Abstract
Background
Faecal calprotectin (FC), a cytosolic protein released by neutrophils (S100 family) in response to inflammation, is a simple, non-invasive test that can be used to differentiate irritable bowel syndrome (IBS) with inflammatory bowel disease (IBD), where there can be considerable symptom overlap.
Aims and methods
The aims of the study were (1) to be able to predict the ability of FC to exclude IBD and determine cut-offs when in remission, (2) to investigate the effects of time and temperature on stability of FC and (3) compare three ELISA kits to measure FC: Buhlmann, PhiCal v1 and PhiCal v2. A total of 311 patients with altered bowel habit were tested for FC; 144 with IBS, 148 with IBD and 19 with other organic causes.
Results
Sensitivity and specificity of FC (with PhiCal v2 kit) to distinguish between functional disorder (IBS) and IBD using cut-off 50 μg/g were 88% and 78%, respectively, with a negative predictive value of 87%. Area under the receiver operating curve was 0.84 (CI 0.78 to 0.90). For those with IBD, FC values below 250 μg/g corresponded with remission of disease with a sensitivity and specificity of 90% and 76%, respectively. Area under the receiver operating curve was 0.93 (CI 0.89 to 0.97). FC was stable once extracted and frozen for up to 2.5 months. Pearson correlation was good between Buhlmann assay and PhiCal v2 (r2 = 0.95).
Conclusions
FC has up to 87% negative predictive value to exclude IBD, and cut-offs less than 250 μg/g had 90% sensitivity to determine remission in IBD. Once frozen, FC is stable and the ELISA monoclonal plates were broadly comparable.
Keywords: IBD, Irritable Bowel Syndrome
Introduction
Faecal calprotectin (FC) is a calcium-binding heterodimer, which is abundant in the cytoplasm of neutrophils.1 Inflammation causes neutrophil activation, which results in calprotectin release proportionate to the degree of inflammation.2 Gastrointestinal inflammation, as in active inflammatory bowel disease (IBD), releases calprotectin into body fluids including faeces.2 3 Manufacturers state that FC remains stable in faeces for up to 5 days3 4 and its use as a non-invasive biomarker in IBD management is gaining popularity. As calprotectin release is proportionate to the degree of inflammation, it may also have a role in monitoring disease activity.5 However, this requires local validation of the various available ELISA kits.
Different monoclonal ELISA kits are available for use in the UK, including the PhiCal (Immundiagnostik Calprotectin ELISA; R-Biopharm, Darmstadt, Germany) and Calprotectin ELISA (Buhlmann, Basel, Switzerland). The specification of the ELISA kits has changed over time as technology has evolved. A new version of the Immundiagnostik Calprotectin (PhiCal v2) ELISA was released in June 2011 replacing PhiCal v1. Previous studies, of each ELISA method, have shown correlation with IBD detection and monitoring of disease activity. Only one study to date has compared polyclonal and monoclonal ELISA for FC detection.6 However, no studies have compared the different sensitivities for disease detection or the reference ranges for disease activity of three monoclonal ELISAs. This is of importance when defining local reference ranges.
The aim of this study was to:
Determine the ability of FC to exclude IBD and optimal cut-offs for IBD in remission
To investigate the effects of time and temperature on stability of FC and
To evaluate three different ELISA kits available commercially in the UK.
Methods
This study was conducted at the University Hospitals Coventry and Warwickshire, which serves a population of approximately 500 000. Ethical approval was not required (as determined by the Local Research Ethics committee) as FC testing was already readily available in the UK.
A total of 311 FC samples were analysed prospectively between September 2010 and August 2012 in whom either a functional gastrointestinal (GI) disease (irritable bowel syndrome (IBS) predominant diarrhoea (IBS-D)) or IBD was suspected in a secondary care setting. As per protocol, all patients with IBS-D fulfilled the Rome 11 criteria (n=144) and details of patients with IBD were recorded (n=148); 57 with ulcerative colitis (UC) and 91 with Crohn's disease. There were 19 with other incidental conditions such as diverticular disease and microscopic colitis. All patients had screening for coeliac disease and anaemia.
Those with IBD had endoscopic/histological and/or radiological confirmation of disease and FC testing within 4 weeks. Demographic and clinical details are shown in table 1. Details of patients with IBD and FC levels are shown in table 2. Active disease was defined by presence of active inflammation on endoscopy, confirmed by histology; remission of disease was defined by quiescent disease on endoscopy and no active inflammation on histology.
Table 1 .
Demographics of patients with IBS and IBD
| Groups (total; n=391) | IBS (n=144) | IBD active (n=88) | IBD remission (n=60) |
|---|---|---|---|
| M:F | 1:1.9 | 1:1.8 | 1:2.9 |
| CRP; mg/L (SD) | 13 (4.6) | 15 (17) | 14 (43) |
| FC results; μg/g (SD) | 34 (69) | 674 (480) | 92 (135) |
CRP, c-reactive protein; FC, faecal calprotectin; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome.
Table 2 .
Details of FC results in patients with IBD (UC and Crohn's) when active and in remission
| Total IBD N=148 |
Crohn's (active) N=57 | Crohn's (remission) N=34 |
UC (active) N=31 |
UC (remission) N=26 |
|---|---|---|---|---|
| FC results; μg/g (SD) | 646 (455) | 96 (147) | 649 (464) | 108 (154) |
FC, faecal calprotectin; IBD, inflammatory bowel disease; UC, ulcerative colitis.
To determine FC stability, stool samples from four random individuals were selected (one without symptoms and three with diarrhoea). These were received in the laboratory at room temperature within 6 h of voiding. Each was homogenised, then split into aliquots for immediate extraction to compare storage at room temperature and at 4°C for up to 3 days. Additionally, aliquots were also stored at 4°C , −20°C and −70°C and extracted at 1 week, 2 weeks and 6 weeks.
For comparison of ELISA kits, a random subset of nine individuals was analysed using three monoclonal ELISA plates; Buhlmann, PhiCal v1 and PhiCal v2. Three aliquots from each individual were analysed with the three ELISA plates in accordance with storage conditions described above. The updated PhiCal v2 (available only since June 2011) offers additional benefits with a shorter incubation period (30 min vs 1 h), room temperature incubation (versus 37°C shaking) and the use of a liquid stable conjugate. This enabled a direct comparison between the old and the new kits by the same manufacturer.
FC sample analysis
For each sample, 100 mg of stool, using a 10 µL inoculation loop was weighed and dispensed into an analysis pot. The exact weight was recorded and 5 mL of extraction buffer added. Samples were vortexed for 30 min, in order to ensure dissolution, and then centrifuged using an Eppendorf centrifuge. The supernatant was removed for analysis by ELISA.
Data analysis and statistics
Data were compiled into Microsoft Excel for analysis and statistics was performed with SPSS V.17. Specificities, sensitivities, positive predictive values (PPVs) and negative predictive values (NPVs), for diagnosing IBD and when in remission, were calculated at different FC cut-off values. Non-parametric testing was applied to determine differences between groups.
Quality assessment
To ensure quality control across the ELISA plates, internal quality controls were run at the end of a plate and compared with the beginning, to minimise interobserver variability. Additionally, samples were also sent for external quality assessment and local results were compared with published data.
Results
To distinguish between IBS (non-organic disease) and IBD (organic disease), FC cut-off of 50 μg/g returned a sensitivity and specificity of 88% and 78%, respectively, with a PPV of 79% and a NPV of 87% (table 3). The area under the receiver operating curve (AUROC) was 0.84 (CI 0.78 to 0.90) (figure 1).
Table 3.
Sensitivity and specificity at different cut-off ranges to distinguish IBS from IBD and IBD in remission compared with active disease
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|
| IBS vs IBD at FC 50 μg/g | 87.5 | 77.7 | 79.2 | 86.5 |
| IBS vs IBD at FC 100 μg/g | 97.2 | 76.3 | 75.3 | 97.4 |
| IBD remission vs active at FC 50 μg/g | 55.2 | 98.9 | 97.0 | 77.4 |
| IBD remission vs active at FC 100 μg/g | 72.4 | 95.6 | 91.3 | 84.3 |
| IBD remission vs active at FC 250 μg/g | 89.7 | 75.6 | 70.3 | 91.9 |
FC, faecal calprotectin; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; NPV, negative predictive value; PPV, positive predictive value.
Figure 1.
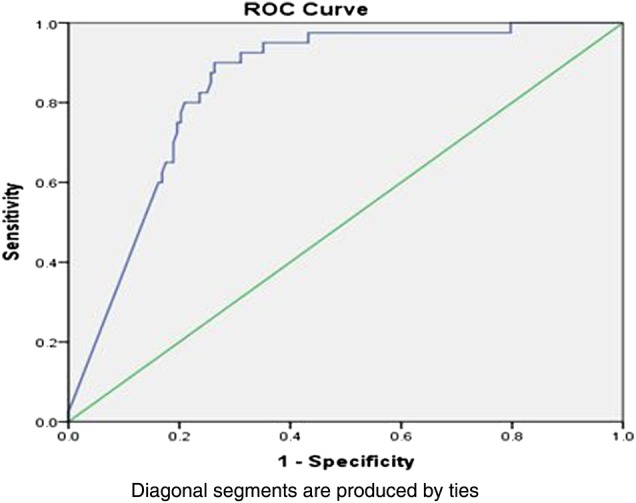
Area under the receiver operating curve showing 0.84 prediction for irritable bowel syndrome versus inflammatory bowel disease using cut-off at 50 μg/g.
However, using cut-off 100 μg/g, sensitivity increased to 97% with a slight fall in specificity to 76% but with a PPV of 75% and a NPV of 97% (table 3). AUROC was 0.88 (CI 0.82 to 0.92) (figure 2).
Figure 2 .
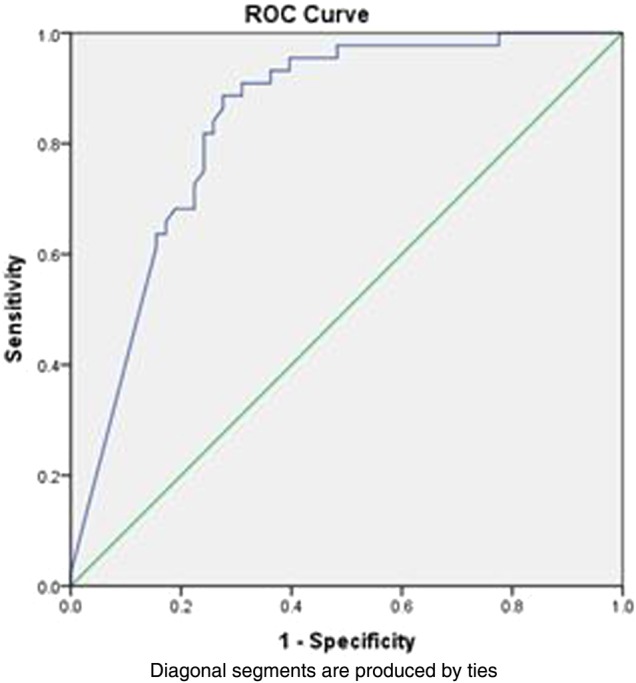
Area under the receiver operating curve showing 0.88 prediction for irritable bowel syndrome versus inflammatory bowel disease using cut-off at 100 μg/g.
For those with IBD, FC values below 250 μg/g correlated with remission of disease with sensitivity and specificity of 90% and 76%, respectively. AUROC was 0.93 (CI 0.89 to 0.97) (figure 3). Remission was confirmed by quiescent disease on endoscopy and no active inflammation on histology.
Figure 3 .
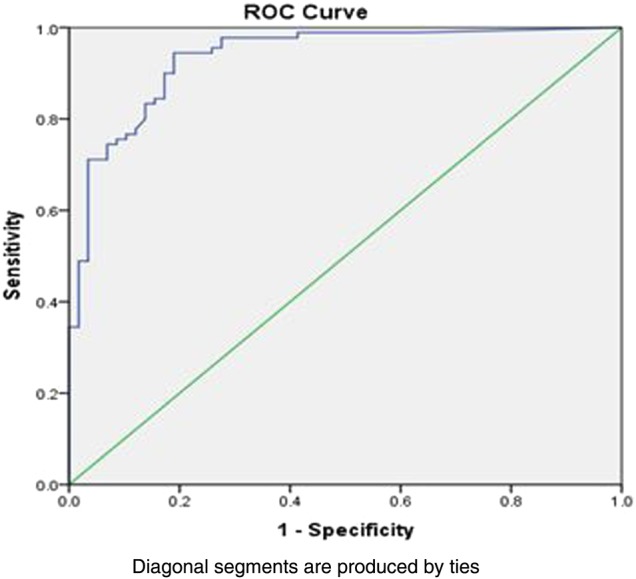
Area under the receiver operating curve (AUROC) for inflammatory bowel disease, active versus remission ROC was 0.93 (CI 0.89 to 0.97).
FC levels fell significantly when stored at room temperature compared with storage at 4°C, especially levels of FC <100 μg/g. If frozen at −20°C, then FC remains stable even prior to extraction into buffer solution for at least 6 weeks (figure 4). However once extracted into buffer solution, it remains stable for up to 2.5 months at −20°C.
Figure 4 .
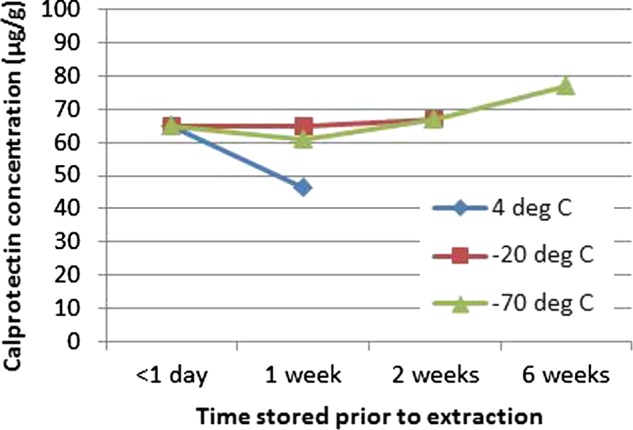
Faecal calprotectin levels based on temperature against time prior to extraction into buffer solution.
Figure 5, shows FC results comparing the three ELISA monoclonal plates which were broadly comparable. There was good correlation between the Buhlmann and the PhiCal v2 results, with r2=0.95 (for FC samples >250 μg/g) and r2=0.72 (for FC samples <250 μg/g). The PhiCal v2 has a higher upper detection limit (840 μg/g) and greater linearity compared with the Buhlmann ELISA (600 μg/g); data not shown. External quality assurance for our results was comparable with the regional reference centre (figure not shown). The cost of ELISA kits are broadly comparable with PhiCal v2 costing ∼£25/test.
Figure 5 .
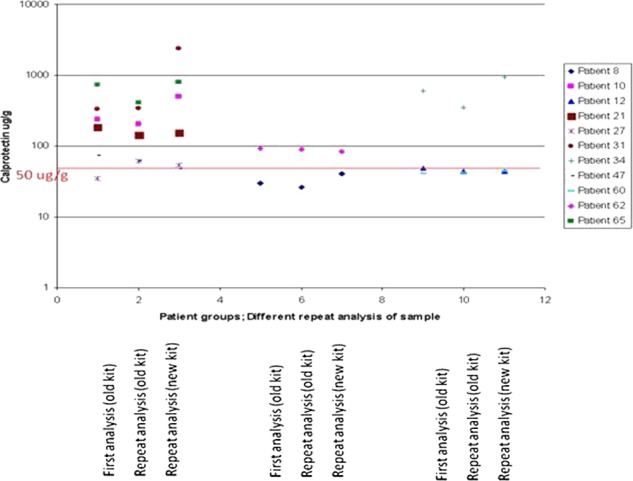
Comparison of faecal calprotectin values with three ELISA kits (Column 1—Buhlmann, Column 2—PhiCal v1 and Column 3—PhiCal v2).
Discussion
A recent systematic review7 and meta-analysis4 have confirmed the utility of FC as a screening tool to identify patients with gut inflammation. Interestingly in the meta-analysis only 30% of adults had confirmatory endoscopic or histological data to confirm inflammation. Our results demonstrate the utility of FC to distinguish non-organic (IBS) from organic (IBD) disease and provide insight into optimal cut-offs to determine when IBD is in remission.
Applying a cut-off level of 50 μg/g demonstrates acceptable sensitivity and specificity to distinguish between IBS and IBD. This does improve further when a cut-off of 100 μg/g is applied. The systemic review by Waugh et al which informed the National Institute of Health and Care Excellence diagnostics assessment group8 suggests most of the evidence is based on a 50 μg/g cut-off which enables reduction in the number of false negatives and is cost-effective.
Previous smaller studies have shown that FC concentrations correlate with endoscopic findings.2 Thus, the ability to quantify FC with different severity levels of inflammation enables monitoring using FC to determine treatment response or failure in subjects with active disease who are initiated on new therapy. This may ultimately reduce the need for repeated endoscopic assessments in active colitis. Damms and Bischoff,9 in 18 patients, have shown that the mean FC level in those with active IBD was 797. Mdel et al,10 in 25 patients with IBD, suggested a cut-off of 217 μg/g to determine remission of IBD. In this study of 148 individuals, we have shown the optimal cut-off to be 250 μg/g with 90% sensitivity and an AUROC of 0.93. FC levels for those with active Crohn's disease and ulcerative colitis were also broadly similar.
FC levels are influenced by storage conditions. Importantly however, once extracted and frozen, FC remains stable. Both manufacturers (Buhlmann and PhiCal v2) recommend a ‘cut-off’ FC level of 50 µg/g, above which all results are deemed positive. Our data also confirms the presence of improved linearity at higher values. This has specific relevance when monitoring disease activity and treatment response in subjects known to have IBD. On a technical note, the PhiCal v2 ELISA kit has a higher upper limit of detection thus reducing the number of dilution steps for high FC values. This would be an important consideration in monitoring IBD disease activity but less so for distinguishing between IBS and organic disease/IBD. Compared with the older PhiCal v1 (no longer available, superseded by PhiCal v2), it has a shorter incubation period and easier application.
It is worth noting that for the same patient groups the Buhlmann ELISA kit consistently produced higher numerical (∼20%) FC results (figure not shown). This has led some departments to consider revising the cut-off value to, for example, 100 μg/g. Intriguingly, both ELISA kits use the same monoclonal antibody, thus the differences must lie in the buffer solution at the time of extraction, reinforcing our views that this appears to be the critical step when analysing FC.
Although all FC testing (even with different ELISA kits) was performed manually, our findings were comparable with other reported series1–6 supporting the validity of our results. Moreover, internal and external quality analyses of our technique were acceptable given the low sample numbers and manual pipetting techniques—within the initial validation cohort of 62 individuals. Subsequent automation for improved precision enabled throughput of larger sample numbers with improved coefficient of variance within our laboratory.
C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are laboratory investigations which are often employed by clinicians in their determination of probability of patients having an underlying organic bowel disease as opposed to a functional disorder. Using a low CRP cut-off is reported to have improved sensitivity, but poorer specificity, which would lead clinicians to unnecessarily investigate, if used in isolation.11 12 ESR has been shown, in other studies, to offer little benefit to the evaluation process for those subjects fulfilling clinical criteria suggestive of IBS.12 Tibble et al reported that abnormal FC had a significantly greater OR using FC to identify IBD (p<0.0001) compared with an elevation in CRP and ESR.13 Our findings support this earlier result by showing greater sensitivity and predictive ability with FC than with ESR and/or CRP to distinguish between functional and organic disorders. This in turn, aids in monitoring of active exacerbations and remission of IBD.
FC is more cost-effective than non-FC strategies in a diagnostic pathway based on a recent systematic evaluation by the Centre for Economic Based Practice 2010 data.14 Although any quality-adjusted life-year gains were likely to be small due to of the low prevalence of IBD and the high sensitivities of all of the tests, considerable savings could still accrue.7 Additionally, a recent study of 3639 patients has suggested that the estimated demand for colonoscopies is reduced by 50% with the 50 μg/g cut-off and by 67% with the 100 μg/g cut-off, equating to €1.57 million and €2.13 million, respectively.15
Overall our data affirms that the use of FC is beneficial in distinguishing between functional GI conditions (IBS) and organic disease (IBD). In those with IBD, a 250 μg/g cut-off aids in determining clinical disease activity. FC testing like β natriuretic polypeptide used in the diagnosis of cardiac failure, is a useful, relatively economical test benefiting primary and secondary care. It helps to sift out those with organic pathology requiring further investigations, and in those with IBD, aids in disease monitoring, avoidance of invasive procedures and reduction of time off work.7 The study population was carefully chosen to allow us to validate symptoms and assess all subjects with endoscopy. Further studies are now needed to assess the use in a primary care population.
What is already known on this topic.
Faecal calprotectin (FC) has a high negative predictive value to distinguish between organic and non-organic disease (functional).
What this study adds.
FC levels fall at room temperature if stored inappropriately ELISA kits to - measure FC are broadly similar; the extraction process it critical.
For those with inflammatory bowel disease (IBD), values of >250μg/g indicate disease activity.
FC values for active IBD are not lower in Crohn's disease compared with ulcerative colitis.
How might it impact on clinical practice in the foreseeable future.
FC testing which is non-invasive, can guide clinicians in managing patients with inflammatory bowel disease.
Footnotes
Contributors: All authors contributed to this work. RPA conceived and designed the study. AD, ZZ, MC, CH contributed to writing of the manuscript and data analysis. CT performed the data analysis of faecal calprotectin. RPA, CH, SS and CN revised the paper for intellectual content. All authors discussed the results and implications and commented on the manuscript at all stages.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Further data is available on request from the corresponding author at R.Arasaradnam@warwick.ac.uk.
References
- 1.Roseth AG, Fagerhol MK, Aadland E, et al. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol 1992;27:793–8. [DOI] [PubMed] [Google Scholar]
- 2.Aomatsu T, Yoden A, Matsumoto K, et al. Fecal calprotectin is a useful marker for disease activity in pediatric patients with inflammatory bowel disease. Dig Dis Sci 2011;56:2372–7. [DOI] [PubMed] [Google Scholar]
- 3.Stríz I, Trebichavský I. Calprotectin—a pleiotropic molecule in acute and chronic inflammation. Physiol Res 2004;53:245–53. [PubMed] [Google Scholar]
- 4.van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 2010;341:c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sipponen T, Kärkkäinen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn's disease and histological findings. Aliment Pharmacol Ther 2008;28:1221–9. [DOI] [PubMed] [Google Scholar]
- 6.Malickova K, Janatkova I, Bortlik M, et al. Calprotectin levels in patients with idiopathic inflammatory bowel disease comparison of 2 commercial tests. Epidemiol Mikrobiol Imunol 2008;57:147–53. [PubMed] [Google Scholar]
- 7.Waugh N, Royle P, Cummins E, et al. Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: systematic review and economic evaluation. Health Tech Assessment 2013;17:xv–xix, 1–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NICE guidelines; Faecal calprotectin diagnostic tests for inflammatory diseases of the bowel; DAP 12; June 2013.
- 9.Damms A, Bischoff SC. Validation and clinical significance of a new calprotectin rapid test for the diagnosis of gastrointestinal diseases. Int J Colorectal Dis 2008;23:985–92. [DOI] [PubMed] [Google Scholar]
- 10.Mdel GS, González R, Iglesias FE, et al. Diagnostic value of faecal calprotectin in predicting an abnormal colonoscopy. Med Clin (Barc) 2006;127:41–6. (abstract in English). [DOI] [PubMed] [Google Scholar]
- 11.Jellema P, van Tulder MW, van der Horst HE, et al. Inflammatory bowel disease: a systematic review on the value of diagnostic testing in primary care . Colorectal Dis 2011;13:239–54. [DOI] [PubMed] [Google Scholar]
- 12.Langhorst J, Elsenbruch S, Koelzer J, et al. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol 2008;103:162–9. [DOI] [PubMed] [Google Scholar]
- 13.Tibble JA, Sigthorsson G, Foster R, et al. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology 2002;123:450–60. [DOI] [PubMed] [Google Scholar]
- 14. CEBP economic report: Value of Calprotectin in screening our IBS 2010. http://www.alphalabs.co.uk/asp/db/documents/cep09026_Calprotectin%20Economic_Jan10.pdf.
- 15.Mindemark M, Larsson A. Ruling out IBD: estimation of the possible economic effects of pre-endoscopic screening with F-calprotectin. Clin Biochem 2012;45: 552–5. [DOI] [PubMed] [Google Scholar]


