Abstract
Objective
Constitutive activation of STAT3 is a hallmark of various human cancers, although an increased pSTAT3 expression in high grade human endometrial cancer has not been reported. In the present study, we examine the expression of STAT family of proteins in endometrial cancer cell lines and the efficacy of HO-3867, a novel STAT3 inhibitor designed in our lab.
Methods
Expression of STAT family proteins was evaluated via western blot. The cell viability, post treatment with HO-3867, was assessed using MTT, cell-cycle profile and annexin assay. In vivo efficacy of HO-3867 was evaluated using xeonograft mice.
Results
Expression of activated STATs was inconsistent among the cell lines and 18 human endometrial cancer specimens tested. While pSTAT3 Tyr705 was not expressed in any of the cell lines, pSTAT3 Ser727 was highly expressed in endometrial cancer cell lines and tumor specimens. HO-3867 decreased the expression of pSTAT3 Ser727 while total STAT3 remained constant; cell viability decreased by 50–80% and induced G2/M arrest in 55% of Ishikawa cells at the G2/M cell cycle checkpoint. There was an increase in p53, a decrease in Bcl2 and Bcl-xL, and cleavage of caspase-3, caspase-7 and PARP. HO-3867 mediated a dosage-dependent inhibition of the growth of xenografted endometrial tumors.
Conclusions
HO-3867 treatment decreases the high levels of pSTAT3 Ser727 in endometrial cancer cells by inducing cell cycle arrest and apoptosis. This suggests a specific role of serine-phosphorylated STAT3, independent of tyrosine phosphorylation in the oncogenesis of endometrial cancer. HO-3867 could potentially serve as an adjunctive targeted therapy.
Keywords: Endometrial cancer, STAT3, HO-3867, STAT3 inhibitor, curcumin analog
Introduction
Endometrial cancer is the most common malignant tumor of the female genital tract. The American Cancer Society, there will be an estimated 52,630 newly diagnosed cases, and 8,590 deaths from endometrial cancer disease in the United States in 2014. While it is the fourth leading cause of cancer in women overall, it is the eighth most common cause of death from disease. This is due to the fact that the majority of endometrial cancer is detected early and often cured with surgery alone or in combination with radiation therapy. However, over the past 15 years the rate of increase in mortality from endometrial cancer has accelerated faster than the incidence of the disease [1–4].
Chemotherapy has been playing a more active role in the management of advanced disease in the past, however response rates range from 25 to 55% only, with a best complete response rate of 22% [5–7]. Rigorous combination chemotherapy significantly improves disease-free survival but with minimal improvement in overall survival at the cost of significant toxicity to healthy, noncancerous cells [8]. Newer targeted agents are currently being developed with the aim of greater specificity towards tumor cells while sparing the normal tissue.
Our laboratory has developed a novel class of anti-cancer drugs, diarylidenylpiperiden-4-ones (DAPs). These include the agents HO-3867 and H-4073, which we have reported upon previously [9–13]. We believe that HO-3867 compound can be modified so as to offer selective protection to normal, healthy cells, while eliminating uterine cancer cells at the same time, by incorporating an antioxidant-promoting moiety within the molecular structure [14–17]. Our previous report showed the anticancer activity of HO-3867 in various cancer cells, and found that its activity is mediated by inhibition of signal transducer and activator of transcription-3 (STAT3) phosphorylation and induction of apoptosis [14, 15]. In this study, we intend to continue our effort to develop novel, and safe synthetic anticancer compounds that are effective in eliminating endometrial cancer cells. By selectively targeting STAT3 and forcing the down regulation of its signaling pathways, cancer cell proliferation can be inhibited, and pro-apoptotic factors will become up regulated.
MATERIALS AND METHODS
Materials
HO-3867 was synthesized in the laboratory [10, 18]. Stock solutions of the compounds were freshly prepared in dimethylsulfoxide (DMSO). Cell-culture medium (RPMI 1640 and DMEM), fetal bovine serum (FBS), antibiotics, sodium pyruvate, trypsin, non-essential amino acids, and phosphate-buffered saline (PBS) were purchased from Gibco (Grand Island, NY). Polyvinylidene fluoride (PVDF) membrane and molecular-weight markers were obtained from Bio-Rad (Hercules, CA). Antibodies against caspase 3, cleaved caspase 3, caspase 7, cleaved caspase 7, PARP, cleaved PARP, pSTAT1, pSTAT3 Tyr705, pSTAT3 Ser727, and pSTAT5, were purchased from Cell Signaling Technology (Beverly, MA). Antibodies specific for STAT3, Actin, cyclin D1, cyclin A, cdk2, cdk5, p35, p21, Bcl-xL and Bcl-2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Enhanced chemiluminescence (ECL) reagents were obtained from Amersham Pharmacia Biotech (GE Healthcare, Piscataway, NJ). All other reagents, of analytical grade or higher, were purchased from Sigma-Aldrich.
Cell lines and cultures
Ishikawa, HEC1B, RL-95-2 andSK-UT-1B endometrial cancer cell lines were used in this study. The cells were grown in RPMI 1640, DMEM, F-12 Hams, or MEBM/RPMI medium supplemented with 5 or 10% FBS, 2% sodium pyruvate, 1% penicillin and 1% streptomycin with or without insulin. Cells were grown in a 75 mm flask to 70% confluence at 37°C in an atmosphere of 5% CO2 and 95% air. Cells were routinely trypsinized (0.05% trypsin/EDTA) and counted using an automated counter (NucleoCounter, New Brunswick Scientific, Edison, NJ).
Cell viability by MTT assay
Endometrial cancer cell viability was determined by a colorimetric assay using MTT. In the mitochondria of living cells, yellow MTT undergoes a reductive conversion to formazan, giving a purple color. Ishikawa, HEC-1B, HEC-1A, AN-3-CA andSK-UT-1B endometrial cancer cells were grown to 80% confluence in 100 mm culture plates, trypsinized, counted and seeded in 96-well plates with an average population of 8,000 cells/well. The cells were then incubated overnight and then treated with either Curcumin 100 μM or HO-3867 1 to 20 μM for 24 h. Cell viability was then calculated. The dose and time of incubation were determined from a set of preliminary experiments. All experiments were done using six replicates and repeated at least three times. Cell viability was expressed as a percentage of MTT viability of untreated cells.
Western blotting
Ishikawa, HEC1B, RL-95-2 andSK-UT-1B endometrial cancer cells were incubated in their respective media. Cell lysates were prepared in non-denaturing lysis buffer. The protein concentration in the lysates was determined using a Pierce detergent-compatible protein assay kit. For Western blotting, 25 to 50 μg of protein lysate per sample was denatured in 2× sample buffer and subjected to SDS-PAGE on a 10% or 12% tris-glycine gel. The separated proteins were transferred to a PVDF membrane and then blocked with 5% nonfat milk powder (w/v) in TBST (10 mM Tris, 100 mM NaCl, 0.1% Tween 20) for 1 h at room temperature or overnight at 4°C. The membranes were incubated with the primary antibodies described above. The bound antibodies were detected with horseradish peroxidase (HRP)-labeled sheep anti-mouse IgG or HRP-labeled donkey anti-rabbit IgG (Amersham) using an enhanced chemiluminescence detection system (ECL Advanced kit). Protein expressions were determined using Image Gauge version 3.45. The protocol was the same for cells treated with DMSO (control) or HO-3867 5 and 10 μM for 24 h. Equal volumes of DMSO (0.1% v/v) were present in both groups.
Immunoprecipitation (IP)
The endometrial cancer cells with and without HO-3867 treatment, were washed with ice-cold PBS and lysed either with lysis buffer 1 (0.05% [vol/vol] NP-40, 15 mM Tris-HCl, pH 7.4, 50 mM NaCl, 5 mM EDTA) supplemented with protease inhibitors. The cells were then centrifuged at 16 000×g for 15 min at 4 °C. The antibody (1 μg) was added to the cell lysate and incubated at 4 °C for 2 h, followed by incubation with Protein A/G PLUS-agarose (Santa Cruz) pre-equilibrated in lysis buffer overnight at 4 °C. Precipitates were washed twice in lysis buffer and three times in ice-cold PBS. Immunoprecipitates were eluted from the agarose by boiling in 2× SDS Gel loading buffer (100 mM Tris-Cl pH 6.8, 4% SDS, 0.2% bromophenol blue, 20% [vol/vol] glycerol, 10% [vol/vol] 2-mercaptoethanol) and subjected to SDS-PAGE and immunoblotting. Immunoblots were imaged with an Epichemi3 Darkroom system (UVP BioImaging Systems).
Cell-cycle analysis
Ishikawa endometrial cancer cells were treated with 5 or 10 μM HO-3867 for 3 and 6 h. Cells were then trypsinized, collected by centrifugation, re-suspended in PBS, and fixed in 70% ethanol at −20°C overnight. After centrifugation, the cells were then washed in PBS and re-suspended in potassium iodide (PI)-staining solution (PBS, PI, RNase). Specimens were incubated in the dark for 30 min at 37°C and then analyzed with the use of an EPICS Profile II flow cytometer (Coulter Corp., Hialeah, FL). All experiments were performed in triplicate.
Apoptosis
Ishikawa cells were cultured in DMEM medium. They were seeded into 100 mm culture dishes and cultured for 24 hours, followed by treatment with varying concentrations (5, and 10μM) of HO-3867 and counted using a NucleoCounter (New Brunswick Scientific, Edison, NJ) after 24, hours of treatment. Apoptotic cells were measured by flow cytometry using Annexin V.
Transfection of Wild-type STAT3 cDNA
The STAT3 overexpression experiments were performed using a wild-type STAT3 cDNA. The FLAG-tagged gene was transfected into Ishikawa endometrial cancer cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. At 24 h after the transfection of the STAT3 gene, HO-3867 (10 μm) was added and incubated for 24 h. The cells were then subjected to a cell-growth assay.
Immunocytochemistry
Ishikawa cells in DMEM medium was seeded onto sterile glass coverslips in 6-well plates with an average population of 50,000 cells/well. After 24 hours of cell culture, the cells were then washed, fixed, and incubated with primary antibody (pSTAT3 Tyr705 and pSTAT3 Ser727) according to a previously described protocol [15].
Patient Samples
Endometrial tumor samples from 10 patients that had undergone initial surgery at The Ohio State University Medical Center were obtained. Samples were homogenized in non-denaturing lysis buffer and subject to western blot analysis as described earlier. The use of human tissues in this study was approved by the Institutional Review Board of The Ohio State University Wexner Medical Center.
Immunohistochemistry
Human endometrial tumor tissues were embedded in OCT medium (Tissue Tek 4583) and stored at −70° C until sectioning. Consecutive, 5 μm tissue sections were obtained for haematoxylin and eosin (H&E) and immunohistochemical (IHC) staining, following previously-described methods [15].
Endometrial tumor xenografts in mice
Cultured ishikawa cancer cells (3 × 10^6 cells in 100 μL of PBS) were subcutaneously injected into the flank of 6-week-old BALB/c nude mice from the National Cancer Institute. The groups were treated using the HO-3867 compound mixed with the animal feed (Harlan Teklad) at two different levels (50 and 100 ppm). The tumor volume was measured at the 5th week, 35 days after the beginning of HO-3867 treatment, the mice were sacrificed and the tumors were resected. The tumor tissues were then subjected to immunoblot analysis, TUNEL assays, and histopathology experiments.
Statistical analysis
Results were expressed as mean ± S.E. Comparisons between groups were made by a Student’s t-test. The significance level was set at p ≤ 0.05.
Results
Expression of pSTAT3 Ser727 in endometrial tumor
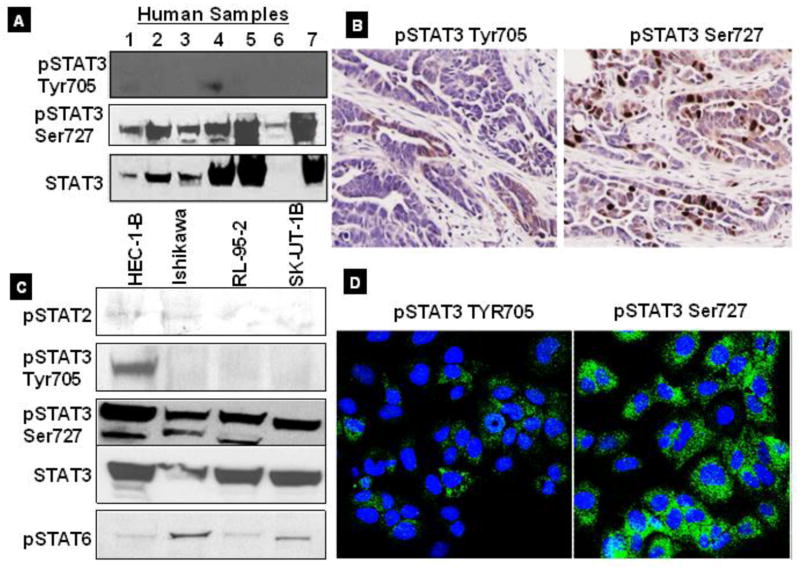
We analyzed the expression of pSTAT3 in cases of endometrial cancer via western blot and immunohistochemistry. Figure 1A shows a representative immunoblot of human tumor samples showing that the expression of pSTAT3 Ser727 is greater than that of pSTAT3 Tyr705 in advanced stage disease. Further, we examined the activated STAT3 expression in human endometrial tumor samples using immunohistochemistry. Figure 1B shows results from immunohistochemical analysis of pSTAT3 expression in endometrial tumors. In advanced stage disease, we found that pSTAT3 Ser727 was highly expressed in endometrial tumor samples. Further, we investigated the expression of other pSTAT proteins (pSTAT2, pSTAT3, and pSTAT6), in various well known endometrial cancer cell lines. pSTAT3 Ser727 is significantly expressed in endometrial cancer cell lines, we did not observe any significant changes in the expression of pSTAT2, and pSTAT6 proteins (Fig. 1C). In addition, we observed that pSTAT3 Ser727 is significantly expressed in cytosolic region in endometrial cancer cells compare with Tyr705 (Fig. 1D). Taken together, our results suggest that pSTAT3 Ser727 is highly expressed in human endometrial tumor tissues and cell lines. It is possible that the high expression of pSTAT3 Ser727 somehow regulates the endometrial cancer cell survival and proliferation.
Figure 1. STAT family expressions in human endometrial cancer.
A, Immunoblot images showed that consistently expression of pSTAT3 Ser727 compared to Tyr705 in human endometrial tumor specimens samples. B, Immunohistochemical analysis of human endometrial tumor specimens, showing increased expression of pSTAT3 Ser727. C, Consistent STAT3 expression in all cell lines compared with other STAT family proteins. Activated form of pSTAT3 Ser727 shows greater expression than pSTAT3 Tyr 705. D, IC staining for pSTAT3 Ser727 and pSTAT3 Tyr705. Confocal images showed that significantly greater density of pSTAT3 Ser727 identified within the cytosol of Ishikawa cell lines.
Effect of HO-3867 on pSTAT3 expression in endometrial cancer cells
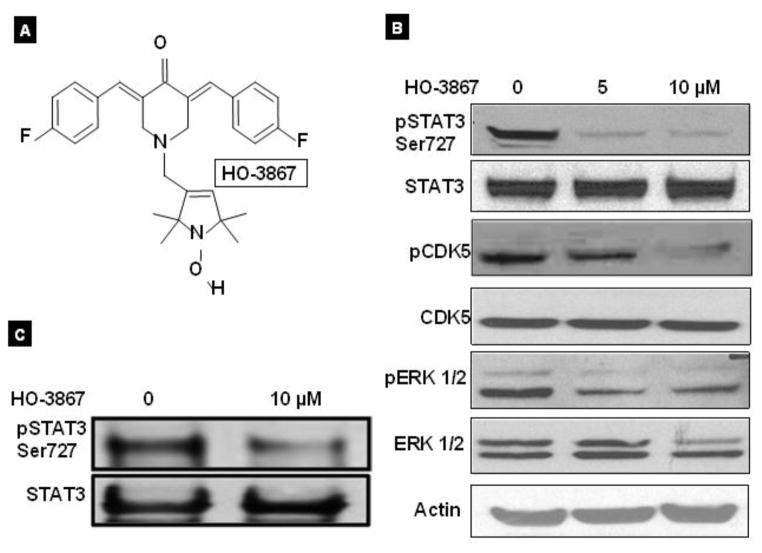
We have previously reported that our novel STAT3 inhibitor of HO-3867 (Fig. 2A) is highly effective in eliminating ovarian cancer cells [10]. Here we examine the effect of HO-3867 on pSTAT3 expression in endometrial cancer cells. Our previous reports have shown that our novel synthetic compound, HO-3867, works, at least in part, via inhibition of the STAT3 signaling pathway in various cancer cell lines [15]. We therefore examined the effects of HO-3867 on endometrial cancer cells. 24 h treatment with either 5 μM or 10 μM of HO-3867 resulted in significantly reduced expression level of pSTAT3 Ser727, without any change in total STAT3 expression (Fig. 2B). Previously, it has been shown that STAT3 is phosphorylated at a Ser727 residue by ERK1/2 and CDK5, a unique member of the CDK family [19–21]. Because we found a drastic decrease in Ser727-phosphorylated STAT3, we investigated for any possible associated decrease in the CDK5 expression. A clear decrease was identified in the expression level of pCDK5 and pERK1/2, without any changes in their total levels, in HO-3867 treated cells (Fig. 2B). These findings suggest that HO-3867 predominantly decreased the expression level of phosphorylated CDK5 and pERK1/2, thereby decreasing the expression level of Ser727-phosphorylated STAT3, which was, at least in part, responsible for the attenuated activity of STAT3. Further, our IP results confirm a significantly reduced expression of pSTAT3Ser727 after treatment with 10μM of HO-3967, although the level of total STAT3 remains unchanged whatsoever (Figure 2C)
Figure 2. HO-3867 selectively target of pSTAT3 Ser727.
A, molecular structure of HO-3867 diarylidenyl piperidone (DAP) backbone conjugated to an N-hydroxypyrroline. B, Western blot of Ishikawa cells treated with HO-3867. There is a significant decrease in expression of pSTAT3 Ser727 in HO-3867 treated cells. There is also decreased expression of pCDK5 and pErk1/2 which are known to complex with pSTAT3. C. Immunoprecipitation assay of Ishikawa cells treated with 10 μM HO-3867. Treated cells show a significant decrease in the amount of pSTAT3 Ser727 present without affecting the total expression of STAT3.
HO-3867 is cytotoxic to various endometrial cancer cell lines
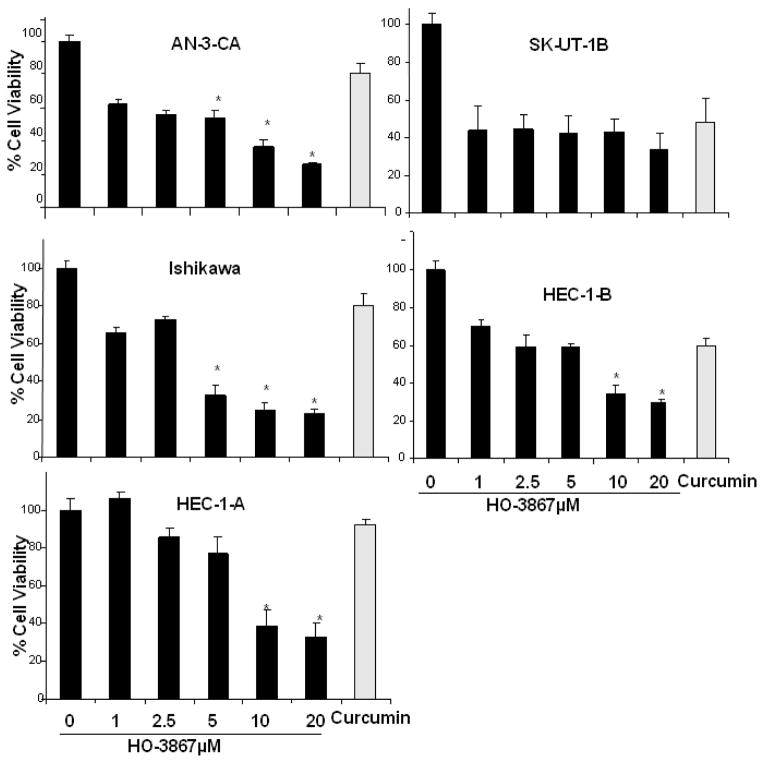
Next we assessed whether the selective targeting of pSTAT3 Ser727 and its target proteins caused endometrial cancer cell growth. The cytotoxic effect of HO-3867 was evaluated and compared with that of curcumin in Ishikawa and other well established human endometrial cancer cell lines. Figure 3 is a graphical representation of cell viability assays performed on various endometrial cancer cells. Cells were treated with increasing concentrations of HO-3867 (1 to 20μM) over the course of 24 hours. The figures show that HO-3867 inhibits cell survival on endometrial cancer cells in a dose-dependent manner. HO-3867 exhibits significantly higher cytotoxicity when compared with curcumin at 100 μM concentration. These results strongly suggest that our novel curcumin analog HO-3867, inhibits endometrial cancer cells growth by targeting STAT3.
Figure 3. Effect of HO-3867 on cell viability.
MTT assay on Ishikawa, AN-3-CA, SK-UT-1B, HEC-1-A and HEC-1B cells incubated in increasing concentrations of HO-3867 (1, 2.5, 5, 10 and 20μM) or curcumin 100 μM for 24 hours. All cell lines show a significant dose-dependent decrease in cell viability. HO-3867 produces a greater effect versus curcumin in AN-3-CA, HEC-1A and Ishikawa cells. The asterisk represents P value less than or equal to 0.05
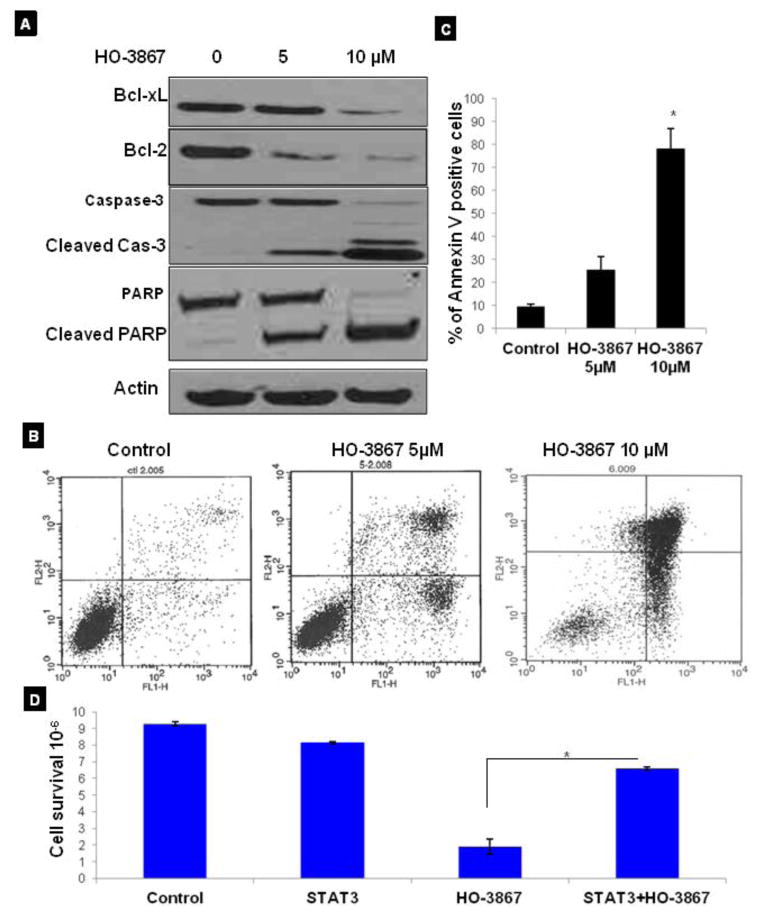
HO-3867 induces G2/M cell cycle arrest and apoptosis in endometrial cancer
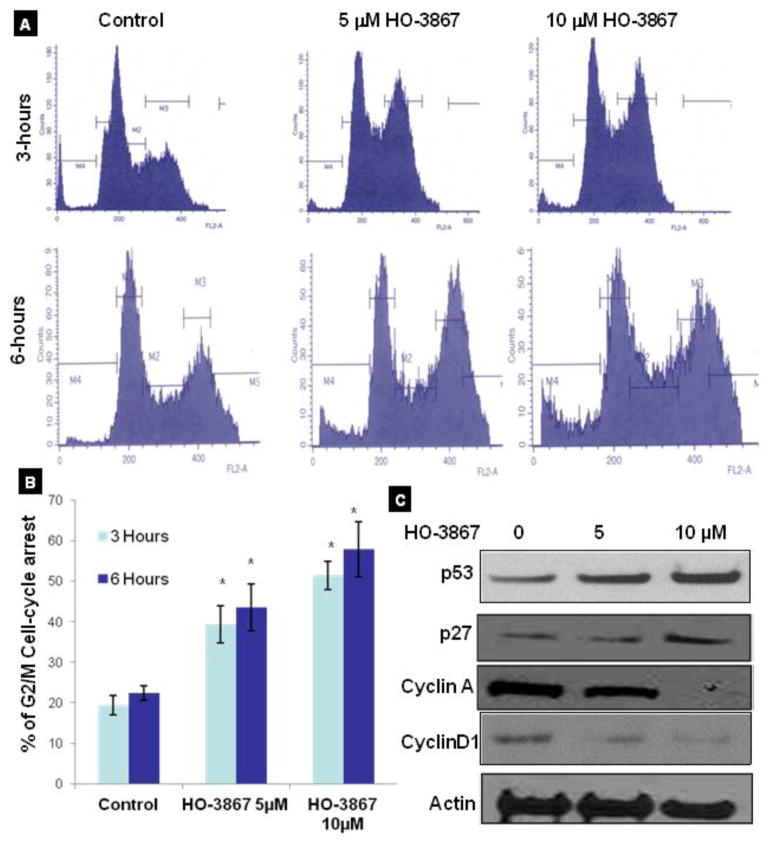
To determine whether the growth inhibition of various endometrial cancer cells by HO-3867 was caused by cell cycle arrest or apoptosis, the cells were treated with 5 and 10 μM of HO-3867 for 3 or 6h. The cells were then fixed, and cell cycle populations were determined by flow cytometry. Our results showed that HO-3867 is selectively induced G2/M cell-cycle arrest in a dose and time-dependent manner (Fig. 4A). The increase in G2/M cell population was greatest at 6 h (Fig. 4B). Further, we examined the effect of HO-3867 on G2/M cell cycle regulatory proteins, including p53, p27, CycA, and CycD1. Treatment with HO-3867 enhanced the levels of p53 and p27 and decreased the expression of Cylin A and Cyclin D1 (Fig. 4C) without any change in p21, cyclin B1, cdk2 and cdk4 (Data not shown here). Generally the arrest of cell cycle progression in cancer cells is associated with the concomitant activation of pro-apoptotic pathways. To determine whether the HO-3867–induced cell cycle arrest led to apoptosis, the expression of activated caspases was probed by Western blotting. Our results showed that down-regulation in the expression of anti-apoptotic proteins like Bcl-2 and Bcl-XL, and activation of the caspase cascade, including cleaved caspase-3, and PARP after treatment with HO-3867 for 24 h. We then evaluated the quantification of apoptosis in treatment with HO-3867 using ANEXIN V. Figures 5B and 5C show the results of annexin V flow cytometry. Treatment of endometrial cancer cells with HO-3867 resulted in a dose-dependent increase in the percentage of cells in early and late apoptosis. In order to determine if decreased cell survival and increased apoptosis was really from inhibition of STAT3, cells were transfected with STAT3 cDNA earlier to treatment with STAT3 inhibitor of HO-3867. Results seen in figure 5D show that cells transfected with STAT3 cDNA had increased cell survival compared to non-transfected cells treated with HO-3867. This infers that endometrial cancer cells transfected with STAT3 cDNA acquired some resistance to HO-3867.
Figure 4. Effect of HO-3867 on cell cycle progression and cell cycle regulatory proteins in endometrial cancer cells.
A, Representative flow cytometry graph for each treatment group, cell cycle arrest assay performed on Ishikawa cells treated with HO-3867 for 3 or 6 hours prior to fixation. Flow cytometry analysis show cells in G2/M phase arrest after 6 hour treatment. Cells treated for 12 hours showed a significant increase in cells undergoing apoptosis. B, G2/M cell cycle distribution as a function of treatment period. Data represent mean S.E. of three independent experiments. * p≤0.05 as compared with control group. C, immunoblot images of cell cycle regulatory molecules p53, p27, CycA, and CycD1, CycA, in ishikawa cells treated with HO-3867 5 and 10 μM.
Figure 5. HO-3867 induces apoptosis in endometrial cancer cells.
A, Western blot of Ishikawa cells treated with 5 or 10 μM HO-3867 for 24 hours. Treated cells show a decrease in anti-apoptosis proteins Bcl-xL and Bcl-2. There is also increased expression in cleaved caspase -7, -3, and cleaved PARP supporting HO-3867 initiates apoptosis in these cells. B, Annexin V assay on ishikawa cell lines after treatment with 5 or 10 μM HO-3867 for 24 hours. A significant increase in the percentage of cells undergoing apoptosis was seen in cancer cell lines after treatment dose. C, ANNEXIN V positive cells as determined by flow cytometry. Data represent mean ± S.E. of three independent experiments.*, p ≤ 0.05 HO-3867 compared with untreated group. (D) HO-3867 treated cells that were transfected with STAT3 cDNA had a significant increase in cell survival from those cells that were not transfected p ≤ 0.05.
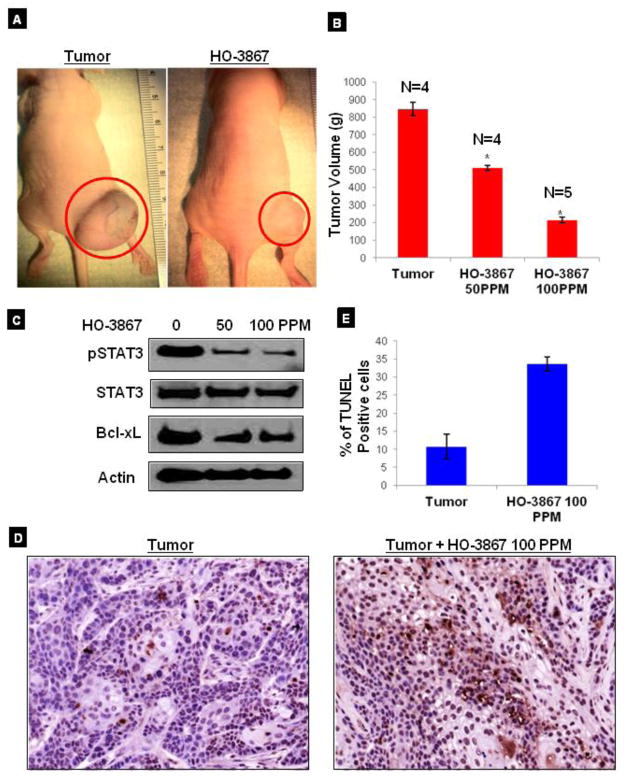
HO-3867 inhibits the growth of xenograft endometrial tumor in mice
Based on our current in vitro results, which showed significant cytotoxicity of HO-3867 towards human endometrial cancer cell lines, we next evaluated the efficacy of HO-3867 in the inhibition of the tumor growth and induction of apoptosis by targeting STAT3 in an in vivo xenograft model of the endometrial cancer. After 4 weeks of HO-3867 treatment, a significant reduction of tumor growth and volume was observed (Fig. 6A & B). After 4 weeks of HO-3867 treatment, the excised tumors showed inhibition of pSTAT3 and decreased expression of other STAT3 target proteins like cyclin D1 and Bcl-xL (Fig. 6C). We further observed a significantly increased the TUNEL-positive staining in tumor tissues treated with HO-3867, when compared to untreated controls (Fig 6D), suggesting that HO-3867 induced apoptosis in vivo. Quantitation of the TUNEL positive cells showed a fourfold increase after HO-3867 treatment in tumor mice as compared to the untreated control (Fig. 6E). The data supports the conclusion that HO-3867 inhibits endometrial cancer through targeting STAT3 and its targeting proteins of cell-cycle and apoptosis.
Figure 6. Effect of HO-3867 on xenograft endometrial tumors.
A, Ishikawa cells were injected into the bilateral flanks of nude mice. Tumor growth images in untreated and HO-3867 treated mice. B, a dose-dependent decrease in the volume (grams) of the xenograft tumors weight is observed following HO-3867 treatments. C, effect of HO-3867 on the expression of pSTAT3 and targeting genes. Immunoblot analysis using tissue lysates of xenograft tumors. The decreased expression of pSTAT3 Ser727, cyclin D1 and Bcl-xL are noted in the HO-3867–treated tumor lysates in a dose-dependent manner. D, TUNEL staining in xenograft tumor and HO-3867 treated tumor. E, Quantification of TUNEL staining in tumor samples: control (11.24%) and HO-3867- treated (34.13%). (p <0.001, n=3).
Discussion
In this study, we showed for the first time, the constitutive expression of pSTAT3 Ser727 in human endometrial tumor tissues and endometrial cancer cell lines. Our novel STAT3 inhibitor of HO-3867 targets pSTAT3 Ser727 resulting in decreased cell proliferation and increased apoptosis in both in vitro and in vivo xenograft endometrial tumor.
STAT3 is a key signaling molecule for many cytokines and growth factor receptors, and has a wide variety of biological functions, including acceleration of cell proliferation and activation of anti-apoptotic proteins. STAT3 is constitutively activated in a number of human tumors, including endometrial cancer, which is why it has attracted much attention as a potential pharmacologic target for treatment [22–24]. There is little pre-existing data on the role of STAT3 in endometrial cancer. A report showed that pSTAT3 is elevated in endometrial cancer cells and specimens. Specifically, pSTAT3 staining was found in 11.8% of grade I, 25.8% of grade 2, and 27.3% of grade 3 endometrial adenocarcinomas [25]. STAT3 is a latent cytoplasmic transcription factor typically activated upon phosphorylation of the Tyr705 residue. There is a second phosphorylation site on the Ser727 residue which is thought to enhance transcriptional activity of the protein [26, 27]. Ser727 is an equally important but less studied phosphorylation site on Stat3. Our current study involving human endometrial cancer cell lines indicates that perhaps the oncogenic potential of STAT3 rests solely on serine phosphorylation as far as endometrial cancer is concerned. We have found the pSTAT3 Tyr705 is not expressed in the majority of the endometrial cancer cell lines tested, whereas pSTAT3 Ser727 is consistently and highly expressed in all of them. A previous study showed significantly higher nuclear localization of p-Stat3-Ser727 in choriocarcinoma [28]. Another recent report has proved that Ser727 phosphorylation is associated with cell survival activity and nuclear translocation of STAT3 in melanoma cells [29]. In addition, this study demonstrated that Ser727 is frequently phosphorylated in the absence of Tyr705 phosphorylation in melanoma cells in vivo, particularly in in situ lesions of acral lentiginous melanoma (ALM) tissues. These results suggest that the overexpression of p-Stat3-Ser727 may play an important role in the pathogenesis of trophoblastic neoplasia and melanoma tumor. Mandal et al [30] have correlated a decreased phosphorylation of S727 with a concomitant increase in phosphorylation at Tyr-705, thereby adding to the invasive capability of glioma cells. Thus, the relatively puzzling contribution of S727 in cancer seems to vary with different kinds and stages, further adding to the mystery.
In this current study, we report that specific phosphorylation of STAT3 Ser727 residue could be a significant contributor to endometrial cancer progression and metastasis. At present, the specific activation or targeting of the STAT3-signaling cascade in endometrial tumors is unknown. Recently, several studies employing genetic and pharmacological approaches to modulate constitutive STAT-3 activity have substantiated the critical role of aberrant STAT-3 activity in malignant transformation and tumor progression, and hence endorse STAT-3 as a novel therapeutic target for cancers[21, 31, 32]. A clinical report has shown that new targeted agents are being investigated alone and with conventional therapy in the treatment of endometrial cancer [33]. Selective targeting of pSTAT3 Ser727 using a novel STAT3 inhibitor could be a potential for use in endometrial and other solid tumors treatment.
Our novel anti-cancer agent of HO-3867 has been shown to be a potent STAT3 inhibitor with anticancer properties in various cancer cells, in vivo ovarian and breast xenograft tumor model. In the present study, first time we have proved that HO-3867 is also selectively targeting the persistent expression of pSTAT3 Ser727 in endometrial cancer. The expression level of Ser727 phosphorylated STAT3 drastically decreased in a dose dependent manner of HO-3867 treatment, followed by a clear down-regulation in the active forms of CDK5 and ERK1/2. CDK5 and ERK have been shown to regulate the phosphorylation of STAT3 on the Ser727 residue in some previous reports [19], suggesting their participation in the oncogenic process through selective regulation of the pSTAT3 Ser727 activity in neoplastic cells. Our results suggest that HO-3867 significantly inhibits the expression level of phosphorylated CDK5, thereby reducing the expression level of Ser727-phosphorylated STAT3, which was, at least in part, responsible for the attenuated activity of STAT3 in endometrial cancer.
There is large body of evidence validating STAT3 as a target for cancer therapy, blocking either Tyr705 or Ser727 sites in various cancer cells. An important downstream effect of Stat3 activation is the Stat3-dependent regulation of several anti-apoptotic genes (Bcl-xL, MCL-1), cell survival proteins (Bcl-2, survivin), cell proliferation (Cyclin D1, D2) and anti-angiogenesis (VEGF) [22, 34–36]. Numerous studies have found these STAT3 dependent down-regulating genes are highly expressed in human cancer, especially in high-grade tumors of ovarian and endometrial origin [37–40]. Our data show that HO-3867 inhibited the cell viability of endometrial cancer cell lines in a dose- and time-dependent manner as determined with the MTT assay. The growth-inhibitory effects of HO-3867 were apparent within 24 h of treatment. In all five endometrial cancer cell lines tested, about 60 to 80% of cell growth inhibition was achieved after 24 hours exposure. Further the HO-3867 induces G2/M cell cycle arrest and apoptosis by modulating STAT3 target genes. Recent evidences have also showed that the nuclear pSTAT3 Ser727 expression is associated with cell survival activity and apoptosis in cancer cells (). Apoptosis can be regulated by activated STAT3 by manipulating the transcription of apoptosis related genes such as caspases, Fas, FAS-L and tumor necrosis factor-related apoptosis-inducing ligand [15, 21, 34]. In addition, our novel STAT3 inhibitor of HO-3867 significantly inhibits the endometrial xenograft tumor growth. The induction of apoptosis in tumor is another approach to limit their uncontrolled proliferation of tumor growth. In the present study, we observed that HO-3867 clearly induces apoptotic death both in vitro and in vivo, at least in part, due to the inhibition of STAT3 target genes. Very recently, we demonstrated that HO-3867 is selectively cytotoxic in ovarian cancer cells while having a less profound effect on normal human cells and tissues that lack constitutively active form of Stat3 [24].
In conclusion, the constitutive expression of pSTAT3 Ser727 could be associated with endometrial tumor cell survival and progression. Our current finding indicates that HO-3867 could effectively suppress the cell survival, induce apoptosis and tumor growth by inhibiting STAT3 phosphorylation. The selective targeting of pSTAT3 Ser727 using our novel STAT3 inhibitor of HO-3867 holds potential for the treatment of endometrial and other solid tumors maintaining high levels of constitutively activated STAT3.
High lights.
The constitutive expression of pSTAT3 Ser727 in human endometrial tumor tissues and endometrial cancer cell lines
HO-3867 could effectively suppress the cell survival and tumor growth by inhibiting STAT3 phosphorylation
Acknowledgments
Grant support
M.D Anderson Cancer Center Uterine SPORE Pilot Grant
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ueda SM, Kapp DS, Cheung MK, Shin JY, Osann K, Husain A, et al. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. American journal of obstetrics and gynecology. 2008;198:218.e1–6. doi: 10.1016/j.ajog.2007.08.075. [DOI] [PubMed] [Google Scholar]
- 2.Dewdney SB, Kizer NT, Andaya AA, Babb SA, Luo J, Mutch DG, et al. Uterine serous carcinoma: increased familial risk for lynch-associated malignancies. Cancer Prev Res (Phila) 2012;5:435–43. doi: 10.1158/1940-6207.CAPR-11-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duong LM, Wilson RJ, Ajani UA, Singh SD, Eheman CR. Trends in endometrial cancer incidence rates in the United States, 1999–2006. J Womens Health (Larchmt) 2011;20:1157–63. doi: 10.1089/jwh.2010.2529. [DOI] [PubMed] [Google Scholar]
- 4.Fuso L, Evangelista A, Pagano E, Piovano E, Perotto S, Mazzola S, et al. Variation in gynecological oncology follow-up practice: attributable to cancer centers or to patient characteristics? A Piedmont Regional Oncology Network Study. Tumori. 2011;97:551–8. doi: 10.1177/030089161109700502. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong K, Randall TC, Polsky D, Moye E, Silber JH. Racial differences in surgeons and hospitals for endometrial cancer treatment. Med Care. 2011;49:207–14. doi: 10.1097/MLR.0b013e3182019123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakkum-Gamez JN, Mariani A, Dowdy SC, Weaver AL, McGree ME, Martin JR, et al. Efficacy of contemporary chemotherapy in stage IIIC endometrial cancer: A histologic dichotomy. Gynecologic oncology. 2014 doi: 10.1016/j.ygyno.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Putten LJ, Hoskins P, Tinker A, Lim P, Aquino-Parsons C, Kwon JS. Population-based treatment and outcomes of Stage I uterine serous carcinoma. Gynecologic oncology. 2014;132:61–4. doi: 10.1016/j.ygyno.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Humber CE, Tierney JF, Symonds RP, Collingwood M, Kirwan J, Williams C, et al. Chemotherapy for advanced, recurrent or metastatic endometrial cancer: a systematic review of Cochrane collaboration. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2007;18:409–20. doi: 10.1093/annonc/mdl417. [DOI] [PubMed] [Google Scholar]
- 9.Selvendiran K, Ahmed S, Dayton A, Ravi Y, Kuppusamy ML, Bratasz A, et al. HO-3867, a synthetic compound, inhibits the migration and invasion of ovarian carcinoma cells through downregulation of fatty acid synthase and focal adhesion kinase. Molecular cancer research: MCR. 2010;8:1188–97. doi: 10.1158/1541-7786.MCR-10-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selvendiran K, Tong L, Bratasz A, Kuppusamy ML, Ahmed S, Ravi Y, et al. Anticancer efficacy of a difluorodiarylidenyl piperidone (HO-3867) in human ovarian cancer cells and tumor xenografts. Molecular cancer therapeutics. 2010;9:1169–79. doi: 10.1158/1535-7163.MCT-09-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selvendiran K, Tong L, Vishwanath S, Bratasz A, Trigg NJ, Kutala VK, et al. EF24 induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by increasing PTEN expression. J Biol Chem. 2007;282:28609–18. doi: 10.1074/jbc.M703796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selvendiran K, Ahmed S, Dayton A, Kuppusamy ML, Rivera BK, Kalai T, et al. HO-3867, a curcumin analog, sensitizes cisplatin-resistant ovarian carcinoma, leading to therapeutic synergy through STAT3 inhibition. Cancer Biol Ther. 2011;12:837–45. doi: 10.4161/cbt.12.9.17713. [DOI] [PubMed] [Google Scholar]
- 13.Dayton A, Selvendiran K, Kuppusamy ML, Rivera BK, Meduru S, Kalai T, et al. Cellular uptake, retention and bioabsorption of HO-3867, a fluorinated curcumin analog with potential antitumor properties. Cancer biology & therapy. 2010;10:1027–32. doi: 10.4161/cbt.10.10.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selvendiran K, Ahmed S, Dayton A, Kuppusamy ML, Tazi M, Bratasz A, et al. Safe and targeted anticancer efficacy of a novel class of antioxidant-conjugated difluorodiarylidenyl piperidones: differential cytotoxicity in healthy and cancer cells. Free radical biology & medicine. 2010;48:1228–35. doi: 10.1016/j.freeradbiomed.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rath KS, Naidu SK, Lata P, Hemant BH, Rivera BK, McCann GA, et al. HO-3867, a safe STAT3 inhibitor, is selectively cytotoxic to ovarian cancer. Cancer research. 2014 doi: 10.1158/0008-5472.CAN-13-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rath KS, McCann GA, Cohn DE, Rivera BK, Kuppusamy P, Selvendiran K. Safe and targeted anticancer therapy for ovarian cancer using a novel class of curcumin analogs. Journal of ovarian research. 2013;6:35. doi: 10.1186/1757-2215-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dayton A, Selvendiran K, Meduru S, Khan M, Kuppusamy ML, Naidu S, et al. Amelioration of doxorubicin-induced cardiotoxicity by an anticancer-antioxidant dual-function compound, HO-3867. The Journal of pharmacology and experimental therapeutics. 2011;339:350–7. doi: 10.1124/jpet.111.183681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalai T, Kuppusamy ML, Balog M, Selvendiran K, Rivera BK, Kuppusamy P, et al. Synthesis of N-substituted 3,5-bis(arylidene)-4-piperidones with high antitumor and antioxidant activity. Journal of medicinal chemistry. 2011;54:5414–21. doi: 10.1021/jm200353f. [DOI] [PubMed] [Google Scholar]
- 19.Fu AK, Fu WY, Ng AK, Chien WW, Ng YP, Wang JH, et al. Cyclin-dependent kinase 5 phosphorylates signal transducer and activator of transcription 3 and regulates its transcriptional activity. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6728–33. doi: 10.1073/pnas.0307606100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courapied S, Sellier H, de Carne Trecesson S, Vigneron A, Bernard AC, Gamelin E, et al. The cdk5 kinase regulates the STAT3 transcription factor to prevent DNA damage upon topoisomerase I inhibition. The Journal of biological chemistry. 2010;285:26765–78. doi: 10.1074/jbc.M109.092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selvendiran K, Koga H, Ueno T, Yoshida T, Maeyama M, Torimura T, et al. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: an implication for the antitumor potential of flavonoids. Cancer research. 2006;66:4826–34. doi: 10.1158/0008-5472.CAN-05-4062. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nature reviews Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Yue P, Page BD, Li T, Zhao W, Namanja AT, et al. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9623–8. doi: 10.1073/pnas.1121606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rath KS, Naidu SK, Lata P, Bid HK, Rivera BK, McCann GA, et al. HO-3867, a Safe STAT3 Inhibitor, Is Selectively Cytotoxic to Ovarian Cancer. Cancer research. 2014;74:2316–27. doi: 10.1158/0008-5472.CAN-13-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CL, Hsieh FC, Lieblein JC, Brown J, Chan C, Wallace JA, et al. Stat3 activation in human endometrial and cervical cancers. British journal of cancer. 2007;96:591–9. doi: 10.1038/sj.bjc.6603597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen Z, Darnell JE., Jr Mapping of Stat3 serine phosphorylation to a single residue (727) and evidence that serine phosphorylation has no influence on DNA binding of Stat1 and Stat3. Nucleic acids research. 1997;25:2062–7. doi: 10.1093/nar/25.11.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–37. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 28.Chan HY, Siu MK, Zhang HJ, Wong ES, Ngan HY, Chan KY, et al. Activated Stat3 expression in gestational trophoblastic disease: correlation with clinicopathological parameters and apoptotic indices. Histopathology. 2008;53:139–46. doi: 10.1111/j.1365-2559.2008.03089.x. [DOI] [PubMed] [Google Scholar]
- 29.Sakaguchi M, Oka M, Iwasaki T, Fukami Y, Nishigori C. Role and regulation of STAT3 phosphorylation at Ser727 in melanocytes and melanoma cells. The Journal of investigative dermatology. 2012;132:1877–85. doi: 10.1038/jid.2012.45. [DOI] [PubMed] [Google Scholar]
- 30.Mandal T, Bhowmik A, Chatterjee A, Chatterjee U, Chatterjee S, Ghosh MK. Reduced phosphorylation of Stat3 at Ser-727 mediated by casein kinase 2 - Protein phosphatase 2A enhances Stat3 Tyr–705 induced tumorigenic potential of glioma cells. Cellular signalling. 2014 doi: 10.1016/j.cellsig.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we? Expert opinion on investigational drugs. 2009;18:45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al Zaid Siddiquee K, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell research. 2008;18:254–67. doi: 10.1038/cr.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bansal N, Yendluri V, Wenham RM. The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer control: journal of the Moffitt Cancer Center. 2009;16:8–13. doi: 10.1177/107327480901600102. [DOI] [PubMed] [Google Scholar]
- 34.Glienke W, Hausmann E, Bergmann L. Targeting STAT3 signaling in pancreatic cancer promotes antiapoptotic gene expression. Pancreas. 2011;40:323–4. doi: 10.1097/MPA.0b013e318204ea7b. [DOI] [PubMed] [Google Scholar]
- 35.Sen M, Joyce S, Panahandeh M, Li C, Thomas SM, Maxwell J, et al. Targeting Stat3 abrogates EGFR inhibitor resistance in cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:4986–96. doi: 10.1158/1078-0432.CCR-12-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu CR, Lee YS, Mahdi RM, Surendran N, Egwuagu CE. Therapeutic targeting of STAT3 (signal transducers and activators of transcription 3) pathway inhibits experimental autoimmune uveitis. PloS one. 2012;7:e29742. doi: 10.1371/journal.pone.0029742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang JZ, Kong XJ, Banerjee A, Muniraj N, Pandey V, Steiner M, et al. STAT3alpha is oncogenic for endometrial carcinoma cells and mediates the oncogenic effects of autocrine human growth hormone. Endocrinology. 2010;151:4133–45. doi: 10.1210/en.2010-0273. [DOI] [PubMed] [Google Scholar]
- 38.Ye F, Chen HZ, Xie X, Ye DF, Lu WG, Ding ZM. Vascular endothelial growth factor (VEGF) and ovarian carcinoma cell supernatant activate signal transducers and activators of transcription (STATs) via VEGF receptor-2 (KDR) in human hemopoietic progenitor cells. Gynecologic oncology. 2004;94:125–33. doi: 10.1016/j.ygyno.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Ye D, Xie X, Chen B, Lu W. VEGF, VEGFRs expressions and activated STATs in ovarian epithelial carcinoma. Gynecologic oncology. 2004;94:630–5. doi: 10.1016/j.ygyno.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 40.Duan Z, Foster R, Bell DA, Mahoney J, Wolak K, Vaidya A, et al. Signal transducers and activators of transcription 3 pathway activation in drug-resistant ovarian cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:5055–63. doi: 10.1158/1078-0432.CCR-06-0861. [DOI] [PubMed] [Google Scholar]