Abstract
Background
Although statin therapy is beneficial for preventing first strokes, the benefit for recurrent stroke and its sub-types remains unknown in Asian populations. The aim of this study is to examine the role of pravastatin in the secondary prevention of stroke in Japanese patients.
Methods
This is a multicenter, randomized, open-label, parallel group study of patients with noncardioembolic ischemic stroke (atherothrombotic infarction, lacunar infarction, and infarction of undetermined etiology). All patients were diagnosed with hyperlipidemia and with a total cholesterol level between 180 and 240 mg/dl at enrollment. Patients in the treatment group receive 10 mg/day of pravastatin, and those in the control group receive no statin treatment. The primary end-point is the recurrence of stroke, including transient ischemic attack. The secondary end-points include the onset of respective stroke sub-types and functional outcomes related to stroke. The patients were enrolled for five-years and will be followed up for five-years.
Results
A total of 1578 eligible patients (age: 66·2 years, men: 68·8%), including 64·2% with lacunar infarction, 25·4% with atherothrombotic infarction, and 10·4% with infarction of undetermined etiology were included in this study. Lipid levels were generally well controlled (total cholesterol: 210·0 mg/dl, low density lipoprotein cholesterol: 129·5 mg/dl) at baseline. In addition, the disability of patients was relatively mild, and cognitive function was preserved in the majority of patients.
Conclusion
This article reports the rationale, design, and baseline features of a randomized controlled trial to assess the effects of statin for the secondary prevention of stroke. Follow-ups of patients are in progress and will end in 2014.
Keywords: recurrence, secondary prevention, statins, stroke
Introduction
Background
Stroke is the leading cause of death and disability in industrialized countries, imposing immeasurable burdens on the society. The reduction of social burden may be most efficiently achieved by preventing first strokes. However, once stroke has occurred, the therapeutic target shifts to the functional improvement and prevention of recurrent stroke. Although common strategies for such secondary prevention include antiplatelet, anticoagulant, and antihypertensive agents 1,2, the prevention strategy must reflect the sub-types of occurred stroke and should be optimized based on the respective etiologies.
3-Hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, called statins, are widely used to improve serum lipid profile. Because of the potent lipid-lowering effects, statins are commonly used to prevent coronary artery disease. In addition to their established value for coronary protection, statins are beneficial for stroke prevention. Indeed, the use of statins has been associated with 20–30% reduction in the risk of stroke in patients with coronary artery disease 3–5. Additionally, statin usage was shown to reduce the risk of stroke by 27% in nearly 20 000 patients at cardiovascular risk 6. Also, in the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese study, statin usage was associated with a 46% reduction in the risk of stroke in hypertensive patients 7. These findings were derived from patients without prior stroke, suggesting a certain benefit of statins for the primary prevention of stroke. However, such a preventive effect has been less robust for the recurrence of stroke. For instance, in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial, including 4731 patients with stroke or transient ischemic attack (TIA), use of a statin was associated with 16% risk reduction for recurrent stroke 8. Also, a meta-analysis involving eight studies and 10 000 patients demonstrated that statin therapy had only a marginal effect in reducing the occurrence of subsequent stroke in patients with prior stroke or TIA 9. Additionally, these findings were obtained from the western people, and whether they apply for the Japanese patients remains to be examined.
Indeed, stroke is a heterogeneous disease with different etiologies, with or without underlying arterial pathologies. Thus, the benefits of statins should reflect the respective stroke sub-types. Particularly, statins are not likely to exert preventive effects in patients with cardioembolic infarction, in which involvement of lipids or atherosclerosis is limited. Given the structural difference between major cerebral arteries and the perforating branches, the effects of statins can differ between atherothrombotic and lacunar infarctions. Moreover, the use of statins might increase the risk of hemorrhagic stroke 10. Nevertheless, the majority of prior studies defined stroke as a whole, with no distinction between sub-types. Consequently, the risk reduction reported in prior studies may have been diluted as a consequence of the combination of all stroke sub-types. In fact, when limited to patients with noncardioembolic infarction, use of statins significantly reduced the risk of recurrent stroke 11. Furthermore, statins have pleiotropic favorable effects on arteries 12–15, including suppression of inflammation 16–18, regression of atherosclerosis 19,20, and improvement of endothelial function 21,22. In addition, Briel et al. demonstrated a stronger association of stroke reduction with statin use than with the extent of lipid reduction 23. Accordingly, pleiotropic vascular protective effects may contribute to the reduction of certain stroke sub-types, requiring further studies to clarify the role of statins in the secondary prevention of stroke. Particularly, elucidation of the effects of statins on respective stroke sub-types can help identify patients who would benefit from these treatments, thereby facilitating a more refined strategy for stroke prevention. Additionally, an examination of these effects in patients with mild hyperlipidemia could reveal whether stroke suppression is induced by lipid reduction or through other pleiotropic effects of statins.
Objectives
Pravastatin, a traditional statin widely prescribed in the clinic, was selected for use in this study to determine whether this drug could reduce the recurrence of stroke with presumed arterial damage in patients with mild hyperlipidemia. In addition, this study evaluates the effects of pravastatin on the onset of respective stroke sub-types and explores the impact of this treatment on the functional outcomes related to stroke. Additionally, concurrent sub-studies are being conducted to assess whether statin usage suppresses chronic inflammation and/or carotid atherosclerosis in the patients enrolled in this study.
Methods
Trial design
This is a multicenter, randomized (1:1), open-label, parallel group study conducted on outpatients with a prior history of stroke (NCT00221104). The study protocol and informed consent form were approved by the institutional review board of each center. All patients received information on the purpose and nature of this study as well as the potential risks and benefits. Thereafter, written informed consent for participation was obtained from each patient. In addition, this study is being conducted under the health insurance system of Japan, in accordance with the Declaration of Helsinki and the Ethical Guideline on Clinical Studies of the Ministry of Health, Labour and Welfare of Japan.
Participants
The current study has enrolled patients aged 45–80 years with a history of noncardioembolic ischemic stroke (atherothrombotic infarction, lacunar infarction, and infarction of undetermined etiology) within the preceding month to three-years. All patients had been previously diagnosed with hyperlipidemia, with serum total cholesterol levels maintained between 180 and 240 mg/dl by treatments other than statins.
Patients were excluded if they had a cerebral infarction of determined rare etiology, (e.g., vertebral artery dissection, fibromuscular dysplasia, and moyamoya disease) or presented an infarction associated with catheterization or surgery. In addition, patients were excluded when use of statins was preferred for the care of coexisting coronary artery disease. Moreover, patients with the following conditions were excluded: hemorrhagic diathesis, coagulopathy, hemorrhagic diseases (e.g., intracerebral hemorrhage, sub-arachnoid hemorrhage, and active peptic ulcer), thrombocytopenia (platelet count ≤100 000/mm3), hepatic dysfunction (aspartate transaminase or alanine transaminase ≥ 100 IU/l), renal dysfunction (serum creatinine ≥2·0 mg/dl), scheduled surgery, and cancer treatment.
Study settings
Patients were enrolled for five-years (from March 2004 to February 2009) from 123 centers across the country. Initially, the enrollment period was three-years, but this period was extended twice due to insufficient accrual of patients. All centers were regional core hospitals with multiple stroke neurologists that provide comprehensive medical services for stroke. Patient follow-up is ongoing and will end in February 2014.
Sub-studies
To explore the pleiotropic effects of the statin, sub-studies focusing on high-sensitivity C-reactive protein (NCT00361699) and carotid artery intima-media thickness (NCT00361530) are concurrently in progress; in these sub-studies, the target measures are centrally evaluated by standardized methods. In addition, for carotid artery evaluation, qualified and accredited sonographers have been employed. Moreover, because of the purported link between statin effects and certain hereditary backgrounds, the evaluation of single-nucleotide polymorphisms (SNPs) was subsequently added to the current study; 396 SNPs are being analyzed in association with stroke and statin effects. The details of these sub-studies will be reported elsewhere.
Interventions
Patients assigned to the pravastatin group receive a daily oral dose of pravastatin of 10 mg. The administration was initiated within one-month after randomization and continued until the final observation or patient's death. When cholesterol levels consistently exceed 240 mg/dl at routine clinical visits, diet and exercise therapies are reinforced. Only when the reinforcement is insufficient, treatments with pravastatin are intensified or other drugs are added, based on decision by the primary physician. However, the use of statins other than pravastatin is prohibited. Patients assigned to the control group receive no statin treatment, although other drugs are administered when necessary. In both groups, hypertension and diabetes mellitus are treated in accordance with clinical practice, without restriction of drug types. Moreover, compliance to the treatment is monitored at every clinical visit.
Outcomes
The primary end-point is the onset of recurrent stroke of any sub-type or TIA. The secondary end-points include the onset of each stroke sub-type, myocardial infarction, vascular accident, death, hospitalization, degree of disability or dependence in daily activities, onset of dementia, and severity of cognitive impairment. Stroke sub-types are diagnosed in accordance with the Treatment of Acute Stroke Trial criteria 24 and defined in accordance with the National Institute of Neurological Disorders and Stroke classification 25. The diagnostic criteria for each stroke sub-type, TIA, myocardial infarction, and vascular accidents are shown in Appendix 1. The degree of disability and dependence in daily activities are evaluated by the Barthel Index (BI) and modified Rankin Scale (mRS). Dementia is diagnosed by the Diagnostic and Statistical Manual of Mental Disorders IIIR criteria. Impairment of cognitive function is assessed in accordance with the Clinical Dementia Rating (CDR) and Mini Mental State Examination (MMSE).
The onset of recurrent stroke, myocardial infarction, vascular accident, death, and hospitalization are monitored at two- and six-months; one-, two, three-, four-, and five-years; and additionally at the completion of this study. Brain magnetic resonance imaging or computed tomography imaging are performed at baseline, at two-years, at the study completion, and when recurrent stroke occurs. In addition, lipid levels are evaluated at the Special Reference Laboratory (SRL, Inc., Tokyo, Japan), which is certified for major lipid measurements in accordance with the Centers for Disease Control and Prevention (Atlanta, GA, USA). Occasionally, intrahospital lipid measurements are conducted at certified study centers.
The primary physician at each study center evaluates each end-point, and a different physician at the same center confirms the evaluation. The central committee annually reviews the data concerning the occurrence of stroke and myocardial infarction, based on information submitted from each center, and judgments are corrected if necessary. Additionally, a source document verification is performed for arbitrarily selected centers.
Hypertension is defined as a blood pressure ≥140/90 mmHg or the use of antihypertensive agents. Diabetes mellitus is diagnosed when any of the following criteria are satisfied: fasting blood glucose ≥126 mg/dl or casual blood glucose ≥200 mg/dl during the past three-months; blood glucose ≥200 mg/dl at two-hours after 75 g OGTT; taking antidiabetic agents; or a previous diagnosis of diabetes mellitus. Hyperlipidemia is defined as total cholesterol ≥220 mg/dl, low density lipoprotein cholesterol ≥140 mg/dl or triglyceride ≥150 mg/dl or by the use of antihyperlipidemic agents.
Sample size
Based on an annual 5% recurrence of stroke (including TIAs) in the control group 26, 25% risk reduction in the pravastatin group and five-years of follow-up, the number of patients required to detect differences between the pravastatin group and the control group was calculated as 1292 for each group (two-tailed 5% significance level, power level of 90%). Assuming that 14% of patients are lost during follow-up, the sample size was set at 1500 in each group and 3000 for the two groups combined.
Interim analyses
The independent data monitoring committee annually reviews the safety data to evaluate the appropriateness of the study continuation. An interim analysis was performed using the Haybittle-Peto 3 SD method 27 in September 2011. Based on the results of the interim analysis, the independent data monitoring committee recommended the continuation of the study.
Randomization
The patients were enrolled through a web-based registration and follow-up system provided by the data center at the Translational Research Informatics Center, Kobe, Japan. After the primary physician obtained patient consent, generally through the support of the clinical research coordinators, access to the system was granted and the physician sent the information required for enrollment. The system automatically evaluated the eligibility of each patient and randomly assigned participants to either the pravastatin or control group (1:1 allocation). When the allocation was performed, the prevalence of stroke sub-types (atherothrombotic infarction vs. other), elevated blood pressure (≥150/90 mmHg vs. not), and diabetes (absence vs. presence) was dynamically balanced between the two groups.
Statistical analysis
Occurrences of predefined events, including stroke, myocardial infarction, and death, are analyzed in accordance with the intention-to-treat principle. The neurological and psychological outcomes are measured in patients for whom such data are available, and last observation carried forward data are used when necessary. The safety analysis set comprises eligible patients who received at least one dose of the study drug.
The cumulative incidences of events are estimated using the Kaplan–Meier method. The cumulative incidence curves for the two groups are compared using a stratified log-rank test. The Cox proportional hazard model is also used to estimate the relative risk (hazard ratio) and the 95% confidence interval, in which risk reduction is expressed as (1 – hazard ratio) × 100%. When events occur two or more times in a patient, the person-years method is used to estimate the incidence and 95% confidence interval.
The BI and mRS are compared between the two groups using the Wilcoxon rank-sum test. For MMSE, a decrease of 5 points or more is defined as cognitive function decline, and the percentage is compared between the two groups using the χ2 test. In patients without dementia at enrollment, the occurrence of dementia and the CDR score are compared between the two groups. Sub-group analyses are based on baseline lipids, blood pressure, the presence of hypertension or diabetes mellitus, and the use of antiplatelet agents. For lipids and blood pressure, the sub-groups were divided into five groups using the baseline data, and the trend for the stroke recurrence risk is being explored. Incidences of treatment discontinuation and serious adverse events are compared using the χ2 test. All analyses are performed using sas software (Cary, NC, USA), and the level of significance is P < 0·05 (two-tailed).
Results
The current study included 1579 patients from 123 participating centers from March 1, 2004 to February 28, 2009. One patient proved to be ineligible after randomization, reducing the total number of eligible patients to 1578. Of these, 1095 and 864 patients, respectively, were enrolled for the concurrent sub-studies on high-sensitivity C-reactive protein and carotid artery intima-media thickness.
The average age of the patients was 66·2 ± 8·5 years old, including 68·8% men (Table 1). In addition, the study sample exhibited a moderate prevalence of hypertension, diabetes, and current smoking. Although all patients had been diagnosed with hyperlipidemia, lipid levels were generally well controlled at baseline. Similarly, blood pressure and fasting blood glucose levels were within the normal ranges, largely due to medication provided at the hospitals. In addition, because all patients had a history of stroke, the majority of the participants were taking antiplatelet agents for the secondary prevention. Moreover, the prevalence of coronary artery disease was relatively low.
Table 1.
Baseline profiles of cardiovascular risk factors
| n | 1578 |
| Age (years) | 66·2 ± 8·5 |
| Gender (male%) | 68·8 |
| Height (cm)/weight (kg) | 160·3 ± 8·7/61·1 ± 10·2 |
| Hypertension (%) | 75·9 |
| Diabetes mellitus (%) | 23·3 |
| Smoking habit (%) | |
| Current smoker/past smoker/nonsmoker/unknown | 16·5/36·9/44·8/0·9 |
| T. Chol/HDL-C/LDL-C (mg/dl) | 210·0 ± 24·7/53·5 ± 15·8/129·5 ± 24·5 |
| Triglyceride (mg/dl) | 142·2 ± 74·2 |
| Systolic/diastolic blood pressure (mmHg) | 137·1 ± 17·8/79·3 ± 11·3 |
| Levels of blood pressure ≥150/90 mmHg (%) | 39·0 |
| Fasting blood glucose (mg/dl) | 117·6 ± 41·0 |
| Use of antiplatelet agents (%) | 90·7 |
| Coronary artery disease (%) | 5·1 |
The data are the mean ± SD for continuous variables. HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; T. Chol, total cholesterol.
Because we enrolled selected stroke patients, the sub-types of stroke comprised 25·4% atherothrombotic infarction, 64·2% lacunar infarction, and 10·4% infarction of undetermined etiology (Table 2). In accordance with the higher prevalence of lacunar infarction, the size of the infarction was small in more than two-thirds (73·1%) of patients. In addition, the infarctions were predominantly located in the perforating artery region and were most often detected in the middle cerebral artery territory (59·0%), followed by the vertebrobasilar artery territory (23·6%). Notably, large infarctions were observed in only 1·2% of patients. Consistent with these results, the disability of each patient was generally mild according to the National Institutes of Health Stroke Scale, BI, and mRS (Fig. 1). Although dementia was diagnosed in 3·2% of patients, cognitive function was preserved in the majority of patients, based on CDR and MMSE.
Table 2.
Baseline profiles of stroke related measures −1
| n | 1578 |
| Sub-types of ischemic stroke (%) | |
| Atherothrombotic/lacunar/undetermined etiology | 25·4/64·2/10·4 |
| Size of infarction* (%) | |
| Small/medium/large | 73·1/20·5/1·2 |
| Location of infarction (%) | |
| Cortical/perforating/both | 18·1/72·3/4·5 |
| Responsible arteries† (%) | |
| ACA/MCA/PCA/VB/BZ | 2·0/59·0/7·5/23·6/2·7 |
| Dementia (%) | 3·2 |
Small, <1·5 cm in diameter; medium, between small and large; large, half or more of the cerebral lobe.
ACA, anterior cerebral artery; BZ, border zone; MCA, middle cerebral artery; PCA, posterior cerebral artery; VB, vertebrobasilar arteries.
Fig 1.
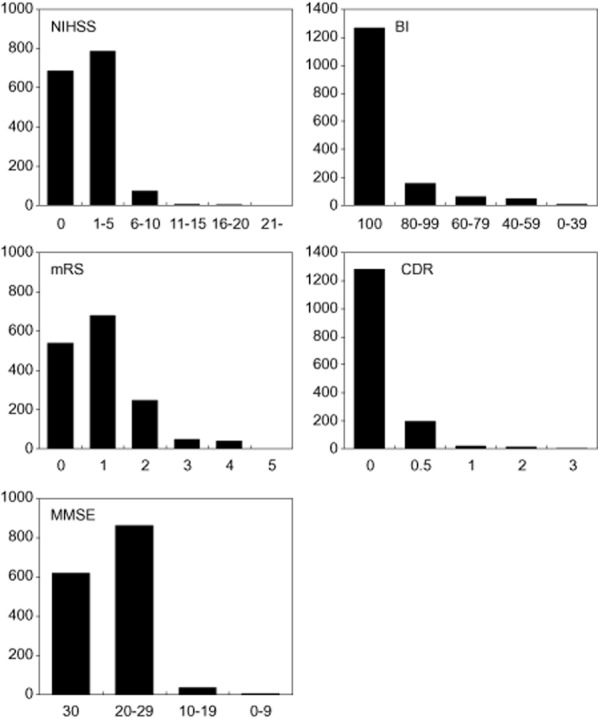
Baseline profiles of stroke-related measures −2.NIHSS, National Institutes of Health Stroke Scale; BI, Barthel Index; mRS, modified Ranking Scale; CDR, Clinical Dementia Rating; MMSE, Mini Mental State Examination. The vertical axes represent the number of patients in respective value ranges.
Discussion
The aim of this study was to evaluate the effects of an HMG-CoA reductase inhibitor for the prevention of recurrent stroke. Patient enrollment was closed at the end of February 2009, and follow-up studies are ongoing. This manuscript reports the study rationale and design as well as baseline features of the patients.
Because pravastatin was used, this study was designed and conducted under the health insurance system of the country. Because use of placebo is not permitted in such a setting, we employed an open-label approach, utilizing clinically prescribed drugs. In addition, this study is conducted as part of the clinical practice, and thus the benefits of individual patients take precedence over the study purposes. For instance, lipid levels must be maintained in clinically acceptable ranges in both the treatment and control groups to reduce cardiovascular risk in individual patients. Similarly, if statins are considered beneficial for the treatment of coexisting coronary stenosis, the usage cannot be prohibited in such patients. Thus, this study was designed under the conditions of the real world of medicine rather than under ideal world conditions.
In the current study, we focused on the vascular protective effects of statins and enrolled stroke patients with presumed arterial damage. Thus, only patients with atherothrombotic infarction, lacunar infarction, or infarction of undetermined etiology were included, where every effort was made to accurately diagnose these stroke sub-types. In addition, we enrolled patients who were previously diagnosed with hyperlipidemia and whose serum total cholesterol levels were maintained between 180 and 240 mg/dl at enrollment because the use or disuse of statins was less likely to jeopardize these patients, allowing for randomization in the clinical practice. Additionally, we excluded patients for whom statins would be prescribed for coronary protection because the inclusion of such patients might increase protocol violation or patient loss in the control group. These factors have substantially narrowed the window of patient enrollments, decreasing the potential candidates for this study.
To treat the patients, we set the dose of pravastatin at 10 mg/day, which is less than that commonly used in the United States and Europe but is the standard in Japan, where lifestyles and hereditary backgrounds are different. In addition, because the study is being conducted as part of the clinical practice, the physicians make every effort to prevent the elevation of lipids in both groups. When serum total cholesterol levels consistently exceed 240 mg/dl during regular patient visits to the clinic, despite the reinforcement of diet and exercise therapies, the dose of pravastatin is increased if the patient is in the treatment group, whereas other types of drugs (except statins) are administered in the control group. Under these conditions, it is reasonable to say that this study is more focused on the pleiotropic effects of statins than on lipid reduction. The use of this approach is consistent with the stronger association of stroke reduction with statin treatment than with the extent of lipid reduction, as reported in previous studies 23,28.
The current study uses stroke recurrence as the primary end-point, including TIA, because the recent advent of thrombolytic or antithrombotic therapies often obscures the separation between stroke and TIA. Among the secondary end-points adopted, the onset of each stroke sub-type is of particular interest, and diagnostic criteria have been clearly defined (Appendix 1). Particularly, determination of the associations between statin use and the onset of respective stroke sub-types could help identify patients that might benefit from this treatment, potentially facilitating a more efficient preventive strategy. However, we must be aware of confounding opinions; the post hoc analysis of SPARCL trial suggested that statin usages were similarly efficacious in preventing recurrent stroke irrespective of the baseline sub-types 29. Thus, this issue is prospectively addressed in the current study, even though the statistical power may not be enough. Other secondary end-points include myocardial infarction, death and hospitalization, consistent with other studies. In addition, several stroke-related functional measures (e.g., BI, mRS, CDR, and MMSE) are included in the secondary end-points. Analyses of the changes in these measures could help determine whether statins are protective against functional deterioration over time. Notably, correlative sub-studies focusing on high-sensitivity C-reactive protein and carotid artery intima-media thickness are being performed concurrently, allowing for further studies on the pleiotropic effects of statins in patients with relatively low lipid levels.
Based on the risk reduction reported in previous studies, we set the sample size at 3000, but due to the narrower window of patient enrollment, this number was not realized. Consequently, 1579 patients were recruited, and 1578 patients were eligible for the current study, roughly corresponding to half of the initial target and decreasing the probability of detecting intergroup differences in stroke recurrence. However, we excluded stroke patients unlikely to benefit from statin treatment, which could enrich the putative risk reduction in the study sample, mitigating the shortage of the statistical power. Indeed, after carefully reviewing the results of interim analysis, the independent data monitoring committee recommended continuation of the study to potentially answer the research questions of this study and concurrent sub-studies. Accordingly, patient follow-ups and evaluations are in progress.
Because all patients previously experienced stroke, the study sample had a moderate prevalence of traditional cardiovascular risk factors (Table 1). Notably, although all patients had been previously diagnosed with hyperlipidemia, the lipid levels were generally well controlled at baseline. Similarly, the levels of blood pressure and fasting blood glucose were within normal ranges, largely due to medications provided at the hospitals. Additionally, the majority of patients were taking antiplatelet agents to prevent stroke recurrence, which should be considered when interpreting the results. The lower prevalence of coronary artery disease may reflect the exclusion of patients requiring statins for coronary protection.
Among the stroke types targeted, lacunar infarction was most prevalent, accounting for two-thirds of patients (Table 2), consistent with the prevalence reported for the Japanese population 30. The lesions were generally small and predominantly located in the perforating branch area of the middle cerebral artery. Atherothrombotic infarction was second most frequent, representing one-fourth of patients. Given the purported arterial protective effects of statins, whether pravastatin suppresses the onset of this stroke sub-type may be of interest. The remaining patients were diagnosed with infarction of undetermined etiology, in which involvement of arterial damage was presumed after comprehensive investigation for atherosclerosis and occult emboli in the systemic vessels. In fact, we made every effort to specify the cause of infarctions and cautiously excluded infarctions of determined etiology. Moreover, large infarctions involving the cerebral cortex were detected in 1·2% of patients, reflecting the outpatient nature of the study sample. Furthermore, the disability of patients was generally mild, which was anticipated because of the high prevalence of lacunar infarction. Although dementia was diagnosed in a small portion of patients, cognitive function was preserved in the majority of patients. Analyses on the relationships between statin use and changes of cognitive function are needed to evaluate the antidementia effects of statins, as previously reported 31.
The current study has certain limitations. First, because it is being conducted as part of the clinical practice, we cannot strictly prohibit the use of statins in the control group, which could potentially increase protocol violation or patient loss. Second, TIA is included in the primary end-point, requiring a caution when interpreting the results of this study. Thus, to allow for a reasonable interpretation, we have clearly defined the diagnostic criteria for respective stroke sub-types and TIA (Appendix 1). Third, the shortage of patient accrual reduces the chance of detecting intergroup differences in stroke recurrence. Indeed, our sample size is substantially smaller than the SPARCL trial (4731 patients with stroke or TIA), which demonstrated the benefit of statin for reducing recurrent stroke (hazard ratio, 0·84; 95% confidence interval, 0·71–0·99) 8. Nonetheless, we are carefully monitoring the onset of each stroke sub-type and evaluating functional measures related to stroke, facilitating extensive analyses regarding the relationships of statin use with stroke sub-types and functional outcomes. Moreover, concurrent sub-studies are ongoing that will facilitate an investigation of the link between pleiotropic effects of statins and stroke recurrence and the role of certain SNPs in these linkages.
Conclusions
This article reported the rationale, design, and baseline features of the Japanese Statin Treatment against Recurrent Stroke (J-STARS). Patient enrollment is closed, and follow-up studies are in progress. The results derived from this study could refine the role of statins in the secondary prevention of stroke.
Acknowledgments
The authors would like to thank all study participants, physicians, comedical staff and co-workers for their assistance in the preparation and execution of this study. In addition, the authors would also like to acknowledge the late Dr Hideo Tohgi for invaluable advice on conceptualization of the study protocol and the late Dr Takeshi Shima for his great help as a member of regional promotion committee.
Registration
This study is registered in ClinicalTrials.gov under NCT00221104.
Organization
The organizational elements of J-STARS are listed in Appendix 2.
Sub-studies
Concurrent with this study, correlative sub-studies on high sensitivity C-reactive protein (1095 patients enrolled) and carotid artery intima-media thickness (864 patients enrolled) are in progress. In addition, the analysis of 396 SNPs was conducted in the current study, and the results will be analyzed in association with statin effects and stroke recurrence.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Definition of endpoints
Appendix 2 J-STARS Group: organizational structure and participants
References
- 1.Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289:2534–2544. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 3.Pearson TA. Primary and secondary prevention of coronary artery disease: trials of lipid lowering with statins. Am J Cardiol. 1998;82:28S–30S. doi: 10.1016/s0002-9149(98)00806-6. [DOI] [PubMed] [Google Scholar]
- 4.Plehn JF, Davis BR, Sacks FM, et al. Reduction of stroke incidence after myocardial infarction with pravastatin: the Cholesterol and Recurrent Events (CARE) study. The Care Investigators. Circulation. 1999;99:216–223. doi: 10.1161/01.cir.99.2.216. [DOI] [PubMed] [Google Scholar]
- 5.White HD, Simes RJ, Anderson NE, et al. Pravastatin therapy and the risk of stroke. N Engl J Med. 2000;343:317–326. doi: 10.1056/NEJM200008033430502. [DOI] [PubMed] [Google Scholar]
- 6.Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 7.Kushiro T, Mizuno K, Nakaya N, et al. Pravastatin for cardiovascular event primary prevention in patients with mild-to-moderate hypertension in the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) Study. Hypertension. 2009;53:135–141. doi: 10.1161/HYPERTENSIONAHA.108.120584. [DOI] [PubMed] [Google Scholar]
- 8.Amarenco P, Bogousslavsky J, Callahan A, 3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 9.Manktelow BN, Potter JF. Interventions in the management of serum lipids for preventing stroke recurrence. Cochrane Database Syst Rev. 2009;(3):CD002091. doi: 10.1002/14651858.CD002091.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henyan NN, Riche DM, East HE, Gann PN. Impact of statins on risk of stroke: a meta-analysis. Ann Pharmacother. 2007;41:1937–1945. doi: 10.1345/aph.1K280. [DOI] [PubMed] [Google Scholar]
- 11.Amarenco P, Labreuche J. Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009;8:453–463. doi: 10.1016/S1474-4422(09)70058-4. [DOI] [PubMed] [Google Scholar]
- 12.Paciaroni M, Bogousslavsky J. Statins and stroke prevention. Expert Rev Cardiovasc Ther. 2009;7:1231–1243. doi: 10.1586/erc.09.106. [DOI] [PubMed] [Google Scholar]
- 13.Sadowitz B, Maier KG, Gahtan V. Basic science review: statin therapy – part I: the pleiotropic effects of statins in cardiovascular disease. Vasc Endovascular Surg. 2010;44:241–251. doi: 10.1177/1538574410362922. [DOI] [PubMed] [Google Scholar]
- 14.Willey JZ, Elkind MS. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors in the treatment of central nervous system diseases. Arch Neurol. 2010;67:1062–1067. doi: 10.1001/archneurol.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Q, Liao JK. Pleiotropic effects of statins. – Basic research and clinical perspectives. Circ J. 2010;74:818–826. doi: 10.1253/circj.cj-10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 17.Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 19.Crouse JR, 3rd, Raichlen JS, Riley WA, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007;297:1344–1353. doi: 10.1001/jama.297.12.1344. [DOI] [PubMed] [Google Scholar]
- 20.Bedi US, Singh M, Singh PP, et al. Effects of statins on progression of carotid atherosclerosis as measured by carotid intimal – medial thickness: a meta-analysis of randomized controlled trials. J Cardiovasc Pharmacol Ther. 2010;15:268–273. doi: 10.1177/1074248410369110. [DOI] [PubMed] [Google Scholar]
- 21.Liu M, Wang F, Wang Y, Jin R. Atorvastatin improves endothelial function and cardiac performance in patients with dilated cardiomyopathy: the role of inflammation. Cardiovasc Drugs Ther. 2009;23:369–376. doi: 10.1007/s10557-009-6186-3. [DOI] [PubMed] [Google Scholar]
- 22.Reriani MK, Dunlay SM, Gupta B, et al. Effects of statins on coronary and peripheral endothelial function in humans: a systematic review and meta-analysis of randomized controlled trials. Eur J Cardiovasc Prev Rehabil. 2011;18:704–716. doi: 10.1177/1741826711398430. [DOI] [PubMed] [Google Scholar]
- 23.Briel M, Studer M, Glass TR, Bucher HC. Effects of statins on stroke prevention in patients with and without coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2004;117:596–606. doi: 10.1016/j.amjmed.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 25.Special report from the National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular diseases III. Stroke. 1990;21:637–676. doi: 10.1161/01.str.21.4.637. [DOI] [PubMed] [Google Scholar]
- 26.Yokota C, Minematsu K, Hasegawa Y, Yamaguchi T. Long-term prognosis, by stroke subtypes, after a first-ever stroke: a hospital-based study over a 20-year period. Cerebrovasc Dis. 2004;18:111–116. doi: 10.1159/000079258. [DOI] [PubMed] [Google Scholar]
- 27.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34:585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uchiyama S, Nakaya N, Mizuno K, et al. Risk factors for stroke and lipid-lowering effect of pravastatin on the risk of stroke in Japanese patients with hypercholesterolemia: analysis of data from the MEGA Study, a large randomized controlled trial. J Neurol Sci. 2009;284:72–76. doi: 10.1016/j.jns.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Amarenco P, Benavente O, Goldstein LB, et al. Results of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial by stroke subtypes. Stroke. 2009;40:1405–1409. doi: 10.1161/STROKEAHA.108.534107. [DOI] [PubMed] [Google Scholar]
- 30.Turin TC, Kita Y, Rumana N, et al. Ischemic stroke subtypes in a Japanese population: Takashima Stroke Registry, 1988–2004. Stroke. 2010;41:1871–1876. doi: 10.1161/STROKEAHA.110.581033. [DOI] [PubMed] [Google Scholar]
- 31.Vaughan CJ. Prevention of stroke and dementia with statins: effects beyond lipid lowering. Am J Cardiol. 2003;91:23B–29. doi: 10.1016/s0002-9149(02)03270-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Definition of endpoints
Appendix 2 J-STARS Group: organizational structure and participants


