Abstract
IMPORTANCE
Older adults have high rates of sleep disturbance, die by suicide at disproportionately higher rates compared with other age groups, and tend to visit their physician in the weeks preceding suicide death. To our knowledge, to date, no study has examined disturbed sleep as an independent risk factor for late-life suicide.
OBJECTIVE
To examine the relative independent risk for suicide associated with poor subjective sleep quality in a population-based study of older adults during a 10-year observation period.
DESIGN, SETTING, AND PARTICIPANTS
A longitudinal case-control cohort study of late-life suicide among a multisite, population-based community sample of older adults participating in the Established Populations for Epidemiologic Studies of the Elderly. Of 14 456 community older adults sampled, 400 control subjects were matched (on age, sex, and study site) to 20 suicide decedents.
MAIN OUTCOMES AND MEASURES
Primary measures included the Sleep Quality Index, the Center for Epidemiologic Studies–Depression Scale, and vital statistics.
RESULTS
Hierarchical logistic regressions revealed that poor sleep quality at baseline was significantly associated with increased risk for suicide (odds ratio [OR], 1.39; 95% CI, 1.14-1.69; P < .001) by 10 follow-up years. In addition, 2 sleep items were individually associated with elevated risk for suicide at 10-year follow-up: difficulty falling asleep (OR, 2.24; 95% CI, 1.27-3.93; P < .01) and nonrestorative sleep (OR, 2.17; 95% CI, 1.28-3.67; P < .01). Controlling for depressive symptoms, baseline self-reported sleep quality was associated with increased risk for death by suicide (OR, 1.30; 95% CI, 1.04-1.63; P < .05).
CONCLUSIONS AND RELEVANCE
Our results indicate that poor subjective sleep quality is associated with increased risk for death by suicide 10 years later, even after adjustment for depressive symptoms. Disturbed sleep appears to confer considerable risk, independent of depressed mood, for the most severe suicidal behaviors and may warrant inclusion in suicide risk assessment frameworks to enhance detection of risk and intervention opportunity in late life.
Suicide is a preventable public health problem and global disease burden, with profound personal, societal, and economic consequences. It accounts for almost 1 million deaths annually and 57% of all violent deaths worldwide.1 In the United States, suicide ranks as a leading causing of death, with a rate of approximately 12.4 deaths per 100 000 individuals.2 In addition, an estimated 25 suicide attempts (100-200 for youth) occur for every death by suicide,3 resulting in more than 400 000 emergency department visits annually.4 Despite unprecedented improvements in awareness and treatment, worldwide suicide rates in the past 50 years have remained alarmingly intractable, and among specific populations in the United States (eg, middle-aged adults), they have increased.5,6 Therefore, the prevention of suicide has been named a national imperative by the Institute of Medicine,7 with recent calls from the US Surgeon General8 to advance research and delineate evidence-based risk factors that reduce stigma, enhance access to care, and influence suicide prevention.
Biological, psychological, and social factors are known to be associated with chronic and acute suicidal risk, including static and dynamic risk factors.9-11 Sleep complaints are listed among the top 10 warning signs of suicide from the Substance Abuse and Mental Health Services Administration,12 and a growing body of research indicates that objective and subjective sleep disturbances such as insomnia, nightmares, and poor sleep quality symptoms may present elevated risk for suicidal ideation, suicide attempts, and death by suicide.13-18 This topic has been reviewed by others.19,20
However, major methodological issues common to this area of research critically limit the validity and generalizability of such findings. Problems include frequent reliance on single-item, retrospective assessments of suicide risk or sleep disturbances (ie, often drawn from depression inventories) and common use of cross-sectional study designs at a single time point. This prevents assessment of the directionality of findings and evaluation of causal risk. Beyond these more basic design issues, a central methodological constraint in this literature includes failure to account for the confounding presence of depression severity. It is critical to determine independence of risk and rule out spurious associations given that sleep disturbance and suicidal symptoms are diagnostic criteria for major depression and considering that major depressive disorder is among the single best predictors of suicide. For these reasons, accounting for depressive symptoms among rigorous, prospective investigations is a necessary test to delineate disturbed sleep as a stand-alone risk factor for suicidal behaviors, independent of depressed mood, vs a correlate of greater depression severity.
Preliminary research addressing such methodological concerns suggests an association between insomnia and nightmare symptoms beyond the influence of depressed mood. This seems true among cross-sectional investigations evaluating suicidal ideation as a primary outcome,17,21,22 in one psychological autopsy study18 retrospectively comparing disturbed sleep among adolescent suicide decedents compared with controls, in a study23 of sleep-related symptoms in the prediction of incident suicide attempts among military personnel, and within an investigation of electroencephalographic sleep parameters among patients having psychosis with and without a history of suicide attempts.24 Even so, comparability across studies remains challenged by gross differences in methodology and diagnostic characteristics. Large-scale investigations, with ability to prospectively evaluate incidence of suicidal behav iors in association w ith subjec tive sleep disturbances, controlling for depressive symptoms, have not been conducted. In addition, no study to date has prospectively evaluated sleep indexes as a unique risk factor for the most severe of suicidal behaviors, death by suicide.
Late life stands apart from other developmental periods insofar as it is characterized by increased prevalence of sleep complaints and disproportionately elevated rates of suicide. Older adults die by suicide at disproportionately higher rates compared with the general population,25 with higher rates likely reflecting increased frailty of older people and greater lethality of methods used for a suicide attempt compared with younger age groups.26 The structure, timing, and consolidation of sleep also change considerably in late life, with greater sleep fragmentation and difficulty maintaining sleep reported, as well as decreased time spent in the deeper stages of sleep, such as slow wave sleep.27,28 Large, multicenter investigations reflect these changes, with clinically significant sleep disruption reported in 57% of older adults.28
The present study investigated whether subjectively assessed sleep disturbance conferred independent risk for suicide compared with control subjects within a population-based, community study of late-life suicide. This article reports on a longitudinal epidemiological study of suicide risk among older adults conducted during a 10-year period. Based on the above findings, disturbed sleep was hypothesized to prospectively predict increased risk for suicide. In line with definitions by Kraemer and colleagues,29 subjectively disturbed sleep quality was expected to emerge as a “variable risk factor” for suicide insofar as risk is both modifiable and precedes the specified outcome (suicide death). Next, poor sleep quality at baseline was expected to significantly predict increased risk for suicide, even after adjustment for concomitant mood symptoms. Finally, in line with research suggesting that difficulty maintaining sleep is especially common in late life,30 we proposed exploratory evaluation of individual sleep items in the prediction of risk.
Methods
Participants and Procedures
Institutional review board approval was obtained for all sites (East Boston [Harvard University], New Haven [Yale University], Iowa County and Washington County [University of Iowa], and Durham, North Carolina [Duke University]) before data collection. Informed written consent was obtained from all participants, and all study procedures were compliant with standard ethical guidelines for research involving humans. Participants were recruited (between 1981 and 1991) as part of a multisite community-based study, the Established Populations for Epidemiologic Studies of the Elderly project. Data collection procedures have been described in detail elsewhere.31,32 The project data set is a longitudinal, cohort study of community elders 65 years or older. Data were collected among 14 1456 participants drawn from the general population (vs a retirement community). Participating sites included (1) New Haven, Connecticut; (2) East Boston, Massachusetts; (3) Iowa County, Iowa, and Washington County, Iowa; and (4) Durham, North Carolina. Response rates ranged from 80% to 85%, with a mean response of 81.5%. Data were collected at 6 time points, with in-person interviews occurring at baseline and at 3 and 6 follow-up years. The present investigation used a nested, case-control design. Cases included those whose cause of death was suicide, as indicated by an International Classification of Diseases, Ninth Revision, code between 950 and 959 on their official death certificate. In total, 21 individuals died by suicide during the 10-year observation period; one suicide decedent received only a partial interview by proxy at baseline, providing insufficient data for final analyses. Therefore, complete data were available for 20 total suicide decedents. For each suicide case, 20 control subjects were randomly selected from the sample, as recommended by Breslow and colleagues.33 Controls were matched to suicide decedents on age, sex, study site, and duration in the Established Populations for Epidemiologic Studies of the Elderly project. Controls were sampled randomly from the cohort completing the follow-up interview closest to the date of suicide, and a direct interview was required for inclusion. The total sample was composed of 420 cases (400 controls and 20 suicide decedents) selected from 14 456 participants.
Measures
Center for Epidemiological Studies–Depression Scale
The Center for Epidemiologic Studies–Depression Scale (CES-D) is a 20-item scale designed to assess the presence of depressive symptoms. The CES-D summed index generates an overall assessment of mood disturbances, with higher scores indicative of elevated depressive symptoms.34 For both cases and controls, baseline severity of depressive symptoms (CES-D total scores) were evaluated in the prediction of increased risk for suicide. Sites used different versions of this instrument. Several sites used the full, 20-item version, whereas 2 others used an abbreviated, 10-item version. To achieve comparability across sites, z scores were calculated for each participant based on the mean (SD) calculated for each site. Results were comparable across versions. Therefore, the 10-item version, administered across all sites, was used to allow for comparisons across sites. Although suicide ideation items are not present in the original CES-D version, one item that assessed sleep disturbance (ie, “my sleep is restless”) was excluded from the CES-D total scores for all analyses.
Sleep Quality Index
The Sleep Quality Index (SQI), a 5-item, investigator-constructed scale, was used to evaluate sleep quality. This scale was administered at the time of the baseline interview. Items on this index assessed difficulty falling asleep, difficulty staying asleep, early morning awakening, daytime sleepiness, and nonrestorative sleep. The frequency of each sleep complaint was assessed on a scale of 1 to 3 (1 indicates most of the time, 2 indicates some of the time, and3 indicates none of the time). Sleep items broadly assessed the frequency and type of sleep disturbance (difficulty falling asleep, difficulty staying asleep, early morning awakening, and nonrestorative sleep) and resulting daytime function (daytime sleepiness), consistent with DSM diagnostic criteria for insomnia disorder. All items were summed to create an overall SQI, with total scores ranging from 5 to 15. For improved interpretability, all items were reverse scored, with higher scores indicative of poorer overall subjective sleep quality. Regarding reliability indexes, coefficient α for this scale was .544. Because Cronbach α may be reduced by brevity of questions alone, inter-item correlation coefficients for the measure were also conducted. These were significant across all but 1 of 10 possible combinations (range, r = 0.11 to r = 0.36). Table 1 summarizes the inter-item correlation matrix. Validated symptom instruments of subjective sleep quality, designed to assess similar insomnia symptom dimensions, show high test-retest reliability and validity, as well as fair to good correspondence to both daily sleep logs and objective sleep parameters.35,36
Table 1.
Intercorrelations Between Variables
| No. | Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|
| 1 | SQI total score | NA | ||||||
|
| ||||||||
| 2 | SQI item 1: difficulty falling asleep | 0.64a | NA | |||||
|
| ||||||||
| 3 | SQI item 2: difficulty staying asleep | 0.70a | 0.30a | NA | ||||
|
| ||||||||
| 4 | SQI item 3: early morning awakening | 0.64a | 0.36a | 0.34a | NA | |||
|
| ||||||||
| 5 | SQI item 4: daytime sleepiness | 0.53a | 0.11b | 0.22a | 0.13b | NA | ||
|
| ||||||||
| 6 | SQI item 5: nonrestorative sleep | 0.46a | 0.23a | 0.11b | 0.12b | 0.03 | NA | |
|
| ||||||||
| 7 | CES-D total score | 0.35a | 0.28a | 0.20a | 0.23a | 0.07 | 0.27a | NA |
|
| ||||||||
| NA | Suicide incidence | 0.14a | 0.13a | 0.09 | 0.05 | 0.07 | 0.15a | 0.14a |
Abbreviations: CES-D, baseline Center for Epidemiologic Studies–Depression Scale; NA, not applicable; SQI, baseline Sleep Quality Index.
P < .01.
P < .05.
Cognitive Impairment
Cognitive functioning at baseline was evaluated using 7 items taken from the 10-item Short Portable Mental Status Questionnaire.37 Items assessed knowledge of day and date, current and previous presidents, mother’s maiden name, current address or phone number, and a serial subtraction task. Participants received one point for each item answered correctly.
Physical Functioning
Physical impairment at baseline was assessed by 3 brief scales. On the Katz Activities of Daily Living Scale,38 participants indicated whether they required help (1 indicates no help, 2 indicates help, and 3 indicates unable) to walk across a room, bathe, groom, dress, eat, get out of bed, and use the toilet. A 3-item measure of gross mobility developed by Rosow and Breslau39 assessed ability (1 indicates able to, and 2 indicates unable to) to do heavy chores, walk up and down a flight of stairs, and walk half a mile. A 4-item physical performance scale based on the work by Nagi40 assessed the degree of difficulty ranging from 1 to 4 (1 indicates no difficulty at all, and 4 indicates just unable to do it) to pull or push large objects, reach one’s arms above shoulder level, write or handle small objects, and stoop, crouch, or kneel.
Vital Statistics Interview
Vital statistics were ascertained using follow-up interviews with participants, relatives, or other contacts, as well as death registrars at local government offices, linkage to the National Death Index, and scanning of local newspaper obituaries. Vital status was known for all participants, and death certificates were obtained for at least 98% at each site during the 10 years. Cause of death as suicide was indicated by an International Classification of Diseases, Ninth Revision, code between 950 and 959 on their official death certificate, and all cases had at least one direct interview.
Data Analytic Plan
Planned Analyses
Using a case-control cohort study design, hierarchical logistic multiple regression analyses were used to examine whether poor self-reported sleep quality at baseline, controlling for baseline depressive symptoms, predicted an increased risk for suic ide. For analysis purposes, partic ipants were recorded as having died by suicide (coded as 1) or not (coded as 0) at follow-up. Death by suicide outcome constituted the dependent variable. Regression block 1 included the CES-D total scores as the independent variable. Regression block 2 included the SQI total scores as the independent variable. It was hypothesized that poorer self-reported sleep (lower SQI scores) at baseline would be significantly associated with death by suicide, even after accounting for depressive symptom severity as a covariate.
A separate, secondary hierarchical logistic multiple regression was next conducted to examine the predictive value of each individual sleep item to risk for death by suicide (ie, difficulty falling asleep, difficulty staying asleep, early morning awakening, daytime sleepiness, and nonrestorative sleep). Death by suicide (yes or no) constituted the dependent variable. Regression block 1 included the CES-D total scores as an independent variable. In separate analyses, regression block 2 included each individual sleep item. These exploratory analyses were conducted to assess the predictive value of each individual SQI item in association with risk for suicide outcome at 10 follow-up years, with the CES-D total scores included as a covariate. Difficulty staying asleep was hypothesized to be significantly associated with suicide risk given its prevalence in late life.
Power
Power was assessed according to the sample size tables for logistic regression provided by Hsieh41 and was based on previous studies42,43 similar in design. It was determined that the proposed analyses had a power exceeding 0.80 (α = .05) to detect an effect size of an odds ratio of 1.3 or higher.
Results
Study Findings
Intercorrelations between variables and descriptive statistics are summarized in Table 1 and Table 2. Participants ranged in age from 66 to 90 years (mean [SD] age, 74.9 [5.6] years). The ethnic composition of this sample was consistent with cross-national data across study sites. Ethnicity and racial categorization options were created by investigators, and identification to a given category was based on participant response as follows: 58.3% white, 19.3% African American, and 1.2% Asian, American Indian, or Hispanic. Ethnicity was unavailable or unreported for one site, which represented 21.2% of the sample. Among suicide decedents, the following racial categories were endorsed: 73.6% white, 5.2% African American, and 5.2% Asian, American Indian, or Hispanic. Among these, 15.7% were unknown or unreported. Ethnicity was not significantly related to study variables. Therefore, it was omitted from primary analyses. The majority (95.0%) of suicide decedents in this sample were male (n = 19). The most common method of suicide was death by firearm (n = 13), although the method of suicide varied considerably across the sample. The percentages of suicide attempts among decedents by method were as follows: firearm (62.0%), hanging (9.5%), cutting (9.5%), poisoning (4.8%), drowning (4.8%), lethal jump (4.8%), and suffocation (4.8%). The mean (SD) time to death in the present study was 4.24 (3.12) years for the sample overall and several years earlier for suicide decedents, at a mean (SD) of 1.75 (1.20) years.
Table 2.
Descriptive Statistics
| Variable | Mean (SD) |
||
|---|---|---|---|
| Controls (n = 400) | Cases (n = 20) | Total Sample (N = 420) | |
| SQI total score, range 5-15 | 8.03 (2.16) | 9.80 (2.86) | 8.12 (2.24) |
|
| |||
| SQI item 1: difficulty falling asleep, range, 0-3 | 1.44 (0.66) | 1.90 (0.85) | 1.46 (0.80) |
|
| |||
| SQI item 2: difficulty staying asleep, range, 0-3 | 1.84 (0.84) | 2.20 (0.89) | 1.85 (0.85) |
|
| |||
| SQI item 3: early morning awakening, range, 0-3 | 1.56 (0.72) | 1.75 (0.85) | 1.57 (0.73) |
|
| |||
| SQI item 4: daytime sleepiness, range 0-3 | 1.86 (0.82) | 2.15 (0.93) | 1.87 (0.83) |
|
| |||
| SQI item 5: nonrestorative sleep, range 0-3 | 1.33 (0.65) | 1.80 (0.89) | 1.35 (0.67) |
|
| |||
| CES-D total score, range 0-10 | 1.33 (1.82) | 3.00 (2.74) | 1.41 (1.91) |
Abbreviations: CES-D, baseline Center for Epidemiological Studies–Depression Scale; SQI, baseline Sleep Quality Index.
Regarding primary analyses, consistent with our hypotheses, results indicated that self-reported sleep quality at baseline significantly predicted increased risk for death by suicide (P < .001). Specifically, the total SQI scores (ie, indicating poorer subjective sleep quality) significantly predicted increased risk for death by suicide during the 10-year period. Table 2 lists the mean SQI total scores for suicide decedents vs controls.
Next, we controlled for the CES-D total scores in the model. In support of our hypothesis, the relationship between poorer sleep quality at baseline and increased risk for death by suicide at 10 follow-up years remained statistically significant (P < .05).
Next, exploratory analyses examined the 5 individual SQI items, each in its own regression block, in the prediction of suicide risk during the 10-year observation period, again controlling for depressive symptoms as indexed by the CES-D total scores. Contrary to expectation, one item (SQI item 5: non-restorative sleep) was statistically significant (P < .05) in its association with increased risk for suicide at follow-up times, accounting for depressed mood. Another item (SQI item 1: difficulty falling asleep) emerged as a unique individual risk factor but as a nonsignificant statistical trend only (P = .08). By comparison, SQI item 2 (difficulty staying asleep), SQI item 3 (early morning awakening), and SQI item4 (daytime sleepiness) were not statistically significant at the individual item level in the prediction of suicide risk (P > .05). Table 3 lists all test statistics, both before and after adjustment for depressive symptoms. Figure 1 shows the mean sleep quality differences between suicide decedents and matched controls.
Table 3.
Model Statistics for Sleep Quality Associated With Suicide Risk at 10 Follow-up Years
| Risk Factor and Model Adjustment | χ2 Wald Statistic | OR (95% CI) | P Value | χ2 Wald Statistica | OR (95% CI)a | P Valuea |
|---|---|---|---|---|---|---|
| SQI total score | 10.87b | 1.39 (1.14-1.69) | .001 | 5.33c | 1.30 (1.04-1.63) | .02 |
|
| ||||||
| SQI item 1: difficulty falling asleep | 7.88b | 2.24 (1.27-3.93) | .005 | 3.11 | 1.76 (0.94-3.29) | .08 |
|
| ||||||
| SQI item 2: difficulty staying asleep | 3.35 | 1.65 (0.96-2.82) | .07 | 1.49 | 1.44 (0.78-2.63) | .23 |
|
| ||||||
| SQI item 3: early morning awakening | 1.37 | 1.40 (0.79-2.49) | .25 | 0.17 | 1.14 (0.60-2.15) | .68 |
|
| ||||||
| SQI item 4: daytime sleepiness | 2.29 | 1.52 (0.88-2.64) | .13 | 1.65 | 1.48 (0.81-2.70) | .20 |
|
| ||||||
| SQI item 5: nonrestorative sleep | 8.43b | 2.17 (1.28-3.67) | .004 | 5.14c | 1.98 (1.09-3.59) | .03 |
Abbreviations: CES-D, Center for Epidemiological Studies–Depression Scale; OR, odds ratio; SQI, Sleep Quality Index.
Adjustment for the CES-D total scores in themodel.
P < .01.
P < .05.
Figure 1. Self-reported Sleep Quality at Baseline and Risk for Death by Suicide at 10 Follow-up Years.
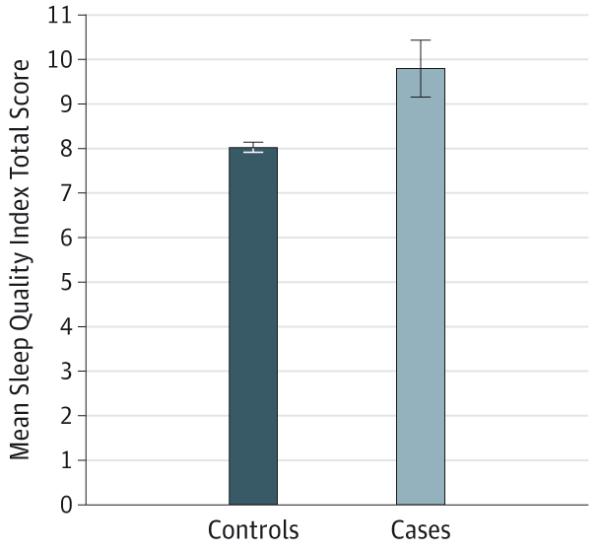
The mean Sleep Quality Index total scores are shown for cases (20 suicide decedents) compared with 400 age and sex–matched control subjects. Higher Sleep Quality Index total scores reflect poorer subjective sleep quality. Error bars represent standard errors. χ 2 = 10.87 (odds ratio, 1.39; 95% CI, 1.14-1.69; P < .001).
Post Hoc Analyses
To further define sleep quality as a predictor of risk, a receiver operator characteristic analysis showed that the SQI total scores significantly distinguished suicide decedents from matched controls (P = .005). This revealed a fair area under the curve of 0.685, with a 95% CI ranging from 0.549 to 0.820 (Figure 2). Next, although the SQI total scores significantly predicted risk after adjustment for the CES-D, both variables were subsequently evaluated within a single regression model for comparison as predictors. This analysis revealed that the SQI accounted for more unique variance in the prediction of suicide risk compared with the CES-D when evaluated within the same model (χ2 = 5.33, P = .02 vs χ2 = 3.94, P = .047, respectively). Even so, based on scientific and clinical rationale (ie, the relationship of these variables to one another, their independent prediction of risk for suicide outcome, and the potential clinical usefulness of their interaction), we explored the extent to which these variables next jointly predicted suicide risk. A significant interaction was observed, wherein high scores on both the CES-D and SQI measures predicted greatest risk for suicide (χ2 = 3.99, P = .046). Finally, with regard to individual sleep items in the prediction of risk for suicide outcome, 2 items reached statistical significance in the initial model (Table 3). Therefore, a subsequent post hoc logistic regression analysis was performed to test whether the 2 items jointly predicted risk to a greater degree compared with each item alone. Omnibus tests revealed that, indeed, the combination of SQI item 1 (difficulty falling asleep) and SQI item 5 (nonrestorative sleep) yielded the largest model statistic (χ2 = 12.18, P = .002) compared with each item evaluated separately in the model.
Figure 2. Self-reported Sleep Quality at Baseline as a Predictor of Risk for Death by Suicide.
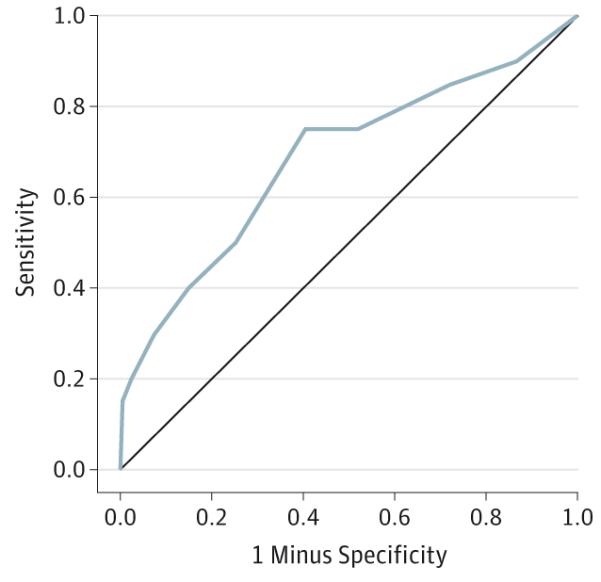
The receiver operating characteristic curve for baseline Sleep Quality Index total scores distinguishes cases (20 suicide decedents) from 400 age and sex–matched control subjects. Higher Sleep Quality Index total scores reflect poorer subjective sleep quality.
Covariate analyses evaluated cognitive and physical disability in the association between sleep quality and suicide outcome. As reported in an earlier study,31 neither cognitive nor physical impairment scores significantly distinguished suicide decedents from matched controls (P > .05). Furthermore, cognitive impairment was not significantly associated with baseline SQI scores (P > .05). Based on linear regression analyses, the 3 brief indexes of physical impairment were positively associated with the SQI, with t = 2.24, β = .28, P = .03 (95% CI, P = .03 to P = .54); t = 4.99, β = .57, P < .001 (95% CI, P = .35 to P = .79); and t = 4.78, β = .20, P < .001 (95% CI, P = .12 to P = .28). However, these relationships were no longer statistically significant after adjustment for the CES-D (P > .05), suggesting that depression may largely account for this link.
Discussion
The aim of the present study was to examine poor subjective sleep quality as an independent risk factor for death by suicide in a population-based study of late life. Results revealed that poor subjective sleep quality at baseline was associated with increased risk for death by suicide at 10 follow-up years. Specifically, those reporting poorer sleep quality (higher SQI total scores) at baseline showed a 1.4 times increased risk for death by suicide (Figure 1). Next, analyses revealed that, when depressive symptoms were entered as a covariate, the relationship between poor sleep quality and risk for suicide death at follow-up times remained statistically significant, supportive of our hypotheses. Controlling for the effects of depressed mood, those with poorer sleep quality at baseline demonstrated a 1.2 times greater risk for suicide death during the 10-year observation period.
These findings converge with past research, diverse in design and samples, indicating associations between sleep disturbances and risk for suicidal behaviors, independent of depressive symptoms or affective disturbance.17,18,21 Remarkably, although both sleep disturbances and depressive symptoms were higher overall among suicide decedents than controls, sleep disturbances outperformed depressive symptoms in the longitudinal prediction of risk for death by suicide 10 years later. This highlights the utility of sleep as an intervention tool and converges with at least one recent study23 showing that a 3-item measure of sleeplessness and general fatigue outperformed symptoms of depression and hopelessness in the prediction of incident suicidal ideation and attempts. Our results nevertheless indicated that the endorsement of higher depressive symptoms and sleep disturbances yielded greatest risk for death by suicide. This demonstrates the importance of assessing disturbed sleep in the presence of other, well-established and robust risk factors for suicide.
The present study contributes to the extant literature in several ways. To our knowledge, to date, it is the first study to evaluate incidence of suicidal behaviors in late life prospectively, as well as in the prediction of risk for the most severe of suicide outcomes, death by suicide. This is in part owing to the time and costs required to prospectively assess death by suicide, which are substantial. For example, during a 34-year period (1966-2000), only 46 studies reported on death by suicide as a primary outcome44; of these, only a small minority evaluated population-based risk. Studies assessing death by suicide as a primary outcome measure include both psychological autopsy studies and population-based community investigations. Although each design provides invaluable information in the delineation of risk factors, longitudinal, community-based investigations (ie, as conducted in the present study) are unique insofar as they collect information directly from the individual, following the outcome (ie, suicide incidence) prospectively over time. Along these lines, this is the only known study to assess poor subjective sleep quality as a risk factor for suicide death within a population-based, prospective study design, controlling for depressed mood. Finally, this study reflects a nationally representative, population-based study of community elders. Older adults die by suicide at disproportionately higher rates compared with the general population and relative to their smaller population demographic size.25,31 An additional strength of the present study is the evaluation of disturbed sleep in association with late-life suicide within a population-based sample of community-dwelling elders (ie, vs those residing in extended care facilities, where the majority of such studies tend to be conducted).
To explore specific sleep complaints in the prediction of suicide risk, we next examined 5 sleep quality symptoms individually in association with suicide risk at 10 follow-up years. Given age-related changes in sleep, difficulty staying asleep was hypothesized to be uniquely associated with increased risk for suicide. Contrary to prediction, 2 sleep quality items emerged as significant individual predictors of risk. Difficulty falling asleep and nonrestorative sleep (and their combination in particular) specifically predicted increased risk for suicide. However, after adjustment for depressive symptoms, this effect reached significance for nonrestorative sleep only. Although different from our hypothesis, cross-sectional studies16,21 are largely consistent with these findings, showing strong associations between general insomnia disturbance and poor sleep quality (ie, reflected by endorsement of nonrestorative sleep) and risk for both suicide ideation and suicide attempts. Future studies are necessary to evaluate how specific types of subjective sleep disturbance may interact to predict increased risk for suicide in late life.
In the present study, poor sleep quality appeared to confer elevated risk for suicide, independent of depressed mood. This highlights the need for additional research that may identify potential explanatory mechanisms in this relationship. Based on the present findings, we propose that deficits in cognitive and emotional processing may play a central role in the link between sleep disturbances and risk for suicide. Both experimental and nonexperimental manipulations of sleep are consistent with this explanation, as well as emerging findings on the role of sleep in cognition and emotion.45 For example, research indicates that sleep fragmentation results in increased emotional reactivity, intensifying negative emotional responses, while blunting positive affect.46 Similarly, sleep deprivation among healthy adults is associated with amplification of amygdala activation, as well as increased reactivity to negative emotions such as anger and fear.47,48 Notably, a night of recovery sleep following sleep deprivation reverses this effect, decreasing amygdala activation and reducing such emotional reactivity.49 Likewise, both emotion regulation and neurocognitive deficits are associated with elevated risk for suicidal behaviors, across age groups and outcome measures.50-54 As one of several possible explanatory pathways, we thus propose that such deficits may lower the threshold for suicidal behaviors by impairing the processing of emotionally salient information and associated neural circuitry. Given their potential clinical utility, additional research investigating such explanatory factors is strongly warranted.
Results from the present study may importantly inform suicide risk assessment and suicide prevention efforts. Older adults are known to visit their primary care physician in the final weeks (45%) and month (73%) prior to suicide death.55 Furthermore, at least one psychological autopsy study18 indicates that disturbed sleep is visible to friends and family members in the weeks and months preceding death. Targeting dis turbed sleep as a visible warning sign of suicide may, in this way, constitute a novel opportunity for improved risk detection, particularly among those at elevated risk. Along these lines, our findings were primarily observed among elderly white men, a demographic group that demonstrates disproportionately higher rates of suicide (14.3 deaths [47.0 deaths for those aged >85 years] per 100 000) compared with the general population (13.8 deaths per 100 000).56 This more narrowed, demographic focus may specifically inform intervention among a group at heightened risk for suicide. Next, suicides in the present study occurred within a mean of approximately 2 years. This suggests that disturbed sleep may confer risk within a relatively acute time frame. Therefore, focus on a factor that is proximal to risk, overrepresented in late life, yet highly modifiable, may constitute a unique opportunity for suicide prevention. Finally, in both uncontrolled and controlled insomnia and nightmare treatment trials, improvements in sleep are associated with large posttreatment symptom reductions in depression and anxiety57-59 and suicidal ideation specifically.60 Such findings highlight the possibility that sleep may serve as a causal risk factor, underscoring the need for additional research.
Several limitations of the present study should be noted. First, self-reported measures of sleep quality were used vs objectively measured sleep complaints. Therefore, it cannot be ruled out that subjective sleep quality may represent an underlying sleep disorder such as chronic insomnia, nightmare disorder, or obstructive sleep apnea. In addition, diagnostic information and other important covariates (eg, chronic pain, substance use, and diverse medical conditions), which may influence sleep quality, were not included in the present study. Therefore, future replication studies to evaluate such variables are recommended. Next, despite a large sample drawn from 14 456 individuals, the present study included 20 suicide decedents, 19 of whom were male. Although this reflects national rates and statistics, with a higher proportion of older men dying by suicide compared with women,51,52 the present results should be interpreted as primarily applicable to men and chiefly white men. Finally, the present study assessed sleep within a large, epidemiological sample using an author-constructed scale of sleep quality. Although multiple symptom domains (ie, frequency, severity, and daytime symptoms) from gold standard insomnia instruments53-55 are represented in the scale, future studies are warranted using validated insomnia symptom measures.
Conclusions
In conclusion, findings from this study indicate that, independent of depressed mood, subjective sleep quality serves as a risk factor for suicide during a 10-year observation period. We suggest that poor subjective sleep quality may therefore represent a useful screening tool and a novel therapeutic target for suicide prevention in late life. Sleep disturbances have been included in the 2013 Veterans Affairs–Department of Defense clinical practice guidelines for patients at risk for suicide,61 and findings from this study suggest rationale for their inclusion in similar evidence-based suicide risk assessment frameworks, intervention strategies, and clinical practice guidelines.
Acknowledgments
Funding/Support: This study was supported in part by grants F31MH080470-01 and K23MH093490 from the National Institutes of Health (Dr Bernert), by grant R49 CE002093 from the Centers for Disease Control and Prevention (Dr Conwell), and by the John Simon Guggenheim Memorial Foundation (Dr Joiner).
Role of the Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.World Health Organization (WHO) Figures and Facts About Suicide. Dept of Mental Health, World Health Organization; Geneva, Switzerland: 1999. [Google Scholar]
- 2.Murphy SL, Xu J, Kochanek MA. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61(4):1–118. [PubMed] [Google Scholar]
- 3.Maris RW. Suicide. Lancet. 2002;360(9329):319–326. doi: 10.1016/S0140-6736(02)09556-9. [DOI] [PubMed] [Google Scholar]
- 4.Doshi A, Boudreaux ED, Wang N, Pelletier AJ, Camargo CA., Jr National study of US emergency department visits for attempted suicide and self-inflicted injury, 1997-2001. Ann Emerg Med. 2005;46(4):369–375. doi: 10.1016/j.annemergmed.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Suicide among adults aged 35-64 years: United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2013;62(17):321–325. [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips JA, Robin AV, Nugent CN, Idler EL. Understanding recent changes in suicide rates among the middle-aged: period or cohort effects? Public Health Rep. 2010;125(5):680–688. doi: 10.1177/003335491012500510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institute of Medicine (IOM) Reducing Suicide: A National Imperative. National Academic Press; Washington, DC: 2002. [Google Scholar]
- 8.Office of the Surgeon General and National Action Alliance for Suicide Prevention . 2012 National Strategy for Suicide Prevention: Goals and Objectives for Action: A Report of the U.S. Surgeon General and of the National Action Alliance for Suicide Prevention. US Dept of Health and Human Services; Washington, DC: 2012. [PubMed] [Google Scholar]
- 9.Mann JJ, Brent DA, Arango V. The neurobiology and genetics of suicide and attempted suicide: a focus on the serotonergic system. Neuropsychopharmacology. 2001;24(5):467–477. doi: 10.1016/S0893-133X(00)00228-1. [DOI] [PubMed] [Google Scholar]
- 10.Rowe JL, Bruce ML, Conwell Y. Correlates of suicide among home health care utilizers who died by suicide and community controls. Suicide Life Threat Behav. 2006;36(1):65–75. doi: 10.1521/suli.2006.36.1.65. [DOI] [PubMed] [Google Scholar]
- 11.Lewinsohn PM, Rohde P, Seeley JR. Adolescent suicidal ideation and attempts: prevalence, risk factors, and clinical implications. Clin Psychol. 1996;3(1):25–46. [Google Scholar]
- 12.Substance Abuse and Mental Health Services Administration, US Department of Health and Human Services Suicide warning signs. 2005 Sep; Accessed July 1, 2014. [Google Scholar]
- 13.Krakow B, Artar A, Warner TD, et al. Sleep disorder, depression, and suicidality in female sexual assault survivors. Crisis. 2000;21(4):163–170. doi: 10.1027//0227-5910.21.4.163. [DOI] [PubMed] [Google Scholar]
- 14.Ağargün MY, Kara H, Solmaz M. Sleep disturbances and suicidal behavior in patients with major depression. J Clin Psychiatry. 1997;58(6):249–251. doi: 10.4088/jcp.v58n0602. [DOI] [PubMed] [Google Scholar]
- 15.Ağargün MY, Kara H, Solmaz M. Subjective sleep quality and suicidality in patients with major depression. J Psychiatr Res. 1997;31(3):377–381. doi: 10.1016/s0022-3956(96)00037-4. [DOI] [PubMed] [Google Scholar]
- 16.Fawcett J, Scheftner WA, Fogg L, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry. 1990;147(9):1189–1194. doi: 10.1176/ajp.147.9.1189. [DOI] [PubMed] [Google Scholar]
- 17.Bernert RA, Joiner TE, Jr, Cukrowicz KC, Schmidt NB, Krakow B. Suicidality and sleep disturbances. Sleep. 2005;28(9):1135–1141. doi: 10.1093/sleep/28.9.1135. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein TR, Bridge JA, Brent DA. Sleep disturbance preceding completed suicide in adolescents. J Consult Clin Psychol. 2008;76(1):84–91. doi: 10.1037/0022-006X.76.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernert RA, Joiner TE. Sleep disturbances and suicide risk: a review of the literature. Neuropsychiatr Dis Treat. 2007;3(6):735–743. doi: 10.2147/ndt.s1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pigeon WR, Pinquart M, Conner K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012;73(9):e1160–e1167. doi: 10.4088/JCP.11r07586. doi:10.4088/JCP.11r07586. [DOI] [PubMed] [Google Scholar]
- 21.Nadorff MR, Nazem S, Fiske A. Insomnia symptoms, nightmares, and suicide risk: duration of sleep disturbance matters. Suicide Life Threat Behav. 2013;43(2):139–149. doi: 10.1111/sltb.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts RE, Roberts CR, Chen IG. Functioning of adolescents with symptoms of disturbed sleep. J Youth Adolesc. 2001;30(1):1–18. [Google Scholar]
- 23.Ribeiro JD, Pease JL, Gutierrez PM, et al. Sleep problems outperform depression and hopelessness as cross-sectional and longitudinal predictors of suicidal ideation and behavior in young adults in the military. J Affect Disord. 2012;136(3):743–750. doi: 10.1016/j.jad.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 24.Keshavan MS, Reynolds CF, Montrose D, Miewald J, Downs C, Sabo EM. Sleep and suicidality in psychotic patients. Acta Psychiatr Scand. 1994;89(2):122–125. doi: 10.1111/j.1600-0447.1994.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) Suicide: facts at a glance. 2012 http://www.cdc.gov/violenceprevention/pdf/suicide_datasheet_2012-a.pdf. Accessed July 1, 2014.
- 26.Conwell Y, Brent D. Suicide and aging, I: patterns of psychiatric diagnosis. Int Psychogeriatr. 1995;7(2):149–164. doi: 10.1017/s1041610295001943. [DOI] [PubMed] [Google Scholar]
- 27.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: a meta-analysis. Arch Gen Psychiatry. 1992;49(8):651–670. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 28.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 29.Kraemer HC, Kazdin AE, Offord DR, Kessler RC, Jensen PS, Kupfer DJ. Coming to terms with the terms of risk. Arch Gen Psychiatry. 1997;54(4):337–343. doi: 10.1001/archpsyc.1997.01830160065009. [DOI] [PubMed] [Google Scholar]
- 30.McCall WV. Sleep in the elderly: burden, diagnosis, and treatment. Prim Care Companion J Clin Psychiatry. 2004;6(1):9–20. doi: 10.4088/pcc.v06n0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turvey CL, Conwell Y, Jones MP, et al. Risk factors for late-life suicide: a prospective, community-based study. Am J Geriatr Psychiatry. 2002;10(4):398–406. [PubMed] [Google Scholar]
- 32.Cornoni-Huntley J, Ostfeld AM, Taylor JO, et al. Established populations for epidemiologic studies of the elderly: study design and methodology. Aging (Milano) 1993;5(1):27–37. doi: 10.1007/BF03324123. [DOI] [PubMed] [Google Scholar]
- 33.Breslow NE, Lubin JH, Marek P, Langholz B. Multiplicative models and cohort analysis. J Am Stat Assoc. 1983;78(381):1–12. [Google Scholar]
- 34.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 35.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 36.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 37.Pfeiffer E. A Short Portable Mental Status Questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 38.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10(1):20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 39.Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21(4):556–559. doi: 10.1093/geronj/21.4.556. [DOI] [PubMed] [Google Scholar]
- 40.Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc. 1976;54(4):439–467. [PubMed] [Google Scholar]
- 41.Hsieh FY. Sample size tables for logistic regression. Stat Med. 1989;8(7):795–802. doi: 10.1002/sim.4780080704. [DOI] [PubMed] [Google Scholar]
- 42.Tanskanen A, Tuomilehto J, Viinamäki H, Vartiainen E, Lehtonen J, Puska P. Nightmares as predictors of suicide. Sleep. 2001;24(7):844–847. [PubMed] [Google Scholar]
- 43.Fujino Y, Mizoue T, Tokui N, Yoshimura T. Prospective cohort study of stress, life satisfaction, self-rated health, insomnia, and suicide death in Japan. Suicide Life Threat Behav. 2005;35(2):227–237. doi: 10.1521/suli.35.2.227.62876. [DOI] [PubMed] [Google Scholar]
- 44.Conner KR, Duberstein PR, Conwell Y, Seidlitz L, Caine ED. Psychological vulnerability to completed suicide: a review of empirical studies. Suicide Life Threat Behav. 2001;31(4):367–385. doi: 10.1521/suli.31.4.367.22048. [DOI] [PubMed] [Google Scholar]
- 45.Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 46.Zohar D, Tzischinsky O, Epstein R, Lavie P. The effects of sleep loss on medical residents’ emotional reactions to work events: a cognitive-energy model. Sleep. 2005;28(1):47–54. doi: 10.1093/sleep/28.1.47. [DOI] [PubMed] [Google Scholar]
- 47.van der Helm E, Gujar N, Walker MP. Sleep deprivation impairs the accurate recognition of human emotions. Sleep. 2010;33(3):335–342. doi: 10.1093/sleep/33.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gujar N, McDonald SA, Nishida M, Walker MP. A role for REM sleep in recalibrating the sensitivity of the human brain to specific emotions. Cereb Cortex. 2011;21(1):115–123. doi: 10.1093/cercor/bhq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Helm E, Yao J, Dutt S, Rao V, Saletin JM, Walker MP. REM sleep depotentiates amygdala activity to previous emotional experiences. Curr Biol. 2011;21(23):2029–2032. doi: 10.1016/j.cub.2011.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dombrovski AY, Butters MA, Reynolds CF, III, et al. Cognitive performance in suicidal depressed elderly: preliminary report. Am J Geriatr Psychiatry. 2008;16(2):109–115. doi: 10.1097/JGP.0b013e3180f6338d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keilp JG, Gorlyn M, Russell M, et al. Neuropsychological function and suicidal behavior: attention control, memory and executive dysfunction in suicide attempt. Psychol Med. 2013;43(3):539–551. doi: 10.1017/S0033291712001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dour HJ, Cha CB, Nock MK. Evidence for an emotion-cognition interaction in the statistical prediction of suicide attempts. Behav Res Ther. 2011;49(4):294–298. doi: 10.1016/j.brat.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 53.Zlotnick C, Donaldson D, Spirito A, Pearlstein T. Affect regulation and suicide attempts in adolescent inpatients. J Am Acad Child Adolesc Psychiatry. 1997;36(6):793–798. doi: 10.1097/00004583-199706000-00016. [DOI] [PubMed] [Google Scholar]
- 54.Kohler CG, Turner TH, Gur RE, Gur RC. Recognition of facial emotions in neuropsychiatric disorders. CNS Spectr. 2004;9(4):267–274. doi: 10.1017/s1092852900009202. [DOI] [PubMed] [Google Scholar]
- 55.Juurlink DN, Herrmann N, Szalai JP, Kopp A, Redelmeier DA. Medical illness and the risk of suicide in the elderly. Arch Intern Med. 2004;164(11):1179–1184. doi: 10.1001/archinte.164.11.1179. [DOI] [PubMed] [Google Scholar]
- 56.WISQARS Web-Based Injury Statistics Query and Reporting System. 2010 http://www.cdc.gov/injury/wisqars/index.html. Accessed June 30, 2014.
- 57.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887–895. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krakow B, Hollifield M, Johnston L, et al. Imagery rehearsal therapy for chronic nightmares in sexual assault survivors with posttraumatic stress disorder: a randomized controlled trial. JAMA. 2001;286(5):537–545. doi: 10.1001/jama.286.5.537. [DOI] [PubMed] [Google Scholar]
- 60.Manber R, Bernert RA, Suh S, Nowakowski S, Siebern AT, Ong JC. CBT for insomnia in patients with high and low depressive symptom severity: adherence and clinical outcomes. J Clin Sleep Med. 2011;7(6):645–652. doi: 10.5664/jcsm.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.US Department of Veterans Affairs VA/DOD clinical practice guidelines: assessment and management of patients at risk for suicide. 2013 http://www.healthquality.va.gov/guidelines/MH/srb/. Accessed July 1, 2014.


