Abstract
Aims
The purpose of this study is to elucidate the clinical manifestations and the current treatment status of cytomegalovirus (CMV) endotheliitis via a large case series obtained from a national survey conducted in Japan.
Methods
The Japan Corneal Endotheliitis Study Group proposed diagnostic criteria for CMV endotheliitis based on a viral examination by PCR of aqueous humour, in combination with clinical manifestations. A national survey was then retrospectively conducted among 1160 members of the Japan Cornea Society. The study reviewed the patient profiles, clinical manifestations, and treatment modalities of individuals who met the diagnostic criteria for CMV endotheliitis.
Results
The study included 109 eyes of 106 patients. Mean patient age was 66.9±10.9 years (85 males (80.2%), 21 females (19.8%)). Patients were commonly diagnosed with anterior uveitis and ocular hypertension prior to confirmation of CMV endotheliitis. Coin-shaped lesions were observed in 70.6%, and linear keratic precipitates in 8.3% of the patients, respectively. 95% of cases were treated with anti-CMV drugs.
Conclusions
CMV endotheliitis is most common in middle-aged and elderly men. CMV endotheliitis should be suspected when patients present with corneal endotheliitis involving coin-shaped lesions accompanied by anterior uveitis and ocular hypertension.
Keywords: Cornea, Infection
Introduction
Corneal endotheliitis is a corneal endothelium-specific inflammation first described by Khodadoust and Attarzadeh in 1982.1 It is characterised by corneal oedema, keratic precipitates (KPs), a mild anterior chamber reaction, and the destruction of corneal endothelium. There are reportedly four clinical forms of cornea endotheliitis, of which, linear corneal endotheliitis is considered the most serious form due to the progressive loss of corneal endothelial cells.2 Corneal endotheliitis is thought to be a viral infection,3 especially in association with herpes simplex virus (HSV),4 5 varicella-zoster virus (VZV)6 and mumps virus.7 However, cases of corneal endotheliitis are often observed to be negative for HSV and VZV, and unresponsive to acyclovir treatment. Such cases are diagnosed as ‘idiopathic corneal endotheliitis’,3 and their prognosis is poor due to the progressive destruction of corneal endothelium that is resistant to antiviral medication.
In 2006, we reported the first case of cytomegalovirus (CMV)-induced corneal endotheliitis in which CMV DNA was detected from the aqueous humour of the patient via PCR, and that showed a positive response to ganciclovir treatment.8 Clinical evidence of CMV endotheliitis has been accumulated,8–24 and the current emphasis is on the importance of early diagnosis and treatment. However, few large series have been reported, and diagnostic criteria for the disease have yet to be established.
The purpose of this study was to elucidate the clinical manifestations, as well as the current status of clinical diagnosis and treatment of CMV endotheliitis via a large case series obtained from the national survey conducted by the Japan Corneal Endotheliitis Study Group (JCESG) in accordance with the diagnostic criteria for CMV endotheliitis instituted by the JCESG.
Methods
Prior to this survey, the JCESG proposed diagnostic criteria for CMV endotheliitis (box 1, figure 1), which were authorised by the Japanese Ministry of Health, Labour and Welfare. Questionnaire surveys on diagnostic criteria were distributed to 1160 members of the Japan Cornea Society. Members with patients who met the diagnostic criteria for CMV endotheliitis were requested to provide relevant data for each patient diagnosed with the disease between November 2004 and November 2011, comprising: demographics, clinical aspects, diagnostic protocol and treatment. This retrospective study was approved by the University Ethical Committees of Kyoto Prefectural University of Medicine, Doshisha University, and each of the other university hospitals associated with the study group.
Box 1 Diagnostic criteria of cytomegalovirus corneal endotheliitis (established by the Japan Corneal Endotheliitis Study Group which was authorised by the Japanese Ministry of Health, Labour and Welfare).
I. Viral examination by PCR of aqueous humour Positive for CMV DNA, but negative for HSV DNA and VZV DNA
-
II. Clinical manifestations
i. Corneal endotheliitis with coin-shaped lesion or linear KPs similar to the rejection line.
- ii. Corneal endotheliitis with localised corneal oedema with KPs associated with two of the following signs:
- recurrent/chronic anterior uveitis
- ocular hypertension/secondary glaucoma
- corneal endothelial cell loss.
Typical CMV endotheliitis: I and II-i
Atypical CMV endotheliitis: I and II-ii
CMV, cytomegalovirus; HSV, herpes simplex virus; KP, keratic precipitate; PCR, polymerase chain reaction; VZV, varicella-zoster virus.
Figure 1.
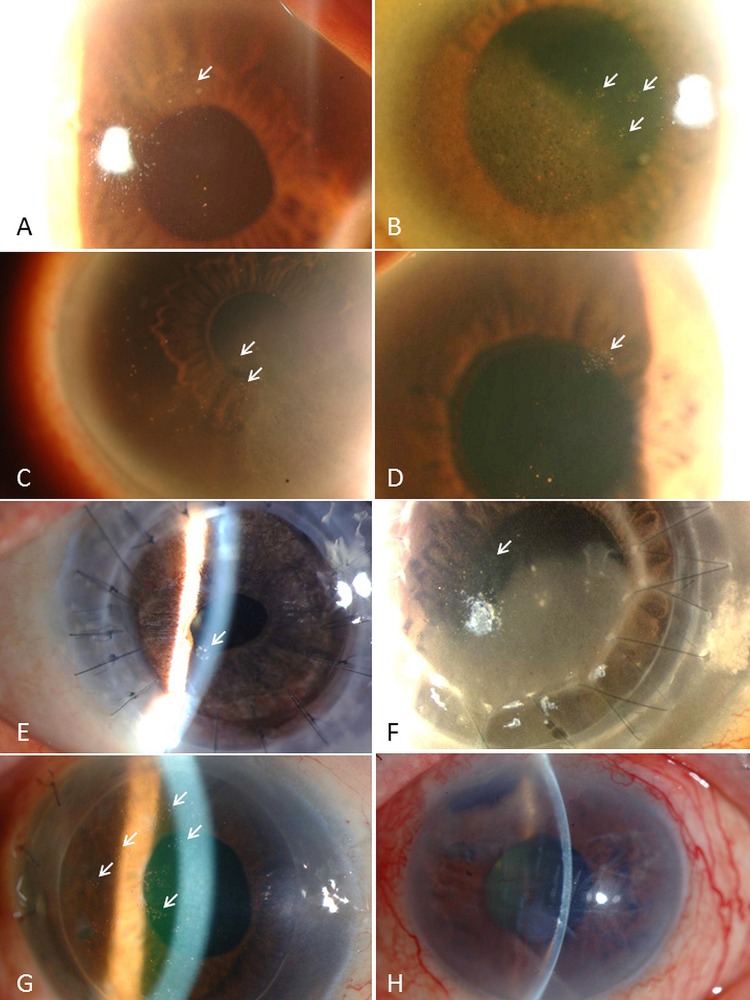
Representative anterior-segment photographs of cytomegalovirus (CMV) corneal endotheliitis. (A–G) Typical CMV endotheliitis: CMV endotheliitis-associated coin-shaped lesions (white arrows). (H) Atypical CMV endotheliitis: CMV endotheliitis not associated with coin-shaped lesions. This case showed corneal oedema with keratic precipitates associated with recurrent anterior uveitis and secondary glaucoma.
Statistical analysis
Statistical analysis was performed using JMP software (V.9.1, SAS Institute, Cary, North Carolina, USA). The association between the duration of disease and best-corrected visual acuity (BCVA), as well as between patient age and corneal endothelial cell density, were analysed via Spearman's test. The effect of anti-CMV medication was analysed via Welch's test. The association between pretreatment and post-treatment BCVA was analysed via paired t test.
Results
A total of 109 eyes of 106 patients were reported from 30 facilities (all hospitals are listed in the online supplementary appendix). Three patients had bilateral eye involvement; 79 were diagnosed as ‘typical CMV endotheliitis’ and 30 were diagnosed as ‘atypical CMV endotheliitis’. The mean follow-up period postdiagnosis of CMV endotheliitis was 27.2±18.8 months.
Patient age and gender
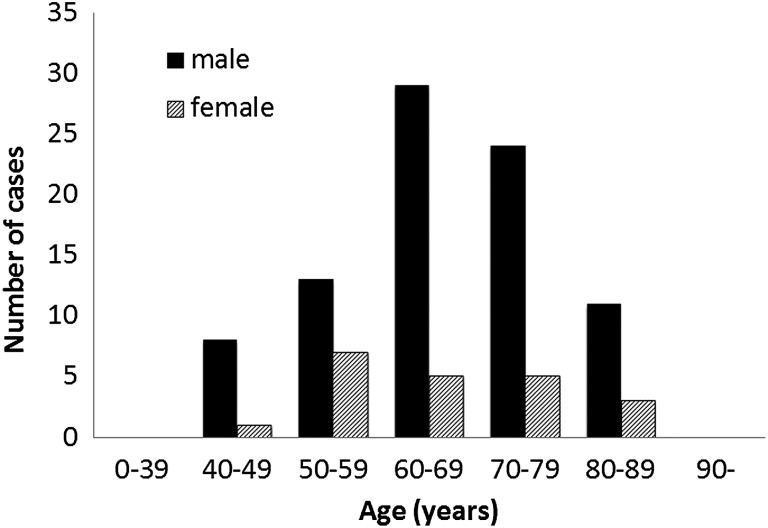
The age and gender of patients diagnosed with CMV endotheliitis is shown in figure 2. Mean age was 66.9±10.9 years (range: 40–85 years). Eighty-five cases (80.2%) were male and 21 (19.8%) cases were female.
Figure 2.
Age and gender of patients with cytomegalovirus (CMV) corneal endotheliitis. Mean patient age was 66.9±10.9 years (range: 40–85 years). Eighty-five cases: (80.2%) were men and 21 (19.8%) cases were women (n=106).
History of systemic and ocular disease
Seventeen patients (16.0%) had been diagnosed with diabetes, 22 (20.8%) with hypertension, and 10 (9.4%) with cancer (under treatment or treated). There were no reports of HIV infection. Almost half the patients had been previously diagnosed with anterior uveitis (48.6%), Posner–Schlossman syndrome (36.7%) or secondary glaucoma/ocular hypertension (39.4%). With regard to surgical treatment prior to the diagnosis of CMV endotheliitis, corneal transplantation was performed in 29 eyes (25.7%), glaucoma surgery in 33 eyes (30.3%) and cataract surgery in 60 eyes (55%).
History of medical treatment prior to diagnosis of CMV endotheliitis
Steroids and immunosuppressive agents
Fifteen patients (14.2%) received systemic administration of steroids, and 105 eyes of 103 patients (97.1%) received topical administration. Three patients who had multiple grafts (three repeated grafts in each case) had received systemic immunosuppressive agents to prevent graft rejection. Two of those patients received systemic cyclosporine and one patient received systemic tacrolimus (FK506).
Antiviral agents
Topical aciclovir ointment was previously used in 35 eyes (32.1%) and oral valaciclovir was used in 28 patients (26.4%). Systemic acyclovir, ganciclovir or valganciclovir were also used in 1 patient each.
Antiglaucoma agents
Seventy-one eyes (65.1%) had undergone treatment with topical antiglaucoma agents. Acetazolamide tablets were also administered to 28 patients (26.4%).
Viral examination
Qualitative and/or quantitative PCR examination using an aqueous sample obtained from each of the 109 eyes (100%) showed positive for CMV DNA, but negative for HSV and VZV DNA (all being diagnostic criteria for CMV endotheliitis). Serum CMV immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies had been tested in 30 and 20 patients, respectively. All the 30 patients (100%) tested positive for CMV IgG antibody and all the 20 patients (100%) tested negative for IgM antibody. Twenty-two patients had been serum tested for CMV pp65 antigen, and all tested negative.
Clinical investigations and ocular manifestations at the diagnosis of CMV endotheliitis
BCVA
Mean BCVA (logMAR) at the time of diagnosis was 0.448. No correlation was found between duration of disease and BCVA at the time of diagnosis (Spearman's test, p=0.402).
Intraocular pressure
Mean intraocular pressure (IOP) was 19.6±9.8 mm Hg (mean±SD). Despite the use of antiglaucoma therapy in 71 eyes and previous glaucoma surgery in 33 eyes, 42 eyes (38.5%) showed high IOP (>22 mm Hg) at the time of diagnosis of CMV endotheliitis.
Corneal endothelial density
Corneal endothelial density (CED) was measured in 62 eyes, and mean CED was 1346.2±729.2 cells/mm2 (mean±SD). CEDs were broadly distributed among the patients, and no correlation was found between patient age and CED at the time of diagnosis (Spearman's test, p=0.513).
Clinical findings at diagnosis
Coin-shaped lesion KPs and corneal oedema were observed in 70.6% and 73.4% of the eyes, respectively. Linear KPs were noted in 8.3% of the eyes, and anterior chamber inflammation was detected in 67.9% of the eyes. Investigation of lens, angle and vitreoretinal abnormality revealed 20 eyes (18.3%) with cataract and 60 eyes (55.0%) that had undergone intraocular lens implantation. Gonioscopic examination results were reported for 33 eyes (30.2%), of which 13 showed some findings such as peripheral anterior synechia, or decreased/increased pigmentation of the trabecular meshwork.
Medical treatment for CMV endotheliitis
In Japan, ganciclovir is available for the treatment of intravenous infusion, and valganciclovir hydrochloride tablets for CMV infection. The results of our survey showed that anti-CMV agents were used in 104 of the total 109 eyes, either systemically, topically, or both. Systemic administration of anti-CMV drugs (either ganciclovir or valganciclovir) was performed in 74 eyes (67.9%), and topical administration of ganciclovir prepared from vials for intravenous infusion11 13 was also used in 82 eyes (75.2%). Fifty-two eyes (47.7%) received both systemic and topical anti-CMV treatment. The effect of anti-CMV treatment was clinically evaluated 1 month after initiation of anti-CMV treatment in 96 eyes, and was scored from 0 to 2— 0: ineffective (no change in corneal findings); 1: partially effective (corneal oedema and KPs improved, yet did not completely disappear); 2: greatly effective (significant improvement of corneal oedema and KPs)) (table 1). Combined anti-CMV therapy (systemic and topical) was more effective than systemic or topical anti-CMV treatment alone (Welch's test, p=0.09), but the difference was non-significant. During the observation period, 39 eyes (36%) showed recurrence of inflammation, and anti-CMV treatment was repeated on those eyes.
Table 1.
Effects of anti-CMV treatment
| Effective (%) | Partially effective (%) | Not effective (%) | |
|---|---|---|---|
| Systemic or topical GCV (n=44) | 52.3 | 34.1 | 13.6 |
| Systemic and topical GCV (n=52) | 67.3 | 26.9 | 5.8 |
CMV, cytomegalovirus; GCV, ganciclovir.
Corneal endothelial function and BCVA at final examination
At the final examination, corneal clarity was successfully retained in 66 eyes (60.6%) without additional corneal transplantation, and the mean corneal endothelial cell density among those cases was 1174.5±69.3 cells/mm2 (mean±SD). Forty-three eyes (39.4%) failed to retain corneal endothelial function during the observation period. Of those, 20 eyes (18.3%) recovered corneal clarity after undergoing corneal transplantation; the remaining 23 eyes (21.1%) with corneal endothelial dysfunction were under consideration for corneal transplantation or were unsuitable for corneal transplantation due to glaucomatous optic disc atrophy. At the final examination, BCVA was evaluated in 107 eyes, and the mean BCVA at follow-up was 0.403 (logMAR). No significant difference was found between pretreatment BCVAs and those at the final examination (paired t test, p=0.376).
Discussion
CMV endotheliitis are most common among middle-aged and elderly men. This finding of male dominancy is consistent with those of previous case-series reports involving non-Japanese populations.9 20–22
We previously reported coin-shaped lesions (or nummular KPs) and linear KPs as characteristic findings of CMV endotheliitis,8 13 and they are often reported in other cases of CMV endotheliitis.9–11 15 17 18 20 23 In this present study, coin-shaped lesions and linear KPs were noted in 70.6% and 8.3% of eyes, respectively. Although we were unable to compare these findings with the incidence in CMV-negative corneal endotheliitis, we consider that coin-shaped lesions and linear KPs are highly typical of CMV endotheliitis. It should be also noted that the presence of coin-shaped lesions and linear KPs can be transient. We expected to identify CMV endotheliitis cases without coin-shaped lesions or linear KPs via the diagnostic criteria for ‘atypical CMV endotheliitis’. In our study, 30 eyes were reported as atypical CMV endotheliitis. Our findings confirmed the existence of overlap cases of CMV corneal endotheliitis and CMV anterior uveitis or inflammatory ocular hypertensive syndrome. The results also revealed that a large number of cases had previously undergone corneal transplantations, indicating the importance of a high index of suspicion for CMV endotheliitis as a cause of graft failure, or a primary cause of corneal endothelial dysfunction that led to the corneal transplantation. Anti-CMV agents had been widely used. When both systemic and topical anti-CMV agents were administered, most patients achieved improvement or complete remission of KPs and corneal oedema. However, 5.8% of cases treated by systemic and topical anti-CMV agents in combination failed to respond to the treatment. Those cases had severe corneal endothelial damage at the time of diagnosis. The data for those patients show that anti-CMV agents are effective for the treatment of active CMV endotheliitis; however, if corneal endothelial damage is severe, CMV endotheliitis often results in irreversible corneal endothelial dysfunction. In terms of visual acuity, no significant improvement in BCVA was found between pretreatment and final examination. We speculate that this study included non-negligible number of patients who had a long history of idiopathic corneal endotheliitis that was resistant to antiherpetic medication. This possibility highlights the need for early recognition and treatment of CMV endotheliitis in order to prevent irreversible corneal endothelial dysfunction. Since this survey was conducted retrospectively, it has limited ability to evaluate the effect of anti-CMV treatment. Further research is needed to evaluate the effect of antiviral treatment by comparing the viral load of aqueous humour before and after treatment via real-time PCR, as some members of the study group have reported previously.15 16 In this retrospective study, we were unable to obtain any Goldmann–Witmer coefficient data for CMV because the anti-CMV IgG titre in aqueous humour was not examined in most of the cases. However, calculation of the Goldmann–Witmer coefficient might be a useful diagnostic tool in addition to the PCR analysis for the diagnosis of CMV endotheliitis, especially in atypical cases.
The pathogenic mechanism of CMV endotheliitis has yet to be defined. Previously, Zhen et al25 reported that anterior chamber-associated immune deviation may play an important role in the pathogenesis of herpetic corneal endotheliitis. One possible background factor for the reactivation of CMV within the anterior chamber is local or systemic use of intensive immunosuppressive medication. It should be noted that topical steroid eye drops were applied to 96.3% of the eyes at the time of CMV endotheliitis diagnosis. Although none of the patients were HIV-positive, we theorise that local immunosuppression might have facilitated CMV reactivation. From another perspective, 25.7% of eyes had previously received corneal transplantation. Although idiopathic corneal endotheliitis (presumably caused by CMV) was reported as the primary diagnosis in most of those cases, the possibility of CMV transmission via donor tissue cannot be excluded.21 However, the suitability of CMV-seropositive donor tissue for corneal transplantation requires further study because serological examination of the donors for CMV was not performed in this study. To prevent recurrence of CMV endotheliitis and to preserve long-term corneal endothelial function, a maintenance therapy using topical anti-CMV agents needs to be developed.
In conclusion, this large case-series study revealed that CMV endotheliitis was common in middle-aged and elderly men. CMV endotheliitis should be suspected, and the performance of PCR of aqueous humour should be evaluated when encountering patients with corneal endotheliitis with coin-shaped lesions or linear KPs, corneal endotheliitis associated with anterior uveitis, elevated IOP, or corneal endothelial cell loss. The findings of this study suggest the effectiveness of antiviral treatment for CMV endotheliitis; however, further study is needed to establish treatment regimens for the disease in order to prevent corneal endothelial dysfunction. To the best of our knowledge, this is the largest case-series of CMV endotheliitis to report the comprehensive clinical features of this newly identified infectious corneal disease.
Supplementary Material
Acknowledgments
The authors wish to thank Drs Kozaburo Hayashi (Kinki University), Chie Sotozono (Kyoto Prefectural University of Medicine), Kazuichi Maruyama (Tohoku University), Hiroko Nakagawa (Kyoto Prefectural University of Medicine), Akiko Fukumoto (Kyoto Prefectural University of Medicine), and Naoki Okumura (Doshisha University)—all of whom are members of the Japan Corneal Endotheliitis Study Group, for collecting the clinical data, and for their useful discussion; Mr John Bush for reviewing the manuscript; and Dr Katsumi Yagi (Senior Researcher, Louis Pasteur Center for Medical Research) for the statistical analysis.
Footnotes
Contributors: Conception and design of the study (NK, TI, SK); analysis and interpretation (NK, TI, TS, AS, YO, MK, DM, YI, TS, KN, HT, SS, MM, SK); writing of the article (NK, TI, YI); critical revision of the article (YO, YI, KN, MM, SK); final approval of the article (NK, TI, ST, AS, YO, MK, DM, YI, TS, KN, HT, SS, MM, SK); data collection (NK, TI, TS, AS, YO, MK, DM, YI, TS, KN, HT, SS, MM, SK); obtaining funding (NK, TI, YO, YI, KN, MM); literature search (NK, TI, YI ); administrative, technical, or logistic support (YI, MM, SK).
Funding: This work was supported in part by Health and Labour Sciences Research Grants (Research on Intractable Diseases) from the Ministry of Health, Labour and Welfare of Japan.
Competing interests: None.
Ethics approval: The University Ethical Committees of Kyoto Prefectural University of Medicine and Doshisha University.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Khodadoust AA, Attarzadeh A. Presumed autoimmune corneal endotheliopathy. Am J Ophthalmol 1982;93:718–22. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki T, Ohashi Y. Corneal endotheliitis. Semin Ophthalmol 2008;23:235–40. [DOI] [PubMed] [Google Scholar]
- 3.Ohashi Y, Kinoshita S, Mano T, et al. Idiopathic corneal endotheliopathy. A report of two cases. Arch Ophthalmol 1985;103:1666–8. [DOI] [PubMed] [Google Scholar]
- 4.Robin JB, Steigner JB, Kaufman HE. Progressive herpetic corneal endotheliitis. Am J Ophthalmol 1985;100:336–7. [DOI] [PubMed] [Google Scholar]
- 5.Ohashi Y, Yamamoto S, Nishida K, et al. Demonstration of herpes simplex virus DNA in idiopathic corneal endotheliopathy. Am J Ophthalmol 1991;112:419–23. [DOI] [PubMed] [Google Scholar]
- 6.Maudgal PC, Missotten L, De Clercq E, et al. Varicella-zoster virus in the human corneal endothelium: a case report. Bull Soc Belge Ophtalmol 1980;190:71–86. [PubMed] [Google Scholar]
- 7.Singh K, Sodhi PK. Mumps-induced corneal endotheliitis. Cornea 2004;23:400–2. [DOI] [PubMed] [Google Scholar]
- 8.Koizumi N, Yamasaki K, Kawasaki S, et al. Cytomegalovirus in aqueous humor from an eye with corneal endotheliitis. Am J Ophthalmol 2006;141:564–5. [DOI] [PubMed] [Google Scholar]
- 9.Chee SP, Bacsal K, Jap A, et al. Corneal endotheliitis associated with evidence of cytomegalovirus infection. Ophthalmology 2007;114:798–803. [DOI] [PubMed] [Google Scholar]
- 10.Shiraishi A, Hara Y, Takahashi M, et al. Demonstration of “owl's eye” morphology by confocal microscopy in a patient with presumed cytomegalovirus corneal endotheliitis. Am J Ophthalmol 2007;143:715–17. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, Hara Y, Uno T, et al. DNA of cytomegalovirus detected by PCR in aqueous of patient with corneal endotheliitis after penetrating keratoplasty. Cornea 2007;26:370–2. [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi Y, Suzuki J, Sakai J, et al. A case of hypertensive keratouveitis with endotheliitis associated with cytomegalovirus. Ocul Immunol Inflamm 2007;15:399–401. [DOI] [PubMed] [Google Scholar]
- 13.Koizumi N, Suzuki T, Uno T, et al. Cytomegalovirus as an etiologic factor in corneal endotheliitis. Ophthalmology 2008;115:292–7. [DOI] [PubMed] [Google Scholar]
- 14.Anshu A, Chee SP, Mehta JS, et al. Cytomegalovirus endotheliitis in Descemet's stripping endothelial keratoplasty. Ophthalmology 2009;116:624–30. [DOI] [PubMed] [Google Scholar]
- 15.Kandori M, Inoue T, Takamatsu F, et al. Prevalence and features of keratitis with quantitative polymerase chain reaction positive for cytomegalovirus. Ophthalmology 2010;117:216–22. [DOI] [PubMed] [Google Scholar]
- 16.Miyanaga M, Sugita S, Shimizu N, et al. A significant association of viral loads with corneal endothelial cell damage in cytomegalovirus anterior uveitis. Br J Ophthalmol 2010;94:336–40. [DOI] [PubMed] [Google Scholar]
- 17.Kandori M, Miyazaki D, Yakura K, et al. Relationship between the number of cytomegalovirus in anterior chamber and severity of anterior segment inflammation. Jpn J Ophthalmol 2013;57:497–502. [DOI] [PubMed] [Google Scholar]
- 18.Shimazaki J, Harashima A, Tanaka Y. Corneal endotheliitis with cytomegalovirus infection of corneal stroma. Eye (Lond) 2010;24:1105–7. [DOI] [PubMed] [Google Scholar]
- 19.Chee SP, Jap A. Immune ring formation associated with cytomegalovirus endotheliitis. Am J Ophthalmol 2011;152:449–53. [DOI] [PubMed] [Google Scholar]
- 20.Hwang YS, Shen CR, Chang SH, et al. The validity of clinical feature profiles for cytomegaloviral anterior segment infection. Graefes Arch Clin Exp Ophthalmol 2011;249:103–10. [DOI] [PubMed] [Google Scholar]
- 21.Lusthaus JA, Kim P, Steller AK, et al. Successful corneal autograft after clearance of anterior chamber cytomegalovirus with oral valganciclovir in a patient with multiple failed corneal allografts. Cornea 2011;30:1054–7. [DOI] [PubMed] [Google Scholar]
- 22.Chee SP, Jap A. Treatment outcome and risk factors for visual loss in Cytomegalovirus endotheliitis. Graefes Arch Clin Exp Ophthalmol 2012;250: 383–9. [DOI] [PubMed] [Google Scholar]
- 23.Chu HY, Sun CC, Chuang WY, et al. Cytomegalovirus associated corneal endotheliitis after penetrating keratoplasty in a patient with Fuchs corneal endothelial dystrophy. Br J Ophthalmol 2012;96:300–1. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi A, Yokogawa H, Higashide T, et al. Clinical significance of owl eye morphologic features by in vivo laser confocal microscopy in patients with cytomegalovirus corneal endotheliitis. Am J Ophthalmol 2012;153:445–53. [DOI] [PubMed] [Google Scholar]
- 25.Zheng X, Yamaguchi M, Goto T, et al. Experimental corneal endotheliitis in rabbit. Invest Ophthalmol Vis Sci 2000;41:377–85. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.