Abstract
BACKGROUND
Female survivors of pediatric Hodgkin lymphoma (HL) who have received chest radiotherapy are at increased risk of breast cancer. Guidelines for early breast cancer screening among these survivors are based on little data regarding clinical outcomes. This study reports outcomes of breast cancer screening with MRI and mammography (MMG) after childhood HL.
METHODS
We evaluated the results of breast MRI and MMG screening among 96 female survivors of childhood HL treated with chest radiotherapy. Outcomes measured included imaging sensitivity and specificity, breast cancer characteristics, and incidence of additional imaging and breast biopsy.
RESULTS
Median age at first screening was 30 years, and the median number of MRI screening rounds was 3. Ten breast cancers were detected in 9 women at a median age of 39 years (range, 24-43 years). Half were invasive and half were preinvasive. The median size of invasive tumors was 8 mm (range, 3-15 mm), and none had lymph node involvement. Sensitivity and specificity of the screening modalities were as follows: for MRI alone, 80% and 93.5%, respectively; MMG alone, 70% and 95%, respectively; both modalities combined, 100% and 88.6%, respectively. All invasive tumors were detected by MRI. Additional investigations were required in 52 patients, (54%), and 26 patients (27%) required breast biopsy, with 10 patients requiring more than 1 biopsy.
CONCLUSIONS
Screening including breast MRI with MMG has high sensitivity and specificity in pediatric HL survivors, with breast cancers detected at an early stage, although it is associated with a substantial rate of additional investigations. Cancer 2014;120:2507–2513. © 2014 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Screening female survivors of pediatric Hodgkin Lymphoma for breast cancer with MRI and mammography detected tumors at an earlier stage than prior studies of mammography alone, although a substantial proportion of women required additional tests for benign imaging findings. The 5-year cumulative incidence of invasive or preinvasive tumors after initiating screening was 10.8%.
Keywords: pediatric Hodgkin lymphoma, breast MRI, screening, second cancer
INTRODUCTION
Female survivors of pediatric Hodgkin's lymphoma (HL) who have received chest radiotherapy (RT) are known to be at increased risk of developing breast cancer.1,2 Consequently, clinical practice guidelines have recommended the early initiation of breast cancer screening among these survivors, with the expectation of reducing the risk of breast cancer–related morbidity and mortality,3–6 although there is evidence that compliance with recommended screening is poor.7,8
A potentially significant limitation of the existing evidence is the scarcity of data regarding the outcome of screening among survivors treated for HL during childhood. It cannot be assumed that outcomes of breast cancer screening among patients treated and screened at older ages will apply to survivors of pediatric HL. The age at which RT is delivered and the attained age of the patient at the time of screening affect both the absolute risk of breast cancerand the imaging characteristics of the breast tissue.9 Although the relative risk of breast cancer may be higher, the absolute risk (which has the greater influence on the outcome of screening) may be lower among younger survivors. Further, the sensitivity of mammography is worse among younger patients,10,11 also potentially influencing the outcome of screening protocols among survivors of childhood HL.
Finally, existing data regarding the effectiveness of breast cancer screening preceded the routine use of MRI, which has emerged as a preferred means of screening high-risk patients.12,13 There are no published data regarding the outcomes of childhood cancer survivors screened with MRI.
To address these issues, we conducted a study of the results of combined MRI and mammographic screening among female pediatric HL patients treated with chest RT, focusing on the characteristics of the detected breast cancers and the need for additional investigations associated with breast MRI screening.
Patients and Methods
Patients were identified from pediatric long-term aftercare clinics at the Princess Margaret Cancer Centre and the Dana-Farber Cancer Institute (DFCI). Patients were identified from pediatric aftercare clinics, HL databases, and survivorships organizations. Patients were included if they had a prior diagnosis of HL before age 20 years, if treatment had included chest RT, and if breast cancer screening had commenced with at least 1 breast MRI. Patients at the Princess Margaret Cancer Centre were screened in a clinical setting if they were >8 years beyond HL therapy or at least age 25, whichever was later, and were recommended to have annual breast MRI, MMG, and clinical examination. Patients from the DFCI were screened as part of a prospective study that included women who were ≥8 years beyond HL therapy and were screened with annual MRI and MMG for 3 years.
Mammography examinations were performed using full-field digital mammography machines (Senographe 2000D or Senographe Essentials, GE Healthcare, Milwaukee, WI; or Selenia Dimensions, Hologic, Bedford, MA). All study participants underwent a 2-view mammographic study of each breast consisting of a craniocaudal and mediolateral oblique view. Additional projections and spot magnification views were used as needed.
Contrast-enhanced MRI examinations were performed at 1.5-T scanner (Signa GE Healthcare, Milwaukee, WI; or Siemens Avanto, Siemens Medical Solutions, Erlangen, Germany) or a 3-T scanner (Siemens Verio) with use of a dedicated surface breast coil. Breast MRI protocol evolved over the course of this study; however, in all cases, it included bilateral fat-suppressed T2-weighted images and both pre- and postcontrast T1-weighted dynamic fat suppressed images. Spatial and temporal resolution were compliant with quality standards of the American College of Radiology.14
Dedicated breast radiologists reviewed MRI and mammographic images. The study was approved by the research ethics boards at the Princess Margaret Cancer Centre and the Dana Faber Cancer Institute.
Data Collection
Patient charts were reviewed and information regarding patient demographics, HL diagnosis and treatment, breast cancer screening, additional investigations performed, breast cancer diagnosis, and treatment were collected. Last follow-up was taken as last clinical review or breast screen imaging, whichever occurred later.
Statistical Analysis
Descriptive statistics were used to describe the clinical features of the cohort, the screening practices, and the additional testing caused by screen-detected findings, as well as the breast cancer outcomes.
True-positive scans were those in which a breast lesion was histologically proven to be an invasive breast cancer or ductal carcinoma in situ (DCIS), and true-negative scans were those that did not show a suspicious breast lesion and no breast cancer was diagnosed in the next 12 months. False-negative scans were those scans that did not detect a suspicious breast lesion, but a breast cancer was detected in the following 12 months, and false-positive scans were those that resulted in a biopsy that was subsequently benign. Additional tests included those tests that were ordered outside the routine annual MMG and MRI and included those ordered immediately and for early screening. The cumulative breast cancer incidence in the study cohort was calculated. Because there was no competing causes of death in the study cohort, the estimated cumulative breast cancer incidence was simply equal to the complement of the Kaplan-Meier estimate of survival probabilities.
RESULTS
Patient Characteristics
In total, 96 of 104 eligible patients (92.3%) were screened with breast MRI from 2005 through 2012. Patient characteristics with regard to initial HL treatment and risk factors for breast cancer are described in Table1. Initial HL diagnosis occurred between 1972 through 2003, and the median age at diagnosis was 15 years (2-19 years). The initial treatment for HL was radiotherapy alone in 31 patients (32.3%) and combined radiation and chemotherapy in 65 patients (67.7%). All patients received mediastinal radiotherapy, with a median dose of 22.5 Gy (range, 14-45 Gy). The median dose for the DFCI patients was 36 Gy (14-45 Gy), and median dose for the Princess Margaret patients was 15 Gy (15-35 Gy). Ninety percent of patients received mantle radiation fields, with 10% receiving mediastinal-involved field radiotherapy excluding the axillae. The most common chemotherapy agents used were mechlorethamine, vincristine, procarbazine, prednisone-doxorubicin (MOPP/ABVD) in 29 patients (30.21%) and ABVD in 12 patients (12.5%). Seventy-eight percent of patients were premenopausal at the time of screening, and 74% had previous exposure to oral contraceptive pills or hormone replacement.
Table 1.
Clinical Characteristics of 96 Female Survivors of Pediatric HL Screened With MRI/MMG
| Age at HL diagnosis (y), median (range) | 15 (2-19) |
| HL treatment, n (%) | |
| Radiation alone | 31 (32.3) |
| Chemotherapy + radiation | 65 (67.7) |
| Radiation type | |
| Mantle, n (%) | 86 (90) |
| Mediastinal, n (%) | 10 (10) |
| Radiation dose (Gy), median (range) | 25.5 (14-45) |
| Chemo therapy, n (%) | |
| None | 31 (32%) |
| Alkylating | 48 (50%) |
| Nonalkylating | 14 (15%) |
| Unknown | 3 (3%) |
| Menopausal status, n (%) | |
| Premenopausal | 75 (78) |
| Postmenopausal | 18 (19) |
| Unknown | 3 (3) |
| Hormone replacement therapy/ | |
| oral contraceptive pill use, n (%) | 71 (74) |
| Yes | 17 (18) |
| No | 8 (8) |
| Unknown | |
| Family history—first-degree relative, n (%) | 4 (4) |
Breast Cancer Screening
At the time that breast MRI screening commenced, 58 patients had had prior breast screening with MMG and/or breast ultrasound (US). The median time from diagnosis of HL to first breast MRI screen was 16 years (range, 5-39 years), and the median age at first MRI screen was 30 years (range, 19-59 years). In total, 274 screening MRIs were performed in 96 patients. The median number of MRI screens was 3 (range, 1-7), and the median number of MMG screens was 3 (range, 0-5). Eleven patients did not receive MMG. Reasons included patient refusal (n = 10) and breast-feeding (n = 1). Eight patients with at least 1 MRI dropped out of screening altogether (reasons not stated), with an overall compliance rate 88 of 104 (84.6%).
Breast Cancer Detection
In the cohort of 96 women, 10 breast cancers were diagnosed in 9 women during 363 person-years of follow-up. The 5-year cumulative incidence of breast cancer from the initiation of screening was 10.8% (95% CI, 3-17.4; Fig. 1). The median screening round at which breast cancer was detected was screening round 2, although 4 patients were diagnosed with breast cancer at the time of the first MRI screen. The median age of breast cancer diagnosis was 39 years (range, 24-43 years), and the median latency period between HL diagnosis and age at breast cancer diagnosis was 21 years (12-27 years). The locations of the breast tumors were as follows: 6 were found in the upper outer quadrant, 2 in the upper inner quadrant, and 2 had an undocumented primary location. Eight patients with a breast cancer detected had received mantle radiotherapy fields, with 1 patient receiving radiation to the mediastinum alone. RT dose was available for 89 patients. Among 61 patients who received >21 Gy to the mediastinum, 8 (13%) were diagnosed with breast cancer, in contrast to 1 of 28 patients (4%) who received <21 Gy (4%); this difference was not statistically significant (P = .17).
Figure 1.
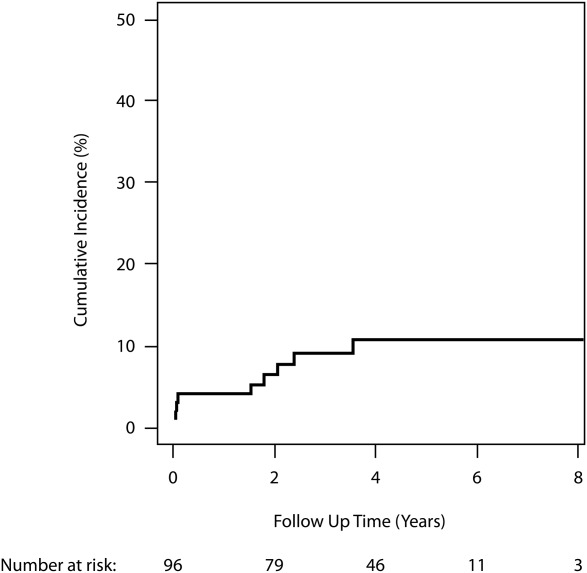
Cumulative incidence of breast cancer starting at time of first MRI screening.
Characteristics of the diagnosed breast cancers are summarized in Table2. Half the tumors diagnosed were invasive, and half were DCIS. The median size of invasive tumor was 8 mm (range, 3-15 mm). All invasive tumors were grade II or III. None of the detected breast cancers was lymph node positive. At last time of follow-up, all patients were alive.
Table 2.
Characteristics of Breast Tumors Detected by MRI/MMG Screening Among 96 Female Survivors of Pediatric HL
| Case | Age at HL Diagnosis (y) | RT Regions and Total Dose | Age at Breast Cancer Diagnosis (y) | Detection Method | Screening Round at Time of Detection | Side | CBE | MMG | US | MRI | Type of Surgery | Histology | T | N | M | Grade | Tumor size (mm) | ER | PR | Her2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | Mantle 25Gy | 39 | MRI MMG | 3 | Left | Neg | Pos | Neg | Pos | Bilat Mx | DCIS | Tis | 0 | 0 | II | 10 | — | — | — |
| 2 | 15 | Mantle 15Gy | 31 | MRI | 1 | Left | Lump | Pos | n/a | Pos | Bilat Mx | IDC | T2 | 0 | 0 | II | 15 | Pos | Neg | Neg |
| 3 | 19 | Mantle + mediastinum 36 Gy | 34 | MMG | 1 | Left | n/a | Pos | n/a | Neg | Bilat Mx | DCIS | Tis | 0 | 0 | II | 3 | Pos | Pos | Equivocal |
| 4 | 12 | Mediastinum 25 Gy | 24 | MRI MMG | 3 | Right | n/a | Pos | Pos | Pos | Bilat Mx | IDC | T1a | 0 | 0 | II | 3 | Pos | Pos | Neg |
| 5 | 19 | Mantle + mediastinum 40Gy | 43 | MRI MMG | 1 | Left | n/a | Pos | Pos | Pos | Bilat Mx | DCIS | Tis | 0 | 0 | II | — | Pos | Pos | Neg |
| 6 | 18 | Mantle 30 Gy + mediastinum 40 Gy | 41 | MRI | 2 | Left | n/a | Neg | Pos | Pos | Bilat Mx | IDC | T1b | 0 | 0 | III | 4 | Neg | Neg | Neg |
| 7 | 19 | Mantle 36 Gy + mediastinum 42 Gy | 40 | MRI MMG | 3 | Right | n/a | Pos | Pos | Pos | Bilat Mx | IDC | T1b | 0 | 0 | III | 8 | Neg | Neg | Neg |
| 8 | 19 | Mantle + mediastinum 40 Gy | 40 | MMG | 4 | Right | n/a | Pos | n/a | Neg | Bilat Mx | DCIS | Tis | 0 | 0 | II | — | Pos | Pos | Neg |
| 9 | 18 | Mantle 30 Gy + mediastinum 40 Gy | 32 | MRI | 1 | Right | n/a | Neg | Neg | Pos | Right Mx | DCIS | Tis | 0 | 0 | II | 5 | Pos | Pos | Pos |
| — | 36 | MRI MMG | 4 | Left | n/a | Pos | n/a | Pos | Left Mx | IDC | T1b | 0 | 0 | III | 10 | Neg | Neg | Neg |
Abbreviations: HL, Hodgkin's lymphoma; RT, radiotherapy; CBE, clinical breast examination; DCIS, ductal carcinoma in situ; Dx, diagnosis; IDC, invasive ductal carcinoma; MMG,= mammography; US, ultrasound; MRI, magnetic resonance imaging; TNM, TNM staging; Mx, right-sided mastectomy; Bilat, bilateral; n/a, not applicable; Neg, negative; Pos, positive.
Of the 10 detected tumors, 5 were visible on both MRI and MMG, 3 were visible only on MRI, and 2 were detected on mammogram alone (both of which were DCIS). The sensitivity of breast MRI was 80.0%, and specificity was 93.5%, and the sensitivity and specificity of MMG were 70.0% and 95.0% respectively. For breast MRI and MMG combined, the sensitivity and specificity were 100% and 88.6%, respectively.
Additional Investigations
Of the 96 patients who were screened, 52 patients (54%) required additional testing outside the planned annual screen. These tests included those ordered immediately following the index screening test as well as early rescreening recommended within 12 months because of findings on the index screening test. Included in the additional tests were 30 MRIs (3 immediate and 27 early), 26 MMGs (18 immediate and 8 early screening), and 65 breast ultrasounds (47 immediate and 18 early screening).
Of all the additional tests, 123 (80%) were prompted by an abnormality on an MRI that required further investigation. The number of patients called back for additional tests was highest at initial-screening MRI and decreased with subsequent breast MRI (Table 3).
Table 3.
Number of Patients Requiring Additional Tests Per Screening Round
| Screening MRI round (n = number at risk) | ||||
|---|---|---|---|---|
| Number of patients (n) | 1 (n = 96) | 2 (n = 81) | 3 (n = 65) | 4 (n = 20) |
| Screening MRI, n (%) | 96 (100%) | 81 (100%) | 65 (100%) | 20 (100%) |
| Screening MMG, n (%) | 72 (75%) | 61 (75%) | 57 (88%) | 9 (45%) |
| Any additional test, n (%) | 34 (35%) | 14 (17%) | 11 (17%) | 2 (10%) |
| Benign biopsy, n (%) | 11 (11%) | 4 (5%) | 3 (5%) | 1 (5%) |
| Positive biopsy, n (%) | 4 (4%) | 1 (1%) | 3 (5%) | 2 (10%) |
Breast biopsy was performed in 26 patients (27%), with 10 patients (10%) requiring more than 1 biopsy. Of the 38 biopsies performed, 28 (74%) had benign pathology, and of the 26 patients who underwent biopsy, 9 had an invasive cancer or DCIS. The number of patients undergoing benign biopsy was highest in the first screening round and decreased in subsequent rounds (Table 3). Similarly, the number of benign biopsies was also highest in the first screening round: among 28 benign biopsies performed across all screening rounds, 20 (71%) were performed in the first screen, and 4, 3, and 1 were performed in rounds 2, 3, and 4, respectively. Of the 28 benign biopsies performed, 17 were prompted by an abnormality on MRI, 6 were a result of an abnormality on MMG, and 5 were a result of an abnormality on both MRI and MMG.
DISCUSSION
To our knowledge, this is the largest study to evaluate the outcome of breast cancer screening among a cohort of at-risk survivors of pediatric HL and the only study in which MRI was employed for screening among all patients studied. When compared with prior reports with non-MRI breast screening,15,16 the breast cancers detected were smaller, more likely to be preinvasive, and less likely to be lymph node positive. Of the breast cancers detected in our study, 50% were preinvasive, with a median invasive size of 8 mm (3-15 mm), and with none associated with lymph node involvement. Terenziani et al reported on 86 pediatric patients treated with chest radiotherapy in which screening imaging was predominantly with MMG and breast ultrasound. Breast MRI screening was also included but only occurred late in the overall screening series. Tumors in that study were larger (median, 10 mm [range, 2-30 mm]), were more frequently node positive (6 of 11 patients), and were less likely to be noninvasive (2 of 11 patients) than the MRI-detected cases reported in our cohort. Howell et al reported the outcome of the UK National breast screening program for HL survivors, in which survivors treated primarily as young adults underwent mammography, with MRI used for young females with mammographically dense breast tissue.17 Interestingly, among 417 patients invited to attend the program, 243 (58%) attended, with 5 breast cancers detected in the program, none with axillary nodal involvement. In contrast, 7 of 13 breast cancers detected among nonattendees involved axillary lymph nodes. Our findings support these results, illustrating the potential benefit of screening, but also the challenge of attracting and retaining high-risk patients in screening programs.
MRI has emerged as the preferred modality for screening women at high risk for breast cancer in other clinical circumstances (eg, BRCA carriers). This is in part attributable to evidence that MMG is less sensitive in women younger than 40 years compared with those 40-49 years old,11 primarily because increased mammographic breast density is known to be inversely related to age. A recent prospective study by Ng et al examined the additional benefit of breast MRI to MMG in screening for patients treated for HL <35 years, with the majority of patients treated as adults.18 This study found that MRI was not more sensitive than MMG, with sensitivity of 68% for MMG and 67% for MRI. Other studies also reporting MRI screening outcomes in older patients have reported that the addition of MRI to mammography increased the detection rate of early invasive cancers.19,20 In our group of pediatric survivors we found that when both invasive and preinvasive tumors were considered, MRI had a sensitivity of 80%, with both cases of false-negative MRIs occurring with preinvasive disease. All cases of invasive breast cancer in our study were detected by breast MRI at an early stage. The finding of a higher sensitivity of MRI in our study could be explained, in part, by the median age of our cohort. Patients had a median age of 30 years when MRI screening was commenced compared with a median age of 37-43 years in prior studies.19,20
The potential carcinogenicity of mammographic radiation exposure has been the subject of concern.21 Based on a quantitative synthesis of epidemiologic and radiobiologic data, Heyes et al estimated that the benefits of mammographic screening outweighed the potential carcinogenicity risks for older average-risk women, but that the potential risks among patients under age 50 suggested a “need for caution.”21 Our view is that the existing risk of breast cancer among these survivors likely eclipses the additional risk caused by mammography. However, given that invasive cancers are reliably detected by MRI alone, some patients may be willing to forgo the added potential to detect DCIS with mammography in order to avoid the additional radiation exposure and discomfort of mammography, and the selection of imaging modality should be made after discussing these issues.
The optimal time to initiate breast cancer screening in childhood HL survivors is uncertain, and clinical practice guidelines vary in their recommendations on this issue. Recommended start times typically include some combination of 8- to 10-year latency from RT and an attained age of 25-30 years.4–6 In our view, the results of this study support earlier start times within this range. Four patients were found to have an existing breast cancer at the time of their first screen, and 3 of these patients were aged 30-35 years at the time of breast cancer detection. The youngest age at which breast cancer was detected was 24 years old. This suggests that initiating screening after age 30 may produce some delay in diagnosis, although we recognize that even the prevalent cancers were detected at an early stage, and our sample size does not permit accurate estimates of the number of patients younger than 30 who would require screening to prevent 1 breast cancer death. Again, the initiation of screening at age 25 potentially leads to >50 years of imaging, and for the reasons indicated above, some patients may reasonably opt to undergo MRI screening only if they have dense breast tissue.
Current evidence suggests that pediatric patients treated with chest radiotherapy have different breast cancer risks than HL patients treated at older ages. For example, population-based studies have shown higher relative risk for breast cancer development in patients who receive radiotherapy between ages 10 and 14 years, with diminishing relative risks with increasing age.1 Different baseline breast cancer risk and breast density may contribute to the differences found in mammographic sensitivity and suggests that screening MRIs in this cohort may be of greater importance when compared with older patients.
Our findings also indicate that an important shortcoming of MRI-based screening among young survivors is the high rate of additional investigations including biopsies that are precipitated by abnormal or ambiguous screening results. More than half the screened patients required an additional investigation, and 27% underwent at least 1 biopsy. Forty-three patients (45%) without breast cancer required additional testing, and 17 patients (20%) had benign biopsies. Our rate of additional investigation is higher than that described in a study of similar patients predominantly screened with breast examination, MMG, and breast ultrasound.22 In that study only 12 patients (14%) without breast cancer required additional testing, and 4 of these patients (5.3%) had benign biopsies.22 Consistent with our findings, the authors of this study found that with the introduction of MRI to their screening program, the number of additional investigations increased. These results are consistent with studies that examined MRI screening in other high-risk settings, where there was a 3- to 5-fold increase in risk of recall for investigation when the ultimate result was a benign lesion. Notably, the frequency of additional screening declined substantially over the first few screening rounds. The rate of additional testing and invasive testing was important to consider in this particular cohort, as additional testing can be anxiety provoking in patients with elevated risk of breast cancer.23 In our experience, it is worthwhile to discuss the risk of additional investigations with patients in advance of initiating screening and to provide appropriate reassurance to allay worry that can be caused by call back for additional investigations and to inform patients that the risk of additional tests declines over the first few rounds of screening. Recent studies suggest that characterization of the metabolic features of MRI-detected breast lesions with proton MR spectroscopy may improve the specificity of MRI and potentially reduce the frequency of imaging-induced biopsy.24
This study has limitations that warrant consideration. Although it is the largest study of MRI breast screening in the target population described to date, it is not possible to make definitive conclusions regarding the merits of this approach with regard to important outcomes such as the prevention of breast cancer deaths. Moreover, subset analyses were underpowered to detect what could be clinically important differences in the breast cancer detection rate that could help to identify the optimal criteria for screening. For example, the crude rate of breast cancer detection among patients prescribed >21 Gy was 3-fold higher than that among patients prescribed lower doses (comparable to those used in contemporary protocols), although this difference was not statistically significant. Given the reduction in breast dose associated with smaller contemporary RT fields for pediatric HL, the findings described here could be enhanced with a more detailed evaluation of the radiation dose-risk relation than we were able to provide. A larger study with longer follow-up would be required to determine the outcomes of screening among pediatric HL survivors treated exclusively with contemporary RT doses and fields. In addition, there was some variability in the timing of screening tests and the use of MMG because of the variety in patient acceptance and schedules. This did not allow side-by-side comparison of the MRI and MMG findings in all screening rounds for all patients or an evaluation of the merit of concurrent versus alternating MRI and MMG. We believe, however, that any implementation of a screening program among survivors would have similar variation in the use and timing of different modalities.
Despite these limitations this study has provided important clinical information to aid in the use of breast MRI screening in the pediatric HL aftercare population. Specifically, clinically significant breast cancers are detected at young ages and aree more likely to be found at earlier stages compared with screening programs that do not include MRI, although at the cost of requiring more additional investigations and biopsy. We would recommend complying with screening guidelines that recommend screening from age 25 years or 8 years following chest RT.5
FUNDING SUPPORT
This work was supported by the Canadian Cancer Society and the Pediatric Oncology Group of Ontario. Dr. Hodgson is supported by a Research Chair from Cancer Care Ontario.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Swerdlow AJ, Cooke R, Bates A, et al. Breast cancer risk after supradiaphragmatic radiotherapy for Hodgkin's lymphoma in England and Wales: a National Cohort Study. J Clin Oncol. 2012;30:2745–2752. doi: 10.1200/JCO.2011.38.8835. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 3.Smith RA, Saslow D, Sawyer KA, et al. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin. 2003;53:141–169. doi: 10.3322/canjclin.53.3.141. [DOI] [PubMed] [Google Scholar]
- 4.Scottish Intercollegiate Guidelines Network. Long term follow up of survivors of childhood cancer. 2004. Guideline no. 76. Available at: http://www.sign.ac.uk/pdf/sign76.pdf. Accessed November 2007.
- 5.Group CsO. Available at: http://www.survivorshipguidelines.
- 6.NCCN. The NCCN Clinical Practice Guidelines in Oncology. Hodgkin's Disease/LymphomaNational Comprehensive Cancer Network. 2008. Available at: http://www.nccn.org.
- 7.Hodgson DC, Grunfeld E, Gunraj N, Del Giudice L. A population-based study of follow-up care for Hodgkin lymphoma survivors: opportunities to improve surveillance for relapse and late effects. Cancer. 116:3417–3425. doi: 10.1002/cncr.25053. [DOI] [PubMed] [Google Scholar]
- 8.Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA. 2009;301:404–414. doi: 10.1001/jama.2008.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cance risk among 5-year survivors of Hodgkin's lymphoma. J Clin Oncol. 2007;25:1489–1497. doi: 10.1200/JCO.2006.09.0936. [DOI] [PubMed] [Google Scholar]
- 10.Smith RA. Breast cancer screening among women younger than age 50: a current assessment of the issues. CA Cancer J Clin. 2000;50:312–336. doi: 10.3322/canjclin.50.5.312. [DOI] [PubMed] [Google Scholar]
- 11.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–175. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 12.Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427–437. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 13.Warner E. Impact of MRI surveillance and breast cancer detection in young women with BRCA mutations. Ann Oncol. 2011;22(Suppl 1):i44–i49. doi: 10.1093/annonc/mdq665. [DOI] [PubMed] [Google Scholar]
- 14.ACR Practice Guideline for the Peformance of Contrast Enhanced Magnetic Resonance Imaging (MRI) of the Breast. 2013. Available at: http://www.acr.org/∼/media/2a0eb28eb59041e2825179afb72ef624.pdf 2013 Accessed October 19,
- 15.Lee L, Pintilie M, Hodgson DC, Goss PE, Crump M. Screening mammography for young women treated with supradiaphragmatic radiation for Hodgkin's lymphoma. Ann Oncol. 2008;19:62–67. doi: 10.1093/annonc/mdm440. [DOI] [PubMed] [Google Scholar]
- 16.Diller L, Medeiros Nancarrow C, Shaffer K, et al. Breast cancer screening in women previously treated for Hodgkin's disease: a prospective cohort study. J Clin Oncol. 2002;20:2085–2091. doi: 10.1200/JCO.2002.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Howell SJ, Searle C, Goode V, et al. The UK national breast cancer screening programme for survivors of Hodgkin lymphoma detects breast cancer at an early stage. Br J Cancer. 2009;101:582–588. doi: 10.1038/sj.bjc.6605215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng AK, Garber JE, Diller LR, et al. Prospective study of the efficacy of breast magnetic resonance imaging and mammographic screening in survivors of Hodgkin lymphoma. J Clin Oncol. 2013;31:2282–2288. doi: 10.1200/JCO.2012.46.5732. [DOI] [PubMed] [Google Scholar]
- 19.Freitas V, Scaranelo A, Menezes R, Kulkarni S, Hodgson D, Crystal P. Added cancer yield of breast magnetic resonance imaging screening in women with a prior history of chest radiation therapy. Cancer. 2013;119:495–503. doi: 10.1002/cncr.27771. [DOI] [PubMed] [Google Scholar]
- 20.Sung JS, Malak SF, Bajaj P, Alis R, Dershaw DD, Morris EA. Screening breast MR imaging in women with a history of lobular carcinoma in situ. Radiology. 2011;261:414–420. doi: 10.1148/radiol.11110091. [DOI] [PubMed] [Google Scholar]
- 21.Heyes GJ, Mill AJ, Charles MW. Mammography-oncogenecity at low doses. J Radiol Prot. 2009;29:A123–A132. doi: 10.1088/0952-4746/29/2A/S08. [DOI] [PubMed] [Google Scholar]
- 22.Terenziani M, Casalini P, Scaperrotta G, et al. Occurrence of breast cancer after chest wall irradiation for pediatric cancer, as detected by a multimodal screening program. Int J Radiat Oncol Biol Phys. 2013;85:35–39. doi: 10.1016/j.ijrobp.2012.03.043. [DOI] [PubMed] [Google Scholar]
- 23.Hutton J, Walker LG, Gilbert FJ, et al. Psychological impact and acceptability of magnetic resonance imaging and X-ray mammography: the MARIBS Study. Br J Cancer. 2011;104:578–586. doi: 10.1038/bjc.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tozaki M, Fukuma E. 1H MR spectroscopy and diffusion-weighted imaging of the breast: are they useful tools for characterizing breast lesions before biopsy? AJR Am J Roentgenol. 2009;193:840–849. doi: 10.2214/AJR.08.2128. [DOI] [PubMed] [Google Scholar]


