need for specific statements here. We appreciate that there are some conditions in which salt restriction may be harmful, and hence the qualifier “unless contraindicated.' These conditions include salt losing nephropathies and those prone to hypotension and volume contraction who do not have heart failure.
Evidence Base
A systematic review of 16 studies addressing salt intake and kidney disease set out to establish whether variations in dietary sodium consumption influence renal outcomes in people with CKD.306 Despite marked heterogeneity, the review suggested that increased salt intake was associated with worsening albuminuria and an increased likelihood of reduction of GFR. Although the quality of the studies included was insufficient to support the authors' hypothesis that increased salt intake is nephrotoxic, the results were strong enough to suggest modest dietary avoidance of salt should be encouraged in people with CKD, especially those with hypertension and/or proteinuria. In a randomized, double-blind, placebo-controlled trial of salt reduction in 40 Afro-Caribbean hypertensives, a salt-restricted diet (approximately 5 g daily) significantly reduced 24-hour urinary protein excretion by 19% and led to a fall in systolic and diastolic BP of 8 mm Hg and 3 mm Hg, respectively.307 The fall in urinary protein excretion correlated with a reduction in urinary sodium excretion, not with BP reduction. Individuals with metabolic syndrome may be especially sensitive to the effects of sodium intake. Hoffman and Cubeddu examined the role of salt intake in increased BP in 109 subjects with metabolic syndrome.308 Salt restriction from an average usual intake of 8.2 g/day to nearly 2.3 g/day reduced the percentage of hypertensive patients from 23.8% to 8.2%. In a six-month prospective controlled trial, 110 patients with GFR categories G4 or G5 (GFR<30 ml/min/1.73 m2) followed a low-sodium (circa 1 g/day) diet as part of either a LPD (0.6 g/kg/day) or very low-protein diet (VLPD) supplemented with essential amino acids (0.35g/kg/day), or a free diet.309 BP fell significantly in the VLPD group from 143±19/84±10 to 128±16/78±7 mm Hg (P<0.0001), despite reduction of antihypertensive drugs. The improved BP correlated with decreased urinary sodium and the authors concluded that the antihypertensive effect was due to reduction of salt intake independent of actual protein intake. Finally, a randomized controlled crossover trial in 52 non-diabetic subjects with CKD compared the effects of a low-sodium diet (target 50 mmol [1.15 g] sodium per day) versus a regular sodium diet (target 200 mmol [4.60 g] sodium per day) on lowering of proteinuria through RAAS blockade.310 The reduction of proteinuria by the addition of a low-sodium diet to angiotensin-converting enzyme inhibition was significantly larger (P<0.001) than the addition of angiotensin receptor blockade to angiotensin-converting enzyme inhibition and similarly the reduction of systolic BP by the addition of a low-sodium diet was significantly larger (P= 0.003) than the addition of angiotensin receptor blockade. The authors concluded that sodium restriction to a level recommended in guidelines was more effective than dual renin-angiotensin blockade for reduction of proteinuria and BP in non-diabetic nephropathy.
International Relevance
Salt intake has been identified as an important driver of high BP in all countries and in adults, thus this recommendation is of international relevance and applicability. The relevance and caveats to children in different situations around the world merits further review.
Implications for Clinical Practice and Public Policy
In most developed countries, a reduction in salt intake can be achieved by a gradual and sustained reduction in the amount of salt added to foods by the food industry. In other countries where most of the salt consumed comes from salt added during cooking or from sauces, a public health campaign is needed to encourage consumers to use less salt. The range of sodium intake associated with improved long-term outcomes is easily achievable and may have the potential to greatly improve health outcomes in patients with CKD around the world.
Areas of Controversy, Confusion, or Non-consensus
While salt restriction is of benefit on a population-basis, and in most people with hypertension and CKD, there may be individual conditions in which salt restriction might be detrimental (e.g., those with salt wasting tubular disorders; those either hypotensive in the absence of heart failure or those prone to volume contraction). Thus while this general statement applies to the majority of people, individualization is required based on clinical circumstance. This may be particularly relevant to children.
Pediatric Considerations
The suggested relative salt restrictions for children with CKD and/or hypertension are age-based with consideration given to both average intakes and upper limit values. The recommended upper limit for sodium intake from which a reduced intake could be derived is provided in Table 26.
Table 26. Recommended Daily Intake of sodium for healthy children.
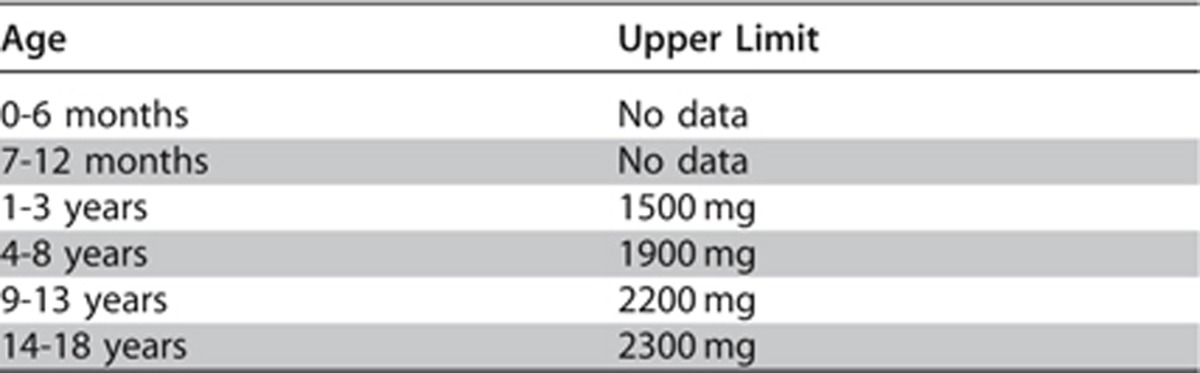
It must also be recognized that children with CKD often have underlying tubular conditions that predispose to numerous electrolyte losses, including sodium. For these children a supplemental rather than restricted sodium intake will be required.
Hyperuricemia
3.1.20: There is insufficient evidence to support or refute the use of agents to lower serum uric acid concentrations in people with CKD and either symptomatic or asymptomatic hyperuricemia in order to delay progression of CKD. (Not Graded)
Hyperuricemia is common in people with CKD and is defined by urate concentrations above 7.0 mg/dl (420 μmol/l) as measured by automated enzymatic (uricase) laboratory methods. Concentrations obtained with colorimetric methods are approximately 1 mg/dl (60 μmol/l) lower. The Work Group believe it important to acknowledge the accumulating body of evidence describing the association of hyperuricemia with CKD and adverse cardiovascular outcomes, and thus list hyperuricemia as a potential contributor to progression. However, at the time of current writing, there is not a reliable body of evidence from which to recommend treatment of hyperuricemia for the specific goal of delaying progression of CKD. Thus, the wording of this ungraded statement is very deliberate.
RATIONALE
Observational data had implicated uric acid in the progression of CKD suggesting that adverse outcomes in people with CKD may be improved by specific uric acid lowering therapy. Small studies using appropriate RCT design have shown reduced left ventricular mass, improved endothelial function, and reduced progression of CKD in people with either symptomatic or asymptomatic hyperuricemia and CKD. Thus, the specific statement is intended to ensure and foster RCTs to properly assess the risk and benefits of uric acid lowering strategies in people with CKD.
Evidence Base
Published data implicate elevated serum uric acid (SUA) concentrations in the progression of CKD.311, 312, 313, 314, 315 Reduction of SUA by allopurinol has been reported to delay progression of CKD in people with both diabetic and nondiabetic CKD.316, 317 Treatment of asymptomatic hyperuricemia has also been reported to improve kidney function even in subjects with normal levels of GFR.318, 319 Both GFR and endothelial function significantly improved in asymptomatic hyperuricemic subjects randomly assigned to 300 mg/day of allopurinol in comparison to placebo.318 A separate double-blind, placebo-controlled, parallel-group study in 67 people with CKD (GFR 30-60 ml/min/1.73 m2) and left ventricular hypertrophy (LVH) randomly assigned subjects to treatment with allopurinol (300 mg/day) or placebo for 9 months.320 In comparison to placebo, the allopurinol-treated subjects had significant reductions in left ventricular mass assessed by magnetic resonance imaging (MRI), and improvements in endothelial function assessed by flow-mediated dilation of the brachial artery and in central arterial stiffness assessed by pulse-wave analysis. Another study randomized 70 subjects with known hyperuricemia or SUA concentrations ≥7.0 mg/dl (≥420 μmol/l) to treatment with either allopurinol monotherapy (100-200 mg/day) or a combination of allopurinol and a citrate preparation (3 g/day).321 SUA concentrations were decreased in both groups but to a significantly lower level by combination treatment. GFR assessed by CrCl increased in the combination therapy group but remained unchanged in those treated with allopurinol alone. Other uric acid lowering agents have also been reported to improve outcomes in people with CKD. In an 8-week, placebo-controlled group comparison of rasburicase and placebo, a single 4.5 mg dose of rasburicase significantly lowered SUA and resulted in a significant improvement in kidney function assessed by CrCl.322 In a post hoc analysis of 1342 patients with type 2 diabetes mellitus and nephropathy participating in the Reduction of Endpoints in Non-Insulin-Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan (RENAAL) trial, Miao et al. examined the relationship between change in SUA concentration after 6 months of treatment with losartan and doubling of SCr or ESRD.323 Baseline SUA was 6.7 mg/dl (400 μmol/l) in placebo and losartan-treated subjects. During the first 6 months, losartan lowered SUA by 0.16 mg/dl (9.5 μmol/l) [95% CI 0.30-0.01; P=0.031] as compared with placebo. The risk of doubling of SCr or ESRD was decreased by 6% (95% CI 10%-3%) per 0.5-mg/dl (30-μmol/l) decrement in SUA during the first 6 months. This effect was independent of other risk markers, including albuminuria.
International Relevance
There are no data to support or refute the importance of hyperuricemia in different geographical reasons or ethnic groups. Further study is needed.
Implications for Clinical Practice and Public Policy
There is insufficient evidence to recommend the use of uric acid lowering agents in asymptomatic individuals for the specific purpose of delaying CKD progression. Further large trials are required to better understand the potential benefit of uric acid lowering for this purpose.
Lifestyle
3.1.21: We recommend that people with CKD be encouraged to undertake physical activity compatible with cardiovascular health and tolerance (aiming for at least 30 minutes 5 times per week), achieve a healthy weight (BMI 20 to 25, according to country specific demographics), and stop smoking. (1D)
RATIONALE
People with CKD have self-reported reduced physical functioning and are not as aerobically fit as the general population. Frailty, impaired physical performance, disability, and geriatric syndromes are common among older adults even with mild kidney disease. Reduced physical functioning and inactivity are associated with increased mortality and poor QOL. Obesity is associated with increased morbidity, mortality, and reduction in life expectancy and leads to an increase in the incidence of diabetes, hypertension, and dyslipidemia. Associations between smoking and CKD suggest that smoking increases the risk of kidney failure and that smoking cessation decreases that risk. Thus, this statement reflects the importance of ensuring lifestyle recommendations.
Evidence Base
CKD patients have a reduced exercise capacity and impaired physical functioning.324, 325, 326 Moreover, reduced physical activity is associated with increased mortality and poor QOL in people with CKD.327, 328, 329 Regular exercise leads to increased exercise capacity, decreased morbidity, and improved health-related QOL.330, 331, 332 Exercise may reduce cardiovascular risk through its beneficial effects on BP, triglycerides, high-density lipoprotein cholesterol (HDL-C), insulin resistance, and glycemic control. In ESRD, exercise has been shown to improve arterial stiffness, BP, cardiorespiratory function, and QOL.333, 334, 335, 336, 337, 338, 339 Less data are available on the beneficial effects of exercise on early CKD. However, as cardiovascular risk gradually increases with both a lower GFR and a higher ACR, it is expected that exercise will also help to prevent progressive CVD in less severe CKD. Indeed, in subjects with GFR categories G3a-G4 (GFR 15–59 ml/min/1.73 m2), long-term exercise training improved physical impairment, arterial stiffness, and health-related QOL.340 It has therefore been argued that exercise training is imperative in CKD patients341 and that support programs including self-monitoring, verbal reinforcement, and motivation should be applied342, 343, 344 in an attempt to prevent the high cardiovascular risk in CKD. A prospective study compared the benefits of 6 months of regular walking in 40 predialysis patients with GFR categories G4-G5 (GFR<30 ml/min/1.73 m2) (20 in an exercising group and 20 patients who continued with usual physical activity for comparison). Improvements noted after 1 month were sustained to 6 months in the 18 of 20 who completed the exercise study. These included improvements in exercise tolerance (reduced exertion to achieve the same activity), weight loss, improved cardiovascular reactivity, avoiding an increase in BP medication and improvements in quality of health and life and uremic symptom scores as assessed by questionnaire.345
In the absence of diabetes, hypertension or other cardiovascular risk factors there has been a lack of evidence to support a causal link between obesity and CKD. Observational studies suggest that obesity is an independent risk factor for CKD.346, 347, 348 The evidence in population studies is conflicting; some studies have failed to link obesity with decreased GFR,349, 350 possibly because BMI in isolation is a poor measure, while others suggest that CKD is independently associated with BMI.351 It has been known for some time that obesity is associated with secondary focal and segmental glomerulosclerosis,352 yet significant associations between obesity and CKD in large observational studies such as The Framingham Heart Study disappear after adjustment for age, gender, and cardiovascular risk factors.353 However, systematic review and meta-analysis of weight loss interventions in CKD have shown weight loss to be associated with significant decrease in proteinuria and systolic BP with no further decrease in GFR in people with CKD during a mean follow-up of 7.4 months.354 A further systematic review drew very similar conclusions. Weight loss interventions were associated with decreased proteinuria and albuminuria by 1.7 g (95% CI 0.7-2.6 g) and 14 mg (95% CI 11-17 mg), respectively (P<0.05).355 Each 1-kg weight loss was associated with 110 mg (95% CI 60-160 mg; P<0.001) decrease in proteinuria and 1.1 mg (95% CI 0.5-2.4 mg; P= 0.011) decrease in albuminuria, respectively, independent of reduction in BP.
Multiple studies document a clear association between smoking and renal damage in the general population, patients with diabetes, and hypertensive patients.356 Smoking is causally linked to cardiovascular events in the general population and is also associated with an increased risk for cardiovascular events in patients with CKD.357, 358, 359, 360 Studies investigating the beneficial effects of smoking cessation on kidney function have all been positive.361, 362, 363, 364, 365
International Relevance
Exercise, weight loss and smoking cessation in CKD are equally important in all countries and thus this recommendation is of international relevance and applicability.
Implications for Clinical Practice and Public Policy
Implementation of this recommendation has no public health cost but has the potential to make far-reaching public health gains both in terms of population health and health-care economics.
Additional dietary advice
3.1.22: We recommend that individuals with CKD receive expert dietary advice and information in the context of an education program, tailored to severity of CKD and the need to intervene on salt, phosphate, potassium, and protein intake where indicated. (1B)
RATIONALE
International Relevance
The importance of lifestyle recommendations and dietary counseling cannot be overstated. It is however recognized that within the context of different countries, health-care systems and jurisdictions, the degree to which these can be implemented is variable. These recommendations serve as a ‘best practice' suggestion.
3.2 COMPLICATIONS ASSOCIATED WITH LOSS OF KIDNEY FUNCTION
People with CKD are prone to develop a variety of complications which reflect loss of endocrine or exocrine function of the kidneys. The incidence and prevalence of these complications increase with severity of CKD as defined predominantly by GFR categories (Table 27).
Table 27. Prevalence of CKD complications by GFR category* derived from CKD cohorts.
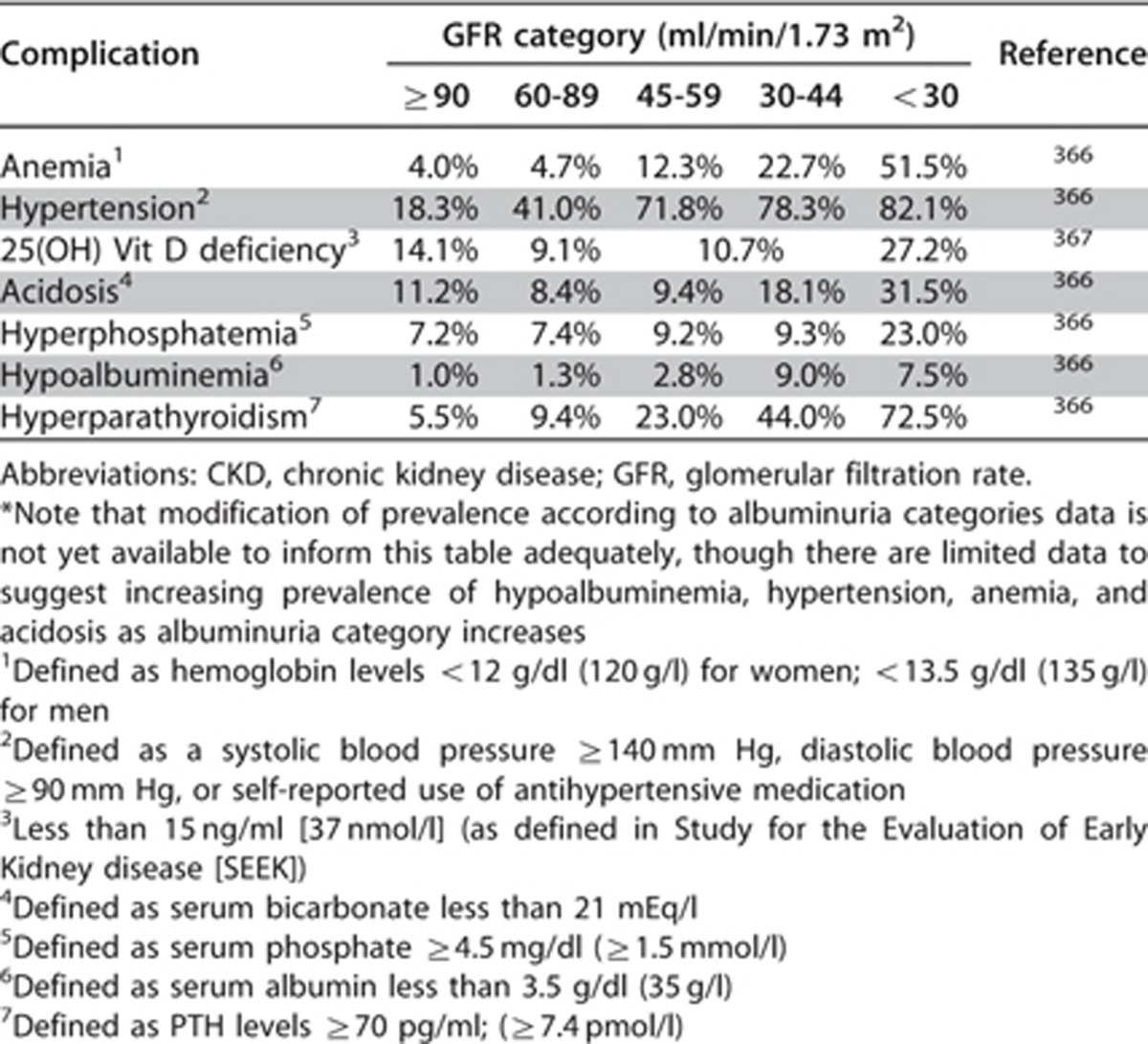
It is beyond the scope of this guideline to describe each of the complications and the proposed treatment options for them in detail as guidance for these conditions can be found in other documents. However, for the purpose of completeness, the key complications and management recommendations for people with CKD are addressed in this section.
In addition to these complications, we have described strategies to delay progression of CKD which are in part predicated on the identification and management of the clinical, metabolic, and hematologic complications. Note that not all people with CKD will have all of the complications and complications may not occur at the same rate or to the same degree in individuals with the same categories of GFR or albuminuria. Nonetheless knowledge of the common complications and treatment options is important in the care of CKD.
Management of Complications
Anemia in CKD
Anemia is an important complication of CKD because it contributes significantly to the heavy symptom burden of CKD. It has a major impact on the lives of people with CKD but it is potentially reversible with appropriate treatment. The guideline statements included here are those we consider to be the key considerations for people with non-dialysis CKD. Interested readers are referred to the KDIGO Clinical Practice Guideline for Anemia in CKD11 for comprehensive guidance on this topic.
Definition and identification of anemia in CKD
3.2.1: Diagnose anemia in adults and children >15 years with CKD when the Hb concentration is <13.0 g/dl (<130 g/l) in males and <12.0 g/dl (<120 g/l) in females. (Not Graded)
3.2.2: Diagnose anemia in children with CKD if Hb concentration is <11.0 g/dl (<110 g/l) in children 0.5-5 years, <11.5 g/dl (115 g/l) in children 5-12 years, and <12.0 g/dl (120 g/l) in children 12-15 years. (Not Graded)
RATIONALE
These statements reflect the need to measure Hb in people with CKD in order to detect if anemia is present, given its association with poor outcomes and its use in prediction models. Anemia is a common occurrence in CKD patients, though variable in its time of presentation and severity within individuals. Thus, the guidelines stress that evaluation and treatment of anemia in people CKD should be undertaken as in other individuals, and emphasize that anemia due to CKD is a diagnosis of exclusion. Furthermore, the guidelines stress that laboratory values used for diagnosis do not imply therapeutic thresholds or targets. For details, consult KDIGO Clinical Practice Guideline for Anemia in CKD.11
Evidence Base
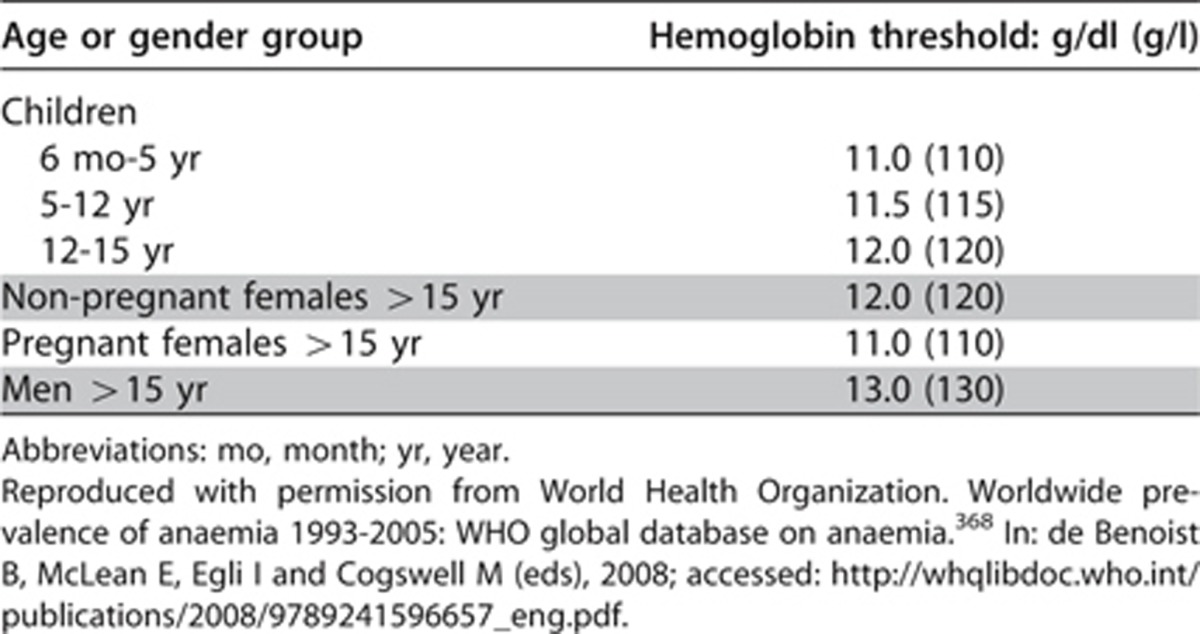
Conventionally anemia is defined as a Hb concentration lower than the established cutoff defined by the WHO.368 Different biological groups have different cutoff Hb values below which anemia is said to be present, ranging from 11 g/dl (110 g/l) for pregnant women and for children between 6 months and 5 years of age, to 12 g/dl (120 g/l) for non-pregnant women, and to 13 g/dl (130 g/l) for men (Table 28).
Table 28. Hemoglobin thresholds used to define anemia.
International Relevance
Globally the prevalence of anemia is 24.8% of the population, much of this is linked to nutritional deficiency exacerbated by infectious disease. However, altitude, race, and smoking will each also have an impact on Hb concentration. Hb concentration can be expected to increase by about 0.6 g/dl (6 g/l) in women and 0.9 g/dl (9 g/l) in men for each 1000 m of altitude above sea level.369 Hb concentrations also vary between races, with African-American individuals consistently showing concentrations 0.5 to 0.9 g/dl (5 to 9 g/l) lower than those of whites or Asian populations.370, 371, 372 Smoking is associated with elevated carboxyhemoglobin levels, which are associated with a compensatory increase in total Hb concentration. Hence, the US Centers for Disease Control and Prevention have recommended a downwards adjustment of 0.3 g/dl (3 g/l) for smokers.373 Thus, specific Hb levels expected for different levels of eGFR should be contextualized within the normal distribution of the population.
Implications for Clinical Practice and Public Policy
The recommended thresholds for diagnosis and evaluation of anemia should not be interpreted as being thresholds for treatment of anemia but simply for the identification of this complication. Practice preferences with respect to treatment strategies should be directed according to local resources.
Evaluation of anemia in people with CKD
- 3.2.3: To identify anemia in people with CKD measure Hb concentration (Not Graded):
- when clinically indicated in people with GFR ≥60 ml/min/1.73 m2 (GFR categories G1-G2);
- at least annually in people with GFR 30-59 ml/min/1.73 m2 (GFR categories G3a-G3b);
- at least twice per year in people with GFR <30 ml/min/1.73 m2 (GFR categories G4-G5).
RATIONALE
The recommendation that patients be evaluated at least annually rests on observations from clinical trials that (in the absence of ESA therapy) the natural history of anemia in patients with CKD is a gradual decline in Hb concentration over time.374, 375, 376 The exact frequency of monitoring of Hb concentration will be influenced by kidney function, underlying disease process, the initial Hb concentration, and the rate of change in Hb concentration. The latter will also be influenced by whether or not anemia is being treated and what type of treatment is undertaken. The statements specifically address the need to measure Hb concentrations at a minimum and are not intended to deter the clinician from more frequent measurements as required for individual circumstances.
The initial evaluation of anemia in CKD is geared towards the exclusion of causes other than those directly related to kidney disease (relative iron and erythropoietin deficiency); see KDIGO Clinical Practice Guideline for Anemia in CKD11 for details.
Evidence Base
Anemia as defined above is found in people with CKD in increasing proportions as GFR declines (Table 27). This is due to a number of reasons, including loss of erythropoietic hormone efficacy, production, substrate deficiency (most notably iron), and other conditions which may contribute to the lack of effective erythropoiesis. For a full description of the evidence behind this statement please refer to the KDIGO Clinical Practice Guideline for Anemia in CKD.11
International Relevance
Frequency of Hb concentration monitoring and the initial evaluation of anemia should not differ by country, except that causes of anemia other than those related to CKD do differ. Nutritional deficiencies in resource-poor areas are common, particularly iron deficiency, which is frequently exacerbated by infectious diseases.
Implications for Clinical Practice and Public Policy
Anemia is associated with increased morbidity, mortality, and consumption of health-care resources. The major health consequences include poor pregnancy outcome, impaired physical and cognitive development, increased risk of morbidity in children, and reduced work productivity in adults.
Treatment of anemia in CKD
The last 30 years have seen a major transition in approach to the treatment of anemia in people with CKD beginning with the introduction of erythropoietin therapy into clinical practice and the subsequent resurgence of interest in iron therapies. The promise of early intervention with anemia treatment in non-dialysis CKD suggested by observational study has been tempered with the reality of risks of adverse cardiovascular outcomes in RCTs. Nevertheless treatment of anemia with iron and ESAs has an important positive role to play in the lives of people with CKD. For the treatment of anemia in people with CKD, we suggest clinicians refer to the KDIGO Clinical Practice Guideline for Anemia in CKD for further details.11
The key points for practitioners to remember include the following:
Work-up for anemia in CKD should include assessment of secondary causes including iron deficiency.
Iron replacement is often effective in anemia of CKD as initial therapy and routes of administration (intravenous or oral) will be determined by clinicians, patient preferences, and local available resources.
ESA therapy is not recommended in those with active malignancy, or recent history of malignancy.
In most people with CKD, ESAs should not be used to intentionally increase the Hb concentration above 11.5 g/dl (115 g/l)
For pediatric patients, the selection of Hb concentration at which ESA therapy is initiated should be individualized after taking into account the potential benefits (e.g., improvement in QOL, school attendance/performance, and avoidance of transfusion) and potential harms.
3.3 CKD METABOLIC BONE DISEASE INCLUDING LABORATORY ABNORMALITIES
Changes in bone mineral metabolism and alterations in calcium and phosphate homeostasis occur early in the course of CKD and progress as kidney function declines (Table 27). These changes are grouped under the umbrella term Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) which includes renal osteodystrophy and extraskeletal (vascular) calcification related to abnormalities of bone mineral metabolism. Renal osteodystrophy is the component of CKD-MBD that is identified and quantified through bone biopsy histomorphometry and includes osteitis fibrosa (hyperparathyroidism), osteomalacia, and adynamic bone disease.
The evidence on which existing recommended guideline treatment targets for serum concentrations of calcium, phosphate, and parathyroid hormone (PTH), and the strategies to achieve these targets, is exclusively observational and thus problematic for that reason. Furthermore, very little of the evidence is derived from patients with non-dialysis CKD. Nevertheless we feel it is important to include here some of the key statements relating to mineral metabolism abnormalities in patients with non-dialysis CKD from the KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) published in 2009.9 These statements will be qualified where new data since the publication of the CKD-MBD guidance have become available.
3.3.1: We recommend measuring serum levels of calcium, phosphate, PTH, and alkaline phosphatase activity at least once in adults with GFR <45 ml/min/1.73 m2 (GFR categories G3b-G5) in order to determine baseline values and inform prediction equations if used. (1C)
RATIONALE
As kidney function declines, abnormalities of serum calcium, phosphate, and circulating hormones related to CKD-MBD progress. These include PTH; 25-hydroxyvitamin D (25(OH)D), 1,25-dihydroxyvitamin D (1,25(OH)2D), and other vitamin D metabolites; fibroblast growth factor-23 (FGF-23); and growth hormone. At the tissue level there is down regulation of vitamin D receptors and resistance to the actions of PTH. Immunohistochemical abnormalities in bone also occur early and generally precede changes in mineral homeostasis. Extraskeletal calcification may result from deranged mineral and bone metabolism and from the therapies used in an attempt to correct these abnormalities. Associations between disorders of mineral metabolism and CVD have widened the focus of CKD-MBD to include abnormal mineral metabolism, abnormal bone, and extraskeletal calcification. Once baseline values have been obtained, the subsequent frequency of testing will be determined on an individual basis by the actual value and any intervention that may be introduced.
Evidence Base
There are observational studies which describe the abnormalities of each of these parameters at relatively high values of eGFR in general and high-risk population cohorts.367, 377, 378 Importantly, abnormalities of calcium and phosphate appear to occur relatively later in the course of CKD than do abnormalities in values of 1,25(OH)2D, 25(OH)D, and PTH. Thus, the recommendation is to evaluate these parameters relatively early in the trajectory of CKD, as an assessment of burden of illness. In dialysis patients, the highest risks for mortality have been reported with combinations of high serum phosphate and calcium together with either high PTH (RR 3.71; 95% CI 1.53-9.03; P= 0.004) or low PTH (RR 4.30; 95% CI 2.01-9.22; P<0.001) compared with the combination of high PTH with normal serum calcium and phosphate which had the lowest mortality and was used as the index category.379 The importance of examining combinations of parameters of mineral metabolism is likely to be no different in patients with less severe CKD, but this has not been tested in non-dialysis populations.
There are also racial differences in the parameters of mineral metabolism. In a multicenter cohort of 227 black and 1633 non-black patients with early CKD, blacks had similar 1,25(OH)2D levels compared with non-blacks but significantly lower levels of 25(OH)D with higher levels of calcium, phosphate, and PTH, and were significantly more likely to have hyperphosphatemia than non-blacks.380 In multivariable analyses adjusted for age, gender, eGFR, BMI, and diabetes, blacks had significantly lower 25(OH)D and higher PTH levels than non-blacks. Examining the relationships between 25(OH)D and PTH in 8415 adult participants (25% black and 24% Mexican-American) in NHANES 2003–2004 and 2005–2006, and the relationship between 25(OH)D and bone mineral density (BMD) in 4206 adult participants (24% black and 24% Mexican-American) in the 2003–2004 NHANES sample, Guitierrez et al. found significant racial differences.381 Blacks and Mexican-Americans had significantly lower 25(OH)D and higher PTH concentrations than whites (P<0.01 for both). Bone mineral density significantly decreased (P<0.01) as serum 25(OH)D and calcium intake declined among whites and Mexican-Americans, but not among blacks (P=0.2).
International Relevance
The association between black race and Hispanics and secondary hyperparathyroidism, independent of known risk factors, suggests that novel mechanisms may contribute to secondary hyperparathyroidism in non-whites with CKD. Testing for these parameters would therefore be informed by the demographics of the population. In different countries and regions, the ability to measure these parameters may vary, thus the authors of the guideline statements appreciate that implementation of regular measurements of all these parameters may not be possible in all jurisdictions.
Implications for Clinical Practice and Public Policy
Given there remains no clear recommendation as to ‘expected' values in CKD nor consensus on thresholds regarding treatment, the testing of PTH, and vitamin D parameters would lead to substantial costs to the health-care system. Abnormal values lead to repeat testing. There are no data to suggest how effective or useful repeated monitoring of abnormal values is, nor what an acceptable interval of monitoring should be to inform care. Laboratory testing for phosphate and calcium is relatively inexpensive, but treatment and ongoing monitoring may be expensive. At the current time, recommendations for testing frequency may be problematic for clinical practice.
Areas of Controversy, Confusion, or Non-consensus
The inter-relationship of calcium, phosphate, and PTH, and the potential impact of vitamin D on these mineral metabolites and extraskeletal calcification remains an area of research and debate among clinicians. Newer research examining the role of FGF-23, an important molecule in phosphate, PTH, and vitamin D homeostasis, has caused many to question the previous focus on PTH values as misplaced. The questions of whether vitamin D therapies are toxic in some or all patients and what values of phosphate are pathologic have yet to be resolved.
Pediatric Considerations
Application of guidelines related to bone health, growth, and CKD in children is extremely complex. Numerous issues arise, including age-related variation in normative values, comparisons across age, sex, size, and the need to account for pubertal changes etc., when one considers the options and targets for evaluation and treatment.
It is recommended that in the application of any of these specific guidelines, the reader carefully reviews the publications as they relate to pediatrics, starting with the following two documents and then accessing the most currently available pediatric CKD resources for the topic(s).
Recommended primary pediatric CKD-MBD resources:
KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 Update382
KDIGO Clinical Practice Guidelines for the Diagnosis, Evaluation, Prevention, and Treatment of CKD-MBD9
3.3.2: We suggest not to perform bone mineral density testing routinely in those with eGFR <45 ml/min/1.73 m2 (GFR categories G3b-G5), as information may be misleading or unhelpful. (2B)
RATIONALE
While there is an appreciation that BMD is measured in many elderly, this statement is intended to highlight for the clinician the fact that the information gained from BMD in those with reduced GFR may be false, leading to either under- or over-treatment. Although fractures rates and fracture-related mortality are elevated in CKD, bone densitometry does not reliably predict fracture risk in patients with GFR <45 ml/min/1.73 m2 and neither does it predict the type of renal osteodystrophy. Thus, BMD measurements do not provide the information usually sought from such testing, which is usually the basis of interventions.
Evidence Base
Decreased bone mass and changes in bone microarchitecture occur early in CKD and worsen with progression of disease such that patients with CKD are at increased risk of bone fracture.383 Bone strength is determined by the density and quality of the bone. Dual-energy x-ray absorptiometry (DXA) scanning measures density of bone but is not able to determine bone quality (cortical and trabecular microarchitecture). Studies using high-resolution peripheral quantitative computed tomography (HR-pQCT) demonstrate abnormalities in the cortical and trabecular microarchitecture of patients with early CKD compared with healthy control subjects.384 Although abnormalities of both DXA and HR-pQCT associate with fractures in patients with CKD, receiver operator characteristic curve analysis suggests that neither technique is predictive of fracture (area under the curve <0.75), although this improved for patients with longer duration of CKD.385 In a cross-sectional study, the combination of these imaging techniques with markers of bone turnover improved prediction of fracture.386
Implications for Clinical Practice and Public Policy
A major component of fracture risk is related to fall risk, thus reduction in fall risk may be achieved through establishment of falls prevention programs. Such programs include medication review; prevention of postural hypotension; cardiac pacing, where appropriate; home hazard assessment and modifications; muscle strengthening and retraining; and treatment of vitamin D deficiency.
Areas of Controversy, Confusion, or Non-consensus
The combination of measurements of bone thickness, BMD of femoral neck, and a history of fracture may be useful to identify CKD patients who might benefit from fracture prevention strategies. Prospective studies are needed to assess the utility of these parameters for fracture prediction in the CKD population.
Treatment of CKD-MBD
Disturbances of calcium, phosphate, vitamin D, and PTH develop early during the course of CKD and are associated with adverse outcomes. Studies of these and other markers of bone mineral metabolism have improved our understanding of disease mechanisms governing adverse outcomes of CKD-MBD but clinical studies have yet to indicate whether or not manipulation of these markers improves patient-level outcomes. In making recommendations for therapeutic targets for mineral metabolism abnormalities, we have been careful not to reach beyond the evidence.
3.3.3: In people with GFR <45 ml/min/1.73m2 (GFR categories G3b-G5), we suggest maintaining serum phosphate concentrations in the normal range according to local laboratory reference values. (2C)
3.3.4: In people with GFR <45 ml/min/1.73m2 (GFR categories G3b-G5) the optimal PTH level is not known. We suggest that people with levels of intact PTH above the upper normal limit of the assay are first evaluated for hyperphosphatemia, hypocalcemia, and vitamin D deficiency. (2C)
RATIONALE
Higher serum phosphate concentrations are associated with mortality and experimental data suggests that serum phosphate concentration is directly related to bone disease, vascular calcification and CVD. Serum phosphate, calcium, and PTH concentrations are all inter-related in patients with CKD. Randomized studies linking manipulation of these parameters to clinical outcomes are lacking but systematic review indicates that earlier phosphate control may help reduce the early clinical consequences of CKD-MBD. Similarly there is insufficient evidence that any specific phosphate binder significantly impacts patient-level outcomes.
Evidence Base
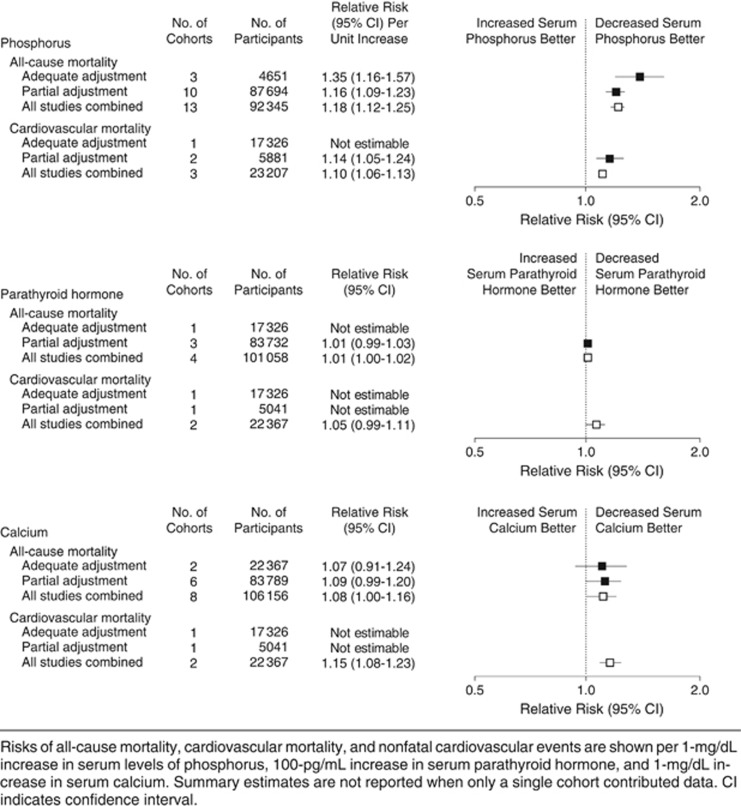
Systematic review of serum concentrations of calcium, phosphate, and PTH and the risk of death and CVD in people with CKD showed that the risk of death increased 18% for every 1 mg/dl (0.33 mmol/l) increase in serum phosphate concentration (RR 1.18; 95% CI 1.12-1.25).387 There was no association seen with either PTH or serum calcium and all-cause mortality (Figure 19). Of the 327,644 subjects included in the review only 16,247 were not receiving dialysis and of these only 8990 were people with GFR <60 ml/min/1.73 m2 not receiving RRT. In these subjects the risk of all-cause mortality for each 1 mg/dl (0.33 mmol/l) increase in serum phosphate concentration was very similar (RR 1.29; 95% CI 1.12-1.48). As with all subjects included there was no evidence of an association between serum calcium concentration and all-cause mortality in people with CKD not receiving RRT (RR 1.02; 95% CI 0.81-1.29). Data for associations of calcium, phosphate, and PTH with cardiovascular death were only available in one of the studies included.
Figure 19.
Summary estimates for risks of all-cause mortality and cardiovascular mortality associated with levels of serum phosphorus, PTH, and calcium. PTH, parathyroid hormone. Reprinted with permission from Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA 2011; 305(11): 1119-1127.387 Copyright © (2011) American Medical Association. All rights reserved. Accessed http://jama.jamanetwork.com/da ta/Journals/JAMA/18301/jrv15003_1119_1127.pdf
In the Multi-Ethnic Study of Atherosclerosis (MESA) study, the associations of serum phosphate concentrations with vascular and valvular calcification were examined in 439 people with GFR <60 ml/min/1.73 m2. Each 1 mg/dl (0.33 mmol/l) increase in serum phosphate concentration was associated with a 21% (P=0.002), 33% (P=0.001), 25% (P= 0.16), and 62% (P=0.007) greater prevalence of coronary artery, thoracic, aortic valve, and mitral valve calcification, respectively.388 The strength of the associations did not differ by age, race, or diabetes. Adjustment for serum concentrations of PTH and 1,25(OH)2D did not alter the strength of the associations.
Factors affecting gastrointestinal phosphate absorption include 1,25(OH)2D, food content, phosphate bioavailability and phosphate binders (natural and prescribed). Sources of dietary phosphate are protein-rich foods, including dairy products, meat, and fish as well as legumes, nuts, chocolates and inorganic phosphate additives such as those found in carbonated drinks. In a non-vegetarian Western diet, over half the dietary intake of phosphate comes from animal protein. Although the phosphate content of plant-derived phosphate is higher than animal derived, its bioavailability in terms of gastrointestinal absorption is lower.389 Inorganic phosphate additives have the highest bioavailability. A number of clinical studies detail benefit from dietary phosphate and protein control in terms of secondary hyperparathyroidism and progression of CKD in people with moderate CKD.390 Few studies have evaluated the impact of dietary phosphate restriction on bone disease or vascular calcification and only one has addressed survival. In people on hemodialysis, a post hoc analysis suggested that more restrictive prescribed dietary phosphate was associated with poorer indices of nutritional status and a greater need for nutritional supplementation.391 There was a stepwise trend toward greater survival with more liberal phosphate prescription, which reached statistical significance among subjects prescribed 1001 to 2000 mg/d and those with no specified phosphate restriction, raising concerns about protein energy malnutrition with dietary phosphate restriction. The means used to achieve phosphate restriction may therefore be important.
Table 29 details the relative cost comparisons of phosphate binders currently in clinical use for which there is observational or study trial data demonstrating their efficacy. Data concerning comparative patient-level outcomes such as mortality are not available.
Table 29. Phosphate binding agents in routine clinical practice and their ranked cost.
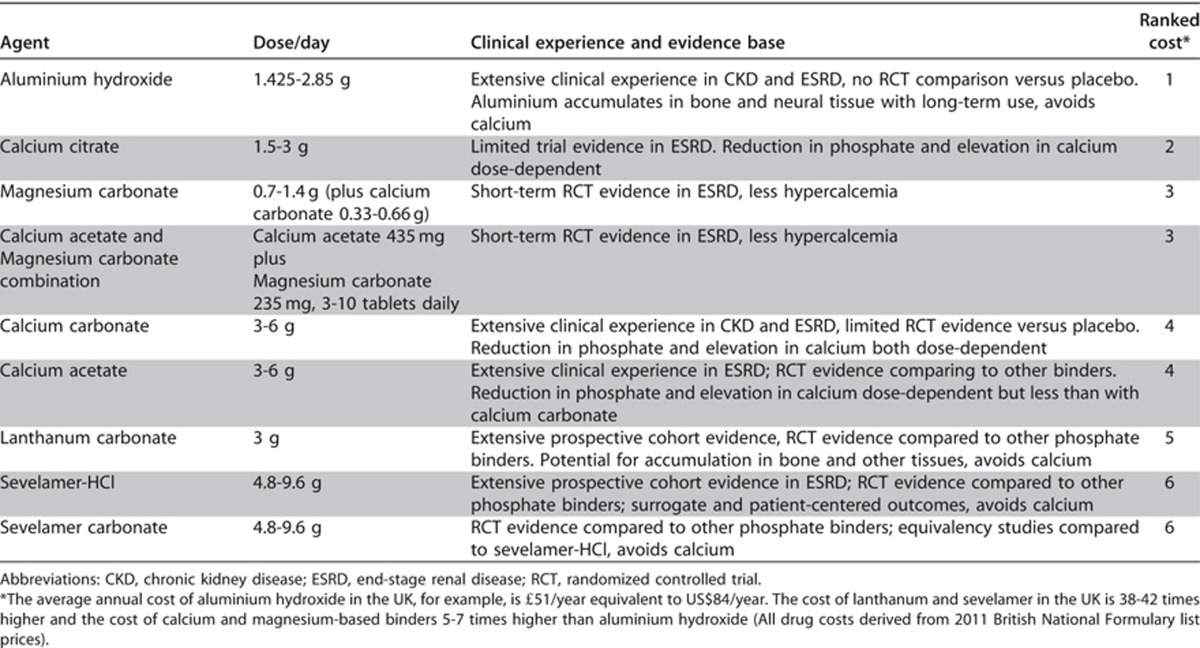
There are a number of agents available for phosphate binding which are listed in the table ranked in order of relative cost, appreciating that both availability and specific costs are country- and era-specific.
A Cochrane meta-analysis considered 60 RCTs or quasi-RCTs (7631 participants) that assessed the effects of various phosphate binders in adults with CKD.392 The authors concluded that all available phosphate-binders reduced serum phosphate concentrations in comparison to placebo but that data to date do not support superiority of novel non-calcium binding agents for patient-level outcomes such as all-cause mortality and cardiovascular end points in CKD.
International Relevance
Availability of different phosphate binders differs around the globe. Thus, recommendations as to specific agents are not possible within the context of these statements. Similarly, dietary phosphate intake may be different around the world, rendering this problem of greater or lesser significance in different jurisdictions. Measurement of specific hormones (PTH, vitamin D) is expensive and may not inform care sufficiently to warrant the expense at this time.
Implications for Clinical Practice and Public Policy
Existing data support prevention of hyperphosphatemia and associated secondary hyperparathyroidism in CKD. In the absence of hypercalcemia, there is no indication to prescribe phosphate-binders that are less cost-effective than calcium-based agents. Current data are insufficient to make recommendations about target levels of serum calcium or PTH concentrations that should be achieved in order to reduce mortality or cardiovascular morbidity in people with CKD not requiring dialysis. Assay variability of PTH and vitamin D remains problematic and this issue is beyond the scope of this document. The practitioner and health-care administrators are advised to appreciate this problem in developing targets for care or thresholds for treatment.
Areas of Controversy, Confusion, or Non-consensus
As per comments above, the data to support levels of laboratory values for interventions, types of interventions, and target values remain problematic. Thus recommendations for therapy remain similarly problematic and practice varies depending on location and resource availability. Likely correlation of symptoms with blood values and addressing laboratory abnormalities within that context is a pragmatic approach at the current time. The non-specialist is asked to seek advice from local experts for best advice for specific individuals.
The KDIGO guidelines on this subject have not been updated at the time of the writing of this CKD guideline. We have attempted to balance current knowledge with published guidance statements in non-CKD populations.
Clarification of Issues and Key points
Clinical trials comparing strategies such as vitamin D replacement, dietary phosphate restriction, phosphate binders, and calcimimetics with placebo are needed to address patient-level outcomes such as mortality and cardiovascular morbidity in people with CKD.
Measurement of vitamin D levels is problematic and expensive and is not advocated here.
Vitamin D supplementation and bisphosphonates in people with CKD
3.3.5: We suggest not to routinely prescribe vitamin D supplements or vitamin D analogs, in the absence of suspected or documented deficiency, to suppress elevated PTH concentrations in people with CKD not on dialysis. (2B)
RATIONALE
This statement is intended to highlight the lack of robust data to support either the measurement or the treatment of vitamin D deficiency in non-dialysis CKD populations. The statement asks the clinician to more fully evaluate the individual situation. The internationally accepted definition of vitamin D deficiency is a blood concentration <20 ng/ml (<50 nmol/l). Low 25(OH)D levels are common in patients with non-dialysis dependent CKD; concentrations of <15 ng/ml (<37 nmol/l) occur in at least 12-15% of patients with CKD and are more prevalent at lower GFR levels, in institutionalized subjects, at extremes of age, and in certain racial groups. Deficiency of 25(OH)D increases fracture risk and is associated with increased mortality. As CKD progresses, levels of 1,25(OH)2D progressively fall and are closely associated with increasing PTH concentrations. In vitamin D-deficient subjects supplementation with vitamin D increases BMD and muscle strength, reduces risk for fractures and falls, and reduces PTH. In the absence of deficiency, treatment with vitamin D and related compounds has not been shown to improve either mortality or cardiovascular outcomes.
Evidence Base
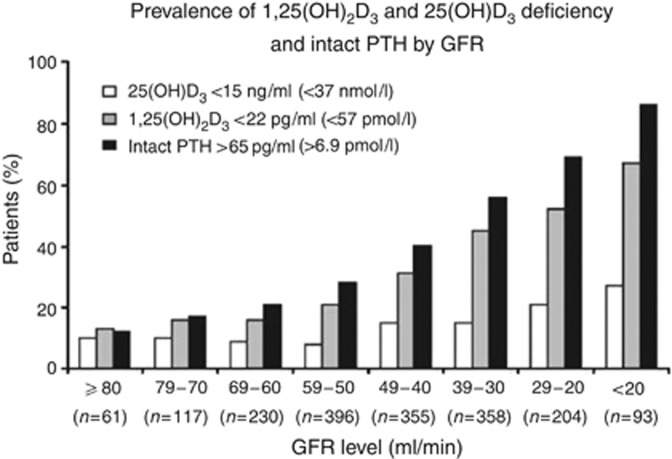
There is a substantial amount of data to support 25(OH)D deficiency in general and CKD populations367, 393 which is likely multifactorial. In addition to 25(OH)D deficiency, note has been made that there is a progressive increase in prevalence of 1,25(OH)2D deficiency with lower GFR category, which occurs earlier than 25(OH)D deficiency (Figure 20).
Figure 20.
Prevalence of deficiency of 1,25(OH)2D3, 25(OH)D3, and secondary hyperparathyroidism by GFR intervals. GFR, glomerular filtration rate; PTH, parathyroid hormone. Adapted by permission from Macmillan Publishers Ltd: Kidney International. Levin A, Bakris GL, Molitch M, et al.367 Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71: 31-38; accessed http://www.nature.com/ki/journ al/v71/n1/pdf/5002009a.pdf
No relationship between 25(OH)D levels and 1,25(OH)2D levels was apparent but there was a strong association between 1,25(OH)2D deficiency and PTH concentration. Of particular note, a higher urinary ACR was associated with lower levels of 1,25(OH)2D at GFR values of <60 ml/min/1.73 m2.
The potential importance of these deficiencies has been illustrated in a number of studies examining the relationship between low levels of 25(OH)D and 1,25(OH)2D and all-cause and cardiovascular mortality in a different cohorts.393, 394
Despite the associations with mortality, systematic review of published data to date on vitamin D supplementation in patients with CKD not on dialysis has only shown an improvement in biochemical end points. A series of publications395, 396 have attempted to summarize the efficacy of vitamin D therapy on biochemical, bone, cardiovascular, and mortality outcomes in people with CKD and not requiring dialysis. No formulation, route, or schedule of vitamin D compound was found to alter the mortality risk or need for dialysis although vitamin D compounds significantly lowered serum PTH concentrations. None of the studies assessed reported outcomes related to CVD, bone disease, or mortality.
International Relevance
The importance of vitamin D deficiency has been addressed in both general and CKD populations. Specific populations have been identified as more likely to be vitamin D deficient depending on cultural and environmental factors; estimates of worldwide prevalence of vitamin D deficiency range from 25-60%. CKD populations within high-risk areas may be particularly vulnerable. The interplay between loss of kidney function and exacerbation of vitamin D deficiency is not known.
Implications for Clinical Practice and Public Policy
Vitamin D supplementation improves biochemical end points similar to active vitamin D analogs with a lower burden of costs and side effects. Measurement of serum 25(OH)D is expensive and in the CKD population with vitamin D deficiency, simple vitamin D replenishment is all that is indicated until new evidence becomes available. Except for education or research purposes, there is no need to measure vitamin D levels in general practice.
Areas of Controversy, Confusion, or Non-consensus
The appropriate dose and formulation of vitamin D, target range for treatment, frequency, route of administration, and safety at different severities of CKD remain to be determined.
3.3.6: We suggest not to prescribe bisphosphonate treatment in people with GFR <30 ml/min/1.73 m2 (GFR categories G4-G5) without a strong clinical rationale. (2B)
RATIONALE
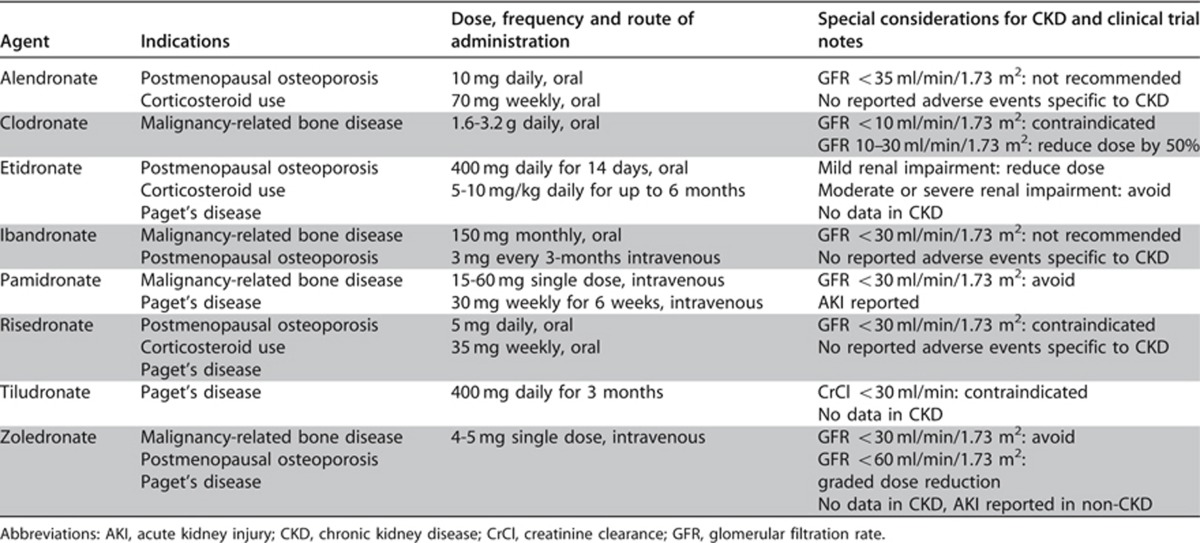
The risk-benefit ratio of bisphosphonates has not been well studied in CKD populations. Indications for bisphosphonate therapy include osteoporosis, corticosteroid therapy, malignant disease and Paget's disease. In people with CKD and GFR categories >60 ml/min/1.73 m2 with osteoporosis and/or at high risk of fractures, and in people with GFRs between 30-60 ml/min/1.73 m2 with normal PTH, osteoporosis and/or at high risk of fracture, treatment should be the same as for the general population (although dose modification may be necessary, see Table 30).
Table 30. Summary data for bisphosphonates and CKD.
In people with CKD at lower categories of GFR (<30-35 ml/min/1.73 m2), the correct diagnosis of osteoporosis becomes increasingly complex and other forms of renal osteodystrophy requiring alternative management strategies to osteoporosis require exclusion before treatment with bisphosphonates is considered. In people with adynamic renal bone disease, more likely at lower GFR categories, there is a lack of evidence of either harm or benefit of bisphosphonates on bone strength or vascular calcification.
Evidence Base
Bisphosphonates increase BMD, reduce bone turnover, and reduce the risk of fragility fractures. The bioavailability of intravenous bisphosphonate formulations is 100% but the bioavailability of oral formulations is only 1-5%. Approximately 50-80% of available bisphosphonate is taken up by bone and the remaining 20-50% is excreted in urine without being metabolized. Although oral bisphosphonates have not been shown to adversely affect kidney function in people with GFRs as low as 15 ml/min/1.73 m2, in post hoc analysis of clinical trial data their safety and efficacy below GFRs of 30 ml/min/1.73 m2 have not been well-validated and intravenous bisphosphonates have been implicated in nephrotoxicity, especially when rapidly infused (Table 30).9, 397, 398 Thus, from a patient's safety perspective, the statement serves to limit exposure of those with abnormal kidney function to these agents.
International Relevance
Given cost and clinical practice variation, the use of bisphosphonates varies around the world. Thus, this statement may be less applicable in different jurisdictions.
Implications for Clinical Practice and Public Policy
Given the widespread use of bisphosphonates in developed countries, especially in older women who are also likely to have some degree of kidney dysfunction, the cessation of bisphosphonates in that group may be problematic. There is a need to monitor clinical practice and understand the implications of this recommendation for large populations, who may or may not be deriving benefit from these agents.
Areas of Controversy, Confusion, or Non-consensus
Despite these concerns, primary care data from the USA suggest that prescription of bisphosphonates is common in people with CKD. Even in the subset with GFR <30 ml/min/1.73 m2, bisphosphonate use was no less prevalent than in the non-CKD population.399 People with CKD in this study were nearly seven times more likely to receive a bisphosphonate than active vitamin D. Similar uncertainty about the efficacy and safety of such treatment for people with CKD was demonstrated in a study of people under the care of a UK renal unit.400 Half of all people prescribed bisphosphonates had a GFR <30 ml/min/1.73 m2 and yet 50% of people with higher GFRs and documented osteoporosis were not prescribed bisphosphonates.
Clarification of Issues and Key Points
Further study is required to elucidate whether or not bisphosphonates, through reduction of bone turnover in people with pre-existing low bone turnover states, would be beneficial or harmful both for bone and vascular calcification.
3.4 ACIDOSIS
The prevalence and severity of metabolic acidosis in people with CKD progressively rises as GFR falls (Table 27). Adaptations in acid excretion by the kidneys initially prevent a fall in serum bicarbonate concentration but as GFR continues to decline below 40 ml/min/1.73 m2, metabolic acidosis commonly develops.
3.4.1: We suggest that in people with CKD and serum bicarbonate concentrations <22 mmol/l treatment with oral bicarbonate supplementation be given to maintain serum bicarbonate within the normal range, unless contraindicated. (2B)
RATIONALE
Serum bicarbonate concentrations less than 22 mmol/l are associated with increased risk of CKD progression and increased risk of death. Conversely, high serum bicarbonate concentrations greater than 32 mmol/l are associated with increased risk of death irrespective of the level of kidney function. Small studies of alkali supplementation have shown reduction in progression of CKD and improved nutritional status in people with CKD. This statement is therefore worded to reflect the potential benefits of oral bicarbonate supplementation to maintain serum bicarbonate concentrations within the normal range, but the word ‘suggest' in the statement reflects the lack of robust evidence base on which to support this statement.
Evidence Base
Chronic metabolic acidosis is associated with increased protein catabolism, uremic bone disease, muscle wasting, chronic inflammation, impaired glucose homeostasis, impaired cardiac function, progression of CKD, and increased mortality.401, 402, 403, 404, 405, 406, 407, 408, 409, 410 The suggestion that treatment of acidosis may be beneficial was first made by Richard Bright,411 later taken up enthusiastically by Arthur Osman412 and further explored by Lyon et al. who conducted a form of crossover study of supplementation with bicarbonate in 17 people with moderate kidney failure and suggested that treatment with alkali might preserve kidney function.413 Rustom et al. described a reduction in proximal renal tubular catabolism and markers of tubular injury after oral NaHCO3 in 11 people with mild/moderate renal impairment (mean 51Cr-EDTA GFR 46.2 ± 6.0 ml/min/1.73 m2) and proteinuria (3.2 ± 0.8 g/24 hours).414 They suggested that oral sodium bicarbonate may protect the proximal renal tubule and help delay kidney disease progression. Mathur et al. randomized 40 subjects with mild to moderate CKD to oral bicarbonate (1.2 mEq/kg of body weight) or placebo for 3 months and suggested that correction of metabolic acidosis significantly attenuates the rise in blood urea.415 A larger study randomly assigned 134 adults with moderate to severe CKD (CrCl 15 to 30 ml/min/1.73 m2) and serum bicarbonate 16 to 20 mmol/l to either supplementation with oral sodium bicarbonate (1.82 ± 0.80 g/day) or standard care for 2 years.416 Primary end points were rate of kidney function decline, the proportion of subjects with rapid decline of kidney function (>3 ml/min/1.73 m2/yr), and ESRD (CrCl <10 ml/min [<0.17 ml/s]). Secondary end points were DPI, normalized protein nitrogen appearance, serum albumin, and mid-arm muscle circumference. Serum bicarbonate increased significantly in the bicarbonate group but despite the associated sodium load there was no difference in BP control, prescription of antihypertensives and loop diuretics, or hospitalization for heart failure compared with the control group. The decline in CrCl was significantly slowed in the bicarbonate group compared with controls (5.93 versus 1.88 ml/min/1.73 m2, P<0.0001), rapid progression was less prevalent (9 versus 45%, RR 0.15; 95% CI 0.06-0.40; P<0.0001), and fewer subjects developed ESRD (6.5 versus 33%, RR 0.13; 95% CI 0.04-0.40; P<0.001). Nutritional parameters also improved significantly with bicarbonate supplementation. In a non-randomized study the effects of supplementation of 1 mmol of bicarbonate equivalent per kg body weight per day for 2 years using oral sodium citrate in 30 subjects with hypertensive nephropathy was compared with 29 control subjects.417 There were no statistically significant differences in baseline demographic data, systolic BP, venous bicarbonate levels, or parameters of kidney function between the two groups. All subjects were receiving angiotensin-converting enzyme inhibition throughout the study and BP was controlled to similar levels in both groups in a 6-month run-in period prior start of study. In those treated with oral sodium citrate, urine endothelin-1 excretion and N-acetyl-β-D glucosaminidase were each significantly lower while the rate of GFR decline was significantly slower after 24 months of treatment compared to the control group.
International Relevance
Alkali supplementation appears to be a promising low-cost, high-benefit adjunct treatment for patients with CKD and may be accessible to all populations. It is not known how prevalent acidosis is among different communities or countries around the world.
Implications for Clinical Practice and Public Policy
The use of sodium bicarbonate or calcium carbonate as a source of alkali should not present financial hardships in most practices or jurisdictions.
Areas of Controversy, Confusion, or Non-consensus
A potential concern regarding treatment of patients with CKD with sodium bicarbonate or sodium citrate is the possible associated sodium loading. A crossover study of 200 mmol/day (17 g/day) of sodium bicarbonate versus 200 mmol/day (12 g/day) of sodium chloride in 10 subjects with severe CKD (CrCl 2.5-16.8 ml/min [0.04-0.28 ml/s]) on an extremely low-sodium diet showed that sodium chloride but not sodium bicarbonate produced an increase in weight and BP.418 A subsequent follow-up study suggested that if dietary sodium chloride intakes are near maximum tolerance, then sodium bicarbonate supplementation should be accompanied by reductions in sodium chloride intake to maintain sodium balance.419 Given the lack of large and long-term trials, many are still not convinced that this is a reasonable recommendation, and is thus controversial. We would like to ensure that individual clinicians are aware of the controversy rather than making no statement at all.
DISCLAIMER
While every effort is made by the publishers, editorial board, and ISN to see that no inaccurate or misleading data, opinion or statement appears in this Journal, they wish to make it clear that the data and opinions appearing in the articles and advertisements herein are the responsibility of the contributor, copyright holder, or advertiser concerned. Accordingly, the publishers and the ISN, the editorial board and their respective employers, office and agents accept no liability whatsoever for the consequences of any such inaccurate or misleading data, opinion or statement. While every effort is made to ensure that drug doses and other quantities are presented accurately, readers are advised that new methods and techniques involving drug usage, and described within this Journal, should only be followed in conjunction with the drug manufacturer's own published literature.
References
- KDIGO CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009. pp. S1–130. [DOI] [PubMed]
- Seliger SL, Zhan M, Hsu VD, et al. Chronic kidney disease adversely influences patient safety. J Am Soc Nephrol. 2008;19:2414–2419. doi: 10.1681/ASN.2008010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy M, Newall R, Borzomato J, et al. Diagnostic accuracy of the urinary albumin: creatinine ratio determined by the CLINITEK Microalbumin and DCA 2000+ for the rule-out of albuminuria in chronic kidney disease. Clin Chim Acta. 2009;399:54–58. doi: 10.1016/j.cca.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Mittalhenkle A, Stehman-Breen CO, Shlipak MG, et al. Cardiovascular risk factors and incident acute renal failure in older adults: the cardiovascular health study. Clin J Am Soc Nephrol. 2008;3:450–456. doi: 10.2215/CJN.02610607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- Hoste EA, Lameire NH, Vanholder RC, et al. Acute renal failure in patients with sepsis in a surgical ICU: predictive factors, incidence, comorbidity, and outcome. J Am Soc Nephrol. 2003;14:1022–1030. doi: 10.1097/01.asn.0000059863.48590.e9. [DOI] [PubMed] [Google Scholar]
- McCullough PA, Wolyn R, Rocher LL, et al. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–375. doi: 10.1016/s0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
- Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- Mehta RH, Grab JD, O'Brien SM, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–2216. doi: 10.1161/CIRCULATIONAHA.106.635573. [DOI] [PubMed] [Google Scholar]
- Thakar CV, Worley S, Arrigain S, et al. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67:1112–1119. doi: 10.1111/j.1523-1755.2005.00177.x. [DOI] [PubMed] [Google Scholar]
- Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844–861. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- Yegenaga I, Hoste E, Van Biesen W, et al. Clinical characteristics of patients developing ARF due to sepsis/systemic inflammatory response syndrome: results of a prospective study. Am J Kidney Dis. 2004;43:817–824. doi: 10.1053/j.ajkd.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989;320:143–149. doi: 10.1056/NEJM198901193200303. [DOI] [PubMed] [Google Scholar]
- Browner WS, Li J, Mangano DT. In-hospital and long-term mortality in male veterans following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA. 1992;268:228–232. [PubMed] [Google Scholar]
- Hou SH, Bushinsky DA, Wish JB, et al. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74:243–248. doi: 10.1016/0002-9343(83)90618-6. [DOI] [PubMed] [Google Scholar]
- Singh P, Rifkin DE, Blantz RC. Chronic kidney disease: an inherent risk factor for acute kidney injury. Clin J Am Soc Nephrol. 2010;5:1690–1695. doi: 10.2215/CJN.00830110. [DOI] [PubMed] [Google Scholar]
- Lafrance JP, Djurdjev O, Levin A. Incidence and outcomes of acute kidney injury in a referred chronic kidney disease cohort. Nephrol Dial Transplant. 2010;25:2203–2209. doi: 10.1093/ndt/gfq011. [DOI] [PubMed] [Google Scholar]
- Chapin E, Zhan M, Hsu VD, et al. Adverse safety events in chronic kidney disease: the frequency of “multiple hits”. Clin J Am Soc Nephrol. 2010;5:95–101. doi: 10.2215/CJN.06210909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertow GM, Christiansen CL, Cleary PD, et al. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med. 1995;155:1505–1511. [PubMed] [Google Scholar]
- Chertow GM, Soroko SH, Paganini EP, et al. Mortality after acute renal failure: models for prognostic stratification and risk adjustment. Kidney Int. 2006;70:1120–1126. doi: 10.1038/sj.ki.5001579. [DOI] [PubMed] [Google Scholar]
- Mehta RL, Pascual MT, Gruta CG, et al. Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol. 2002;13:1350–1357. doi: 10.1097/01.asn.0000014692.19351.52. [DOI] [PubMed] [Google Scholar]
- Paganini EP, Larive B, Kanagasundaram NS. Severity scores and outcomes with acute renal failure in the ICU setting. Contrib Nephrol. 2001. pp. 181–195. [DOI] [PubMed]
- Uchino S, Bellomo R, Morimatsu H, et al. External validation of severity scoring systems for acute renal failure using a multinational database. Crit Care Med. 2005;33:1961–1967. doi: 10.1097/01.ccm.0000172279.66229.07. [DOI] [PubMed] [Google Scholar]
- Waikar SS, Curhan GC, Wald R, et al. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17:1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- Khosla N, Soroko SB, Chertow GM, et al. Preexisting chronic kidney disease: a potential for improved outcomes from acute kidney injury. Clin J Am Soc Nephrol. 2009;4:1914–1919. doi: 10.2215/CJN.01690309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouque D, Laville M. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst Rev. 2009. p. CD001892. [DOI] [PubMed]
- Fouque D, Laville M, Boissel JP, et al. Controlled low protein diets in chronic renal insufficiency: meta-analysis. BMJ. 1992;304:216–220. doi: 10.1136/bmj.304.6821.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasiske BL, Lakatua JD, Ma JZ, et al. A meta-analysis of the effects of dietary protein restriction on the rate of decline in renal function. Am J Kidney Dis. 1998;31:954–961. doi: 10.1053/ajkd.1998.v31.pm9631839. [DOI] [PubMed] [Google Scholar]
- Pedrini MT, Levey AS, Lau J, et al. The effect of dietary protein restriction on the progression of diabetic and nondiabetic renal diseases: a meta-analysis. Ann Intern Med. 1996;124:627–632. doi: 10.7326/0003-4819-124-7-199604010-00002. [DOI] [PubMed] [Google Scholar]
- Robertson L, Waugh N, Robertson A. Protein restriction for diabetic renal disease. Cochrane Database Syst Rev. 2009. p. CD002181. [DOI] [PMC free article] [PubMed]
- Menon V, Kopple JD, Wang X, et al. Effect of a very low-protein diet on outcomes: long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis. 2009;53:208–217. doi: 10.1053/j.ajkd.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Knight EL, Stampfer MJ, Hankinson SE, et al. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138:460–467. doi: 10.7326/0003-4819-138-6-200303180-00009. [DOI] [PubMed] [Google Scholar]
- Chaturvedi S, Jones C. Protein restriction for children with chronic renal failure. Cochrane Database Syst Rev. 2007. p. CD006863. [DOI] [PubMed]
- National Kidney Foundation. KDOQI clinical practice guideline for diabetes and chronic kidney disease: 2012 Update. Am J Kidney Dis. 2012;60:850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Zinman B, Cleary PA, et al. Modern-day clinical course of type 1 diabetes mellitus after 30 years' duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983-2005) Arch Intern Med. 2009;169:1307–1316. doi: 10.1001/archinternmed.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- Ichikawa H, Nagake Y, Takahashi M, et al. What is the best index of glycemic control in patients with diabetes mellitus on hemodialysis. Nihon Jinzo Gakkai Shi. 1996;38:305–308. [PubMed] [Google Scholar]
- Joy MS, Cefalu WT, Hogan SL, et al. Long-term glycemic control measurements in diabetic patients receiving hemodialysis. Am J Kidney Dis. 2002;39:297–307. doi: 10.1053/ajkd.2002.30549. [DOI] [PubMed] [Google Scholar]
- Nakao T, Matsumoto H, Okada T, et al. Influence of erythropoietin treatment on hemoglobin A1c levels in patients with chronic renal failure on hemodialysis. Intern Med. 1998;37:826–830. doi: 10.2169/internalmedicine.37.826. [DOI] [PubMed] [Google Scholar]
- Ng JM, Cooke M, Bhandari S, et al. The effect of iron and erythropoietin treatment on the A1C of patients with diabetes and chronic kidney disease. Diabetes Care. 2010;33:2310–2313. doi: 10.2337/dc10-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Chujo K, Yamada M, et al. Lower value of glycated haemoglobin relative to glycaemic control in diabetic patients with end-stage renal disease not on haemodialysis. Ann Clin Biochem. 2012;49:68–74. doi: 10.1258/acb.2011.011161. [DOI] [PubMed] [Google Scholar]
- Vos FE, Schollum JB, Coulter CV, et al. Assessment of markers of glycaemic control in diabetic patients with chronic kidney disease using continuous glucose monitoring. Nephrology (Carlton) 2012;17:182–188. doi: 10.1111/j.1440-1797.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Executive summary: Standards of medical care in diabetes--2012. Diabetes Care. 2012;35 (Suppl 1:S4–S10. doi: 10.2337/dc12-s004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Burton C, Mishra SI, Fink JC, et al. An in-depth review of the evidence linking dietary salt intake and progression of chronic kidney disease. Am J Nephrol. 2006;26:268–275. doi: 10.1159/000093833. [DOI] [PubMed] [Google Scholar]
- Swift PA, Markandu ND, Sagnella GA, et al. Modest salt reduction reduces blood pressure and urine protein excretion in black hypertensives: a randomized control trial. Hypertension. 2005;46:308–312. doi: 10.1161/01.HYP.0000172662.12480.7f. [DOI] [PubMed] [Google Scholar]
- Hoffmann IS, Cubeddu LX. Increased blood pressure reactivity to dietary salt in patients with the metabolic syndrome. J Hum Hypertens. 2007;21:438–444. doi: 10.1038/sj.jhh.1002153. [DOI] [PubMed] [Google Scholar]
- Bellizzi V, Di Iorio BR, De Nicola L, et al. Very low protein diet supplemented with ketoanalogs improves blood pressure control in chronic kidney disease. Kidney Int. 2007;71:245–251. doi: 10.1038/sj.ki.5001955. [DOI] [PubMed] [Google Scholar]
- Slagman MC, Waanders F, Hemmelder MH, et al. Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: randomised controlled trial. BMJ. 2011;343:d4366. doi: 10.1136/bmj.d4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo G, Venanzi S, Verdura C, et al. Association of uric acid with change in kidney function in healthy normotensive individuals. Am J Kidney Dis. 2010;56:264–272. doi: 10.1053/j.ajkd.2010.01.019. [DOI] [PubMed] [Google Scholar]
- Iseki K, Ikemiya Y, Inoue T, et al. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642–650. [PubMed] [Google Scholar]
- Mok Y, Lee SJ, Kim MS, et al. Serum uric acid and chronic kidney disease: the Severance cohort study. Nephrol Dial Transplant. 2012;27:1831–1835. doi: 10.1093/ndt/gfr530. [DOI] [PubMed] [Google Scholar]
- Wen CP, David Cheng TY, Chan HT, et al. Is high serum uric acid a risk marker or a target for treatment? Examination of its independent effect in a large cohort with low cardiovascular risk. Am J Kidney Dis. 2010;56:273–288. doi: 10.1053/j.ajkd.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Yamada T, Fukatsu M, Suzuki S, et al. Elevated serum uric acid predicts chronic kidney disease. Am J Med Sci. 2011;342:461–466. doi: 10.1097/MAJ.0b013e318218bd89. [DOI] [PubMed] [Google Scholar]
- Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388–1393. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu YP, Leung KT, Tong MK, et al. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Kanbay M, Huddam B, Azak A, et al. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6:1887–1894. doi: 10.2215/CJN.11451210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbay M, Ozkara A, Selcoki Y, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39:1227–1233. doi: 10.1007/s11255-007-9253-3. [DOI] [PubMed] [Google Scholar]
- Kao MP, Ang DS, Gandy SJ, et al. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2011;22:1382–1389. doi: 10.1681/ASN.2010111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito J, Matsuzawa Y, Ito H, et al. The alkalizer citrate reduces serum uric Acid levels and improves renal function in hyperuricemic patients treated with the xanthine oxidase inhibitor allopurinol. Endocr Res. 2010;35:145–154. doi: 10.3109/07435800.2010.497178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaguarnera M, Vacante M, Russo C, et al. A single dose of rasburicase in elderly patients with hyperuricaemia reduces serum uric acid levels and improves renal function. Expert Opin Pharmacother. 2009;10:737–742. doi: 10.1517/14656560902781972. [DOI] [PubMed] [Google Scholar]
- Miao Y, Ottenbros SA, Laverman GD, et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011;58:2–7. doi: 10.1161/HYPERTENSIONAHA.111.171488. [DOI] [PubMed] [Google Scholar]
- Johansen KL. Exercise and chronic kidney disease: current recommendations. Sports Med. 2005;35:485–499. doi: 10.2165/00007256-200535060-00003. [DOI] [PubMed] [Google Scholar]
- Johansen KL, Chertow GM, Ng AV, et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;57:2564–2570. doi: 10.1046/j.1523-1755.2000.00116.x. [DOI] [PubMed] [Google Scholar]
- Padilla J, Krasnoff J, Da Silva M, et al. Physical functioning in patients with chronic kidney disease. J Nephrol. 2008;21:550–559. [PubMed] [Google Scholar]
- Beddhu S, Baird BC, Zitterkoph J, et al. Physical activity and mortality in chronic kidney disease (NHANES III) Clin J Am Soc Nephrol. 2009;4:1901–1906. doi: 10.2215/CJN.01970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill DN, Torrance GW, Taylor DW, et al. Measurement of quality of life in end-stage renal disease: the time trade-off approach. Clin Invest Med. 1987;10:14–20. [PubMed] [Google Scholar]
- DeOreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis. 1997;30:204–212. doi: 10.1016/s0272-6386(97)90053-6. [DOI] [PubMed] [Google Scholar]
- Booth FW, Gordon SE, Carlson CJ, et al. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol. 2000;88:774–787. doi: 10.1152/jappl.2000.88.2.774. [DOI] [PubMed] [Google Scholar]
- Dickinson HO, Mason JM, Nicolson DJ, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;24:215–233. doi: 10.1097/01.hjh.0000199800.72563.26. [DOI] [PubMed] [Google Scholar]
- Stewart KJ. Exercise training and the cardiovascular consequences of type 2 diabetes and hypertension: plausible mechanisms for improving cardiovascular health. JAMA. 2002;288:1622–1631. doi: 10.1001/jama.288.13.1622. [DOI] [PubMed] [Google Scholar]
- Daul AE, Schafers RF, Daul K, et al. Exercise during hemodialysis. Clin Nephrol. 2004;61 Suppl 1:S26–S30. [PubMed] [Google Scholar]
- Deligiannis A. Cardiac adaptations following exercise training in hemodialysis patients. Clin Nephrol. 2004;61 Suppl 1:S39–S45. [PubMed] [Google Scholar]
- Liu SH, C LC, Yeh SH, et al. Effect of exercise training on hemodialysis. Journal of the Formosan Medical Association. 2002;6:129–142. [Google Scholar]
- Mustata S, Chan C, Lai V, et al. Impact of an exercise program on arterial stiffness and insulin resistance in hemodialysis patients. J Am Soc Nephrol. 2004;15:2713–2718. doi: 10.1097/01.ASN.0000140256.21892.89. [DOI] [PubMed] [Google Scholar]
- Ouzouni S, Kouidi E, Sioulis A, et al. Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin Rehabil. 2009;23:53–63. doi: 10.1177/0269215508096760. [DOI] [PubMed] [Google Scholar]
- Storer TW, Casaburi R, Sawelson S, et al. Endurance exercise training during haemodialysis improves strength, power, fatigability and physical performance in maintenance haemodialysis patients. Nephrol Dial Transplant. 2005;20:1429–1437. doi: 10.1093/ndt/gfh784. [DOI] [PubMed] [Google Scholar]
- Vaitkevicius PV, Fleg JL, Engel JH, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- Mustata S, Groeneveld S, Davidson W, et al. Effects of exercise training on physical impairment, arterial stiffness and health-related quality of life in patients with chronic kidney disease: a pilot study. Int Urol Nephrol. 2011;43:1133–1141. doi: 10.1007/s11255-010-9823-7. [DOI] [PubMed] [Google Scholar]
- Szromba C, Thies MA, Ossman SS. Advancing chronic kidney disease care: new imperatives for recognition and intervention. Nephrol Nurs J. 2002;29:547–559. [PubMed] [Google Scholar]
- Chen PY, Huang YC, Kao YH, et al. Effects of an exercise program on blood biochemical values and exercise stage of chronic kidney disease patients. J Nurs Res. 2010;18:98–107. doi: 10.1097/JNR.0b013e3181dda726. [DOI] [PubMed] [Google Scholar]
- Tobita I, Suzuki S, Kobayashi T, et al. A programme to encourage participation of haemodialysis patients in an exercise regimen. J Ren Care. 2009;35:48–53. doi: 10.1111/j.1755-6686.2009.00079.x. [DOI] [PubMed] [Google Scholar]
- van Vilsteren MC, de Greef MH, Huisman RM. The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in The Netherlands: results of a randomized clinical trial. Nephrol Dial Transplant. 2005;20:141–146. doi: 10.1093/ndt/gfh560. [DOI] [PubMed] [Google Scholar]
- Kosmadakis GC, John SG, Clapp EL, et al. Benefits of regular walking exercise in advanced pre-dialysis chronic kidney disease. Nephrol Dial Transplant. 2012;27:997–1004. doi: 10.1093/ndt/gfr364. [DOI] [PubMed] [Google Scholar]
- Hall JE, Crook ED, Jones DW, et al. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci. 2002;324:127–137. doi: 10.1097/00000441-200209000-00003. [DOI] [PubMed] [Google Scholar]
- Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:550–562. doi: 10.2215/CJN.04071206. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen X, Song Y, et al. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73:19–33. doi: 10.1038/sj.ki.5002586. [DOI] [PubMed] [Google Scholar]
- Hobbs H, Farmer C, Irving J, et al. Is high body mass index independently associated with diminished glomerular filtration rate? An epidemiological study. J Ren Care. 2011;37:148–154. doi: 10.1111/j.1755-6686.2011.00231.x. [DOI] [PubMed] [Google Scholar]
- Mohsen A, Brown R, Hoefield R, et al. Body mass index has no effect on rate of progression of chronic kidney disease in subjects with type 2 diabetes mellitus J Nephrol 2011. 0. [DOI] [PubMed]
- Burton JO, Gray LJ, Webb DR, et al. Association of anthropometric obesity measures with chronic kidney disease risk in a non-diabetic patient population. Nephrol Dial Transplant. 2012;27:1860–1866. doi: 10.1093/ndt/gfr574. [DOI] [PubMed] [Google Scholar]
- Kasiske BL, Napier J. Glomerular sclerosis in patients with massive obesity. Am J Nephrol. 1985;5:45–50. doi: 10.1159/000166902. [DOI] [PubMed] [Google Scholar]
- Hsu CY, McCulloch CE, Iribarren C, et al. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- Navaneethan SD, Yehnert H, Moustarah F, et al. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4:1565–1574. doi: 10.2215/CJN.02250409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshinnia F, Wilt TJ, Duval S, et al. Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant. 2010;25:1173–1183. doi: 10.1093/ndt/gfp640. [DOI] [PubMed] [Google Scholar]
- Orth SR, Hallan SI. Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients--absence of evidence or evidence of absence. Clin J Am Soc Nephrol. 2008;3:226–236. doi: 10.2215/CJN.03740907. [DOI] [PubMed] [Google Scholar]
- Jungers P, Massy ZA, Nguyen Khoa T, et al. Incidence and risk factors of atherosclerotic cardiovascular accidents in predialysis chronic renal failure patients: a prospective study. Nephrol Dial Transplant. 1997;12:2597–2602. doi: 10.1093/ndt/12.12.2597. [DOI] [PubMed] [Google Scholar]
- Muntner P, He J, Astor BC, et al. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16:529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- Myllymaki J, Syrjanen J, Helin H, et al. Vascular diseases and their risk factors in IgA nephropathy. Nephrol Dial Transplant. 2006;21:1876–1882. doi: 10.1093/ndt/gfl062. [DOI] [PubMed] [Google Scholar]
- Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- Chase HP, Garg SK, Marshall G, et al. Cigarette smoking increases the risk of albuminuria among subjects with type I diabetes. JAMA. 1991;265:614–617. [PubMed] [Google Scholar]
- Gambaro G, Bax G, Fusaro M, et al. Cigarette smoking is a risk factor for nephropathy and its progression in type 2 diabetes mellitus. Diabetes Nutr Metab. 2001;14:337–342. [PubMed] [Google Scholar]
- Hallan SI, Orth SR. Smoking is a risk factor in the progression to kidney failure. Kidney Int. 2011;80:516–523. doi: 10.1038/ki.2011.157. [DOI] [PubMed] [Google Scholar]
- Sawicki PT, Didjurgeit U, Muhlhauser I, et al. Smoking is associated with progression of diabetic nephropathy. Diabetes Care. 1994;17:126–131. doi: 10.2337/diacare.17.2.126. [DOI] [PubMed] [Google Scholar]
- Sung RS, Althoen M, Howell TA, et al. Excess risk of renal allograft loss associated with cigarette smoking. Transplantation. 2001;71:1752–1757. doi: 10.1097/00007890-200106270-00009. [DOI] [PubMed] [Google Scholar]
- Inker LA, Coresh J, Levey AS, et al. Estimated GFR, albuminuria, and complications of chronic kidney disease. J Am Soc Nephrol. 2011;22:2322–2331. doi: 10.1681/ASN.2010111181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemiaIn: de Benoist B, McLean E, Egli I, and Cogswell M (eds),2008
- Beall CM, Goldstein MC. Hemoglobin concentration of pastoral nomads permanently resident at 4,850-5,450 meters in Tibet. Am J Phys Anthropol. 1987;73:433–438. doi: 10.1002/ajpa.1330730404. [DOI] [PubMed] [Google Scholar]
- Cresanta JL, Croft JB, Webber LS, et al. Racial difference in hemoglobin concentration of young adults. Prev Med. 1987;16:659–669. doi: 10.1016/0091-7435(87)90049-1. [DOI] [PubMed] [Google Scholar]
- Meyers LD, Habicht JP, Johnson CL. Components of the difference in hemoglobin concentrations in blood between black and white women in the United States. Am J Epidemiol. 1979;109:539–549. doi: 10.1093/oxfordjournals.aje.a112712. [DOI] [PubMed] [Google Scholar]
- Pan WH, Habicht JP. The non-iron-deficiency-related difference in hemoglobin concentration distribution between blacks and whites and between men and women. Am J Epidemiol. 1991;134:1410–1416. doi: 10.1093/oxfordjournals.aje.a116046. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. CDC criteria for anemia in children and childbearing-aged women. MMWR Morb Mortal Wkly Rep. 1989;38:400–404. [PubMed] [Google Scholar]
- Levin A, Djurdjev O, Thompson C, et al. Canadian randomized trial of hemoglobin maintenance to prevent or delay left ventricular mass growth in patients with CKD. Am J Kidney Dis. 2005;46:799–811. doi: 10.1053/j.ajkd.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- Roger SD, McMahon LP, Clarkson A, et al. Effects of early and late intervention with epoetin alpha on left ventricular mass among patients with chronic kidney disease (stage 3 or 4): results of a randomized clinical trial. J Am Soc Nephrol. 2004;15:148–156. doi: 10.1097/01.asn.0000102471.89084.8b. [DOI] [PubMed] [Google Scholar]
- Moranne O, Froissart M, Rossert J, et al. Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol. 2009;20:164–171. doi: 10.1681/ASN.2008020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalotti JA, Uribarri J, Chen SC, et al. Trends in mineral metabolism: Kidney Early Evaluation Program (KEEP) and the National Health and Nutrition Examination Survey (NHANES) 1999-2004. Am J Kidney Dis. 2008;51:S56–S68. doi: 10.1053/j.ajkd.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Stevens LA, Djurdjev O, Cardew S, et al. Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: evidence for the complexity of the association between mineral metabolism and outcomes. J Am Soc Nephrol. 2004;15:770–779. doi: 10.1097/01.asn.0000113243.24155.2f. [DOI] [PubMed] [Google Scholar]
- Gutierrez OM, Isakova T, Andress DL, et al. Prevalence and severity of disordered mineral metabolism in Blacks with chronic kidney disease. Kidney Int. 2008;73:956–962. doi: 10.1038/ki.2008.4. [DOI] [PubMed] [Google Scholar]
- Gutierrez OM, Farwell WR, Kermah D, et al. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22:1745–1753. doi: 10.1007/s00198-010-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Kidney Foundation. KDOQI clinical practice guideline for nutrition in children with CKD: 2008 update. Am J Kidney Dis. 2009;53:S1–124. doi: 10.1053/j.ajkd.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Nickolas TL, Leonard MB, Shane E. Chronic kidney disease and bone fracture: a growing concern. Kidney Int. 2008;74:721–731. doi: 10.1038/ki.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetta J, Boutroy S, Vilayphiou N, et al. Early impairment of trabecular microarchitecture assessed with HR-pQCT in patients with stage II-IV chronic kidney disease. J Bone Miner Res. 2010;25:849–857. doi: 10.1359/jbmr.090831. [DOI] [PubMed] [Google Scholar]
- Nickolas TL, Stein E, Cohen A, et al. Bone mass and microarchitecture in CKD patients with fracture. J Am Soc Nephrol. 2010;21:1371–1380. doi: 10.1681/ASN.2009121208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickolas TL, Cremers S, Zhang A, et al. Discriminants of prevalent fractures in chronic kidney disease. J Am Soc Nephrol. 2011;22:1560–1572. doi: 10.1681/ASN.2010121275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305:1119–1127. doi: 10.1001/jama.2011.308. [DOI] [PubMed] [Google Scholar]
- Adeney KL, Siscovick DS, Ix JH, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol. 2009;20:381–387. doi: 10.1681/ASN.2008040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257–264. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist MK, Chiarelli G, Lim L, et al. Early initiation of phosphate lowering dietary therapy in non-dialysis chronic kidney disease: a critical review. J Ren Care. 2009;35 Suppl 1:71–78. doi: 10.1111/j.1755-6686.2009.00064.x. [DOI] [PubMed] [Google Scholar]
- Lynch KE, Lynch R, Curhan GC, et al. Prescribed dietary phosphate restriction and survival among hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:620–629. doi: 10.2215/CJN.04620510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaneethan SD, Palmer SC, Vecchio M, et al. Phosphate binders for preventing and treating bone disease in chronic kidney disease patients. Cochrane Database Syst Rev. 2011. p. CD006023. [DOI] [PubMed]
- Mehrotra R, Kermah D, Budoff M, et al. Hypovitaminosis D in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1144–1151. doi: 10.2215/CJN.05781207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaneethan SD, Schold JD, Arrigain S, et al. Low 25-hydroxyvitamin D levels and mortality in non-dialysis-dependent CKD. Am J Kidney Dis. 2011;58:536–543. doi: 10.1053/j.ajkd.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandula P, Dobre M, Schold JD, et al. Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol. 2011;6:50–62. doi: 10.2215/CJN.03940510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SC, McGregor DO, Craig JC, et al. Vitamin D compounds for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev. 2009. p. CD008175. [DOI] [PubMed]
- Miller PD. The kidney and bisphosphonates. Bone. 2011;49:77–81. doi: 10.1016/j.bone.2010.12.024. [DOI] [PubMed] [Google Scholar]
- Torregrosa JV, Ramos AM. Use of bisphosphonates in chronic kidney disease] Nefrologia. 2010;30:288–296. doi: 10.3265/Nefrologia.pre2010.Apr.10320. [DOI] [PubMed] [Google Scholar]
- Bhan I, Dubey A, Wolf M. Diagnosis and management of mineral metabolism in CKD. J Gen Intern Med. 2010;25:710–716. doi: 10.1007/s11606-010-1316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney AE, Leonard N, McCloskey MC, et al. Bisphosphonate prescribing in chronic kidney disease. J R Coll Physicians Edinb. 2009;39:4–9. [Google Scholar]
- Ayus JC, Krothapalli RK. Effect of bicarbonate administration on cardiac function. Am J Med. 1989;87:5–6. doi: 10.1016/s0002-9343(89)80475-9. [DOI] [PubMed] [Google Scholar]
- Bailey JL, Wang X, England BK, et al. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J Clin Invest. 1996;97:1447–1453. doi: 10.1172/JCI118566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domrongkitchaiporn S, Pongskul C, Sirikulchayanonta V, et al. Bone histology and bone mineral density after correction of acidosis in distal renal tubular acidosis. Kidney Int. 2002;62:2160–2166. doi: 10.1046/j.1523-1755.2002.00656.x. [DOI] [PubMed] [Google Scholar]
- Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant. 2009;24:1232–1237. doi: 10.1093/ndt/gfn633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak RH. Effect of metabolic acidosis on insulin action and secretion in uremia. Kidney Int. 1998;54:603–607. doi: 10.1046/j.1523-1755.1998.00023.x. [DOI] [PubMed] [Google Scholar]
- Menon V, Tighiouart H, Vaughn NS, et al. Serum bicarbonate and long-term outcomes in CKD. Am J Kidney Dis. 2010;56:907–914. doi: 10.1053/j.ajkd.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Mitch WE, Price SR. Mechanisms activated by kidney disease and the loss of muscle mass. Am J Kidney Dis. 2001;38:1337–1342. doi: 10.1053/ajkd.2001.29249. [DOI] [PubMed] [Google Scholar]
- Navaneethan SD, Schold JD, Arrigain S, et al. Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2395–2402. doi: 10.2215/CJN.03730411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael KL, Wei G, Baird BC, et al. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int. 2011;79:356–362. doi: 10.1038/ki.2010.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SN, Abramowitz M, Hostetter TH, et al. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis. 2009;54:270–277. doi: 10.1053/j.ajkd.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright R.Reports of Medical Cases, Selected with a View of Illustrating the Symptoms and Cure of Diseases by a Reference to Morbid Anatomy, Volume 1. London: Brown & Green.1827 [PMC free article] [PubMed]
- Osman AA. The value of alkalis in the treatment of chronic nephritis. Lancet. 1930;2:945–959. [Google Scholar]
- Lyon DM, Dunlop DM, Stewart CP. The alkaline treatment of chronic nephritis. Lancet. 1931;2:1009–1013. [Google Scholar]
- Rustom R, Grime JS, Costigan M, et al. Oral sodium bicarbonate reduces proximal renal tubular peptide catabolism, ammoniogenesis, and tubular damage in renal patients. Ren Fail. 1998;20:371–382. doi: 10.3109/08860229809045124. [DOI] [PubMed] [Google Scholar]
- Mathur RP, Dash SC, Gupta N, et al. Effects of correction of metabolic acidosis on blood urea and bone metabolism in patients with mild to moderate chronic kidney disease: a prospective randomized single blind controlled trial. Ren Fail. 2006;28:1–5. doi: 10.1080/08860220500461187. [DOI] [PubMed] [Google Scholar]
- de Brito-Ashurst I, Varagunam M, Raftery MJ, et al. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20:2075–2084. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phisitkul S, Khanna A, Simoni J, et al. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int. 2010;77:617–623. doi: 10.1038/ki.2009.519. [DOI] [PubMed] [Google Scholar]
- Husted FC, Nolph KD, Maher JF. NaHCO3 and NaC1 tolerance in chronic renal failure. J Clin Invest. 1975;56:414–419. doi: 10.1172/JCI108107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husted FC, Nolph KD. NaHCO3 and NaCl tolerance in chronic renal failure II. Clin Nephrol. 1977;7:21–25. [PubMed] [Google Scholar]