Abstract
Purpose
Abuse and misuse of prescription opioids are serious public health problems. Abuse-deterrent formulations are an intervention to balance risk mitigation with appropriate patient access. This study evaluated the effects of physicochemical barriers to crushing and dissolving on safety outcomes associated with extended-release oxycodone (ERO) tablets (OxyContin) using a national surveillance system of poison centers. Other single-entity (SE) oxycodone tablets and heroin were used as comparators and to assess substitution effects.
Methods
The National Poison Data System covering all US poison centers was used to measure changes in exposures in the year before versus the 2 years after introduction of reformulated ERO (7/2009–6/2010 vs 9/2010–9/2012). Outcomes included abuse, therapeutic errors affecting patients, and accidental exposures.
Results
After ERO reformulation, abuse exposures decreased 36% for ERO, increased 20% for other SE oxycodone, and increased 42% for heroin. Therapeutic errors affecting patients decreased 20% for ERO and increased 19% for other SE oxycodone. Accidental exposures decreased 39% for ERO, increased 21% for heroin, and remained unchanged for other SE oxycodone. During the study period, other interventions to reduce opioid abuse occurred, for example, educational and prescription monitoring programs. However, these have shown small effects and do not explain a drop for ERO exposures but not for other opioids.
Conclusions
After ERO reformulation, calls to poison centers involving abuse, therapeutic errors affecting patients, and accidental exposures decreased for ERO, but not for comparator opioids. Abuse-deterrent formulations of opioid analgesics can reduce abuse, but switching to other accessible non abuse-deterrent opioids might occur. © 2013 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons, Ltd.
Keywords: opioid abuse, abuse deterrence, poison centers, oxycodone, heroin, patient outcomes, pharmacoepidemiology
INTRODUCTION
Abuse and misuse of prescription opioids are serious public health problems that result in a significant public health burden.1–4 However, opioid analgesics are recommended for treatment of serious, persistent pain after non-pharmacologic therapies, and non-opioid medications have been used.5–9 Extended-release opioid analgesics provide important pain relief for ∼4 million patients in the USA per year. Risk management of opioid analgesics is therefore an important public health priority.10 Abuse-deterrent formulations are a potentially effective approach to minimize abuse of prescription opioids while preserving analgesic benefits for patients.11,12 The US FDA has stated “FDA considers the development of these products a high public health priority.”13 However, little data exist evaluating outcomes of these formulations in the real world.
Prescription opioids are abused through oral ingestion (with or without tampering), inhalation (“snorting”), injection, and smoking. Non-oral routes of administration (ROAs) increase the abuse potential of opioids because of rapid absorption of drug.11,12,14 A progression of opioid abuse occurs whereby users initially abuse orally, then progress to injection or snorting by the time of admission to substance abuse treatment.12,15–17
OxyContin® is an extended-release oxycodone (ERO) formulation to treat moderate-to-severe chronic pain.18 It has been widely abused,19–21 especially by non-oral ROAs.22–25 ERO was reformulated with physicochemical barriers to breaking, crushing, or dissolving intended to deter abuse. Reformulated ERO was approved in the USA in April 2010. On 9 August 2010, the manufacturer stopped shipments of original ERO and started shipping only reformulated ERO. Pre-approval studies had demonstrated that reformulated ERO is bioequivalent to original ERO when taken orally, is harder to extract oxycodone from,26 and has less liking to abusers.27 Postmarketing studies have reported reductions in abuse of reformulated ERO among individuals assessed in substance abuse treatment centers.28 However, increases in heroin abuse among the subpopulation with diagnosed dependence on prescription opioids have been reported,29 but the effects of introducing a potential abuse-deterrent formulation of a widely used opioid-analgesic on the general population have not been evaluated.
This study used the National Poison Data System (NPDS) maintained by the American Association of Poison Control Centers to assess the effects of reformulated ERO on abuse. Calls to poison centers are strongly correlated with poisoning mortality as identified on death certificates for opioids and may be used for timely surveillance of mortality.30 Changes in exposures for ERO from 1 year before to 2 years after reformulated ERO were evaluated to test the hypothesis that reformulating ERO tablets would reduce calls to poison centers due to problems with ERO (i.e., “exposures”). These changes were compared with trends for two comparator opioids: other single-entity (SE) oxycodone products (excluding OxyContin) and heroin. Comparators were used to differentiate between ERO-specific and general opioid trends, and to measure increases in other opioids as reported in a national surveillance system.
In addition to abuse, tablets with barriers to crushing, chewing, or breaking could reduce serious adverse events from accidental exposures among young children who put tablets in their mouth by impeding their chewing or crushing the tablet in a way that releases a potentially fatal dose inadvertently. Tablets with barriers to crushing, chewing, or breaking could also reduce serious adverse events among patients exposed to doses intended for sustained release that are delivered as immediate release, an event that the boxed warning in the product label states can lead to fatal respiratory depression.31 Therefore, this study also tested the hypothesis that reformulating ERO tablets would reduce calls to poison centers due to (i) therapeutic errors affecting patients and (ii) accidental exposures. This study is part of a formal postmarketing commitment program required by the FDA, and the results, raw data, and sas programming code have been submitted to the FDA.
METHODS
Data from the NPDS, a surveillance system that captures 99.8% of poison exposures reported to all poison centers in the USA,31 were used to examine trends in exposures reported to poison centers per quarter for ERO, SE oxycodone excluding ERO, and heroin. Changes were compared from the 1 year preceding (3Q2009–2Q2010) to the 2 years following (4Q2010–3Q2012) reformulated ERO, with 3Q2010 considered a transition period.
Given the imprecise ability to differentiate between original and reformulated ERO, both original and reformulated ERO were included in ERO trends. Because of the categories that the poison centers use for aggregate measures across drugs in a group, the SE oxycodone group consisted of both immediate-release SE oxycodone and generic extended-release oxycodone, but excluded brand extended-release oxycodone (OxyContin).
Exposures reported to poison centers are classified into reasons, including intentional abuse, unintentional therapeutic errors, unintentional general exposures, and adverse reactions.32,33 Intentional abuse is defined as an exposure resulting from the intentional, improper, or incorrect use of a substance in order to gain a high, euphoric effect or some other psychotropic effect. Unintentional therapeutic errors (errors affecting patients) are defined as an exposure resulting from unintentional deviation from a proper therapeutic regimen that results in wrong dose, incorrect ROA, administration to the wrong person, or administration of the wrong substance. Unintentional general (accidental) exposures are those not defined in another category and include exposures resulting from children accidentally swallowing adults' medicine, which could be fatal.
Poisson regression was used to calculate percent change and 95% confidence intervals (CI) in the average number of exposures per quarter. To facilitate comparisons across different kinds of exposures, the 1-year period prior to reformulated ERO was used as a baseline, and the change from baseline was calculated. The latter was excluded from pre-reformulation versus post-reformulation change measures but included in figures and trends. Rates adjusted for population size and number of prescriptions dispensed were calculated using data from US census 2010 and IMS Health, respectively. Changes in the slope of trends in exposures before and after reformulated ERO, between 2007 and 3Q2012, were assessed using Poisson regression with a single spline knot at the end of 3Q2010 to provide a piecewise linear function.34,35 Analyses were performed using sas v9.2 (SAS Institute, Inc., Cary, NC, USA) and MS Excel 2010.
RESULTS
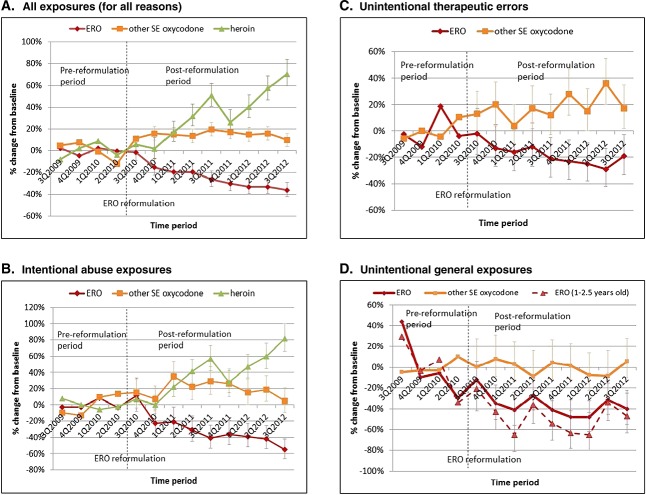
Compared with the one-year baseline period before reformulation of ERO, ERO exposure types decreased, including all, abuse, therapeutic errors, and accidental exposures (Figure 1). In contrast, exposures for other SE oxycodone products increased or remained unchanged during the same period. Heroin abuse increased sharply starting 4 to 6 months after reformulation.
Figure 1.
Changes in calls to poison centers reporting problems from exposure to brand extended-release oxycodone (ERO), other single-entity (SE) oxycodone products, and heroin by exposure type between 3Q2009 and 3Q2012
All exposures for ERO declined 26% (693 to 509 per quarter), but increased 15% for other SE oxycodone (from 1449 to 1670) and increased 37% for heroin (589 to 807) from the pre-reformulation to post-reformulation period (Table 1). Abuse exposures for ERO decreased 36% (130 to 83), increased 20% for other SE oxycodone (229 to 273), and increased 42% (356 to 505) for heroin. Unintentional therapeutic errors affecting patients for ERO decreased 20% (161 to 129) and increased 19% (223 to 265) for other SE oxycodone. Unintentional general (accidental) exposures for ERO decreased 39% (75 to 46), changed 0% (189 to 189) for other SE oxycodone, and increased 21% for heroin (22 to 27). The majority (63%) of unintentional general exposures were among children 1 to 2½ years of age. In the age category of 12 to 29.9 months of age, accidental exposures decreased by 51% (95%CI: −60%, −40%) from before to after reformulation. Adverse reactions for ERO decreased 34% and increased 15% for other SE oxycodone.
Table 1.
Changes in the number of extended-release oxycodone (ERO) exposures per quarter reported to US poison centers from 1 year before to 2 years after introduction of reformulated ERO by reasons for exposure
| Pre-reformulation (average per quarter) | Post-reformulation (average per quarter) | Change (post–pre) % | 95%CI | p value | |
|---|---|---|---|---|---|
| Extended-release oxycodone | |||||
| All exposures | 692.8 | 509.4 | −26 | (−28, −20) | <0.0001 |
| –Intentional | 390.5 | 292.5 | −25 | (−26, −16) | <0.0001 |
| –Abuse | 130.3 | 83.3 | −36 | (−40, −23) | <0.0001 |
| –Suspected suicide | 182.3 | 143.1 | −21 | (−26, −10) | <0.0001 |
| –Misuse | 51.3 | 40.4 | −21 | (−29, 2) | 0.0076 |
| –Unintentional | 242.5 | 180.9 | −25 | (−31, −18) | <0.0001 |
| –Misuse | 5.3 | 3.9 | −26 | (−58, 37) | 0.2826 |
| –General | 75.0 | 45.9 | −39 | (−49, −29) | <0.0001 |
| –Therapeutic errors | 161.3 | 129.4 | −20 | (−26, −9) | <0.0001 |
| –Adverse reactions | 29.8 | 19.5 | −34 | (−50, −17) | 0.0005 |
| –Withdrawal | 13.5 | 4.5 | −67 | (−74, −37) | <0.0001 |
| –Unknown | 42.8 | 38.3 | −11 | (−24, 12) | 0.244 |
| Other single-entity oxycodone | |||||
| All exposures | 1448.8 | 1670.3 | 15 | (12, 20) | <0.0001 |
| –Intentional | 887.5 | 1042.4 | 17 | (14, 24) | <0.0001 |
| –Abuse | 228.5 | 273.4 | 20 | (13, 33) | <0.0001 |
| –Suspected suicide | 477.8 | 553.4 | 16 | (10, 23) | <0.0001 |
| –Misuse | 104.0 | 119.6 | 15 | (5, 34) | 0.0172 |
| –Unintentional | 427.8 | 471.4 | 10 | (3, 16) | 0.0009 |
| –Misuse | 8.5 | 12.0 | 41 | (2, 129) | 0.0840 |
| –General | 189.5 | 189.4 | 0 | (−9, 10) | 0.9882 |
| –Therapeutic errors | 223.0 | 264.5 | 19 | (7, 26) | <0.0001 |
| –Adverse reactions | 74.3 | 85.8 | 15 | (1, 34) | 0.0382 |
| –Withdrawal | 14.3 | 14.9 | 4 | (−26, 44) | 0.7899 |
| –Unknown | 125.3 | 153.6 | 23 | (11, 36) | 0.0005 |
| Heroin | |||||
| All exposures | 587.3 | 806.5 | 37 | (22, 35) | <0.0001 |
| –Intentional | 527.3 | 726.1 | 38 | (22, 36) | <0.0001 |
| –Abuse | 355.8 | 505.1 | 42 | (24, 41) | <0.0001 |
| –Suspected suicide | 100.8 | 130.4 | 29 | (9, 39) | <0.0001 |
| –Misuse | 46.5 | 60.4 | 30 | (3, 47) | 0.0025 |
| –Unintentional | 28.0 | 34.5 | 23 | (−6, 49) | 0.0624 |
| –Misuse | 2.0 | 3.5 | 75 | (−61, 107) | 0.1627 |
| –General | 22.3 | 27.0 | 21 | (−6, 57) | 0.1245 |
| –Therapeutic errors | 0.8 | 0.6 | −17 | (−83, 498) | 0.8029 |
| –Adverse reactions | 2.0 | 7.9 | 294 | (60, 629) | 0.0003 |
| –Withdrawal | 15.8 | 18.1 | 15 | (−28, 37) | 0.3520 |
| –Unknown | 35.0 | 44.5 | 27 | (5, 55) | 0.0161 |
Prescriptions for ERO decreased 2% in the first and 9% in the second year after reformulation from the year pre-reformulation, while prescriptions for other SE oxycodone products increased 51% by 3Q2012 (Figure 2) from the year pre-reformulation. Changes from baseline in ERO exposures had consistent decreases for number of exposures per quarter, population-adjusted rates, and prescription-adjusted rates. However, changes in prescription-adjusted rates for other SE oxycodone differed from exposure numbers and population-adjusted rates because of a 50% increase in the number of prescriptions. Therapeutic errors for ERO declined steadily after reformulation, except for a spike in 3Q2012 (Figure 3), while those for other SE oxycodone increased in number.
Figure 2.
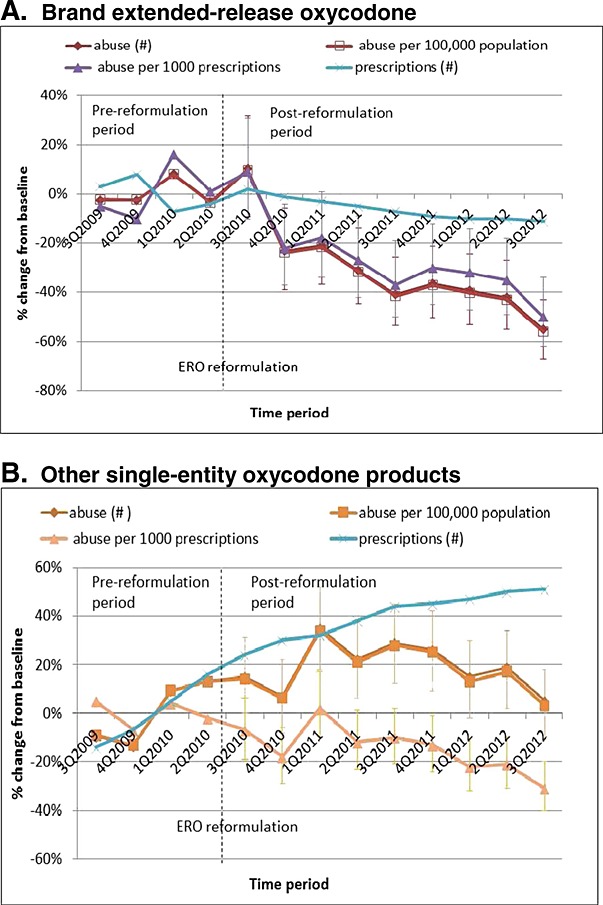
Changes in abuse exposures and number of prescriptions from 1 year preceding to 2 years following extended-release oxycodone (ERO) reformulation
Figure 3.
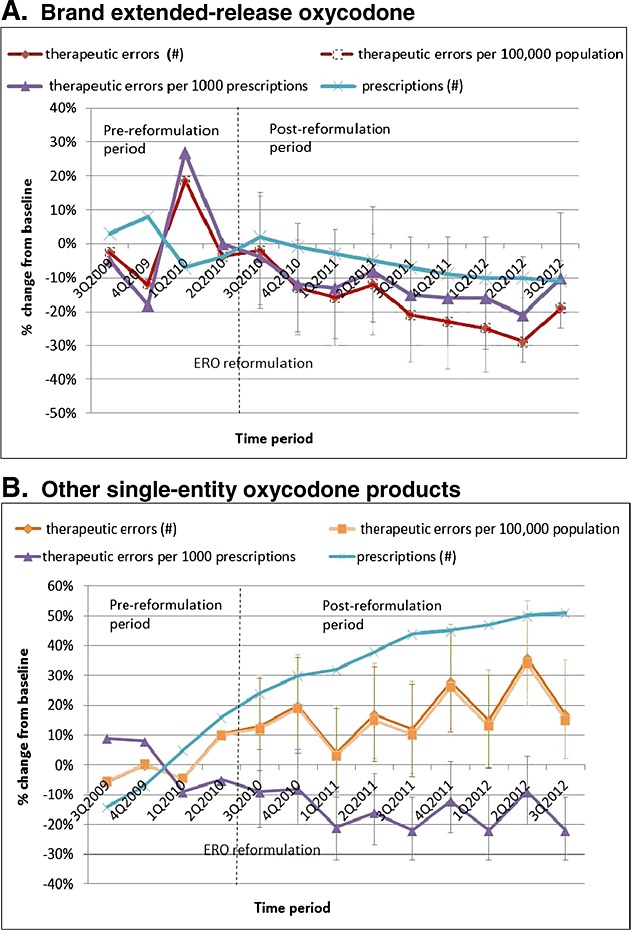
Changes in unintentional therapeutic errors and number of prescriptions from 1 year preceding to 2 years following extended-release oxycodone (ERO) reformulation
In general, changes in exposures were greater for higher ERO dosage strengths than lower (Table 2). For all, abuse, suicide, and accidental exposures, decreases were greater with increasing dose. The largest decreases for the 80 mg dose were seen for abuse and accidental exposures. For other exposure types, decreases were greater for doses ≥ 40 mg versus < 40 mg. In contrast to findings for other dosage strengths, for the 80 mg dosage strength, changes in prescription-adjusted rates differed from those for population-adjusted rates because of large declines in 80 mg prescriptions in the post-reformulation period. After reformulation, ERO 80 mg prescriptions decreased 31%, ERO 40–60 mg prescriptions decreased 15%, and ERO 10–30 mg prescriptions increased 7%. Significant reductions in prescription-adjusted rates occurred for all, abuse, suicide, therapeutic error, accidental, and adverse reaction ERO exposures.
Table 2.
Change in average number of extended-release oxycodone exposures per quarter by tablet strengths from pre- (July 2009 to June 2010) to post-reformulation of OxyContin (October 2010 to September 2012)
| Pre-reformulation (3Q2009–2Q2010) | Post-reformulation (4Q2010–3Q2012) | % change in numbers | % change per 100 000 population | % change per 1000 prescriptions | 95%CI for Rx adjusted rates | p value for Rx adjusted rates | |
|---|---|---|---|---|---|---|---|
| All exposures | |||||||
| All strengths | 692.8 | 509.4 | −26% | −27% | −21% | (−25, −17) | <0.0001 |
| 10–30 mg | 412.3 | 345.3 | −16% | −17% | −22% | (−27, −17) | <0.0001 |
| 40–60 mg | 155.8 | 95.9 | −38% | −39% | −28% | (−35, −20) | <0.0001 |
| 80 mg | 111.5 | 64.9 | −42% | −43% | −15% | (−25, −4) | 0.0102 |
| Abuse | |||||||
| All strengths | 130.3 | 83.3 | −36% | −37% | −31% | (−39, −23) | <0.0001 |
| 10–30 mg | 77.0 | 60.0 | −22% | −23% | −27% | (−37, −16) | <0.0001 |
| 40–60 mg | 21.0 | 10.1 | −52% | −52% | −43% | (−58, −23) | 0.003 |
| 80 mg | 28.5 | 11.8 | −59% | −59% | −40% | (−54, −21) | 0.0002 |
| Intentional misuse | |||||||
| All strengths | 51.3 | 40.4 | −21% | −22% | −15% | (−29, 1) | 0.0625 |
| 10–30 mg | 30.3 | 27.0 | −11% | −12% | −17% | (−33, 4) | 0.1039 |
| 40–60 mg | 12.0 | 7.8 | −35% | −36% | −24% | (−48, 11) | 0.1518 |
| 80 mg | 8.3 | 5.5 | −33% | −34% | −3% | (−38, 52) | 0.8975 |
| Suspected suicide | |||||||
| All strengths | 182.3 | 143.1 | −21% | −23% | −16% | (−23, −7) | 0.0003 |
| 10–30 mg | 132.0 | 110.8 | −16% | −17% | −22% | (−30, −13) | <0.0001 |
| 40–60 mg | 24.3 | 18.4 | −24% | −25% | −11% | (−31, 15) | 0.3763 |
| 80 mg | 20.5 | 13.3 | −35% | −36% | −6% | (−29, 26) | 0.6802 |
| Unintentional therapeutic errors affecting patients | |||||||
| All strengths | 161.3 | 129.4 | −20% | −21% | −14% | (−22, −5) | 0.0032 |
| 10–30 mg | 72.0 | 72.4 | 1% | −1% | −6% | (−19, 8) | 0.3614 |
| 40–60 mg | 61.8 | 36.4 | −41% | −42% | −31% | (−42, −18) | <0.0001 |
| 80 mg | 25.5 | 19.8 | −23% | −24% | 13% | (−12, 45) | 0.3437 |
| Unintentional general/accidental exposures | |||||||
| All strengths | 75.0 | 45.9 | −39% | −40% | −34% | (−44, −23) | <0.0001 |
| 10–30 mg | 41.8 | 29.1 | −30% | −31% | −35% | (−47, −21) | <0.0001 |
| 40–60 mg | 18.0 | 10.4 | −42% | −43% | −32% | (−51, −7) | 0.0156 |
| 80 mg | 14.8 | 6.4 | −57% | −57% | −37% | (−57, −8) | 0.0154 |
| Adverse reactions | |||||||
| All strengths | 29.8 | 19.5 | −34% | −35% | −30% | (−44, −11) | 0.0040 |
| 10–30 mg | 20.3 | 12.6 | −38% | −38% | −42% | (−57, −22) | 0.0003 |
| 40–60 mg | 7.3 | 4.5 | −38% | −45% | −27% | (−55, 19) | 0.2065 |
| 80 mg | 2.3 | 2.4 | 6% | −20% | 54% | (−30, 240) | 0.2881 |
Heroin abuse exposures increased steadily between 2007 and 3Q2012 (Figure 4). However, results from spline regression indicate that there was a significant increase in slope, from 3.4% quarterly increase (95%CI: 2.7% to 4.0%) pre-reformulation versus 5.8% quarterly increase (95%CI: 5.0% to 6.7%) post-reformulation. The net pre versus post increase in heroin abuse was 2.3% (95%CI: 1.0% to 3.7%) quarterly. ERO abuse, in contrast, had a flat slope quarterly slope pre-reformulation (quarterly change of 0.3%, 95%CI: −0.7%, 1.4%) and a downward slope post-reformulation of −8.0% (−9.7%, −6.3%) so that the net pre versus post decrease in ERO abuse was −8.3% (95%CI: −10.6%, −6.0%) quarterly.
Figure 4.
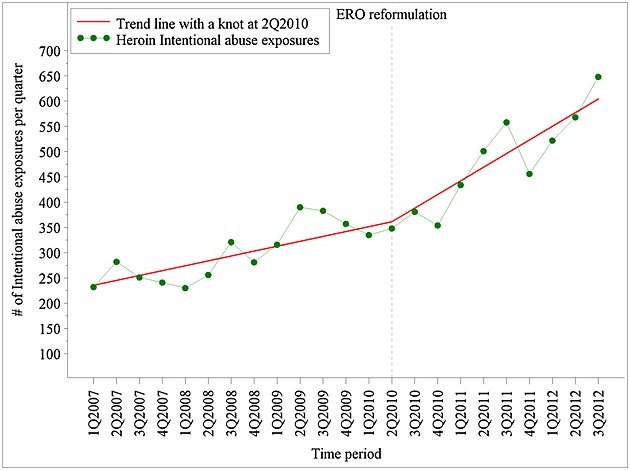
Trends in intentional abuse exposures for heroin before and after extended-release oxycodone (ERO) reformulation—raw data and linear spline trend lines
DISCUSSION
The current study uses data from a national surveillance system of exposures reported to poison centers and extends previously published findings of the impact of ERO on abuse in two recent studies of patients in substance abuse treatment programs. Cicero et al. reported results of sequential cross-sectional surveys of patients with diagnosed opioid dependence/addiction in the USA showing that the proportion of patients using to “get high in the past 30 days at least once” fell from 47.4% to 30.0% (p < 0.001), whereas heroin use increased from 10% to 20%.29 Butler et al. used a sentinel surveillance sample of 140 496 individuals assessed for substance abuse treatment in the USA and reported that abuse of reformulated ERO was 41% lower (95%CI: 44% to 37%) than historical abuse for original ERO, with oral abuse 17% lower (95%CI: 23% to 10%) and non-oral abuse 66% lower (95%CI: 69% to 63%).28
Over the 3-year period surrounding the ERO reformulation, there were large changes in the number and rates of poison center exposures associated with oxycodone in the USA. The number of exposures for brand ERO (OxyContin) decreased steadily after the reformulation was introduced. Marked decreases occurred in all, abuse, suicide, therapeutic errors, accidental, and adverse reaction ERO exposures, and were sustained for the 2-year period after reformulation. In contrast, exposures for other SE oxycodone increased or remained unchanged after reformulation, suggesting that changes were specific to reformulated ERO. Overall, abuse exposures for ERO decreased 36% in the 2 years after reformulation, but those associated with 80 mg tablets decreased 59%. These results are consistent with the intended role of physicochemical barriers to breaking, crushing, or dissolving to reduce the desirability of tablets for the purpose of abuse, and higher dosage strengths being preferred for abuse.
The number of therapeutic errors affecting patients decreased 20%, and those associated with 40–60 mg and 80 mg doses decreased 41% and 23%, respectively.
Therapeutic errors include “unintentional deviation from a proper therapeutic regimen that results in wrong dose.” Reformulation reduces the release of higher than intended doses from tablets that may be crushed, chewed, or dissolved by patients for ease of ingestion or crushed by health care providers for ease of administration to patients. The product label warns that it is important to instruct patients to swallow tablets intact because life-threatening respiratory depression may occur with use of OxyContin even when not abused if tablets are crushed, dissolved, or chewed, causing rapid release and absorption of potentially fatal doses of oxycodone.18 This is corroborated by the significant decrease after reformulation in medication errors, including fatal errors, reported to the manufacturer from patients crushing, chewing, or dissolving tablets (unpublished data).
Accidental exposures, which include unintentional ingestion by children gaining access to these substances for nonmedical use, decreased 39%, and decreased 57% for the 80 mg dose. Pre-reformulation, 63% of accidental exposures occurred among children 1 to 2½ years of age, and these exposures decreased 51% post-reformulation. These results support the hypothesis that tablets with barriers to crushing, chewing, or breaking reduce serious adverse events in young children (who inadvertently put tablets in their mouth) by preventing chewing or breaking the tablet to release a potentially fatal dose.
Abuse exposures for heroin increased steadily before and after reformulation, but the rate of increase was on average 2.3% greater quarterly after reformulation. This increase is consistent with reports of patients preferring heroin or other prescription opioids over reformulated ERO.29
While reductions in ERO exposures occurred, there are other possible explanations for the decreases besides the effects of reformulation. These include the passive surveillance system of poison centers, changes in reporting processes to poison centers, possible misclassification at the poison centers between original and reformulated ERO exposures, reductions in ERO prescriptions, changes in the population covered, and secular trends in prescription opioid abuse and misuse due to other interventions.
The NPDS depends on calls reported to poison centers and can under-represent the occurrence of exposures. To address potential bias from changes in reporting over time, comparator groups of other opioids were used. It is unlikely that changes in reporting of exposures accounted for the observed changes, because the direction of changes observed for ERO and comparator opioids differed, and trends in exposures for ERO and other SE oxycodone were relatively stable prior to reformulation.
To address potential misclassification between original and reformulated ERO exposures, this analysis assessed changes in exposures for all brand ERO formulations, regardless of original or reformulation report, even though poison center staff obtain specifics of products from callers.36 As a result, ERO exposures underestimated the impact of reformulation by including original ERO exposures in the post-reformulated ERO period. The greater decline in ERO exposures in more recent periods, but not for comparator opioids, is consistent with decreasing availability of original ERO. Prescriptions filled at pharmacies for original ERO constituted 7.4%, 1.8%, and 0.6% of total ERO prescriptions in January 2011, June 2011, and December 2011, respectively.
To address the potential impact of reductions in ERO prescriptions, prescription-adjusted analyses were conducted. Changes in ERO prescription-adjusted rates were similar to changes in number of exposures. To address changes in population covered, population-adjusted rates were calculated.
During the current study period, there have been a number of other interventions intended to reduce prescription opioid abuse, such as educational, prescription monitoring, overdose prevention, and drug take-back programs. Preliminary evaluation of state prescription monitoring programs suggests they have a positive impact on opioid abuse/misuse37; however, their effect on drug overdose fatality rates is unclear.38 These programs have been generally voluntary, intermittently conducted, and reported either no impact38 or an effect of slowing the increasing trend in poison center exposures,37 but have not been reported to cause reductions in poison center exposures. The FDA Risk Evaluation and Mitigation Strategy (REMS) for the opioid class of extended-release and long-acting opioids was approved in July 2012 with continuing education training of healthcare providers beginning in March 2013.10 Any effects of the REMS would be after the time frame of the current study. Overdose prevention and drug take-back programs have been applied to all opioid analgesics, including ERO and other oxycodone products. Therefore, other interventions do not account for the large drop in exposures for ERO and increases for other oxycodone products.
The strengths of this study include the national scale, analyses of exposures by reasons (e.g., abuse, therapeutic errors, and accidental exposures), analyses of changes by dosage strengths, inclusion of heroin changes relative to pre-existing trends, and assessment of time trends using a quasi-experimental design.
Data sources to assess the impact of reformulation on abuse and medication errors are limited. Administrative claims databases provide data on opioid overdose but rarely identify the opioid associated with the overdose. Mortality databases (e.g., National vital statistics) do not differentiate between immediate-release and extended-release oxycodone formulations. Federally funded emergency department surveillance studies (e.g., Drug Abuse Warning Network) are not publically available and have a limited sampling frame, or do not include emergency departments associated with abuse or illicit drugs (e.g., National Electronic Injury Surveillance System-All Injury Program). The NPDS is therefore an important data source for assessing formulation-specific effects.
In conclusion, abuse, therapeutic errors affecting patients, and accidental exposures reported to US poison centers decreased for ERO after reformulation but not for comparator opioids. These findings are consistent with other published reports and anticipated effects, and indicate that this abuse-deterrent formulation has reduced abuse and adverse outcomes from misuse. However, reformulating one product's tablets will not treat opioid addiction, and substitution of other available opioids is likely to occur. However, findings in this report provide proof of concept that physicochemical barriers can reduce abuse and misuse of tablets with such barriers. More consistent applications of abuse-deterrent properties to prescription opioids and addressing heroin availability may be necessary to maximize the public health benefit. These data support FDA's statement that development of abuse-deterrent formulations is a high public health priority. Abuse-deterrent formulations may be a valuable risk management tool for opioid analgesics so that innovation, policing, regulation, and education39 can be combined to mitigate the risks and improve the benefit-risk balance of opioid analgesics.
CONFLICT OF INTEREST
All authors on this manuscript are employees of the company that manufactures one of the products (OxyContin) assessed in this study.
KEY POINTS.
A national surveillance system of all US poison centers (the National Poison Data System) was used to evaluate the effects of reformulating extended-release oxycodone tablets with physicochemical barriers to crushing and dissolving on abuse and safety outcomes.
The number of abuse, therapeutic errors, and accidental exposures reported to US poison centers decreased for extended-release oxycodone (ERO) and increased for other SE oxycodone (both extended-release and immediate-release SE oxycodone) and heroin after reformulation of ERO, consistent with anticipated effects.
The results indicate that formulating opioid products with barriers to tampering can reduce their abuse by certain modes while preserving analgesic benefit to patients.
However, the results also suggest that reformulating one opioid alone to have abuse-deterrent characteristics will not achieve the desired public health benefit if established opioid abusers can easily switch to other available non-abuse-deterrent opioids.
Acknowledgments
This study was sponsored by Purdue Pharma L.P. Dr Paul Coplan, Hrishikesh Kale, Lauren Sandstrom, Dr Craig Landau, and Dr Howard Chilcoat are employees of Purdue Pharma L.P. Purdue Pharma manufactures ERO (OxyContin). Writing assistance was provided by Dr Margi Goldstein.
REFERENCES
- 1.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657–659. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- 2.Birnbaum HG, White AG, Reynolds JL, et al. Estimated costs of prescription opioid analgesic abuse in the United States in 2001: a societal perspective. Clin J Pain. 2006;22:667–676. doi: 10.1097/01.ajp.0000210915.80417.cf. [DOI] [PubMed] [Google Scholar]
- 3.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–1983. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 4.White AG, Birnbaum HG, Mareva MN, et al. Direct costs of opioid abuse in an insured population in the United States. J Manag Care Pharm. 2005;11:469–479. doi: 10.18553/jmcp.2005.11.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 7.Department of Veterans' Affairs/Department of Defense (VA/DoD) Clinical practice guideline: management of opioid therapy for chronic pain. 2010.
- 8.National Opioid Use Guideline Group (NOUGG) Canadian guideline for safe and effective use of opioids for chronic non-cancer pain. 2010.
- 9.American Academy of Pain Medicine. Use of opioids for the treatment of chronic pain. 2013. Mar, Available at: http://www.painmed.org/files/use-of-opioids-for-the-treatment-of-chronic-pain.pdf.
- 10.FDA News Release. FDA introduces new safety measures for extended-release and long-acting opioid medications. 2012. Jul 09, Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm310870.htm [30 March 2013]
- 11.Raffa RB, Pergolizzi JV. Opioid formulations designed to resist/deter abuse. Drugs. 2010;70(13):1657–1675. doi: 10.2165/11537940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Katz N, Dart RC, Bailey E, Trudeau J, Osgood E, Paillard F. Tampering with prescription opioids: nature and extent of the problem, health consequences, and solutions. Am J Drug Alcohol Abuse. 2011;37:205–217. doi: 10.3109/00952990.2011.569623. [DOI] [PubMed] [Google Scholar]
- 13.FDA Guidance for Industry. Abuse-deterrent opioids — evaluation and labeling. 2013. Jan 09, Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM334743.pdf.
- 14.National Drug Intelligence Center. OxyContin diversion and abuse. 2001. Jan, http://www.justice.gov/archive/ndic/pubs/651/abuse.htm [23 February 2012]
- 15.Hays L, Kirsh KL, Passik SD. Seeking drug treatment for OxyContin abuse: a chart review of consecutive admissions to a substance abuse treatment facility in Kentucky. J Natl Compr Canc Netw. 2003;1:423–428. doi: 10.6004/jnccn.2003.0035. [DOI] [PubMed] [Google Scholar]
- 16.Hays LR. A profile of OxyContin addiction. J Addict Dis. 2004;23(4):1–9. doi: 10.1300/J069v23n04_01. [DOI] [PubMed] [Google Scholar]
- 17.Butler SF, Black RA, Grimes Serrano JM, Wood ME, Budman SH. Characteristics of prescription opioid abusers in treatment: prescription opioid use history, age, use patterns and functional severity. J Opioid Manag. 2010;6(4):239–252. doi: 10.5055/jom.2010.0022. [DOI] [PubMed] [Google Scholar]
- 18.OxyContin [full prescribing information] Stamford, CT: Purdue Pharma L.P.; 2012. http://app.purduepharma.com/xmlpublishing/pi.aspx?id=o [25 January 2013] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) CDC grand rounds: prescription drug overdoses — a U.S. epidemic. Morb Mortal Wkly Rep. 2012;61(01):10–13. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6101a3.htm?s_cid=mm6101a3_w [12 December 2012] [PubMed] [Google Scholar]
- 20.Cone EJ, Fant RV, Rohay JM, et al. Oxycodone involvement in drug abuse deaths: a DAWN-based classification scheme applied to an oxycodone postmortem database containing over 1000 cases. J Anal Toxicol. 2003;27:57–67. doi: 10.1093/jat/27.2.57. [DOI] [PubMed] [Google Scholar]
- 21.Dhalla IA, Mamdani MM, Sivilotti ML, Kopp A, Qureshi O, Juurlink DN. Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. CMAJ. 2009;181:891–896. doi: 10.1503/cmaj.090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler SF, Black RA, Cassidy TA, Dailey TM, Budman SH. Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm Reduct J. 2011;8:29–45. doi: 10.1186/1477-7517-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler SF, Budman SH, Licari A, et al. National addictions vigilance intervention and prevention program (NAVIPPRO™): a real-time, product-specific public health surveillance system for monitoring prescription drug abuse. Pharmacoepidemiol Drug Saf. 2008;17:1142–1154. doi: 10.1002/pds.1659. [DOI] [PubMed] [Google Scholar]
- 24.Katz N, Fernandez K, Chang A, Benoit C, Butler SF. Internet-based survey of nonmedical prescription opioid use in the United States. Clin J Pain. 2008;24(6):528–535. doi: 10.1097/AJP.0b013e318167a087. [DOI] [PubMed] [Google Scholar]
- 25.Passik SD, Hays L, Eisner N, Kirsh KL. Psychiatric and pain characteristics of prescription drug abusers entering drug rehabilitation. J Pain Palliat Care Pharmacother. 2006;20(2):5–13. [PubMed] [Google Scholar]
- 26.Cone EJ, Giordano J, Weingarten B. An iterative model for in vitro laboratory assessment of tamper deterrent formulations. Drug and Alcohol Depend. 2013;131(1–2):100–105. doi: 10.1016/j.drugalcdep.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Sellers EM, Harris SC, Perrino PJ, Colucci SV. Comparative assessment of tampering potential and recreational drug user preferences for opioid formulations. Palm Springs, CA, USA: College on Problems of Drug Dependence 74 Annual Meeting; 2012. Jun, Relative attractiveness of reformulated OxyContin. [Google Scholar]
- 28.Butler SF, Cassidy TA, Chilcoat H, et al. Abuse rates and routes of administration of reformulated extended-release oxycodone: initial findings from a sentinel surveillance sample of individuals assessed for substance abuse treatment. J Pain. 2013;14(4):351–358. doi: 10.1016/j.jpain.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Cicero TJ, Ellis MS, Surratt HL. Effect of abuse-deterrent formulation of OxyContin. N Engl J Med 367. 2012;2:187–189. doi: 10.1056/NEJMc1204141. [DOI] [PubMed] [Google Scholar]
- 30.Dasgupta N, Davis J, Jonsson Funk M, Dart R. Using poison center exposure calls to predict methadone poisoning deaths. PLoS One. 2012;7(7):e41181. doi: 10.1371/journal.pone.0041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC) Using the National Poisoning Data System for public health surveillance — a collaborative effort of the Centers for Disease Control and Prevention and the American Association of Poison Control Centers. Department of Health and Human Services; 2005. Jul, CDC fact sheet. www.cdc.gov/nceh/hsb/chemicals/pdfs/npds.pdf. [13 February 2013] [Google Scholar]
- 32.Bronstein AC, Spyker DA, Cantilena LR, Rumack BH, Dart RC. 2011 Annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 29th Annual Report. Clin Toxicol. 2012;50:911–1164. doi: 10.3109/15563650.2012.746424. [DOI] [PubMed] [Google Scholar]
- 33.American Association of Poison Control Centers. National Poison Data System (NPDS) Data Dictionary; (2012.9.21) 2012. pp. 24–41.
- 34.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6(4):356–365. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Philadelphia (PA) Lippincott: Williams & Wilkins; 2008. p. 410. 3rd edn. [Google Scholar]
- 36.Bronstein AC, Spyker DA, Cantilena LR, Jr, Rumack BH, Dart RC. 2011 Annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 29th Annual Report. Clin Toxicol (Phila) 2012;50(10):911–1164. doi: 10.3109/15563650.2012.746424. [DOI] [PubMed] [Google Scholar]
- 37.Reifler LM, Droz D, Bailey JE, et al. Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 2012;13(3):434–42. doi: 10.1111/j.1526-4637.2012.01327.x. [DOI] [PubMed] [Google Scholar]
- 38.Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 2011;12(5):747–54. doi: 10.1111/j.1526-4637.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 39.Office of National Control Policy. Epidemic. Responding to America's prescription drug abuse crisis. 2011. Available at: http://www.whitehouse.gov/ondcp/prescription-drug-abuse [30 March 2013]