Abstract
Background
Postural balance and potentially fall risk increases among older adults living with neurological diseases, especially Parkinson's disease (PD). Since conventional therapies, such as levodopa or deep brain stimulation may fail to alleviate or may even worsen balance, interest is growing in evaluating alternative PD therapies.
Objective
The purpose of the current study was to assess improvement in postural balance in PD patients following electro-acupuncture (EA), as an alternative therapy.
Methods
Fifteen aging adults (70.2 ± 7.3 years) with idiopathic PD and 44 healthy age- matched participants (74.6 ± 6.5 years) were recruited. PD participants were randomly assigned (with a ratio of 2 to 1) to an intervention (n=10) or to a control group (n=5). The intervention group received a 30-minute EA treatment on a weekly basis for three weeks, while the control group received a sham treatment. Outcomes were assessed at baseline and after the final therapy. Measurements included balance assessment, specifically ratio of medial-lateral (ML) center of gravity (COG) sway to anterior-posterior (AP) sway (COGML/AP) and ankle-to-hip sway during eyes-open, eyes-closed, and eyes-open dual-tasks trials, Unified Parkinson's Disease Rating Scale (UPDRS), and quality of life, concerns for fall, and pain questionnaires.
Results
No difference was observed for assessed parameters between intervention and control groups in baseline. After treatment, improvement in balance performance was observed in the intervention group. Compared with a healthy population, PD patients prior to treatment had larger COGML/AP sway with more dependency on upper-body movements for maintaining balance. Following EA therapy, COGML/AP sway reduced by 31% and Ankle/Hip sway increased by 46% among different conditions (p = 0.02 for dual-task condition). The clinical rating revealed an overall improvement (p < 0.01) in the activity of daily living (UPDRS part II, 46%) and motor examination (UPDRS part III, 40%). There was significant reduction (p < 0.02) in the specific items regarding UPDRS fall status (67%), and rigidity (48%). Changes were small and non-significant in the controls (p > 0.29).
Conclusions
This pilot study demonstrated improvement in rigidity and balance following EA. These preliminary results suggest EA could be a promising alternative treatment for balance disturbance in PD.
Keywords: Parkinsonism, acupuncture, postural control, rigidity, ankle-strategy, hip-strategy, anterior-posterior sway, medial-lateral sway
Introduction
The number of older adults aged 65 and older is projected to double, by 2050 [1]. Despite extensive efforts for improving elders’ quality of life, fall-induced injury is still a major source of morbidity and mortality [2]. In addition to aging, the risk of fall can increase in the presence of neurological diseases. Stolze et al. reported that falls in neurological patients are twice as frequent as in age-matched healthy populations, with the most frequently diagnosed falls in Parkinson's disease (PD) patients [3]. Although a direct objective predictor of falling risk has not yet been discovered, several research studies have identified a strong association between poor postural balance and increased risk of falling. Abnormal postural sway measured by the range of sway, for example, has been introduced as a significant independent predictor of recurrent falls [4,5], or as a distinguishable factor among fallers and non-fallers [5,6].
PD is the second most common neurodegenerative disease worldwide, and is classically characterized by four cardinal features: tremor, bradykinesia, rigidity, and postural instability. Loss of balance control, which might significantly be associated with falls, is an inevitable consequence of the disease, and one of the most important mediators of quality of life [7,8]. Unfortunately, with common treatments such as levodopa or deep brain stimulation, balance control may not improve, or may even worsen [9-11]. In light of the significant limitations of conventional therapy, interest in complementary and alternative therapies for PD is growing. Exercise regimens and motor trainings, for example, have demonstrated significant enhancement in balance-related performance, functional mobility, activity of daily living, and ultimately a reduction in risk of fall [12-15]. However, in advanced stage of PD, patients may not be able to perform regular exercise. It is notable then that in addition to exercise as an alternative therapy for PD, acupuncture emerging as one of the most popular of these alternatives [16-18]. Studies have demonstrated that acupuncture is the most frequent alternative treatment [16,17], especially for improving sleep, quality of life, and motor performance [18,19]. However, in the majority of acupuncture studies quality of outcome measures that can objectively identify relevant functional improvements are inadequate [20]. Most of the existing studies used subjective, global scales (e.g., Unified Parkinson's Disease Rating Scale - UPDRS) covering a broad range of PD symptoms, which are inappropriate to quantify specific changes such as compromised postural balance. Although, wearable inertia sensors have been used to differentiate balance deficits in PD patients compared to healthy population [21], to our knowledge, balance improvement in PD patients following acupuncture has not been objectively quantified using the wearable technology .
The purpose of the current study was to assess improvement in postural balance in PD patients following electro-acupuncture (EA) therapy in a small sample size of PD patients as a proof of concept. Poor balance control in PD patients compared to healthy populations has been previously linked to differences in direction of body sway and type of strategy (hip- or ankle-strategy). Compared to healthy older adults, PD patients may have more, or sometimes less, body sway during postural balance [22-24]; thus using total body sway is not reliable for measuring compromised balance in PD patients [25]. Of note here, PD patients more consistently exhibit less anterior-posterior (AP) body sway and more medial-lateral (ML) sway as compared with age-matched healthy individuals [26]. Further, previous work suggests that PD patients have a higher tendency to use the hip joint for maintaining balance (hip-strategy), instead of ankle joints (ankle-strategy), primarily as a result of increased ankle stiffness and compromised reflexive response of ankle muscles [27,28]. Consequently, to test the efficacy of EA treatment in improving balance in PD participants, we hypothesized: 1) a reduction in ratio of ML/AP body sway; and 2) an increase ratio of ankle rotations/hip rotations. As a secondary aim, we compared ratio of ML/AP body sway and ankle/hip rotations between PD and healthy samples to confirm the differences between these two groups.
Methods
Participants
Aging adults aged 55 years or older with idiopathic PD diagnosed by movement disorder specialists based on the UK Brain Bank criteria were recruited from the University of Arizona Neurology Clinic. Participants were excluded if they were diagnosed with any type of neurological disorder other than PD or if they have prior experience of EA therapy. A healthy, non-frail sample (measured by Fried index [29]) of community-dwelling older adults aged 65 years or older were recruited from University of Arizona geriatric clinics. As mentioned above large variability of balance behaviors exist among PD population, therefore, healthy aging adults were recruited here, as a sample, in order to compare balance behaviors between healthy and PD samples prior to EA. Elders with major mobility or balance disorders, including PD, were excluded, as were those unable to walk a distance of 20 meters without walking assistance. The study was approved by the University of Arizona Institutional Review Board and written informed consent was obtained from all subjects before participation.
Study Design
The study was designed as a patient and assessor blinded, placebo-controlled, intervention pilot study. PD Participants were randomly assigned (with ratio of 2 to 1) to the intervention (n=10) or control groups (n=5). Since the primary focus of the current study was to explore balance improvement in PD patients following EA, larger number of PD participants were devoted to the intervention group. Each participant in the intervention group went through a 30-minute EA treatment once a week for three weeks, while participants in the control group received a weekly 30-minute sham treatment. Outcomes were assessed in “off medication stage” (>12 hours after their last PD medication dose) at baseline and after the final therapy. In addition, balance parameters prior to treatment were compared with an age-matched healthy population to assess impaired postural balance behaviors prior to treatment. To control for placebo effect a similar experimental protocol was explained to all participants and in the beginning of the experiment they were informed that they might be in either an intervention group or a control group.
Measurements included balance, SF-12 health survey [30], short Falls Efficacy Scale-International (SHORT FES-I) [31], and visual analog scale (VAS) for pain [32]. The Unified Parkinson's Disease Rating Scale (UPDRS) [33] was also performed by a trained examiner. Furthermore, participants were screened for cognition status using Mini-Mental State Examination (MMSE) [34] and for disease stage using Hoehn and Yahr staging [35] at baseline. Fall status and rigidity was quantified using UPDRS sub-score at baseline and after treatment. Fall status was also quantified using subjective evaluation within UPDRS, and rigidity with joint stiffness examination performed by the UPDRS examiner.
Electro-acupuncture procedure
A comprehensive and systematically applied EA regimen, consisting of scalp and body acupuncture, with a focus on improving balance and gait for PD patients was designed and administered by (HL), who is double-certified by the American Board of Medical Acupuncture and the American Board of Psychiatry and Neurology. Sterile disposable, surgical stainless steel acupuncture needles (Seirin, L type, Japan), measuring 0.25mm in diameter, 40-50 mm in length, were used for acupuncture, and electric stimulators (ITO ES-130, Japan) were used for EA stimulation. Real EA was performed at the following acupuncture points: GV20 (Baihui), GV14 (Dazhui) on the midline and bilateral Foot Motor Sensory Area, Balance Area, bilateral ST36 (Zusanli), LI4 (Hegu), GB34 (Yanglingquan), LR3 (Taichong), KI3 (Taixi), SP6 (Sanyinjiao), BL40 (Weizhong). Electrical stimulation was applied for 30 minutes at a frequency of 4 Hz or 100 Hz with intensity just below the level that induces visible muscle contraction. The acupuncture points and frequencies were selected based on previous work [18,19,36,37]. Sham acupuncture was performed for the control group with insertion of needles just under the skin at non-acupuncture points (the same for all subjects) at scalp, neck, shoulder, upper and lower extremities, and was stimulated in a similar fashion but with minimal intensity compared to real EA (just turning on the light of the stimulator). To assure that all participants in both intervention and control group received similar sensation during the experimental procedure, the EA procedure was controlled to have the same duration with acupuncture needle placement as close as possible in both the intervention group and the control group.
Experimental procedure
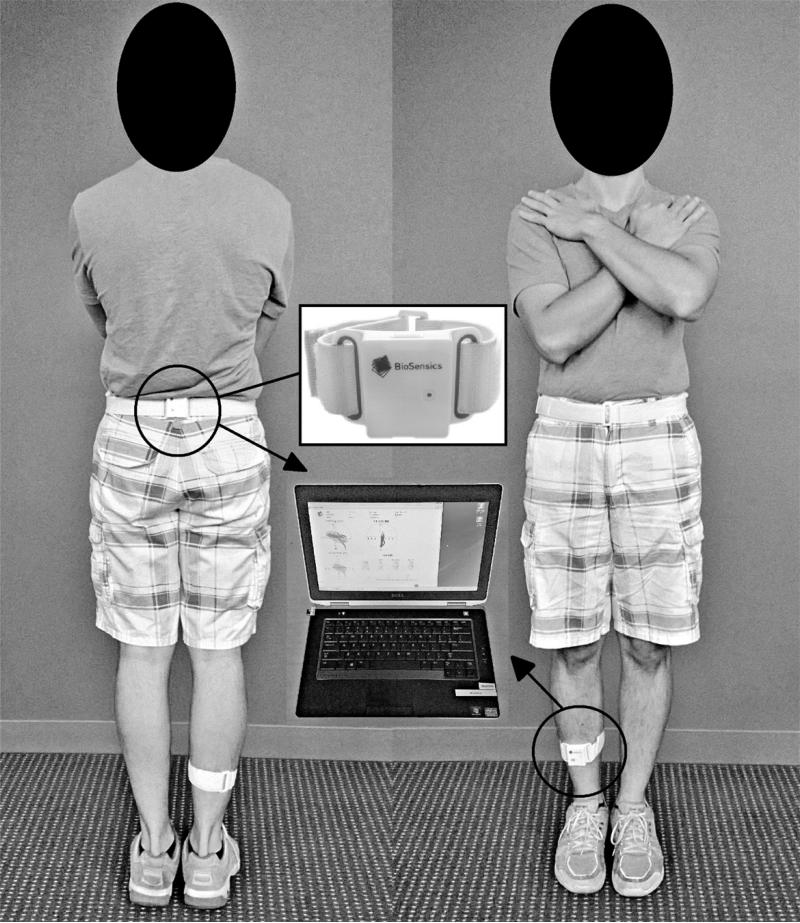
Each participant performed six 30-second trials of balance assessment, in each trial participants stood upright with their feet as close together as possible but without touching each other, and with their arms crossed. Participants were asked to cross their arms during balance tests to minimize the effect of arm movements on center of gravity (COG) displacements. In the first two trials, participants were instructed to keep their eyes open (eyes-open trials), with no target being specified. In the third and fourth trials participants kept their eyes closed (eyes-closed trials). In the fifth and sixth trials, participants were instructed to countdown from a random specified number while maintaining their balance with their eyes open (dual-task trials). In each trial the COG was estimated following identical procedures reported in our earlier study using wearable sensors [38,39]. Briefly, two sensors (Figure 1), each including a triaxial accelerometer, triaxial gyroscope, and a triaxial magnometer, were used to estimate three-dimensional angles of the ankle and hip joints (BalanSensTM, BioSensics LLC, Boston, MA). A two-link inverted pendulum model of the human body was then used to calculate the COG from AP and ML angles during body movements.
Figure 1.
Two wireless sensors are attached to shank and trunk to provide three-dimensional angles of ankle and hip joints in real-time, and to ultimately estimate COG.
Balance outcome measures
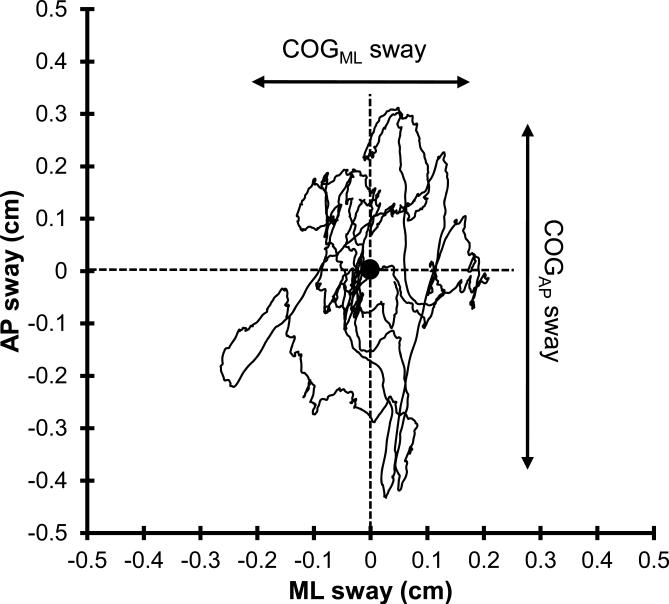
Outcome measures include body sway parameters: COGAP sway, COGML sway, COGML/AP sway, Ankle sway, Hip sway, and Ankle/Hip sway. The sway ranges in AP and ML directions were defined as COGAP sway and COGML sway, respectively, after excluding outliers, which were estimated by calculating 5 and 95 percentiles of COG data (Figure 2) [38]. The COGML/AP sway was then calculated as the ratio of the sway range in ML over AP direction (i.e., COGML/AP sway = COGML sway / COGAP sway). COGML/AP was measured here, since according to previous studies, PD patients show larger COGAP sway and less COGML sway compared to a healthy age-matched population [22,26]. As such, an increased value of COGML/AP demonstrates compromised balance in PD patients. Ankle sway was calculated as the product of range of ankle rotations in AP and ML direction; similarly, hip sway was calculated as the product of hip ranges of rotation in AP and ML direction. To evaluate improvement in using ankle-strategy instead of hip-strategy, the ratio of Ankle sway over Hip sway was calculated as Ankle/Hip sway. A larger magnitude of Ankle/Hip sway reveals an improvement in using ankle joints for maintaining balance in PD patients.
Figure 2.
A sample of stabilogram plot from a non-frail participant. The figure illustrates a larger body sway in AP compared to ML direction.
Statistical Analysis
Comparison between healthy and PD group for baseline balance parameters was performed using unpaired t-test (or Mann–Whitney U test for non-parametric samples). Comparison between intervention and control groups for participant's characteristics and study outcomes were also performed using unpaired t-test (or Mann–Whitney U test for non-parametric samples) except for participant's gender, for which, Chi-square test was used. Effect of EA on balance data were compared using paired t-test (or Mann–Whitney U test for non-parametric samples) for intervention and control PD groups by comparing pre- and post-data.
For outcomes that were significantly improved in the intervention group, between group (intervention vs. control), comparisons of changes in parameters were performed using mixed ANOVA models, considering age and body mass index (BMI) as covariates. Association between participants’ characteristics and improvement in outcome measures following EA, was assessed using linear regression models. Only parameters with the highest effect size were considered here as dependent variables, and baseline values of each parameter, age, disease stage, and BMI were used, in separate models, independent variables. Summary results are presented as means (standard errors – SE or standard deviation – SD). All analyses were done using JMPTM (Version 10, SAS Institute Inc., Cary, NC), and statistical significance was concluded when p < 0.05.
Results
Participants
Fifteen participants with PD (age 70.2 ± 7.3 years; BMI 27.5 ± 6.5 kg/m2) and 44 healthy aging adults (age 74.6 ± 6.5 years; BMI 25.7 ± 4.5 kg/m2) were recruited. Demographic information of participants is reported in Table 1 and 2.
Table 1.
Mean (SD or percentage) values of participant demographic information and pre-EA balance behaviors. The symbol * indicates a significant difference between intervention and control groups.
| Intervention | Control | Total | p-value | |
|---|---|---|---|---|
| Number (% of total) | 10 (67%) | 5 (33%) | 15 | - |
| Male (% of the group) | 6 (60%) | 2 (40%) | 8 (53%) | 0.46 |
| Age (SD) (yr) | 69.8 (4.5) | 71.0 (11.7) | 70.2 (7.3) | 0.78 |
| Stature (SD) (cm) | 164.6 (10.1) | 163.6 (13.5) | 164.3 (10.9) | 0.87 |
| Body mass (SD) (kg) | 74.3 (10.4) | 76.4 (23.8) | 74.9 (15.3) | 0.81 |
| BMI (SD) (kg/m2) | 27.5 (4.1) | 28.1 (6.5) | 27.5 (6.5) | 0.85 |
| MMSE score (SD) | 24.9 (6.7) | 27.2 (1.1) | 25.7 (5.6) | 0.47 |
| Disease stage (SD) | 3.0 (1.0) | 2.9 (0.7) | 2.9 (0.9) | 0.92 |
| COGap (SD) (cm) † | 0.50 (0.34) | 0.69 (0.51) | 0.61 (0.40) | 0.38 ‡ |
| COGML (SD) (cm) † | 0.61 (0.39) | 0.82 (0.20) | 0.70 (0.41) | 0.18 ‡ |
| cogML/AP (SD) † | 1.42 (0.60) | 1.66 (0.86) | 1.54 (0.68) | 0.42 ‡ |
| Ankle (SD) (deg) † | 2.83 (3.48) | 3.91 (2.54) | 3.50 (3.82) | 0.18 ‡ |
| Hip (SD) (deg) † | 1.81 (1.93) | 2.44 (0.98) | 2.19 (2.05) | 0.11 ‡ |
| Ankle/Hip (SD) † | 1.38 (0.43) | 1.59 (0.75) | 1.50 (0.53) | 0.26 ‡ |
| UPDRS - Fall | 0.9 (1.3) | 0.8 (0.8) | 0.9 (1.1) | 0.88 |
| UPDRS - Rigidity | 6.4 (5.1) | 7.4 (4.5) | 6.7 (4.8) | 0.72 |
| UPDRS I | 5.3 (3.5) | 3.3 (2.4) | 4.6 (3.2) | 0.28 |
| UPDRS II | 18.0 (9.7) | 17.0 (5.7) | 17.7 (8.4) | 0.84 |
| UPDRS III | 35.1 (15.3) | 34.2 (12.3) | 34.8 (13.9) | 0.91 |
Average values among different conditions (i.e. eyes-open, eyes-closed, and dual task) were compared between intervention and control samples.
Smallest p-value among conditions (i.e. eyes-open, eyes-closed, and dual task)
Table 2.
Mean (SD or percentage) values of participant demographic information. For balance parameters mean (SD) values across eyes-open and eyes-closed trials are presented. The symbol * indicates a significant difference.
| PD | Healthy | 95% CI | p-value | ||
|---|---|---|---|---|---|
| Number (% of total) | 15 | 44 | - | - | - |
| Male (% of the group) | 8 (53%) | 7 (16%) | 0.26 | 1.57 | <.01* |
| Age (SD) (yr) | 70.2 (7.3) | 74.6 (6.5) | −0.21 | 3.71 | 0.08 |
| Stature (SD) (cm) | 164.3 (10.9) | 159.1 (6.8) | −0.04 | 0.01 | 0.29 |
| Body mass (SD) (kg) | 74.9 (15.3) | 66.1 (15.8) | −7.92 | 0.15 | 0.06 |
| BMI (kg/m2) | 27.5 (6.5) | 25.7 (4.5) | −2.38 | 0.36 | 0.15 |
| MMSE score (SD) | 25.7 (5.6) | 29.2 (1.1) | 0.64 | 1.52 | <.0001* |
| COGAP (SD) (cm) | 0.61 (0.40) | 1.32 (0.68) | 0.22 | 0.52 | <.0001* |
| COGML (SD) (cm) | 0.70 (0.41) | 0.67 (0.35) | −0.12 | 0.09 | 0.77 |
| COGML/AP (SD) | 1.54 (0.68) | 0.57 (0.32) | −0.52 | −0.31 | <.0001* |
| Ankle (SD) (deg) | 3.50 (3.82) | 7.87 (6.85) | 0.63 | 3.88 | <.01* |
| Hip (SD) (deg) | 2.19 (2.05) | 5.13 (3.73) | 0.65 | 2.26 | <.001* |
| Ankle/Hip (SD) | 1.50 (0.53) | 1.66 (1.01) | −0.15 | 0.33 | 0.45 |
CI: Confidence Intervals
Balance: Comparison between healthy and PD sample
Comparing the PD and healthy samples showed no significant differences in demographic information, except for gender and MMSE score (Table 2). Although these differences were observed, two groups were still considered demographically comparable, since no significant effect of gender or MMSE score was observed in balance outcome measures in either healthy or PD groups (p > 0.17). Overall, a larger amount of sway was noticeable among the healthy group compared to PD participants; COGAP, Ankle, and Hip sway were significantly larger among healthy population by 116%, 125%, and 134%, respectively (p < 0.01); while COGML was comparable between two groups (p = 0.77). COGML/AP sways were also significantly smaller (144%) among the healthy compared to PD group (p < 0.0001).
Balance: Comparison between intervention and control group in PD sample
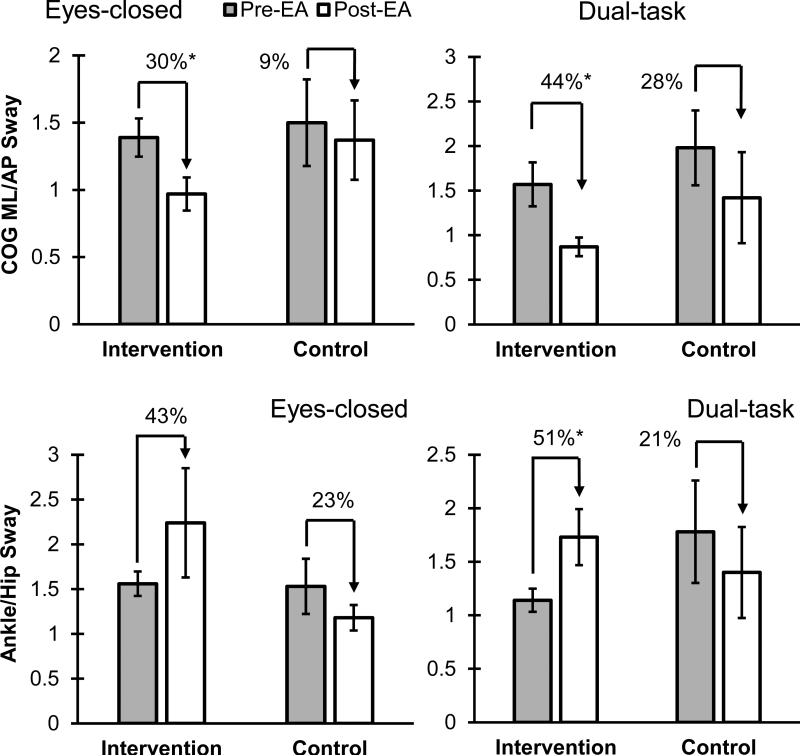
No significant difference was observed in participants’ demographic information, disease stage, or MMSE between intervention and control groups (p > 0.47) (see Table 1 for details). Further, comparison of outcome measures (balance and subjective evaluations) at the baseline showed no significant difference between the intervention and control groups (p > 0.11). After treatment, improvement in balance performance was observed in intervention group; overall, COGML/AP sway reduced by 31%, and Ankle/Hip sway increased by 46% among different conditions (see Figure 3 and Table 3 for details). The pre- and post-EA difference in the intervention group was more pronounced during the dual task condition. Although improvement in balance performance was also observed in some of the outcome measures in the control group, changes were small and non-significant (p > 0.29). Comparing balance parameter improvement between intervention and control groups showed that improvement in balance parameters was significant only for Ankle/Hip sway (p = 0.02) (Table 4). Regression models, however, showed no significant correlation (p > 0.13 and R2 < 27) between Ankle/Hip sway improvement with disease stage, or baseline values of Ankle/Hip sway.
Figure 3.
Ratio of COGML/AP and Ankle/Hip sway in intervention and control group pre- and post-EA during eyes-closed and dual-task conditions. The symbol * indicates a significant difference. Mean values and SE were illustrated.
Table 3.
Mean (SD) and pre- and post-EA comparison of balance parameters in eyes-open, eyes-closed, and dual task conditions for intervention and control group. The symbol * (grey cells) indicates a significant difference.
| Sway Parameter | Condition | Intervention |
Control |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-EA | Post-EA | Difference | 95% CI | p-value | Pre-EA | Post-EA | Difference | 95% CI | p-value | ||||
| COGAP (cm) | Eyes-open | 0.57 (0.45) | 0.73 (0.55) | 28% ↑ | −0.31 | 0.64 | 0.76 | 0.73 (0.46) | 0.81 (0.58) | 11% ↑ | −0.69 | 0.86 | 0.81 |
| Eyes-closed | 0.55 (0.33) | 0.82 (0.63) | 49% ↑ | −0.21 | 0.75 | 0.25 | 0.67 (0.44) | 0.78 (0.45) | 15% ↑ | −0.54 | 0.75 | 0.72 | |
| Dual-task | 0.39 (0.24) | 0.88 (0.66) | 127% ↑ | −0.01 | 0.98 | 0.04* | 0.67 (0.63) | 0.94 (0.62) | 40% ↑ | −0.64 | 1.18 | 0.35 | |
| COGML (cm) | Eyes-open | 0.65 (0.52) | 0.51 (0.30) | 21% ↓ | −0.28 | 0.54 | 0.55 | 0.79 (0.25) | 0.74 (0.20) | 6% ↓ | −0.28 | 0.38 | 0.6 |
| Eyes-closed | 0.68 (0.45) | 0.63 (0.42) | 8% ↓ | −0.36 | 0.46 | 0.79 | 0.77 (0.23) | 0.83 (0.17) | 7% ↑ | −0.35 | 0.24 | 0.66 | |
| Dual-task | 0.49 (0.19) | 0.57 (0.34) | 17% ↑ | −0.35 | 0.18 | 0.59 | 0.89 (0.11) | 0.87 (0.27) | 2% ↓ | −0.31 | 0.35 | 0.87 | |
| COGML/AP | Eyes-open | 1.29 (0.57) | 1.03 (0.62) | 20% ↓ | −0.31 | 0.81 | 0.29 | 1.49 (0.91) | 1.24 (0.61) | 17% ↓ | −0.90 | 1.41 | 0.92 |
| Eyes-closed | 1.39 (0.45) | 0.97 (0.39) | 30% ↓ | 0.03 | 0.82 | 0.04* | 1.50 (0.72) | 1.37 (0.66) | 9% ↓ | −0.88 | 1.15 | 0.77 | |
| Dual-task | 1.57 (0.78) | 0.87 (0.33) | 44% ↓ | 0.12 | 1.28 | 0.02* | 1.98 (0.94) | 1.42 (1.14) | 28% ↓ | −0.98 | 2.10 | 0.42 | |
| Ankle (deg) | Eyes-open | 3.54 (5.66) | 3.21 (3.86) | 9% ↓ | −4.26 | 4.93 | 0.94 | 3.45 (2.370 | 3.68 (2.35) | 7% ↑ | −3.20 | 3.67 | 0.86 |
| Eyes-closed | 3.31 (3.38) | 5.24 (6.79) | 58% ↑ | −3.24 | 7.11 | 0.76 | 3.81 (1.38) | 4.29 (1.35) | 13% ↑ | −1.51 | 2.47 | 0.35 | |
| Dual-task | 1.65 (1.39) | 3.52 (3.47) | 114% ↑ | −0.70 | 4.46 | 0.05* | 4.47 (3.86) | 4.19 (2.55) | 6% ↓ | −5.18 | 4.63 | 0.53 | |
| Hip (deg) | Eyes-open | 1.97 (2.67) | 1.61 (1.70) | 18% ↓ | −1.77 | 2.49 | 0.5 | 2.19 (1.02) | 2.54 (1.64) | 16% ↑ | −1.71 | 2.42 | 0.7 |
| Eyes-closed | 2.11 (2.24) | 2.41 (2.76) | 14% ↑ | −2.07 | 2.68 | 0.88 | 2.78 (1.26) | 3.87 (1.69) | 39% ↑ | −1.12 | 3.30 | 0.29 | |
| Dual-task | 1.35 (0.87) | 1.95 (1.57) | 44% ↑ | −0.63 | 1.81 | 0.16 | 2.35 (0.67) | 3.12 (1.23) | 33% ↑ | −0.75 | 2.30 | 0.29 | |
| Ankle/Hip | Eyes-open | 1.44 (0.51) | 2.08 (1.73) | 45% ↑ | −0.62 | 1.90 | 0.33 | 1.46 (0.49) | 1.51 (0.40) | 4% ↑ | −0.61 | 0.71 | 0.86 |
| Eyes-closed | 1.56 (0.43) | 2.24 (1.93) | 43% ↑ | −0.73 | 2.08 | 0.31 | 1.53 (0.69) | 1.18 (0.32) | 23% ↓ | −1.20 | 0.50 | 0.35 | |
| Dual-task | 1.14 (0.34) | 1.73 (0.83) | 51% ↑ | −0.04 | 1.21 | 0.02* | 1.78 (1.07) | 1.40 (0.95) | 21% ↓ | −1.86 | 1.10 | 0.53 | |
Table 4.
Mean (SD) changes after the treatment in balance and UPDRS parameters in intervention and control group. The symbol * (grey cells) indicates a significant difference between changes in parameters among two groups after treatment. Symbol ↓ indicates a reduction and ↑ an increase in each parameter following the treatment.
| Parameter | Intervention | Control | 95% CI | p-value | Effect Size | |
|---|---|---|---|---|---|---|
| COGAP - Eyes-open | 0.2 (0.6) ↑ | 0.1 (0.8) ↑ | −1.0 | 0.9 | 0.85 | 0.14 |
| COGAP - Eyes-closed | 0.3 (0.6) ↑ | 0.1 (0.5) ↑ | −0.9 | 0.5 | 0.60 | 0.36 |
| COGAP - Dual-task | 0.5 (0.7) ↑ | 0.3 (0.4) ↑ | −0.8 | 0.4 | 0.42 | 0.37 |
| COGML - Eyes-open | 0.1 (0.3) ↓ | 0.1 (0.3) ↓ | −0.3 | 0.4 | 0.61 | 0.30 |
| COGML - Eyes-closed | 0.1 (0.5) ↓ | 0.1 (0.3) ↑ | −0.4 | 0.6 | 0.63 | 0.49 |
| COGML - Dual-task | 0.1 (0.3) ↑ | 0.1 (0.2) ↓ | −0.4 | 0.2 | 0.47 | 0.78 |
| COGML/AP - Eyes-open | 0.3 (0.7) ↓ | 0.3 (1.2) ↓ | −1.4 | 1.4 | 0.99 | 0.01 |
| COGML/AP - Eyes-closed | 0.4 (0.6) ↓ | 0.1 (0.6) ↓ | −0.5 | 1.5 | 0.41 | 0.50 |
| COGML/AP - Dual-task | 0.7 (0.7) ↓ | 0.6 (0.8) ↓ | −0.8 | 1.1 | 0.74 | 0.13 |
| Ankle - Eyes-open | 0.3 (5.1) ↓ | 0.2 (2.8) ↑ | −3.8 | 5.0 | 0.78 | 0.12 |
| Ankle - Eyes-closed | 1.9 (6.5) ↑ | 0.5 (2.2) ↑ | −6.4 | 3.5 | 0.54 | 0.51 |
| Ankle - Dual-task | 1.9 (3.3) ↑ | 0.3 (2.1) ↓ | −5.2 | 0.9 | 0.15 | 0.79 |
| Hip - Eyes-open | 0.4 (2.2) ↓ | 0.4 (1.6) ↑ | −1.5 | 2.9 | 0.50 | 0.42 |
| Hip - Eyes-closed | 0.3 (3.4) ↑ | 1.1 (2.5) ↑ | −2.7 | 4.2 | 0.63 | 0.27 |
| Hip - Dual-task | 0.6 (1.6) ↑ | 0.8 (1.4) ↑ | −1.6 | 2.0 | 0.83 | 0.13 |
| Ankle/Hip - Eyes-open | 0.6 (1.5) ↑ | 0.1 (0.4) ↑ | −1.7 | 0.5 | 0.28 | 0.46 |
| Ankle/Hip - Eyes-closed | 0.7 (1.8) ↑ | 0.3 (0.5) ↓ | −2.4 | 0.4 | 0.06 | 0.76 |
| Ankle/Hip - Dual-task | 0.6 (0.7) ↑ | 0.4 (0.6) ↓ | −1.8 | 0.2 | 0.02* | 1.53 |
| SF-12 (PCS) | 0.6 (6.4) ↑ | 1.0 (7.5) ↓ | −10.8 | 7.8 | 0.71 | 0.23 |
| SF-12 (MCS) | 3.7 (11.2) ↑ | 0.5 (3.4) ↑ | −11.6 | 5.2 | 0.42 | 0.39 |
| Short FES-I | 2.2 (3.9) ↓ | 1.8 (3.8) ↑ | −0.8 | 8.8 | 0.09 | 1.04 |
| VAS | 1.1 (3.0) ↓ | 1.2 (2.8) ↓ | −3.6 | 3.4 | 0.95 | 0.03 |
| UPDRS - Fall | 0.6 (0.8) ↓ | 0.2 (0.5) ↓ | −0.6 | 0.3 | 0.39 | 0.59 |
| UPDRS - Rigidity | 3.1 (3.1) ↓ | 0.8 (2.8) ↑ | −3.9 | 0.0 | 0.05* | 1.32 |
| UPDRS I | 2.6 (2.1) ↓ | 1.8 (2.7) ↑ | −7.6 | −1.1 | <.01* | 1.82 |
| UPDRS II | 7.2 (5.5) ↓ | 0.4 (3.4) ↓ | −5.9 | −0.5 | 0.02* | 1.49 |
| UPDRS III | 16.0 (6.2) ↓ | 3.0 (5.2) ↑ | −13.2 | −5.7 | <.001* | 3.30 |
Subjective evaluations and UPDRS: Comparison between intervention and control group in PD sample
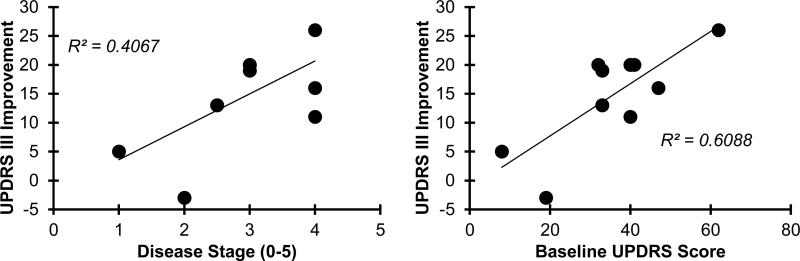
The clinical rating revealed significant improvements (p < 0.01) in the activity of daily living (UPDRS part II, 46%) and motor examination (UPDRS part III, 40%). There was significant reduction (p < 0.02) in the specific items regarding fall status (67%), and rigidity (48%). (Table 5). Although changes were not significant, Short FES-I and VAS scores also reduced by 15% and 44% following treatment in the intervention group (p > 0.26). No significant improvement was observed in the control group pre- and post-EA (p > 0.27). Comparison between the intervention and control groups for improvement in UPDRS scores showed significant differences in rigidity, UPDRS II, and UPDRS III (p < 0.05) (Table 4). Furthermore, results from regression models demonstrated significant positive correlations (Figure 4) between UPDRS III improvement and disease stage (r = 0.65, p = 0.04), and between UPDRS III improvement and UPDRS III baseline values (r=0.81, p < .01).
Table 5.
Mean (SD) and pre- and post-EA comparison of subjective evaluations and UPDRS parameters for intervention and control group. The symbol * (grey cells) indicates a significant difference.
| Parameter | Intervention |
Control |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-EA | Post-EA | Difference | 95% CI | p-value | Pre-EA | Post-EA | Difference | 95% CI | p-value | |||
| SF-12 (PCS) | 39.7 (11.7) | 40.3 (14.1) | 1% ↑ | −11.6 | 12.8 | 0.82 | 34.7 (9.4) | 33.7 (5.3) | 3% ↓ | −12.6 | 10.7 | 0.85 |
| SF-12 (MCS) | 43.4 (12.6) | 47.1 (8.5) | 8% ↑ | −6.5 | 13.9 | 0.45 | 47.2 (7.3) | 47.7 (7.9) | 1% ↑ | −10.7 | 11.6 | 0.93 |
| Short FES-I | 17.2 (6.8) | 14.6 (6.9) | 15% ↓ | −9.0 | 3.8 | 0.41 | 16.6 (5.0) | 18.4 (4.0) | 11% ↑ | −4.9 | 8.5 | 0.55 |
| VAS | 2.5 (3.2) | 1.4 (2.4) | 44% ↓ | −3.7 | 1.5 | 0.26 | 3.4 (3.3) | 2.2 (3.0) | 35% ↓ | −5.8 | 3.4 | 0.5 |
| UPDRS-Fall | 0.9 (1.3) | 0.3 (0.7) | 67% ↓ | −1.6 | 0.4 | 0.02* | 0.8 (0.8) | 0.6 (0.5) | 25% ↓ | −1.3 | 0.9 | 0.37 |
| UPDRS-Rigidity | 6.4 (5.1) | 3.3 (2.8) | 48% ↓ | −8.0 | 0.5 | 0.01* | 7.4 (4.5) | 8.2 (4.0) | 11% ↑ | −6.1 | 8.3 | 0.55 |
| UPDRS I | 5.3 (3.5) | 2.7 (2.3) | 49% ↓ | −5.5 | 0.6 | <.01* | 3.3 (2.4) | 5.1 (4.2) | 55% ↑ | −3.4 | 7.0 | 0.21 |
| UPDRS II | 18.0 (9.7) | 10.8 (6.6) | 46% ↓ | −10.6 | −3.8 | <.0001* | 17.0 (5.7) | 16.6 (7.9) | 9% ↑ | −3.3 | 2.5 | 0.8 |
| UPDRS III | 35.1 (15.3) | 19.1 (10.9) | 40% ↓ | −12.1 | −19.8 | <.01* | 34.2 (12.3) | 37.2 (11.6) | 2% ↑ | −1.6 | 7.6 | 0.27 |
Figure 4.
Correlation between UPDRS III improvement and disease stage, and between UPDRS III improvement and UPDRS III baseline values.
Discussion
Alterations in body sway following EA
In confirmation of our hypotheses, we observed improvements in postural balance in PD patients following EA. Overall, results demonstrate an increased sway following EA, especially in AP direction. Comparing the PD patients with the control age-matched group, Horak et al., observed that PD patients had very small sway area, and related this small sway to a larger joint and muscle stiffness, and overall “rigidity” in PD patients [22]. Conversely, other studies showed larger amounts of sway (area and velocity of sway) in PD patients compared to their healthy age-matched group [23,24]. According to previous evidence, combinations of the cardinal motor signs may differ among PD patients; one PD patient might develop joint rigidity, while limb tremor is more noticeable for another patient [40]. This may lead to a bimodal balance pattern (either very large or very small sway) in PD patients. To account for this variability in balance among those with PD, we compared balance parameters with an age-matched healthy population prior to treatment initiation. Our comparison demonstrated a smaller sway in PD patients in AP direction. After EA, COGAP sway increased by up to 127%, to a value closer to healthy individuals, which can be considered a significant improvement in balance among those in the intervention group.
Other studies, have suggested that the interaction or relationship between ML and AP sway is a more valid measure of balance impairment, especially in PD patients [26,41]. Healthy adults show a larger AP compared to ML sway (COGML/AP < 0.6 among different conditions – see Table 2). In the current study, we observed that PD patients had a large mean (SD) COGML/AP ratio of 1.5 (0.7) prior to the treatment, with an increasing trend by switching from eyes-open to eyes-closed, and from eyes-closed to dual-task conditions. The mean (SD) value of COGML/AP reduced to 0.9 (0.5) in the intervention group following EA treatment. The observed COGML/AP improvement can be explained based on the structure of lower extremity joints and their corresponding balance control mechanism.
An improvement in COGML/AP could result from either increased COGAP or reduction in COGML; however, overall increase in COGAP was dominant here in COGML/AP reduction. Comparing the COGAP and COGML, previous studies suggested that in healthy individuals body sway is larger in AP as compared to ML direction, mainly due to the inherent structural mechanism of ankle and hip joints [42,43]. The human balancing mechanism activates plantar flexor and dorsiflexor muscles of ankle and flexor and extensor hip muscles to control body sway in AP direction, and as such, muscle groups in both legs provide torques in a similar direction. This mechanism helps to overcome the larger flexibility in AP (compared to ML) direction that occurs because of the hinge-like structure of ankle and tibiotarsal joints. In contrast, a different controlling mechanism exists to balance the human body in ML direction using a cancellation strategy. Invertor/evertor muscles of ankle and abductor/adductor muscles of hip work in opposite directions on the left and right sides. Therefore, to provide the balance in ML direction, the controlling mechanism works with loading and unloading of bilateral muscles, instead of increasing the level of muscle activity [43]. In this context, the effect of EA treatment was to increase ankle and hip joints compliance during quiet standing, especially in the AP direction. These results were in agreement with clinical evaluation of rigidity (UPDRS part III-22), which showed a 48% reduction following EA treatment.
Alterations in Ankle/Hip sway following EA
An increased ratio of Ankle/Hip sway, as expected in our second hypothesis, was evident in the intervention group. In almost all conditions (except eyes-open hip sway), both ankle and hip sway increased following treatment; the amount of increase was, however, larger for ankle joint sway. Accordingly, an increase in Ankle/Hip sway suggests an increase in using ankle-strategy instead of hip-strategy for maintaining balance following the treatment. Deterioration in using ankle-strategy was suggested in previous studies [26], and within the current approach using wearable sensors we were able to accurately evaluate the Ankle/Hip sway as an indicator of the ratio of using ankle-strategy over hip-strategy. Comparing PD and healthy groups, we observed that ankle sway was smaller in PD participants. Accordingly, increased ankle-strategy use following EA in the intervention group suggests improvement in balance. To understand how an increased use of ankle-strategy (instead of hip-strategy) can improve the balance, one should consider the differences in these two strategies and their roles in maintaining balance.
To maintain balance on a flat support, healthy individuals dominantly use ankle-strategy. In the condition of short surface with lack of firmness, or with a lack of sensory feedback, hip-strategy assists in maintaining balance [44]. Similarly, as a result of neuromuscular complications, muscle weakness or limitation in range of motion of the ankle joint, larger compensatory motion from the hip and trunk is required to correct the posture during upright standing [45]. Existing evidence indicates that PD patients have altered postural reflexes in their lower extremities, defined by delayed reflex onsets especially in the ankle joint [27,28]. Additionally, reduced muscle strength and rigidity in lower extremities, which are associated with PD [22,46], can lead to smaller ankle-strategy use, even in simple upright standing on a flat surface. Inability to make full use of ankles for balancing and high dependency on upper-body movements, therefore, may cause an increased risk of falling in PD patients [46]. The results following EA, demonstrate an increased Ankle/Hip sway, which may improve balance with EA intervention. However, it is not clear whether EA improved only rigidity or both rigidity and reflexive responses of the ankle here. In future studies, reflexive responses could be measured using electromyography to address this limitation.
Limitations, summary of findings, and future directions
One limitation of the current study is the small number of participants, and, therefore, the current results, while encouraging, should be considered preliminary results, which demonstrate the proof of concept for EA benefits in balance in PD patients. These results need to be confirmed in a larger sample size in future research. Although, balance improvement was observed in almost all individuals in the intervention group, as a result of small sample size, differences in balance improvement between intervention and control groups was observed only in Ankle/Hip sway. We detected improvement in balance behaviors mostly in dual-task condition. Based on minimum estimated effect size here (0.37), to observe significant improvement in main balance parameters (i.e., COMML/AP and Ankle/Hip sway) for intervention group following EA treatment in other balance conditions a sample size of 48 participants is required (power of 80% and α = 0.05). Similarly, to detect differences in balance improvement between the intervention and control groups in most of main outcome measures in eyes-closed and dual-task conditions a sample size of 51 participants in each group is required. In addition, the improvements in balance behaviors were observed only immediately after EA treatment. Retention of therapy and long term effect of acupuncture on balance is, therefore, still unclear, and will be addressed in our future studies. Moreover, our limiting of the duration of the treatment to three weeks (three sessions), and the potential beneficial effect of a longer duration of treatment and gradual improvements in balance should be assessed later. In addition, future research should address benefit of EA on gait and reduction of risk of falling, as well as prospective falls.
A key challenge linked to using wearable sensors to estimate COG is that, unlike camera motion analysis system or force platform that use a fixed landmark reference (i.e. room reference axis), the axis of the sensor is highly dependent on how it is attached to the body and how it rotates during body motion. To address this challenge, we used a quaternion model as described earlier [38] to better estimate rotations and correct the change in landmark reference of wearable sensors. Further, we used a low-pass filtering algorithm to minimize signal drifting. Another limitation of this study is using a two-link inverted-pendulum model to estimate COG, assuming that motions around other body joints during balance test are negligible compared to motions around hip and ankle joints. In our previous study [47], we demonstrated that this assumption produces an acceptable agreement (r > 0.8) for the estimation of COG trajectory, when it is compared to full biomechanical model of human body as estimated by camera motion analysis system.
Despite these limitations, this randomized controlled study provides the proof of concept for potential benefits of non-pharmaceutical based EA therapy on enhancing balance in aging adults living with PD. Preliminary results revealed that people at advanced stages of PD, with poorer UPDRS III score, may benefit even more from EA treatment. Results indicate that benefit from EA on balance enhancement was independent of baseline balance in our study. Moreover, a high sensitivity of detecting balance problems was observed in a dual-task condition, which might arise from the inherent neurological sources of PD progression. This was in agreement with UPDRS I subjective evaluations, that showed a significant improvement in “mental, behavior, and mood” following EA treatment (see Table 4 and 5). Previous research also demonstrated exacerbation of motor performance, specifically during gait, in PD patients while performing in a dual-task condition [48]. Accordingly, balance improvement observed here might, to some extent, be related to improvement in cognitive behaviors in the PD sample after the treatment. Overall, performing hard cognitive tasks might increase the risk of falling in PD patients, and future studies should aim on studying reasons behind poor balance in dual-task conditions among PD patients and potential exercise regimens for its improvement. In contrast to dual-task conditions of balance testing, small differences were observed in eyes-open and eyes-closed conditions. Further, UPDRS measures were more sensitive in detecting improvements in PD patients after EA treatment.
Conclusion
Despite the fact that current conclusions should be confirmed in a larger sample size, the current study demonstrates proof of concept for improvement in PD patients’ balance following EA. These improvements were characterized by reduction in COGML/AP sway and enhancement in using ankle-strategy following treatment, which may be caused by reduced rigidity and increased reflexive responses of lower limb muscles. These results were in agreement with subjective evaluations of participants regarding reduced risk of fall and rigidity, and with improvement in motor examination and activity of daily living within the UPDRS test. In these trials the dual-task was found to be the most sensitive condition for evaluating enhancement in postural balance following EA treatment. Finally, with the current wearable sensor technology we were able to quantify postural sway in the ankle and hip joints separately. Using the wearable sensor technology, all the measurements were performed in a clinical setting within a short duration of time to allow for several measurements.
Acknowledgement
This study was partially supported by an STTR-Phase II Grant (Award Number 2R42AG032748) from the National Institute on Aging, and the Arizona Center on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health. We thank Stephan Karp for helping with data collection.
References
- 1.Vincent GK, Velkoff VA. US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2010. The next four decades: The older population in the united states: 2010 to 2050. [Google Scholar]
- 2.Kannus P, Parkkari J, Koskinen S, Niemi S, Palvanen M, Järvinen M, Vuori I. Fall-induced injuries and deaths among older adults. JAMA: the journal of the American Medical Association. 1999;281:1895–1899. doi: 10.1001/jama.281.20.1895. [DOI] [PubMed] [Google Scholar]
- 3.Stolze H, Klebe S, Zechlin C, Baecker C, Friege L, Deuschl G. Falls in frequent neurological diseases. Journal of neurology. 2004;251:79–84. doi: 10.1007/s00415-004-0276-8. [DOI] [PubMed] [Google Scholar]
- 4.Thapa PB, Gideon P, Brockman KG, Fought RL, Ray WA. Clinical and biomechanical measures of balance fall predictors in ambulatory nursing home residents. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1996;51:M239–M246. doi: 10.1093/gerona/51a.5.m239. [DOI] [PubMed] [Google Scholar]
- 5.Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. Journal of Gerontology. 1994;49:M72–M84. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- 6.Lajoie Y, Gallagher S. Predicting falls within the elderly community: Comparison of postural sway, reaction time, the berg balance scale and the activities-specific balance confidence (abc) scale for comparing fallers and non-fallers. Archives of gerontology and geriatrics. 2004;38:11–26. doi: 10.1016/s0167-4943(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 7.Dennison AC, Noorigian JV, Robinson KM, Fisman DN, Cianci HJ, Moberg P, Bunting-Perry L, Martine R, Duda J, Stern MB. Falling in parkinson disease: Identifying and prioritizing risk factors in recurrent fallers. Am J Phys Med Rehabil. 2007;86:621–632. doi: 10.1097/PHM.0b013e311611583. [DOI] [PubMed] [Google Scholar]
- 8.Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in parkinson disease. Neurology. 2010;75:116–124. doi: 10.1212/WNL.0b013e3181e7b688. [DOI] [PubMed] [Google Scholar]
- 9.Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: Scaling, set, and tone. J Neurophysiol. 1996;75:2380–2396. doi: 10.1152/jn.1996.75.6.2380. [DOI] [PubMed] [Google Scholar]
- 10.Rocchi L, Carlson-Kuhta P, Chiari L, Burchiel KJ, Hogarth P, Horak FB. Effects of deep brain stimulation in the subthalamic nucleus or globus pallidus internus on step initiation in parkinson disease: Laboratory investigation. Journal of neurosurgery. 2012;117:1141–1149. doi: 10.3171/2012.8.JNS112006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St George RJ, Carlson-Kuhta P, Burchiel KJ, Hogarth P, Frank N, Horak FB. The effects of subthalamic and pallidal deep brain stimulation on postural responses in patients with parkinson disease. Journal of neurosurgery. 2012;116:1347–1356. doi: 10.3171/2012.2.JNS11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen NE, Sherrington C, Paul SS, Canning CG. Balance and falls in parkinson's disease: A meta analysis of the effect of exercise and motor training. Movement Disorders. 2011;26:1605–1615. doi: 10.1002/mds.23790. [DOI] [PubMed] [Google Scholar]
- 13.Crizzle AM, Newhouse IJ. Is physical exercise beneficial for persons with parkinson's disease? Clinical Journal of Sport Medicine. 2006;16:422–425. doi: 10.1097/01.jsm.0000244612.55550.7d. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic parkinson's disease. Archives of physical medicine and rehabilitation. 2003;84:1109–1117. doi: 10.1016/s0003-9993(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 15.Gobbi LT, Oliveira-Ferreira MD, Caetano MJD, Lirani-Silva E, Barbieri FA, Stella F, Gobbi S. Exercise programs improve mobility and balance in people with parkinson's disease. Parkinsonism & related disorders. 2009;15:S49–S52. doi: 10.1016/S1353-8020(09)70780-1. [DOI] [PubMed] [Google Scholar]
- 16.Lökk J, Nilsson M. Frequency, type and factors associated with the use of complementary and alternative medicine in patients with parkinson's disease at a neurological outpatient clinic. Parkinsonism & related disorders. 2010;16:540–544. doi: 10.1016/j.parkreldis.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Rajendran PR, Thompson RE, Reich SG. The use of alternative therapies by patients with parkinson's disease. Neurology. 2001;57:790–794. doi: 10.1212/wnl.57.5.790. [DOI] [PubMed] [Google Scholar]
- 18.Shulman LM, Wen X, Weiner WJ, Bateman D, Minagar A, Duncan R, Konefal J. Acupuncture therapy for the symptoms of parkinson's disease. Movement disorders. 2002;17:799–802. doi: 10.1002/mds.10134. [DOI] [PubMed] [Google Scholar]
- 19.Lee MS, Shin BC, Kong JC, Ernst E. Effectiveness of acupuncture for parkinson's disease: A systematic review. Movement Disorders. 2008;23:1505–1515. doi: 10.1002/mds.21993. [DOI] [PubMed] [Google Scholar]
- 20.Lee MS, Shin BC, Kong JC, Ernst E. Effectiveness of acupuncture for parkinson's disease: A systematic review. Mov Disord. 2008;23:1505–1515. doi: 10.1002/mds.21993. [DOI] [PubMed] [Google Scholar]
- 21.Mancini M, Horak FB. The relevance of clinical balance assessment tools to differentiate balance deficits. European journal of physical and rehabilitation medicine. 2010;46:239. [PMC free article] [PubMed] [Google Scholar]
- 22.Horak F, Nutt J, Nashner L. Postural inflexibility in parkinsonian subjects. Journal of the neurological sciences. 1992;111:46–58. doi: 10.1016/0022-510x(92)90111-w. [DOI] [PubMed] [Google Scholar]
- 23.Adkin A, Bloem B, Allum J. Trunk sway measurements during stance and gait tasks in parkinson's disease. Gait & posture. 2005;22:240–249. doi: 10.1016/j.gaitpost.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Ickenstein GW, Ambach H, Klöditz A, Koch H, Isenmann S, Reichmann H, Ziemssen T. Static posturography in aging and parkinson's disease. Frontiers in aging neuroscience. 2012:4. doi: 10.3389/fnagi.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul SS, Canning CG, Sherrington C, Fung VS. Reproducibility of measures of leg muscle power, leg muscle strength, postural sway and mobility in people with parkinson's disease. Gait & Posture. 2012;36:639–642. doi: 10.1016/j.gaitpost.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell S, Collin J, De Luca C, Burrows A, Lipsitz L. Open-loop and closed-loop postural control mechanisms in parkinson's disease: Increased mediolateral activity during quiet standing. Neuroscience letters. 1995;197:133–136. doi: 10.1016/0304-3940(95)11924-l. [DOI] [PubMed] [Google Scholar]
- 27.Bloem B. Postural instability in parkinson's disease. Clinical neurology and neurosurgery. 1992;94:41–45. doi: 10.1016/0303-8467(92)90018-x. [DOI] [PubMed] [Google Scholar]
- 28.Bloem B, Van Dijk J, Beckley D, Roos R, Remler M, Bruyn G. Altered postural reflexes in parkinson's disease: A reverse hypothesis. Medical hypotheses. 1992;39:243–247. doi: 10.1016/0306-9877(92)90116-t. [DOI] [PubMed] [Google Scholar]
- 29.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G. Frailty in older adults evidence for a phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Medical care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Kempen GI, Yardley L, Van Haastregt JC, Zijlstra GR, Beyer N, Hauer K, Todd C. The short fes-i: A shortened version of the falls efficacy scale-international to assess fear of falling. Age and ageing. 2008;37:45–50. doi: 10.1093/ageing/afm157. [DOI] [PubMed] [Google Scholar]
- 32.Langley G, Sheppeard H. The visual analogue scale: Its use in pain measurement. Rheumatology international. 1985;5:145–148. doi: 10.1007/BF00541514. [DOI] [PubMed] [Google Scholar]
- 33.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R. Movement disorder society-sponsored revision of the unified parkinson's disease rating scale (mds updrs): Scale presentation and clinimetric testing results. Movement disorders. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 34.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 35.Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK. Movement disorder society task force report on the hoehn and yahr staging scale: Status and recommendations the movement disorder society task force on rating scales for parkinson's disease. Movement Disorders. 2004;19:1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 36.Lam YC, Kum WF, Durairajan SSK, Lu JH, Man SC, Xu M, Zhang XF, Huang XZ, Li M. Efficacy and safety of acupuncture for idiopathic parkinson's disease: A systematic review. The Journal of Alternative and Complementary Medicine. 2008;14:663–671. doi: 10.1089/acm.2007.0011. [DOI] [PubMed] [Google Scholar]
- 37.Yeo S, Lim S, Choe IH, Choi YG, Chung KC, Jahng GH, Kim SH. Acupuncture stimulation on gb34 activates neural responses associated with parkinson's disease. CNS neuroscience & therapeutics. 2012;18:781–790. doi: 10.1111/j.1755-5949.2012.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Najafi B, Horn D, Marclay S, Crews RT, Wu S, Wrobel JS. Foot technology, part 1 of 2: Assessing postural control and postural control strategy in diabetes patients using innovative and wearable technology. Journal of diabetes science and technology. 2010;4:780. doi: 10.1177/193229681000400403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Najafi B, Bharara M, Talal TK, Armstrong DG. Advances in balance assessment and balance training for diabetes. Diabetes Management. 2012;2:293–308. [Google Scholar]
- 40.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for parkinson disease. Archives of neurology. 1999;56:33. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 41.Stylianou AP, McVey MA, Lyons KE, Pahwa R, Luchies CW. Postural sway in patients with mild to moderate parkinson's disease. International Journal of Neuroscience. 2011;121:614–621. doi: 10.3109/00207454.2011.602807. [DOI] [PubMed] [Google Scholar]
- 42.Collins JJ, De Luca CJ. Open-loop and closed-loop control of posture: A random-walk analysis of center-of-pressure trajectories. Experimental Brain Research. 1993;95:308–318. doi: 10.1007/BF00229788. [DOI] [PubMed] [Google Scholar]
- 43.Winter DA. Human balance and posture control during standing and walking. Gait & posture. 1995;3:193–214. [Google Scholar]
- 44.Runge C, Shupert C, Horak F, Zajac F. Ankle and hip postural strategies defined by joint torques. Gait & Posture. 1999;10:161–170. doi: 10.1016/s0966-6362(99)00032-6. [DOI] [PubMed] [Google Scholar]
- 45.Horak FB. Clinical measurement of postural control in adults. Physical Therapy. 1987;67:1881–1885. doi: 10.1093/ptj/67.12.1881. [DOI] [PubMed] [Google Scholar]
- 46.Bloem BR, Van Vugt J, Beckley DJ. Postural instability and falls in parkinson's disease. Advances in neurology. 2001;87:209. [PubMed] [Google Scholar]
- 47.Najafi B, Lee-Eng J, Marclay S, Wrobel J, Goebel4 R. Estimation of center of mass trajectory using wearable sensors during golf swing. Sensors. 2014 Under Review. [PMC free article] [PubMed] [Google Scholar]
- 48.O'Shea S, Morris ME, Iansek R. Dual task interference during gait in people with parkinson disease: Effects of motor versus cognitive secondary tasks. Physical Therapy. 2002;82:888–897. [PubMed] [Google Scholar]